Abstract
Reactive species of oxygen and nitrogen have been collectively implicated in pulmonary oxygen toxicity, but the contributions of specific molecules are unknown. Therefore, we assessed the roles of several reactive species, particularly nitric oxide, in pulmonary injury by exposing wild-type mice and seven groups of genetically altered mice to >98% O2 at 1, 3, or 4 atmospheres absolute. Genetically altered animals included knockouts lacking either neuronal nitric oxide synthase (nNOS−/−), endothelial nitric oxide synthase (eNOS−/−), inducible nitric oxide synthase (iNOS−/−), extracellular superoxide dismutase (SOD3−/−), or glutathione peroxidase 1 (GPx1−/−), as well as two transgenic variants (S1179A and S1179D) having altered eNOS activities. We confirmed our earlier finding that normobaric hyperoxia (NBO2) and hyperbaric hyperoxia (HBO2) result in at least two distinct but overlapping patterns of pulmonary injury. Our new findings are that the role of nitric oxide in the pulmonary pathophysiology of hyperoxia depends both on the specific NOS isozyme that is its source and on the level of hyperoxia. Thus, iNOS predominates in the etiology of lung injury in NBO2, and SOD3 provides an important defense. But in HBO2, nNOS is a major contributor to pulmonary injury, whereas eNOS is protective. In addition, we demonstrated that nitric oxide derived from nNOS is involved in a neurogenic mechanism of HBO2-induced lung injury that is linked to central nervous system oxygen toxicity through adrenergic/cholinergic pathways.
Keywords: normobaric oxygen toxicity, hyperbaric oxygen toxicity, superoxide dismutase, glutathione peroxidase 1, neurogenic pulmonary oxygen toxicity
The Lung has a Limited Window of tolerance for oxygen, and pulmonary toxicity occurs when the partial pressure of inspired oxygen exceeds 0.5 atmospheres absolute (ATA) for a sufficient time. For example, prolonged exposure to oxygen pressures up to 1.0 ATA (normobaric oxygen or NBO2) results in diffuse pulmonary damage with extensive inflammation, accumulation of pleural fluid, disruption of the alveolar membrane, and eventually impaired gas exchange, respiratory failure, and death (3, 10, 26). When oxygen pressure exceeds 1 ATA (hyperbaric oxygen or HBO2), the development of lung injury is accelerated; but if O2 pressure remains below 2 ATA, morphological and biochemical patterns of damage remain similar to those observed after prolonged exposure to NBO2 (8, 13).
However, as inspired oxygen pressures exceed 2 ATA, a different pathophysiology emerges. At 2.5 ATA or above, lung injury develops even more rapidly than at lower levels of hyperbaric oxygenation, with little or no pleural fluid or inflammation. Instead, patchy congestion of the pulmonary vasculature or focally distributed intra-alveolar hemorrhage appears, especially in animals that exhibit convulsions from central nervous system (CNS) oxygen toxicity (4, 5, 21, 23, 36, 41, 48, 51).
As for normobaric oxygen toxicity, it is widely accepted that diffuse alveolar-capillary injury is a direct effect of oxygen, as reactive oxygen species (ROS) overwhelm the lung’s antioxidant defenses and alter cellular metabolism, signaling, and function (14, 15, 22, 32). Prolonged NBO2 also elevates the production of reactive nitrogen species (RNS) in lung tissue, particularly nitric oxide (NO), which can be cytotoxic and can also combine with superoxide anion () to form highly reactive peroxynitrite (ONOO−) (24, 33, 37, 43, 46). Paradoxically, NO may also help defend against pulmonary oxygen toxicity in its role as a signaling molecule (1, 6).
Pulmonary tolerance to normobaric hyperoxia is associated with antioxidant enzymes in the lung, including mitochondrial superoxide dismutase (SOD2), extracellular superoxide dismutase (SOD3), catalase (CAT), cytosolic glutathione peroxidase 1 (GPx1), glutathione reductase, glucose 6-phosphate dehydrogenase, and the peroxiredoxins (9, 11, 18, 25, 38, 50). Thus, many biomolecules, particularly ROS, RNS, and antioxidant enzymes, are involved in the pathophysiology of lung damage in NBO2 and in the defense against it, but their individual roles have not been fully established.
