Abstract
Trypsin-catalyzed stable isotope 16O/18O-labeling of the C-terminal carboxyl groups of peptides is increasingly used in shotgun proteomics for relative peptide/protein quantitation. However, precise quantitative measurements are often complicated by residual trypsin that can catalyze the back-exchange of 18O with 16O after labeling. Here, we demonstrate through a detailed evaluation that boiling the peptide sample for 10 minutes provides a simple means for completely quenching residual trypsin activity and preventing oxygen back-exchange in 18O-labeled samples. We also observed that the presence of organic solvents such as acetonitrile made quenching trypsin activity less efficient. Finally, current 18O-labeling methods that typically employ immobilized trypsin result in significant sample losses due to non-specific binding of peptides on the resin, making their application toward smaller biological samples increasingly impractical. We present here an improved 18O-labeling protocol that is more applicable to microscale biological samples by using solution-phase trypsin instead of immobilized trypsin to overcome the non-specific sample loss issue encountered with the use of immobilized trypsin. The ability to generate stably 18O-labeled samples without back-exchange should enable more effective applications of 18O-labeled toward large-scale biomarker discovery and validations where an 18O-labeled sample can be used as a common reference for quantitation.
Keywords: Mass spectrometry, LC-MS, proteomics, sample preparation, 18O-labeling, back-exchange, boiling
Introduction
Biological studies that investigate the differential regulation of proteins with mass spectrometry (MS) have utilized various isotopic labeling methods for quantitative comparisons1. Trypsin-catalyzed stable isotope 18O-labeling at C-terminal carboxyl groups of tryptic peptides has been increasingly applied for quantitative proteomics studies in various biological systems2-7. 18O-labeling is simple, cost-effective, and flexible in its ability to specifically label all tryptic peptides originating from any type of sample; this labeling approach does have several limitations that have prevented its broad applications in biological studies. These limitations include potentially incomplete labeling, back-exchange from 18O to 16O, and sample losses during labeling. The issue of varying peptide labeling efficiencies has been largely addressed by performing a trypsin-catalyzed labeling after digestion rather than during digestion3, 5, 8, where nearly all peptides can reach oxygen exchange equilibrium with optimized incubation time and enzyme concentration.
Perhaps the most significant limitation is the diminished quantitative accuracy due to oxygen “back-exchange” where 18O is replaced with 16O after labeling through a residual trypsin-catalyzed reaction when the sample is placed into a buffer containing 16O water. If trypsin with residual activity is present, 18O-labeled samples that have been combined with their 16O-labeled counterparts can undergo significant back-exchange, making it problematic to accurately quantify the relative abundance differences between the 18O- and 16O-labeled samples. Therefore, trypsin must either be removed or inactivated after labeling so that the quantitative accuracy can be maintained.
Traditional protocols that do not fully inactivate or remove trypsin depend upon sample analysis being performed immediately after labeling to decrease the extent of back-exchange that occurs8, 9. This requirement is inconvenient for many situations (e.g., automated, high-throughput LC-MS analysis) and makes the application of the 18O-labeling for large-scale study sets impractical. Recent attempts have been made to minimize this back-exchange by inactivating trypsin by cysteine alkylation8 and by using immobilized (IM) trypsin rather than solution-phase (SP) trypsin for digestion and/or labeling because it can be easily and rapidly separated from the sample by centrifugation or filtering10. However, these modifications can lead to significant sample losses, which can be detrimental for very small samples of microgram amounts that are commonly found in clinical studies. For example, alkylating tyrpsin requires an SPE clean-up that results in significant sample losses11. More importantly, Sevinsky et al10 observed that the alkylation method still resulted in near-complete back-exchange. Nonspecific loss also occurs when removing IM trypsin from the sample by centrifugation or employing a molecular weight cutoff filter. Since IM trypsin is added to the sample in a volume-dependent manner instead of in an enzyme-to-substrate ratio like SP trypsin, the use of immobilized trypsin during digestion requires a relatively large amount of IM trypsin because samples are generally diluted prior to digestion to ensure that reagents used for denaturing, reducing, and alkylating do not interfere with trypsin activity12. Therefore, SP trypsin is more often used for digestion while IM trypsin is more commonly employed for post-digestion labeling where the sample volume is minimal, lowering sample loss and cost3.
