Abstract
Objective
Experimental studies suggest that adipose inflammation is etiologically linked to obesity-induced systemic disease. Our goal was to characterize the state of inflammation in human fat in relation to vascular function and metabolic parameters in obese individuals.
Methods and Results
We collected subcutaneous abdominal fat in 77 obese subjects (BMI ≥30 kg/m2) and quantified adipose macrophage population using targeted immunohistochemistry. Brachial artery vasodilator function was examined using high-resolution vascular ultrasound. In 50 subjects, an inflamed adipose phenotype characterized by tissue macrophage accumulation in crown-like structures was associated with systemic hyperinsulinemia and insulin resistance (HOMA-IR 5.5 ± 4.5 vs. 2.6 ± 1.9, p=0.002), and impaired endothelium-dependent, flow-mediated vasodilation (8.5 ± 4.4% vs. 10.8 ± 3.8%, p<0.05), as compared to subjects with quiescent non-inflamed adipose architecture (n=27). Macrophage retention in fat was linked to upregulated tissue CD68 and TNF-α mRNA expression in addition to increased plasma hs-CRP.
Conclusions
In a cohort of obese subjects, we demonstrate that proinflammatory changes in adipose tissue are associated with systemic arterial dysfunction and insulin resistance. These findings suggest that adipose inflammation may be linked to vascular injury and increased cardiovascular risk in obese subjects.
Keywords: obesity, endothelium, inflammation, insulin, vasculature
Obesity represents a disease state characterized by chronic subclinical inflammation linked to increased risk of type-2 diabetes and atherosclerosis.1,2 While the stimulus or source for persistent immune activation remains unclear, fat tissue is increasingly being recognized as an important hotbed of metabolic activity, and significant source of proatherogenic and proinflammatory adipocytokines that orchestrate metabolic and vascular dysfunction.3,4 Animal studies suggest that adipose tissue macrophage (ATM) activity is functionally intertwined with systemic disease mechanisms.5–7 The pathogenic link is supported by pharmacogenetic studies demonstrating that attenuation of ATM influx alters cytokine production and improves insulin sensitivity.8,9 From a clinical perspective, inflammatory changes in fat have not been commonly investigated in human disease nor examined in the context of functional cardiovascular abnormalities.
Inflammatory mechanisms are critical to all stages of cardiovascular disease progression and play a causal role in vascular endothelial dysfunction that represents a crucial early event in atherosclerosis and subsequent coronary heart disease (CHD) events.10,11 These mechanisms are, in part, supported by local and systemic release of inflammatory cytokines that mediate activation of neutrophils, monocytes, and T-cells, promote lipid-laden foam cell accumulation, weaken atherosclerotic plaque stability, and impair nitric oxide (NO)-mediated endothelium-dependent arterial vasodilation.12,13 While obesity is broadly linked with vascular diathesis, considerable heterogeneity in arterial function exists between individuals with excess fat mass that may be relevant to cardiovascular risk.14 The extent to which variability in inflammation of adipose stores relates to systemic vascular phenotype is unknown. The goal of the study was to examine the association between the state of inflammation in human fat and vascular and metabolic parameters in obese individuals.
Methods
Study Subjects
We enrolled consecutive obese men and women with a body mass index (BMI) ≥30 kg/m2 (range 32–78 kg/m2), age ≥18 years, receiving care at the Boston Medical Center Nutrition and Weight Management Center. This high-volume ambulatory center provides outpatient comprehensive dietary, medical, behavioral, or surgical treatments to promote lifestyle modification and weight loss. Patients with unstable medical conditions such as active coronary syndromes, congestive heart failure, systemic infection, malignancy, or pregnancy were excluded. All subjects gave written, informed consent and the study was approved by the Boston University Medical Center Institutional Review Board.
Vascular studies
Vascular ultrasound studies of brachial artery vasoreactivity were performed in a temperature-controlled room with subjects lying supine in a fasting state. Studies were performed during a weight-stable period prior to initiation of weight loss intervention, and vasoactive medications held 24 hours prior to ultrasound examination. Trained sonographers examined brachial artery vasomotor responses using a noninvasive, standardized method of ultrasound imaging as previously described, using a Toshiba Powervision 6000 system.15,16 Flow-mediated dilation (FMD) and nitroglycerin-mediated dilation (NMD) of the brachial artery were examined as measures of endothelium-dependent and - independent dilation, respectively. Brachial artery FMD responses were examined following a 5-minute cuff occlusion in an upper arm position above the antecubital crease. Pulsed-Doppler flow velocity signals at baseline and after cuff deflation quantified measures of reactive hyperemia. Sublingual nitroglycerin (0.4 mg) was omitted if the subject declined or had a history of migraines, blood pressure <100 mmHg, previous adverse reaction to nitrates, or used phosphodiesterase type-5 medications. An investigator blinded to clinical information performed all off-line analyses of digitized end-diastolic images.