Although ROS and RNS are acknowledged as major factors in lung injury induced by NBO2, it is not clear whether they are also involved in the pathogenesis of pulmonary toxicity in hyperbaric oxygen. Recently, we reported that suppression of NOS activity in HBO2 can prevent the seizures of CNS oxygen toxicity and protect against hyperoxic lung injury as well, suggesting that the CNS and pulmonary toxicities of hyperoxia are linked (13). Moreover, we found that the effects on pulmonary oxygen toxicity of either the nonselective NOS inhibitor NG-nitro-l-arginine methyl ester (l-NAME) or the selective neuronal NOS (nNOS) inhibitor 7-nitroindazole do not differ significantly, suggesting that nNOS may have a major role in the pulmonary injury that occurs in HBO2, particularly if seizures occur (13). However, this study shed no light on the contribution of NO derived from endothelial NOS (eNOS) or inducible NOS (iNOS), or on the relative contributions of each of the three NOS isoforms to pulmonary damage in HBO2. Therefore, in the present study we compared the contributions to pulmonary damage or protection in normobaric and hyperbaric oxygen of endothelial, neuronal, and inducible NOS by using knockout (KO) mice deficient in each of these NOS isoforms. We also used two transgenic variants having altered levels of eNOS activity (S1179D and S1179A mice). In addition to studying the toxic and protective effects of NO, we also examined the roles of SOD3 and GPx1 in pulmonary oxygen toxicity by using mice deficient in these major pulmonary antioxidant enzymes.
MATERIALS AND METHODS
Experimental animals
The C57BL/6 strain of wild-type (WT) mice was used in this study, as were mutants of this strain lacking normal function of either endothelial NOS (eNOS−/−), neuronal NOS (nNOS−/−), inducible NOS (iNOS−/−), extracellular SOD (SOD3−/−), or glutathione peroxidase 1 (GPx1−/−).
In addition, we used two novel transgenic variants, S1179A and S1179D (2), that have altered levels of eNOS activity due to substitutions at Ser1179. In WT animals, eNOS is reversibly activated by phosphorylation at this site; but in S1179A mutants, Ser1179 is converted to alanine, which cannot be phosphorylated, and activation cannot occur (34). Therefore, eNOS activity in transgenic S1179A mice is lower than that in WT mice. In S1179D mutants, by contrast, substitution of Ser1179 with aspartate results in phosphomimetic activity, nearly equivalent to that seen in WT eNOS that is activated by phosphorylation at Ser1179 (31). Transgenic mice (S1179A and S1179D) were derived from eNOS−/− mice that had been back-crossed to C57BL/6 background for 10 generations and from C3H mice carrying the S1179A or S1179D transgene. Thus, C3H mice were mated with eNOS−/− mice to obtain heterozygous eNOS knock-out mice, and those offspring carrying the transgenes were interbred to obtain homozygous eNOS knockout mice with either the S1179A or S1179D transgene. The vascular responses to ACh, sodium nitroprusside, and phenylephrine of the eNOS−/− littermates from these matings that did not contain the S1179A or S1179D transgenes were indistinguishable from those of the eNOS KO mice on a C57BL/6 background (2).
Standard procedures were used to assure uniformity of the various mutant strains. The genetic identity of breeders was determined after selective gene amplification by PCR. The SOD3-deficient C57BL/6 mice were bred at Duke University from a colony developed at Umeå University in Sweden (7). These animals were crossbred at the Duke Vivarium with C57BL/6 WT mice to yield heterozygotes, which were then crossed to produce homozygous KO and WT breeding pairs; the latter were used as controls. The SOD3−/− mice are completely without SOD3 but do show normal activities of the SOD1 and SOD2 isozymes (7). GPx1−/− KO were bred at Duke University from C57BL/6 breeding pairs that were a gift from Ye-Shih Ho of Wayne State University (17). KO for iNOS were obtained from Jackson Laboratories and were originally developed at Burroughs Wellcome (29). KO strains for eNOS and nNOS and the transgenic strains S1179A and S1179D were developed and bred at Massachusetts General Hospital (2, 19, 20). All NOS null mutants were backcrossed to the C57BL/6 WT strain for more than 10 generations to provide stable phenotypes.