We have previously reported that 18O back-exchange can be prevented by boiling the sample for 10 minutes to quench residual trypsin activity prior to labeling using immobilized trypsin3, 13. More recently, similar heat-inactivation methods were also reported14. However, the effectiveness of this procedure for quenching trypsin activity and preventing back-exchange has not been assessed in detail. Effective prevention of back-exchange is especially important for large-scale quantitative proteomics, where 18O-labeling is ideally suited for generating a labeled “universal” reference sample and large-scale quantitative discovery proteomics. As we recently reported, this can be achieved by comparing each sample to the labeled reference without the need to label individual biological samples15. In this work, we report a detailed assessment of the effect of boiling on 18O-labeling stability. Our data demonstrate that trypsin activity can be quenched by the boiling procedure and the 18O-labeled sample is stable without any observable back-exchange following a week at room temperature. We also describe an improved protocol for minimizing sample loss while simultaneously preventing back-exchange.
Experimental
Materials and Reagents
All chemicals, materials, and reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise specified. Solution-phase porcine trypsin was obtained from Promega (Madison, WI), while the immobilized trypsin was provided by Applied Biosystems (Foster City, CA).
Digestion of Bovine Serum Albumin
Lyophilized bovine serum albumin (BSA) was suspended in 25 mM NH4HCO3 buffer, pH 7.8, and its concentration determined using a BCA assay (Pierce Biotechnology, Inc., Rockford, IL). The sample was denatured and reduced with 8 M urea and 10 mM DTT at 37 °C for one hour followed by alkylation with 40 mM of iodoacetamide at 37 °C for one hour in the dark. The sample was then diluted 6-fold with 50 mM NH4HCO3 buffer, pH 7.8, and digested with 1:50 porcine trypsin:sample (w/w) for 3 hours at 37°C. The digested sample was then cleaned with 1 mL C18 columns with 50 mg bed weight on a vacuum manifold. The columns were conditioned with 3 mL 100% methanol and 2 mL 0.1% trifluoroacetic acid (TFA). Following sample addition, the columns were washed with 4 mL of 95:5 H2O:acetonitrile, 0.1% TFA and the sample eluted with 1 mL of 80:20 acetonitrile:H2O, 0.1% TFA. The sample was then concentrated, but not to complete dryness, in a SpeedVac (Thermo Fisher Scientific, Rockford, IL) to remove the acetonitrile and TFA prior to boiling.
18O-Labeling Using Immobilized Trypsin
150 μg peptide aliquots were either not boiled or boiled in a water bath for 10 minutes. Immediately following boiling, samples were snap-frozen in liquid nitrogen, placed on ice for 5 minutes, or left at room temperature to cool. All samples were dried to completion in a vacuum concentrator. The peptide samples were redissolved in 20 μL 100% acetonitrile, followed by the addition of 100 μL of 50 mM NH4HCO3 buffer in H218O, pH 7.8. Then, 1 μL of 1 M CaCl2 and 5 μL of immobilized trypsin were added. The samples were briefly vortexed to mix. The tubes were wrapped in parafilm and placed in a thermomixer for 5 hours at 30 °C, shaking at 1350 rpm. The reaction was stopped with 5 μL of formic acid and vortexed briefly. The immobilized trypsin was removed from the sample with centrifugation at 16 k g for 2 minutes. The immobilized trypsin pellet was then washed twice with freshly made 50 μL 60% methanol, 1% formic acid. The supernatant from all washing steps were collected and spun again to ensure that there was no remaining immobilized trypsin. Samples were concentrated in a vacuum concentrator and their amount determined with a BCA assay. Samples were diluted to 0.15 μg/μL with 25 mM NH4HCO3 buffer in H216O, pH 7.8, and stored at -80°C until MS analysis.
18O-Labeling Using Solution-Phase Trypsin
The dried 150 μg peptide samples were redissolved in 20 μL 100% acetonitrile and sonicated for 10 seconds. 100 μL of 50 mM NH4HCO3 buffer in H218O, 1 μL of 1 M CaCl2, and solution-phase trypsin dissolved in H218O at a 1:200, 1:100, 1:50, or 1:25 trypsin:peptide ratio (w/w) were added to the samples and vortexed to mix. The tubes were wrapped in parafilm and placed in a thermomixer for 5 hours at 37 °C, shaking at 450 rpm. The reaction was stopped by boiling the sample in a 100 °C water bath for 10 minutes, followed by immediate snap-freezing in liquid N2. Once the samples were thawed, 5 μL formic acid was added. Sample concentration was determined with a BCA assay.