Subcutaneous adipose tissue collection
In the same subjects, we collected subcutaneous adipose tissue (SAT) via percutaneous needle biopsy or during gastric bypass surgery. Abdominal subcutaneous fat biopsies were performed lateral to the umbilicus using standard sterile technique. Briefly, the region was carefully inspected for anatomical landmarks, draped, sterily prepped using alcohol and betadine, and locally anaesthetized with 2 ml 2% lidocaine. Through a small superficial 0.5 cm skin incision, adipose tissue was collected via a 3-hole cannula needle. Subcutaneous abdominal tissue was also directly harvested in a cohort of bariatric subjects during planned operative procedure. All tissue samples were stored in formalin or promptly frozen in liquid nitrogen to be stored at −80° C. Each subject provided a single biopsy specimen for analysis. Tissue samples were then used for immunohistochemical and functional analyses using established methods described in the manuscript.
Adipose tissue immunohistochemistry
Immunohistochemical stains were performed in the Department of Anatomic Pathology at Boston Medical Center. Infiltration of macrophage cell populations into adipose tissue was characterized using cell-specific stains targeted to CD68 (predilute antibodies from DakoCytomation Corporation, Carpinteria, California, USA). Briefly, 5 micron thick adipose tissue sections were fixed and loaded onto Biogenex I-6000 machine for incubation with primary antibodies. Multilink biotinylated secondary antibody was then allowed to react for 30 minutes at room temperature. Slides were then washed with PBS and placed in diaminobenzidine solution and microscopically examined for a positive reaction and counterstained with hematoxylin. All samples were evaluated in a blinded fashion by the pathologist (LJ) for the presence (+) or absence (−) of macrophage crown-like structures (CLS) in fat biopsy tissue as described in previous clinical studies.17,18 CLS status was assessed following examination of all fields available per slide at high-power field (HPF) magnification using light microscopy. Each subject-specific biopsy sample yielded a mean of 15 ± 7 HPFs for analysis per slide. Subjects were dichotomously categorized as being CLS+ if distinct adipose tissue macrophage clusters were present in any examined HPF, or CLS− if clusters were completely absent in all histological fields for a given subject. Additionally, total macrophage counts from all slide fields were quantified and indexed to number of fields examined for each subject.
Quantification of gene expression using real-time PCR
Subcutaneous tissue biopsy specimens from human subjects provide samples for ex vivo analysis of cytokine mRNA expression. Briefly, following collection, biopsy specimens were placed in ice-cold saline, and transported to the laboratory within 15 minutes. Samples were cleaned by removing visible blood vessels and clots, immersed in RNA preserving solution (RNAlater, Sigma-Aldrich, St. Louis, MO, USA.), and stored at −20°C. Total RNA was isolated from fat tissue via Ambion Inc (Austin, TX, USA) and cDNA synthesized by reverse transcription. SYBR-Green based real-time polymerase chain reaction (PCR) was used to analyze TNF-α and CD68 mRNA expression. Results were analyzed in reference to an endogenous house-keeping gene. PCR reaction was performed using an Applied Biosystems 7500 Real-time PCR system.
Anthropometric and Metabolic Measures
Concomitant with vascular and immunohistochemical studies, clinical characteristics including blood pressure, heart rate, height, weight, BMI, and waist circumference were recorded for each subject. Biochemical analyses including lipids, glucose, insulin, homeostasis model assessment of insulin resistance (HOMA-IR) were quantified from blood samples collected in a fasting state using standard methods provided by the Boston Medical Center clinical chemistry laboratories. Plasma adipocytokine levels were measured using immunoassay kits for TNF-α (Invitrogen, Carlshad, CA, USA); high-sensitivity C-reactive protein (CRP) (MP Biomedicals, Orangeburg, NY, USA); osteopontin (R&D Systems, Minneapolis, MN, USA); leptin (ALPCO Diagnostics, Salem, NH, USA); MCP-1 (RayBiotech, Norcross, GA, USA); and high-molecular weight (HMW) adiponectin (Millipore, St. Charles, MO, USA).
Statistical Analysis
Analyses were completed using SPSS for Windows, version 12.1 (SPSS Inc., Chicago, IL, USA). Data are presented as mean ± SD, unless otherwise indicated. Categorical group differences were examined using the Chi-square test or Fisher’s exact test as appropriate. Kolgomorov-Smirnov tests were used to determine whether continuous variables were normally distributed. Student’s t-tests and Mann U Whitney tests were utilized to examine relationships between adipose inflammatory status and continuous variables. Correlations between vascular parameters and clinical data were examined using Pearson’s or Spearman methods. For all analyses, P value <0.05 was considered statistically significant.