Oxygen exposures
All studies were performed using awake, freely moving mice according to protocols approved by the Duke University Institutional Animal Care and Use Committee, using three different O2 exposures: 1 ATA for 72 h, 3 ATA for 6 h, and 4 ATA for 2 h. To be able to compare the results of this study with those of our earlier published observations (13), we used the same levels of hyperoxia, 1 ATA and 3 ATA O2. Additional exposures at 4 ATA O2 were used to evaluate the role of NOS isoforms in lung injury associated with oxygen seizures since, unlike rats, mice did not routinely manifest seizures at 3 ATA O2.
For normobaric exposures, mice were placed in a sealed acrylic chamber with free access to food and water, with O2 monitored continuously (series 570 O2 Analyzer, Servomex) and kept at or above 98%. Gas flow through the chamber was maintained at ~12 l/min, sufficient to allow complete gas exchange every 5 min and to keep the CO2 level close to that of room air. The temperature in the chamber was 22–24°C and relative humidity 60–70%.
For hyperbaric exposures, mice were placed in a glove-bag apparatus inside a large pressure chamber (Duke Center for Hyperbaric Medicine and Environmental Physiology) at either 3 ATA for 6 h or at 4 ATA for 2 h. Inspired oxygen concentration was maintained above 99% and CO2 limited to 0.03%. The temperature in the chamber was 22–24°C, with relative humidity ~60%.
Behavioral observations
Mice were visually monitored throughout all hyperoxic exposures for behavioral signs of CNS toxicity, including restlessness, excessive cleaning movements, changed breathing patterns, shaking, and convulsions.
Pleural fluid and bronchoalveolar lavage
Immediately after hyperoxic exposure, each mouse was killed with an overdose of halothane, chest and peritoneal cavities were carefully opened, and the volume of pleural fluid was measured by syringe. Bilateral bronchoalveolar lavage (BAL) was performed with 1 ml of sterile, non-pyrogenic PBS. BAL fluid (BALF) was analyzed for markers of lung injury, including total cell count, lactate dehydrogenase (LDH) activity, and total protein content. Cell counts (cells/ml of BALF) were made with a hemocytometer (Neubauer) immediately after lung lavage. LDH activity and total protein content were measured in the supernatant (after centrifugation at 3,500 g for 5 min at 4°C) as indicators of damage to the blood-air boundary. The catalytic activity of LDH in cell-free BALF was evaluated as the amount of l-lactate oxidized at 30°C, per unit time, from reduction of NAD+. Total protein content was measured with the bicinchoninic acid assay using bovine serum albumin as a standard (42).
Experimental design
In the first two series of experiments, WT and five groups of KO mice (eNOS−/−, nNOS−/−, iNOS−/−, GPx1−/−, and SOD3−/−) were exposed to O2 at either 1 ATA for 72 h or 3 ATA for 6 h to evaluate the role of each of the three NOS isoforms and of two antioxidant enzymes (SOD3 and GPx1) in pulmonary oxygen toxicity in mice under hyperoxic conditions.
In a third series of experiments, we verified whether NO derived from either eNOS or nNOS is involved in the pulmonary injury associated with HBO2-inducd convulsions. For this, WT, eNOS−/−, and nNOS−/− mice as well as S1179A and S1179D transgenic mice were exposed to 4 ATA for 2 h. Indices of lung injury were determined in animals with and without oxygen convulsions. We did not use other groups of KO animals in this series because in a preliminary study, we found no significant differences in indices of pulmonary damage in those animals compared with WT mice. To confirm the protective role of NO derived from eNOS, we assessed lung injury in eNOS−/− mice exposed to O2 at 4 ATA for 2 h, immediately after pretreatment with the NO donor S-nitroso-N-acetylpenicillamine (SNAP; 0.1 mg/kg ip).
In a fourth series of experiments, we explored the link between autonomic nervous system activity and endothelial or neuronal NOS in 3 ATA HBO2 by comparing lung injury in WT, eNOS−/−, and nNOS−/− mice, after inhibition of adrenergic receptors by propranolol (5 mg/kg ip) or cholinergic receptors by tiotropium bromide (10 mg/kg ip).