This experiment was repeated, but acetonitrile was not added to the samples. Instead, samples were sonicated for 10 seconds after the addition of 100 μL 50 mM NH4HCO3 buffer in H218O, pH 7.8. All of the other steps were the same.
LC-MS Analysis
All labeled samples were analyzed by an in-house automated 4-column reverse phase high pressure liquid chromatography (HPLC) system16 and subjected to a 100 minute gradient using mobile phases consisting of 0.1% formic acid in water (A) and 0.1% formic acid in acetonitrile (B). After 20 minutes of 100% mobile phase A, mobile phase B was increased exponentially over the remaining 80 minutes to a final concentration of 70%. The columns were 360 μm outer diameter, 75 μm inner diameter, and 70 cm long fused silica capillaries (Polymicro Technologies, Phoenix, AZ) slurry-packed with 3 μm C18 Jupiter bonded particles with 300 Ǻ pores (Phenomenex, Torrence, CA). 2.4 kV was applied to fused silica capillary tips made in-house (360 μm outer diameter and 50μm inner diameter) to produce electrospray ionization. The samples were introduced into an LTQ-Orbitrap XL (Thermo Fisher Scientific, Rockford, IL) with an electrospray ionization interface manufactured in-house and analyzed as described previously17.
Results & Discussion
18O back-exchange after labeling
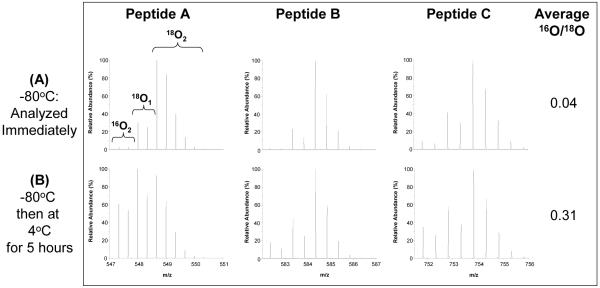
In typical post-digestion 18O-labeling studies, proteins are first cleaved by trypsin in water and then the peptide products are subsequently dried and resuspended in 18O-enriched water containing immobilized (IM) trypsin, which catalyzes 18O-labeling3, 7. The use of a catalytic enzyme immobilized on beads facilitates the enzyme removal and reduces the extent of back-exchange; however, if the residual trypsin introduced from the initial digestion step is not inactivated, back-exchange can still be problematic for samples that are not analyzed immediately after labeling or thawing. To illustrate this problem, tryptically-digested BSA samples were labeled with 18O using IM trypsin and the residual trypsin from the initial solution-phase (SP) digestion was not inactivated by any means. The samples were stored at -80 °C immediately after labeling. One aliquot was thawed and immediately analyzed and another aliquot was analyzed after being stored at 5 h at 4 °C. As shown in Figure 1, the sample analyzed immediately after thawing showed minimal unlabeled 16O species, thus only a relatively low degree of back-exchange occurred since -80 °C inhibited trypsin activity. However, significant back-exchange was observed for the sample stored for 5 h at 4 °C prior to analysis based on the intensities of the 16O-labeled peptide peaks. 16O/18O abundance ratios can be determined using the equations described in Qian et al3 as a quantitative measure of back-exchange. The average 16O/18O abundance ratios for the three peptides for Figure 1A and 1B were calculated as 0.04 and 0.31, respectively, suggesting nearly 30% of peptide molecules were exchanged back to 16O after just several hours at 4 °C. Back-exchange can be minimized by immediate sample analysis, but limits the application of the 16O/18O-labeling method toward large-scale study sets.
Figure 1.
18O back-exchange following sample storage prior to LC-MS analysis. (A) An 18O-labeled sample was stored at -80°C and an aliquot was analyzed immediately after thawing. (B) Another aliquot of the same sample was analyzed after five hours at 4°C. The average 16O/18O abundance ratio for peptides A, B, and C is presented in the column to the right of each condition. The amino acid sequences of peptides A, B, and C depicted in all figures are KVPQVSTPTLVEVSR, LVNELTEFAK, and EYEATLEECCAK, respectively.