Results
Clinical and histological data
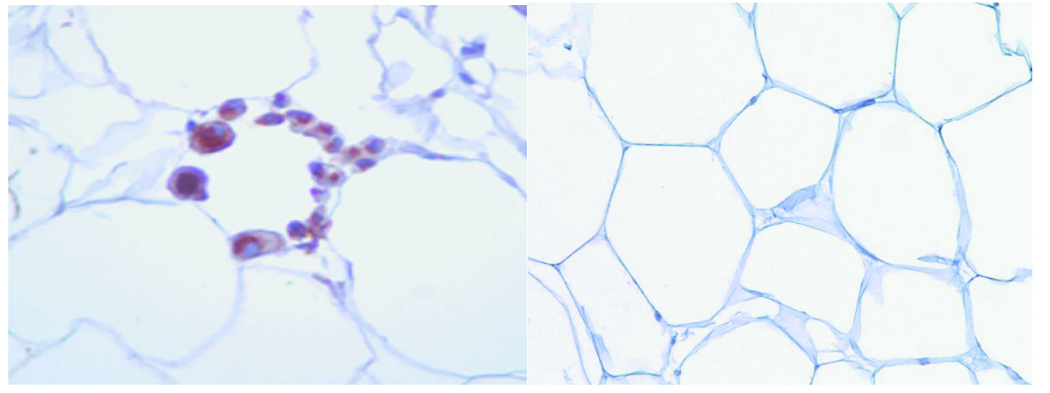
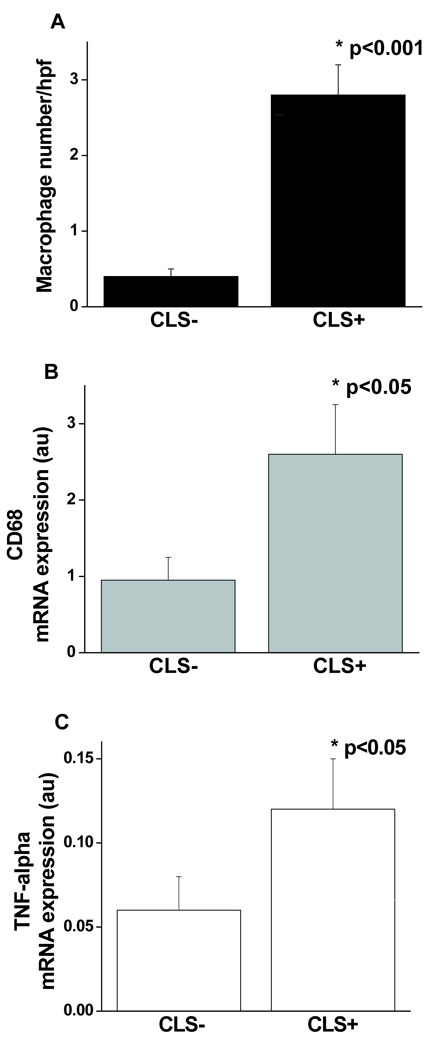
A total of 77 patients (mean age 44 ± 10 yr, 94% female) completed the study. All subjects were obese with average BMI 44 ± 8 kg/m2 (range 32–78 kg/m2) and waist circumference (WC) 110 ± 14 cm (68 –154 cm). A large number of individuals were severely obese with 71% of the population exhibiting at least class 3 obesity (BMI ≥ 40 kg/m2). We characterized subcutaneous adipose tissue (SAT) histology using targeted immunohistochemistry for CD68 as a macrophage marker and identified primarily two architectural phenotypes within adipose reserves. In 50 subjects, we observed the presence of aggregated macrophages assembled into ring patterns or crown-like structures (CLS+) buttressed around adipocytes as shown in Figure 1. This organized macrophage pattern has been previously described in human fat and represents a hallmark of localized chronic inflammation.17,19 In contrast, in 27 obese subjects inflammatory activity in SAT was quiescent with absence of any macrophage crowning (CLS−). Total adipose macrophage count/HPF was significantly higher in the CLS+ group (p<0.001) (Figure 2A), and correlated with HOMA-IR (r= 0.33, p=0.009), insulin (r= 0.24, p= 0.048), and plasma adiponectin (r= −0.37, p= 0.004).
Figure 1.
Left panel: Representative light microscopic histology of CLS+ human subcutaneous abdominal fat. Aggregates of CD68 immunoreactive macrophages (brown color) are organized in crown-like structures around individual adipocytes as a hallmark of localized chronic inflammation in adipose tissue. Right panel: CLS− adipose tissue with absence of macrophage rings.
Figure 2.
(A) Fat tissue macrophage density quantified by immunohistochemistry was significantly higher in CLS+ subjects (p<0.001) which was confirmed by (B) RT-PCR CD68 gene expression (p<0.05). (C) TNF-α mRNA expression in fat quantified by RT-PCR was significantly higher in CLS+ subjects (p=0.035). Data are presented as mean ± SE.