Statistical analysis
Results are expressed as means ± SE. Significance was evaluated with commercially available software (Statview, Calabasas, CA) using ANOVA and Scheffeé’s post hoc test. Unpaired t-tests enabled comparison of absolute changes in the biochemical indices of lung injury in hyperoxia with respect to their control values in the same strains of mice breathing room air. Differences were considered significant if P < 0.05.
RESULTS
Animal behavior in hyperoxia
Mice exposed to NBO2 began to huddle together after 24 h; their breathing slowed after 48 h; and after 72 h, 1 of 12 WT and 2 of 11 SOD3−/−mice died. We did not analyze mortality statistically because the death rates were too low in both groups.
All mice exposed to 3 ATA O2 survived the 6-h exposure with no signs of excitability or seizures but did manifest tachypnea. Some of the mice exposed to 4 ATA O2 exhibited spasticity, rigidity, or convulsions (see below).
Lung injury in normobaric and hyperbaric oxygen
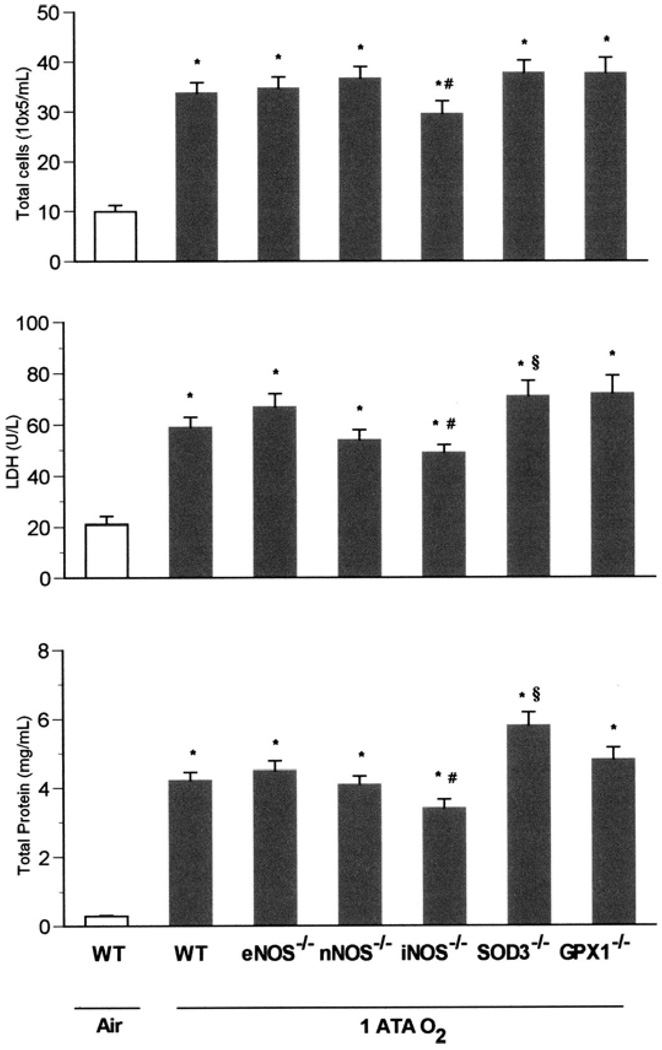
Baseline lung measurements made on all KO strains breathing room air were no different from those of WT mice breathing air (data not shown). Based on this observation, we compared indices of lung injury in KO strains in NBO2 and HBO2 vs. WT mice breathing air. After exposure to NBO2 for 72 h, WT and all mutant strains demonstrated significant lung injury compared with WT mice breathing air (Fig. 1). Intergroup comparisons revealed that the iNOS KO sustained less pulmonary injury than did either the WT animals or the other mutants studied. By contrast, SOD3−/− mice showed more injury, with significant increases in BALF total protein compared with WT and the other mutant strains as well as significantly higher LDH activity and total cells than in iNOS−/− animals.
Fig. 1.