Evaluation of the effectiveness of boiling in quenching trypsin activity and preventing back-exchange
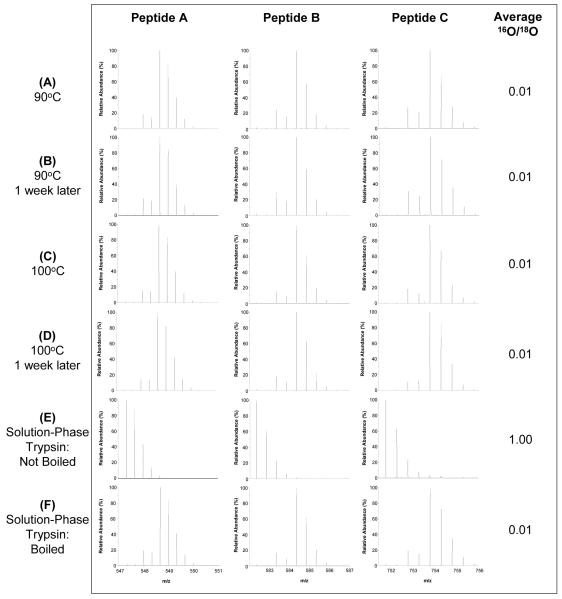
Protein denaturation using heat has long been applied in sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE)18. We have reported that solution-phase trypsin activity can be quenched through boiling the sample for 10 min prior to labeling, thus preventing 18O back-exchange3, 13; however, the effectiveness of this procedure has not been demonstrated. Here, we aimed to perform a more detailed assessment of the effectiveness of boiling in quenching trypsin activity by comparing the extent of back-exchange before and after one week of sample storage at room temperature. Figure 2 shows that samples that were heated at 90 °C or boiled at 100 °C before labeling and immediately cooled in liquid nitrogen did not experience back-exchange. We also tested whether back-exchange occurred after a long storage time for those boiled samples. As shown in Figure 2B and 2D, no back-exchange was observed for samples stored at one week at room temperature prior to LC-MS analysis, which is an extreme storage condition used in this experiment simply to illustrate the effects of sample processing on back-exchange. However, samples should be stored at -80 °C after labeling to ensure sample integrity.
Figure 2.
The effectiveness in preventing oxygen back-exchange by boiling. (A-D) Samples were heated or boiled for 10 minutes at 90°C or 100°C, respectively, before labeling and immediately snap-frozen in liquid N2. Samples were analyzed immediately after labeling and again after 1 week at room temperature. (E) An aliquot of non-boiled solution-phase trypsin was added to the boiled sample in 2D after labeling. (F) An aliquot of boiled solution-phase trypsin was added to the boiled sample in 2D after labeling.
The average 16O/18O abundance ratio for all peptides in Figure 2A - D was 0.01, suggesting the presence of ∼1% of 16O species in these labeled peptides. It should be noted that some 16O is introduced into the sample during 18O-labeling in our protocol since CaCl2 and immobilized trypsin are dissolved in H216O, while the H218O used for labeling is ∼95 - 97%. Due to this, 100% labeling efficiency is not expected; rather, based on the amount of H216O present, the maximum labeling efficiency when the equilibrium is reached, there will be approximately 0.5-1% of peptides are unlabeled, which agrees well with our observed average 16O/18O abundance ratio of 0.01.
To further illustrate the effectiveness of boiling in quenching trypsin activity, freshly prepared boiled or non-boiled solution-phase trypsin aliquots in a 1:50 trypsin:peptide ratio (w/w) were added to the samples. As shown in Figure 2E and 2F, complete back-exchange was observed in the aliquot with non-boiled trypsin added while no significant back-exchange was observed for the aliquot containing boiled trypsin. The data demonstrate that the trypsin activity is completely quenched by this procedure and 18O-labeled samples are stable for long storage periods, especially given that the samples are often stored at low temperatures (-20 or -80 °C) prior to analyses.
In addition, we tested different methods of cooling the samples (i.e., snap-freezing in liquid N2, placing on ice for 5 minutes, placing at room temperature for 5 minutes) after boiling to see if this affected trypsin’s ability to re-form into its catalytic tertiary structure and, therefore, regain its activity for catalyzing back-exchange. However, the temperature at which the sample is cooled does not affect the back-exchange (data not shown), which also demonstrates that trypsin does not recover its activity after being boiled for 10 minutes.