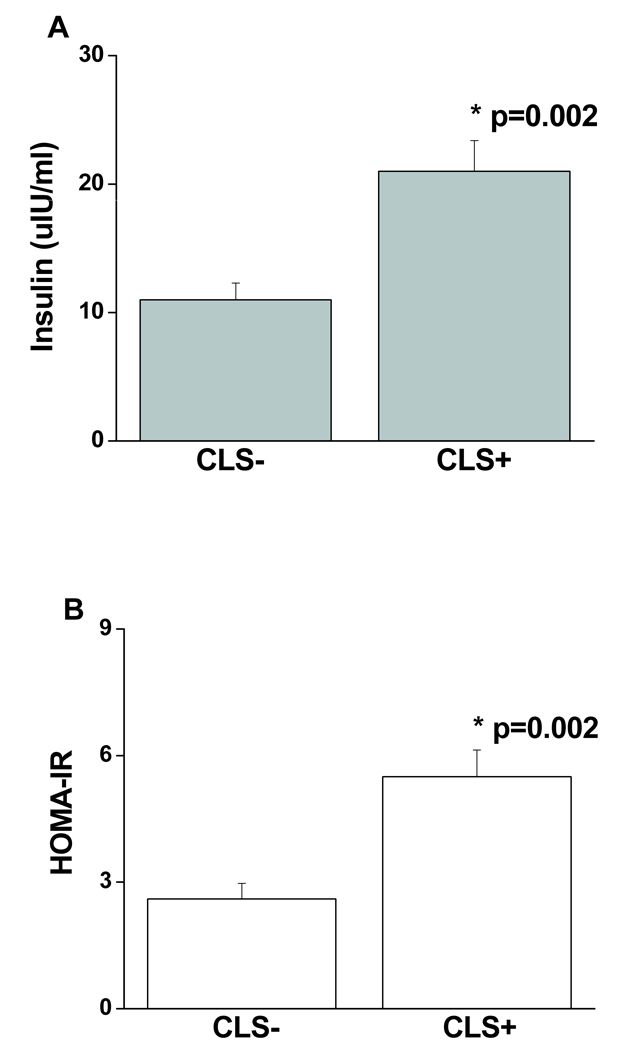
Clinical and metabolic parameters stratified by CLS status are displayed in Table 1. As shown, there were no significant differences in adiposity parameters or plasma lipids between groups. In these severely obese subjects, we observed a high clinical prevalence of metabolic syndrome in both the CLS+ (71%) and CLS− (67%) groups (p=0.66). In contrast, CLS+ subjects exhibited significantly higher insulin levels (21 ± 17 vs. 11 ± 7 uIU/ml, p=0.002) and insulin resistance assessed by HOMA-IR (5.5 ± 4.5 vs. 2.6 ± 1.9, p=0.002) as compared to the CLS− group (Figure 3). There were no group differences in use of statin medications, non-steroidal anti-inflammatory drugs, ACE-inhibitor or angiotensin receptor blocker use, hypoglycemic agents, or post-menopausal status (Table 1).
Table 1.
Clinical and metabolic parameters stratified by CLS status.
| CLS− (n=27) |
CLS+ (n=50) |
P | |
|---|---|---|---|
| Age (years) | 44 ± 9 | 43 ± 11 | 0.68 |
| BMI (kg/m2) | 45 ± 7 | 44 ± 9 | 0.32 |
| WC (cm) | 108 ± 14 | 110 ± 14 | 0.55 |
| LDL-C (mg/dl) | 125 ± 30 | 124 ± 33 | 0.42 |
| HDL-C (mg/dl) | 50 ± 8 | 47 ± 12 | 0.06 |
| TG (mg/dl) | 113 ± 53 | 139 ± 76 | 0.13 |
| Glucose (mg/dl) | 96 ± 17 | 104 ± 29 | 0.17 |
| Systolic BP (mmHg) | 129 ± 14 | 129 ± 11 | 0.92 |
| Female (%) | 92% | 96% | 0.65 |
| Diabetes mellitus (%) | 29 | 28 | 0.88 |
| TZD (%) | 4 | 8 | 0.65 |
| Metformin (%) | 11 | 14 | 0.92 |
| ACEI/ARB (%) | 26 | 28 | 0.85 |
| Statin (%) | 15 | 16 | 1.0 |
| NSAID (%) | 4 | 16 | 0.15 |
| Post-menopausal (%) | 19 | 20 | 0.88 |
ACEI = Angiotensin converting enzyme inhibitor
ARB = Angiotensin receptor blocker
TZD = Thiazolidenedione
NSAID = Non-steroidal anti-inflammatory drug
Figure 3.
Metabolic parameters stratified by fat tissue CLS status. (A) CLS+ subjects exhibited significantly higher plasma insulin concentrations (p=0.002), and (B) insulin resistance assessed by HOMA-IR (p=0.002) as compared to the CLS− group. Data are presented as mean ± SE.
Vascular function data
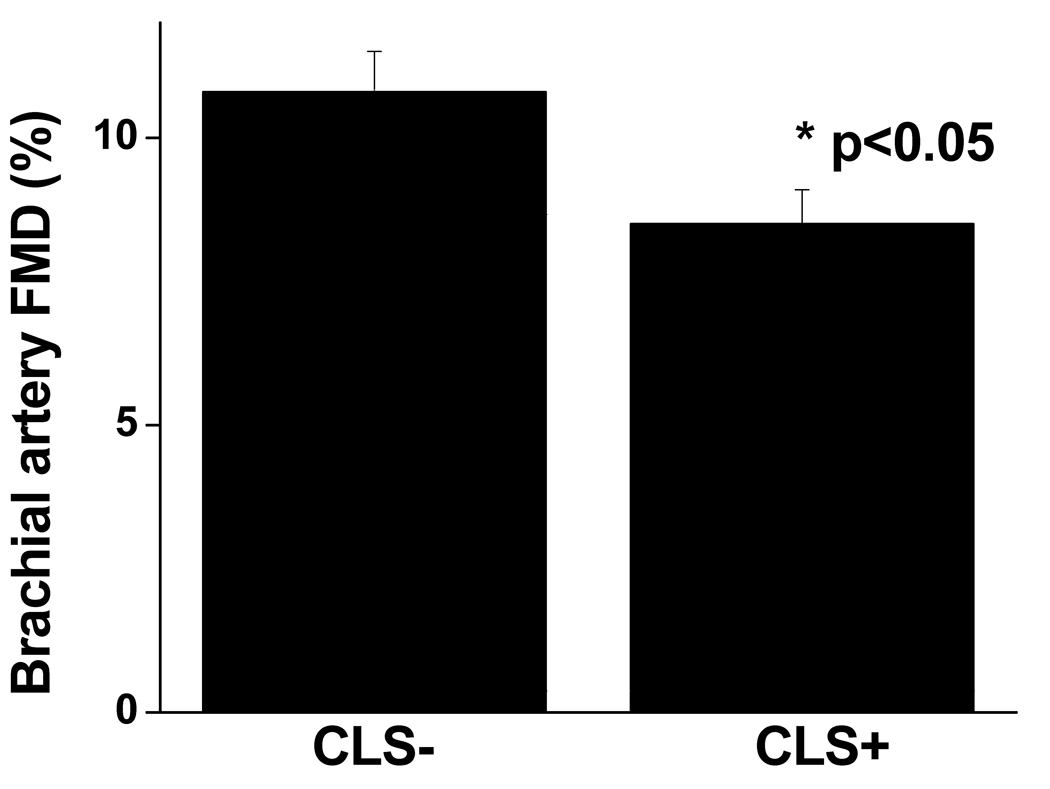
For the entire population, mean brachial artery flow-mediated dilation (FMD) was 9.3 ± 4.3% and nitroglycerin-mediated dilation was 12.2 ± 4.8%. As shown in Figure 4, brachial artery FMD was significantly impaired in the CLS+ vs. CLS− group (8.5 ± 4.4% vs. 10.8 ± 3.8%, respectively, p<0.05). Additional brachial artery parameters are displayed in Table 2. Nitroglycerin-mediated, endothelium-independent vasodilation which represents vascular smooth muscle responsiveness to nitric oxide was similar in both groups. Similarly, there were no group differences in measures of hyperemic forearm blood flow. Thus the findings suggest that the observed vascular impairment associated with an inflamed fat phenotype was linked to abnormalities in conduit artery endothelial function. Correlations between FMD and HOMA-IR were (−0.14, p=0.28) and insulin (−0.13, p=0.29).
Figure 4.
Brachial artery flow-mediated (FMD) dilation stratified by adipose CLS status. Subjects with an inflamed fat phenotype (CLS+) had significantly worse endothelium-dependent brachial artery vasodilation (p=0.02) as compared to the CLS− group. Data are presented as mean ± SE.
Table 2.
Vascular parameters stratified by CLS status.
| CLS− (n=27) |
CLS+ (n=50) |
P | |
|---|---|---|---|
| Nitroglycerin-mediated dilation (%) | 11.4 ± 3.7 | 12.6 ± 5.4 | 0.57 |
| Hyperemic flow (ml/min) | 1168 ± 376 | 1127 ± 437 | 0.89 |
| Hyperemic flow increase (%) | 668 ± 323 | 611 ± 313 | 0.46 |
Adipose gene expression and plasma cytokines
We investigated whether histologic findings of inflammation in SAT are associated with functional upregulation of tissue cytokine expression and measures of systemic inflammation. As shown in Figure 2B and 2C, adipose CD68 and TNF-α mRNA expression were significantly greater in CLS+ fat tissue. While BMI correlated strongly with plasma leptin (r=0.47, p<0.001) and MCP-1 (r=0.34, p=0.005) in this cohort of subjects, there were no significant differences between the CLS+ and CLS− groups in plasma TNF-α (2.5 ±1.5 vs. 1.5 ± 1 pg/ml, p=0.17); osteopontin (23.5 ± 21 vs.19.8 ± 20.8 ng/ml, p=0.46); leptin (11.3 ± 7.3 vs. 11 ± 5.9 ng/ml, p=0.93); or MCP-1 (53 ± 21 vs. 57 ± 21 pg/ml, p=0.53). In contrast, plasma hs-CRP was significantly higher in subjects with inflamed fat phenotype (7.2 ± 3.9 vs. 4.8 ± 3.4 mg/L, p=0.02) along with a strong trend for lower HMW adiponectin in this group (12.8 ± 9.8 vs.17.8 ± 11.7 ng/ml, p=0.07). Adiponectin negatively correlated with insulin (r = − 0.39, p=0.002) and HOMA (r= −0.38, p=0.003).
Discussion
In the present study, we demonstrated that histologic findings of inflammation in human fat characterized by macrophage infiltration in distinct crown-like patterns was associated with upregulated tissue cytokine gene expression, systemic inflammation, insulin resistance, and vascular endothelial dysfunction. Our data demonstrate that human fat depots exhibit variable degrees of inflammation and suggest that adipose immune activity may be linked to a pathogenic phenotype associated with metabolic and vascular dysfunction. To our knowledge, our findings are among the first clinical studies linking adipose inflammation to systemic arterial dysfunction that may identify obese individuals at increased cardiovascular risk.