An improved 18O-labeling protocol with solution-phase trypsin
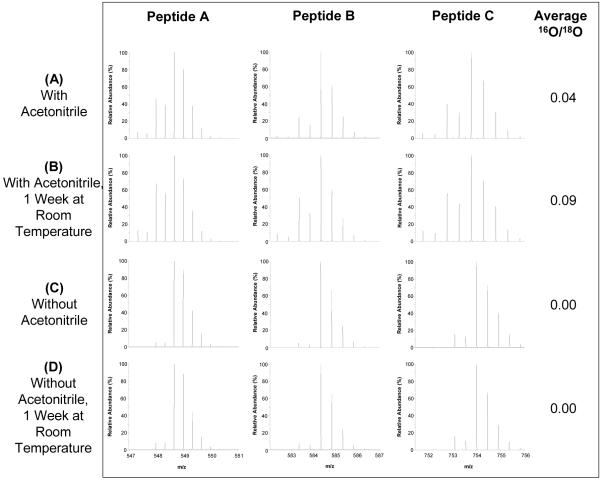
Immobilized (IM) trypsin is commonly employed during the labeling step because it can be separated from the sample through centrifugation or filtering to minimize the potential for trypsin-catalyzed back-exchange. However, the use of IM trypsin inevitably introduces sample loss when the IM trypsin is removed due to non-specific binding, thus preventing the application of 18O-labeling to small biological samples (e.g., low μg protein quantity). For example, the average peptide recovery using the protocol outlined in the Experimental section is ∼70%. Such non-specific loss can also affect the reproducibility of labeling. We next investigated whether solution-phase trypsin can be used to achieve the same effective labeling since it can be completely quenched by the boiling procedure. By using SP trypsin, the inherent problems associated with removing immobilized trypsin from the sample (i.e., irreproducibility and sample loss) are circumvented. Figure 3C and 3D show that complete labeling with an average 16O/18O abundance ratio of 0.00 was observed before and after one week of storage at room temperature without the use of acetonitrile and with a 1:50 trypsin:peptide ratio (w/w), a trypsin level that would not interfere with analysis. Furthermore, the average sample recovery was ∼97%. The 16O/18O abundance ratios for 34 randomly selected peptides before and after one week at room temperature was manually calculated. No difference in the 16O/18O abundance ratios (ranging from 0.00 to 0.02) was observed for these peptides, again illustrating the nearly complete labeling efficiency without back-exchange.
Figure 3.
18O-labeling using solution-phase trypsin and the effect of organic cosolvents on 18O-labeling efficiency and back-exchange. After labeling using solution-phase trypsin, samples were boiled at 100°C followed by a 5% formic acid (v/v) addition. They were analyzed immediately after labeling and again after 1 week at room temperature.
Interestingly, we observed that samples that included 16% (v/v) of organic solvents, particularly acetonitrile, for labeling using solution-phase trypsin showed slightly lower labeling efficiency (Fig. 3A, 3B) compared to those samples without organic solvents (Fig. 3C, 3D) when the same boiling procedure was applied after labeling. In addition, there is clear evidence that back-exchange occurred after one week at room temperature for the sample that contained acetonitrile (Fig. 3B). Average 16O/18O abundance ratios for Figure 3A and 3B are 0.04 and 0.09, respectively, suggest that up to 10% back-exchange occurred under these conditions. The data illustrate that the boiling procedure does not completely quench trypsin activity in the presence of organic cosolvents. This incomplete quenching was not observed for labeling using immobilized trypsin because the boiling step was performed prior to labeling when the sample was in a buffer that did not contain organic solvents, which allowed complete quenching of residual SP trypsin during boiling and the immobilized trypsin was effectively removed by centrifugation. However, samples that were labeled using IM trypsin and boiled after the IM trypsin was removed also experienced incomplete trypsin quenching and significant back-exchange because the IM trypsin pellets were washed with methanol and the boiled sample contained 26% (v/v) methanol and 9% (v/v) acetonitrile. Clearly, the presence of organic cosolvents decreases the ability of boiling to completely quench trypsin activity.