Obesity is rapidly emerging as a major public health issue worldwide. Excess fat is associated with several risk factors for coronary heart disease including hypertension, dyslipidemia, and impaired glucose tolerance.20,21 These risk factors share a common pathophysiology linked to chronic inflammation that plays a critical role in the pathogenesis of atherosclerosis. The biological connection is supported by numerous clinical studies demonstrating that inflammatory biomarkers consistently identify patients at increased cardiovascular risk.10,22 A key component of the atherogenic process involves activation of the vascular endothelium in response to circulating factors including oxidized-LDL, free fatty acids, interleukins, TNF-α, and CRP that stimulate expression of adhesion molecules, toll-like receptors, and monocyte chemoattractant protein-1 (MCP-1) promoting atherosclerotic changes in the vascular wall and loss of NO bioaction.11–13 A proatherogenic milieu supported by endothelial dysfunction is causally linked to risk of myocardial infarction and stroke.23–25 In this regard, adipose phenotype and associated adipocytokine overexpression amplified by tissue macrophage buildup may lead to a heightened systemic state of inflammation and endothelial activation. Our findings of a specific impairment in arterial function with altered adipose metabolism builds conceptual basis for a pathogenic adipose-vascular axis, and prompts speculation that functional changes originating in extravascular fat stores may be linked to CHD risk.
The association between insulin resistance and adipose inflammation represents validation of prior data from rodent models and adds to limited information available in humans. Macrophage-driven inflammation of fat precedes and temporally correlates with development of insulin resistance in mouse models of dietary or genetic obesity.5 Direct local effects of TNF-α on adipocyte biology provides a link to mechanisms of insulin resistance in these models, although as in our study, a firm association between insulin sensitivity and plasma TNF-α levels has been variable.3,26,27 Additional cytokines including IL-6, iNOS, MCP-1, ICAM-1 activated by NF-κB and JNK-dependent pathways and sustained cross-talk between adipocytes, macrophages, and vascular endothelial cells likely amplify a localized process to induce a broader systemic state of inflammation associated with metabolic and vascular dysfunction. These basic mechanisms are in line with clinical studies demonstrating impaired endothelium-dependent dilation with insulin resistance syndromes and conversion of the endothelium to a proinflammatory phenotype.28 In the present study however, correlation of arterial responses with insulin resistance was modest in this uniformly severely obese population, an observation that has been consistent across other studies that examined this issue in the obese.29,30 This can be partly explained by the high prevalence of abnormal HOMA-IR in these subjects thus limiting its discriminatory power. Other cytokine products and adipose-derived modulators of vascular biology may play a more significant role in governing vascular phenotype in extreme obesity.
While clinical studies have described macrophage crown-like structures in fat, we have specifically associated this phenotype with multi-level physiological dysfunction.17–19,31 While isolated ATMs in addition to ring aggregates were present through adipose tissue, we did not identify dense infiltration patterns encompassing up to half of fat population as described in leptin-deficient or diet-induced obese mice.6 As scattered immune cells are ubiquitously present throughout various organs and carry out tissue-specific functions, it is plausible that ring-like accumulation patterns represent more advanced stages of an inflammatory response and thus more closely relate to systemic activation, as supported by increased plasma CRP in our study. The stimulus for macrophage influx into fat is largely unknown and likely involves a complex pathophysiology that includes toxic adipocyte hypertrophy and dysfunction; tissue hypoxia; oxidative stress; and cytokine cross-talk with increased local expression of TNF-α, C-C motif chemokine receptor-2 (CCR2), and MCP-1.8,17 Recent data suggest that macrophage crowns localize selectively to sites of adipocyte necrosis suggesting that adipocyte death may modulate tissue inflammation.17,32 ATM accumulation in different fat reserves has been associated with metabolic dysfunction,9,33 and the literature is contentious vis-à-vis depot-specific links to cardiometabolic risk. While metabolic abnormalities are primarily ascribed to visceral fat, recent data from the Framingham Heart Study demonstrate that both subcutaneous and visceral loads relate to atherogenic biomarkers and each depot may individually play a role in total adiposity burden.34,35
In the present study we focused primarily on macrophage markers though chemokines involved in T cell recruitment are also linked to components of the metabolic syndrome, suggesting that the inflammatory response involves a complex interplay of various immune cell types.36 A seminal finding in our study was the striking inter-individual difference in adipose histology and gene expression, independent of BMI, prompting speculation that for any given degree of adiposity, a higher state of immune activity in fat is linked to a more proatherogenic profile. In this context, the extent to which characterization of inflammatory burden may add to the global risk assessment of obese patients deserves further investigation. We find it thus plausible that intrinsic genetic or physiological alterations that render individuals more susceptible to inflammatory activation in association with weight gain also place them at increased cardiometabolic risk.