The effects of organic solvents and aqueous-organic cosolvent mixtures on protein structure have been documented19, 20. Proteins are generally known to be denatured in organic solvents, yet enzyme activity is frequently greater in the presence of low levels of organic cosolvents when compared to their activity in pure aqueous buffer20. To our knowledge, there are no detailed reports of enzyme activity in organic cosolvents after being boiled at 100°C. In addition, mixtures of acetonitrile with water and acetonitrile with methanol form positive azeotropes that lower the minimum boiling points of the resulting solutions to 76.5 °C and 63.5 °C, respectively21, 22. Using a thermostat probe, we measured the temperature of boiling 10 mL solutions with 16% (v/v) acetonitrile or 26% (v/v) methanol and 9% (v/v) acetonitrile after being placed in a boiling water bath. The formation of an acetonitrile/water azeotrope resulted in a boiling point of 87 °C while the mixture of acetonitrile/water and acetonitrile/methanol azeotropes boiled at 83 °C. Our results indicate that the combined effects of organic cosolvent stabilization and decreased boiling points do not completely inactivate trypsin. Complete quenching of trypsin activity can be achieved, however, when the sample is boiled for ten minutes in a water bath when organic solvents such as methanol and acetonitrile are excluded.
Optimized 18O-labeling protocol
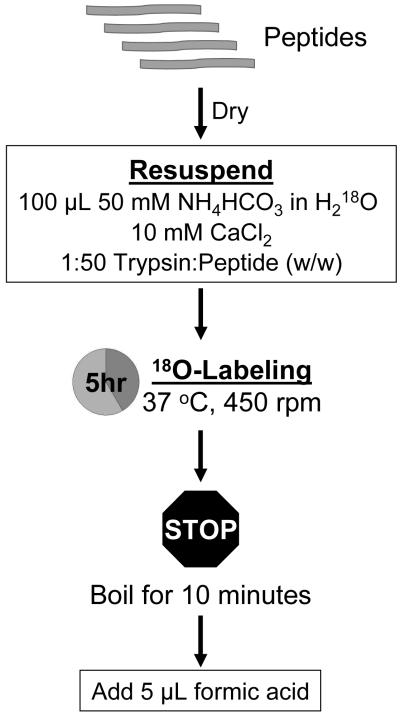
Following this detailed assessment, we present a simple optimized labeling protocol that minimizes sample loss, prevents back-exchange, and provides better reproducibility than our previously reported protocol using immobilized trypsin3, 13 (Figure 4). After the sample is tryptically digested and dried to completion, the peptides are resuspended in 100 μL 50 mM NH4HCO3 in H218O and mixed by brief sonication. Upon the addition of 10 mM CaCl2 and 1:50 solution-phase trypsin:peptide (w/w), the sample is incubated at 37 °C for 5 hours. The labeling reaction is stopped by boiling the sample in a water bath for 10 minutes. Finally, 5 μL formic acid is added to further inhibit any possible residual trypsin activity23.
Figure 4.
Optimized 18O-Labeling Workflow.
In addition to 18O-labeling, the procedure can also be applied toward 16O-labeling using H216O for comparative analyses. If the 18O-labeled sample will be used as the reference for quantitation, the labeling efficiency of the 18O-labeled sample should be verified to ensure accurate quantitation and the unlabeled sample must be boiled for 10 minutes to inactivate trypsin activity prior to mixing the 16O and 18O-labeled samples in a 1:1 ratio.
Conclusions
While thermal inactivation of trypsin has been previously reported, this study provides the first thorough evaluation of the effectiveness of boiling on quenching trypsin activity and preventing back-exchange. Our data demonstrate that complete prevention of 18O back-exchange can be reproducibly achieved by boiling the peptide sample for 10 minutes followed by a 5% formic acid (v/v) addition. In addition, the implementation of solution-phase trypsin during the labeling procedure provides more reproducible labeling and better sample recovery when compared to immobilized trypsin where non-specific loss inevitably occurs. Our improved protocol with solution-phase trypsin for labeling is particularly useful for labeling very small biological or clinical samples where stable isotope labeling experiments have been traditionally infeasible. The ability to generate stably labeled 18O samples without back-exchange will also enable investigations that involve a large number of samples for biomarker discovery and validation using the 18O-labeled reference concept15.
Acknowledgements
The authors thank the National Institute of General Medical Sciences (NIGMS, Large Scale Collaborative Research Grants U54 GM-62119-02) and NIH National Center for Research Resources (RR018522) for support of portions of this research and the Environmental Molecular Sciences Laboratory (EMSL) at Pacific Northwest National Laboratory (PNNL), a national scientific user facility sponsored by the U.S. Department of Energy’s (DOE) Office of Biological and Environmental Research, for the development and use of the instrumentation applied in this research. PNNL is a multi-program national laboratory operated by Battelle Memorial Institute for the DOE under Contract DE-AC05-76RL01830.
Abbreviations
- BCA
bicinchoninic acid
- BSA
bovine serum albumin
- DTT
dithiothreitol
- HPLC
high pressure liquid chromatography
- IAA
iodoacetamide
- m/z
mass-to-charge ratio
- IM
immobilized
- SDS-PAGE
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SP
solution-phase
- SPE
solid phase extraction
References
- 1.Ong SE, Mann M. Mass spectrometry based proteomics turns quantitative. Nat Chem Biol. 2005;1(5):252–62. doi: 10.1038/nchembio736. [DOI] [PubMed] [Google Scholar]
- 2.Miyagi M, Rao KC. Proteolytic 18O-labeling strategies for quantitative proteomics. Mass Spectrom Rev. 2007;26(1):121–36. doi: 10.1002/mas.20116. [DOI] [PubMed] [Google Scholar]
- 3.Qian WJ, Monroe ME, Liu T, Jacobs JM, Anderson GA, Shen Y, Moore RJ, Anderson DJ, Zhang R, Calvano SE, Lowry SF, Xiao W, Moldawer LL, Davis RW, Tompkins RG, Camp DG, 2nd, Smith RD. Quantitative proteome analysis of human plasma following in vivo lipopolysaccharide administration using 16O/18O labeling and the accurate mass and time tag approach. Mol Cell Proteomics. 2005;4(5):700–9. doi: 10.1074/mcp.M500045-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petyuk VA, Qian WJ, Chin MH, Wang H, Livesay EA, Monroe ME, Adkins JN, Jaitly N, Anderson DJ, Camp DG, 2nd, Smith DJ, Smith RD. Spatial mapping of protein abundances in the mouse brain by voxelation integrated with high throughput liquid chromatography mass spectrometry. Genome Res. 2007;17(3):328–36. doi: 10.1101/gr.5799207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heller M, Mattou H, Menzel C, Yao X. Trypsin catalyzed 16O-to-18O exchange for comparative proteomics: tandem mass spectrometry comparison using MALDI-TOF, ESI-QTOF, and ESI-ion trap mass spectrometers. J Am Soc Mass Spectrom. 2003;14(7):704–18. doi: 10.1016/S1044-0305(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 6.Chin MH, Qian WJ, Wang H, Petyuk VA, Bloom JS, Sforza DM, Lacan G, Liu D, Khan AH, Cantor RM, Bigelow DJ, Melega WP, Camp DG, 2nd, Smith RD, Smith DJ. Mitochondrial dysfunction, oxidative stress, and apoptosis revealed by proteomic and transcriptomic analyses of the striata in two mouse models of Parkinson’s disease. J Proteome Res. 2008;7(2):666–77. doi: 10.1021/pr070546l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fenselau C, Yao X. Proteolytic labeling with 18O for comparative proteomics studies: preparation of 18O-labeled peptides and the 18O/16O peptide mixture. Methods Mol Biol. 2007;359:135–42. doi: 10.1007/978-1-59745-255-7_9. [DOI] [PubMed] [Google Scholar]
- 8.Staes A, Demol H, Van Damme J, Martens L, Vandekerckhove J, Gevaert K. Global differential non gel proteomics by quantitative and stable labeling of tryptic peptides with oxygen 18. J Proteome Res. 2004;3(4):786–91. doi: 10.1021/pr049956p. [DOI] [PubMed] [Google Scholar]