Our present study has several limitations. We examined fat histology exclusively in obese subjects since securing tissue was technically safer and more feasible in this clinical study. Comparing present findings to a lean group may be the focus of future investigation. In addition, we relied on a single biopsy site in order to minimize subject discomfort, and it remains plausible that multi-site sampling could have influenced subject categorization. However, our findings are in line with prior clinical studies demonstrating that individuals with lesser degrees of metabolic dysfunction appear to have lower inflammatory burden.37 Waist-to-hip ratio assessment was not available owing to lack of hip measurements, although waist circumference was similar in both groups. We used HOMA to assess in vivo insulin sensitivity but acknowledge that it only represents an approximation of systemic insulin resistance.38 Lastly, we found no group differences in plasma adipocytokines other than hs-CRP. Our present study may not be powered to detect such differences or plasma concentrations may not adequately reflect local tissue biology.39
In summary, in a cohort of obese subjects we have demonstrated that proinflammatory changes in subcutaneous adipose tissue characterized by macrophage infiltration and upregulated cytokine expression are associated with systemic endothelial dysfunction and insulin resistance. These findings suggest that inflammation in adipose tissue may be linked to systemic arterial injury and increased cardiovascular risk in obese subjects.
Acknowledgements
Sources of Funding
This work was supported by National Institutes of Health grants to Dr. Gokce (R01 HL074097 and HL084213).
Footnotes
Disclosures
None
References
- 1.Olshansky SJ, Passaro DJ, Hershow RC, Layden J, Carnes BA, Brody J, Hayflick L, Butler RN, Allison DB, Ludwig DS. A potential decline in life expectancy in the United States in the 21st century. N Engl J Med. 2005;352:1138–1145. doi: 10.1056/NEJMsr043743. [DOI] [PubMed] [Google Scholar]
- 2.Baker JL, Olsen LW, Sorensen TA. Childhood body-mass index and the risk of coronary heart disease in adulthood. N Engl J Med. 2007;357:2329–2337. doi: 10.1056/NEJMoa072515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu HY, Barnes GT, Yang Q, Tan Q, Yang DS, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wellen KE, Hotamisligil GS. Obesity-induced inflammatory changes in adipose tissue. J Clin Invest. 2003;112:1785–1788. doi: 10.1172/JCI20514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2006;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Gregorio GB, Yao-Borengasser A, Rasouli N, Varma V, Lu T, Miles LM, Ranganathan G, Peterson CA, McGehee RE, Kern PA. Expression of CD68 and macrophage chemoattractant protein-1 genes in human adipose and muscle tissues - Association with cytokine expression, insulin resistance, and reduction by pioglitazone. Diabetes. 2005;54:2305–2313. doi: 10.2337/diabetes.54.8.2305. [DOI] [PubMed] [Google Scholar]
- 10.Libby P, Ridker PM. Inflammation and atherothrombosis - From population biology and bench research to clinical practice. J Am Coll Cardiol. 2006;48:A33–A46. [Google Scholar]
- 11.Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction - Testing and clinical relevance. Circulation. 2007;115:1285–1295. doi: 10.1161/CIRCULATIONAHA.106.652859. [DOI] [PubMed] [Google Scholar]
- 12.Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. [DOI] [PubMed] [Google Scholar]
- 13.Huang AL, Vita JA. Effects of systemic inflammation on endothelium-dependent vasodilation. Trends Cardiovasc Med. 2006;16:15–20. doi: 10.1016/j.tcm.2005.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benjamin EJ, Larson MG, Keyes MJ, Mitchell GF, Vasan RS, Keaney JF, Lehman BT, Fan SX, Osypiuk E, Vita JA. Clinical correlates and heritability of flow-mediated dilation in the community - The Framingham Heart Study. Circulation. 2004;109:613–619. doi: 10.1161/01.CIR.0000112565.60887.1E. [DOI] [PubMed] [Google Scholar]
- 15.Gokce N, Keaney JF, Jr, Frei B, Holbrook M, Olesiak M, Leeuwenburgh C, Heinecke JW, Vita JA. Long-term ascorbic acid administration reverses endothelial vasomotor dysfunction in patients with coronary artery disease. Circulation. 1999;99:3234–3240. doi: 10.1161/01.cir.99.25.3234. [DOI] [PubMed] [Google Scholar]
- 16.Vita JA. Nitric oxide-dependent vasodilation in human subjects. Methods Enzymol. 2002;359:186–200. doi: 10.1016/s0076-6879(02)59183-7. [DOI] [PubMed] [Google Scholar]
- 17.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang SP, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 19.Kolak M, Westerbacka J, Velagapudi VR, Wagsater D, Yetukuri L, Makkonen J, Rissanen A, Hakkinen AM, Lindell M, Bergholm R, Hamsten A, Eriksson P, Fisher RM, Oresic M, Yki-Jarvinen H. Adipose tissue inflammation and increased ceramide content characterize subjects with high liver fat content independent of obesity. Diabetes. 2007;56:1960–1968. doi: 10.2337/db07-0111. [DOI] [PubMed] [Google Scholar]
- 20.McTigue K, Larson JC, Valoski A, Burke G, Kotchen J, Lewis CE, Stefanick ML, Van Horn L, Kuller L. Mortality and cardiac and vascular outcomes in extremely obese women. JAMA. 2006;296:79–86. doi: 10.1001/jama.296.1.79. [DOI] [PubMed] [Google Scholar]
- 21.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291:2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 22.Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, Hsia J, Gersh BJ, Rifai N, Ridker PM, Pfeffer MA, Braunwald E. Prognostic significance of the Centers for Disease Control/American Heart Association high-sensitivity C-reactive protein cut points for cardiovascular and other outcomes in patients with stable coronary artery disease. Circulation. 2007;115:1528–1536. doi: 10.1161/CIRCULATIONAHA.106.649939. [DOI] [PubMed] [Google Scholar]
- 23.Gokce N, Keaney JF, Jr, Menzoian JO, Watkins M, Hunter L, Duffy SJ, Vita JA. Risk stratification for postoperative cardiovascular events via noninvasive assessment of endothelial function. Circulation. 2002;105:1567–1572. doi: 10.1161/01.cir.0000012543.55874.47. [DOI] [PubMed] [Google Scholar]
- 24.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA. Prognostic value of coronary vascular endothelial dysfunction. Circulation. 2002;106:653–658. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 25.Targonski PV, Bonetti PO, Pumper GM, Higano ST, Holmes DR, Jr, Lerman A. Coronary endothelial dysfunction is associated with an increased risk of cerebrovascular events. Circulation. 2003;107:2805–2809. doi: 10.1161/01.CIR.0000072765.93106.EE. [DOI] [PubMed] [Google Scholar]
- 26.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose Expression of Tumor-Necrosis-Factor-Alpha - Direct Role in Obesity-Linked Insulin Resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased Adipose-Tissue Expression of Tumor-Necrosis-Factor-Alpha in Human Obesity and Insulin-Resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction: implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams IL, Chowienczyk PJ, Wheatcroft SB, Patel A, Sherwood R, Momin A, Shah AM, Kearney MT. Effect of fat distribution on endothelial-dependent and endothelial-independent vasodilatation in healthy humans. Diabetes Obes Metab. 2006;8:296–301. doi: 10.1111/j.1463-1326.2005.00505.x. [DOI] [PubMed] [Google Scholar]
- 30.Arcaro G, Zamboni M, Rossi L, Turcato E, Covi G, Armellini F, Bosello O, Lechi A. Body fat distribution predicts the degree of endothelial dysfunction in uncomplicated obesity. Int J Obes Relat Metab Disord. 1999;23:936–942. doi: 10.1038/sj.ijo.0801022. [DOI] [PubMed] [Google Scholar]
- 31.Cancello R, Tordjman J, Poitou C, Guilhem G, Bouillot JL, Hugol D, Coussieu C, Basdevant A, Bar Hen A, Bedossa P, Guerre-Millo M, Clement K. Increased infiltration of macrophages in omental adipose tissue is associated with marked hepatic lesions in morbid human obesity. Diabetes. 2006;55:1554–1561. doi: 10.2337/db06-0133. [DOI] [PubMed] [Google Scholar]
- 32.Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 33.Harman-Boehm I, Bluher M, Redel H, Sion-Vardy N, Ovadia S, Avinoach E, Shai I, Kloting N, Stumvoll M, Bashan N, Rudich A. Macrophage infiltration into omental versus subcutaneous fat across different populations: Effect of regional adiposity and the comorbidities of obesity. J Clin Endocrinol Metab. 2007;92:2240–2247. doi: 10.1210/jc.2006-1811. [DOI] [PubMed] [Google Scholar]
- 34.Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, Vasan RS, Murabito JM, Meigs JB, Cupples LA, D'Agostino RB, O'Donnell CJ. Abdominal visceral and subcutaneous adipose tissue compartments - Association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116:39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- 35.Pou KM, Massaro JM, Hoffmann U, Vasan RS, Maurovich-Horvat P, Larson MG, Keaney JF, Meigs JB, Lipinska I, Kathiresan S, Murabito JM, O'Donnell CJ, Benjamin EJ, Fox CS. Visceral and subcutaneous adipose tissue volumes are cross-sectionally related to markers of inflammation and oxidative stress - The Framingham Heart Study. Circulation. 2007;116:1234–1241. doi: 10.1161/CIRCULATIONAHA.107.710509. [DOI] [PubMed] [Google Scholar]
- 36.Matter CM, Handschin C. RANTES (regulated on activation, normal T cell expressed and secreted), inflammation, obesity, and the metabolic syndrome. Circulation. 2007;115:946–948. doi: 10.1161/CIRCULATIONAHA.106.685230. [DOI] [PubMed] [Google Scholar]
- 37.Karelis AD, Faraj M, Bastard JP, St Pierre DH, Brochu M, Prud'Homme D, Rabasa-Lhoret R. The metabolically healthy but obese individual presents a favorable inflammation profile. J Clin Endocrinol Metab. 2005;90:4145–4150. doi: 10.1210/jc.2005-0482. [DOI] [PubMed] [Google Scholar]
- 38.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol-Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 39.Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T, Aszmann O, Ludvik B, Silberhumer GR, Prager G, Stulnig TM. Osteopontin expression in human and murine obesity: Extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology. 2008;149:1350–1357. doi: 10.1210/en.2007-1312. [DOI] [PubMed] [Google Scholar]