- 9.Liu H, Zhang Y, Meng L, Qin P, Wei J, Jia W, Li X, Cai Y, Qian X. Non-gel-based dual 18O labeling quantitative proteomics strategy. Anal Chem. 2007;79(20):7700–7. doi: 10.1021/ac0709302. [DOI] [PubMed] [Google Scholar]
- 10.Sevinsky JR, Brown KJ, Cargile BJ, Bundy JL, Stephenson JL., Jr. Minimizing back-exchange in 18O/16O quantitative proteomics experiments by incorporation of immobilized trypsin into the initial digestion step. Anal Chem. 2007;79(5):2158–62. doi: 10.1021/ac0620819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stewart II, Thomson T, Figeys D. 18O labeling: a tool for proteomics. Rapid Commun Mass Spectrom. 2001;15(24):2456–65. doi: 10.1002/rcm.525. [DOI] [PubMed] [Google Scholar]
- 12.Viswanatha T, Liener IE. The inhibition of trypsin. III. Influence of urea. J Biol Chem. 1955;215(2):777–85. [PubMed] [Google Scholar]
- 13.Liu T, Qian WJ, Strittmatter EF, Camp DG, 2nd, Anderson GA, Thrall BD, Smith RD. High throughput comparative proteome analysis using a quantitative cysteinyl peptide enrichment technology. Anal Chem. 2004;76(18):5345–53. doi: 10.1021/ac049485q. [DOI] [PubMed] [Google Scholar]
- 14.Storms HF, van der Heijden R, Tjaden UR, van der Greef J. Considerations for proteolytic labeling optimization of 18O incorporation and prohibition of back-exchange. Rapid Commun Mass Spectrom. 2006;20(23):3491–7. doi: 10.1002/rcm.2738. [DOI] [PubMed] [Google Scholar]
- 15.Qian WJ, Liu T, Petyuk VA, Gritsenko MA, Petritis BO, Polpitiya AD, Kaushal A, Xiao W, Finnerty CC, Jeschke MG, Jaitly N, Monroe ME, Moore RJ, Moldawer LL, Davis RW, Tompkins RG, Herndon DN, Camp DG, Smith RD. Large-Scale Multiplexed Quantitative Discovery Proteomics Enabled by the Use of an (18)O-Labeled “Universal” Reference Sample. J Proteome Res. 2009;8(1):290–299. doi: 10.1021/pr800467r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shen Y, Zhao R, Belov ME, Conrads TP, Anderson GA, Tang K, Pasa Tolic L, Veenstra TD, Lipton MS, Udseth HR, Smith RD. Packed capillary reversed phase liquid chromatography with high performance electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry for proteomics. Anal Chem. 2001;73(8):1766–75. doi: 10.1021/ac0011336. [DOI] [PubMed] [Google Scholar]
- 17.Qian WJ, Kaleta DT, Petritis BO, Jiang H, Liu T, Zhang X, Mottaz HM, Varnum SM, Camp DG, 2nd, Huang L, Fang X, Zhang WW, Smith RD. Enhanced detection of low abundant human plasma proteins using a tandem IgY12 supermix immunoaffinity separation strategy. Mol Cell Proteomics. 2008 doi: 10.1074/mcp.M800008-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald TP, Sugrue RJ. The use of two dimensional SDS PAGE to analyze the glycan heterogeneity of the respiratory syncytial virus fusion protein. Methods Mol Biol. 2007;379:97–108. doi: 10.1007/978-1-59745-393-6_7. [DOI] [PubMed] [Google Scholar]
- 19.Klibanov AM. Improving enzymes by using them in organic solvents. Nature. 2001;409(6817):241–6. doi: 10.1038/35051719. [DOI] [PubMed] [Google Scholar]
- 20.Tyagi R, Batra R, Gupta MN. Amorphous enzyme aggregates: Stability toward heat and aqueous organic cosolvent mixtures. Enzyme and Microbial Technology. 1999;24(56):348–354. [Google Scholar]
- 21.McKinney JD. RAPID ANALYSIS FOR AFLATOXINS IN COTTONSEED PRODUCTS WITH SILICA GEL CARTRIDGE CLEANUP. Journal of the American Oil Chemists Society. 1981;58(12):A935–A937. [Google Scholar]
- 22.Zhou M, Persin M, Sarrazin J. Methanol removal from organic mixtures by pervaporation using polypyrrole membranes. Journal of Membrane Science. 1996;117(1 2):303–309. [Google Scholar]
- 23.Smillie LB, Neurath H. Reversible inactivation of trypsin by anhydrous formic acid. J Biol Chem. 1959;234(2):355–9. [PubMed] [Google Scholar]