Pulmonary oxygen toxicity in 1 atmospheres absolute (ATA) O2. Wild-type (WT), eNOS−/−, nNOS−/−, iNOS−/−, SOD3−/−, and GPx1−/−mice (n = 11, 11, 10, 8, 9, and 8, respectively) exposed to normobaric hyperoxia (NBO2) for 72 h demonstrated severe increases in the measured indices of pulmonary injury compared with WT mice (n = 5) breathing room air (*P < 0.05 vs. WT in air). Scheffé’s post hoc test revealed that iNOS knockouts sustained significantly less pulmonary injury than did WT animals after exposure to NBO2 (#P ≤ 0.05 vs. WT in NBO2). By contrast, SOD3−/− mice showed more injury, with significant increases in bronchoalveolar lavage fluid (BALF) total protein and LDH activity (§P < 0.05 vs. WT in NBO2).
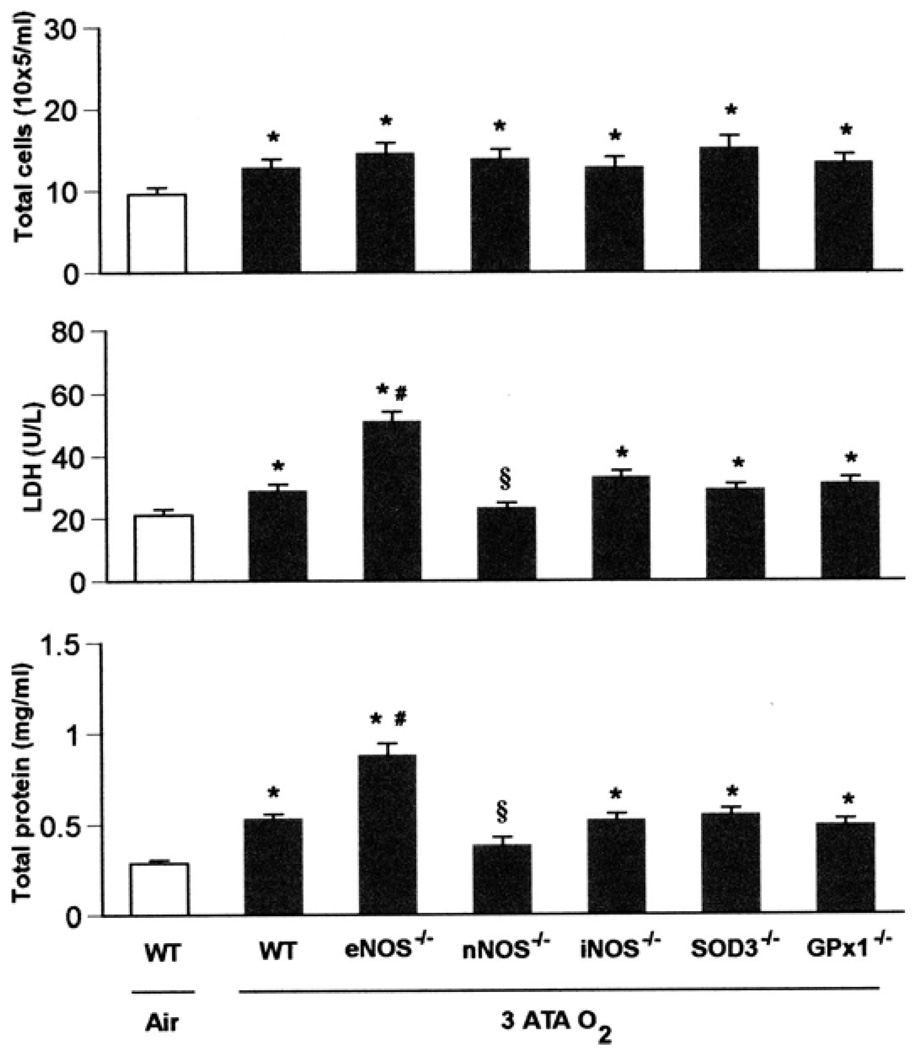
Mice exposed to 3 ATA for 6 h also showed lung injury, but much less than in mice exposed to normobaric oxygen for 72 h (Fig. 2). Among KO groups exposed to this level of HBO2, nNOS−/− mice were the most resistant to lung injury, whereas eNOS−/− mutants were the most vulnerable. Three groups of KO (GPx1−/−, SOD3−/−, and iNOS−/−) showed no differences compared with WT mice (Fig. 2).
Fig. 2.
Pulmonary oxygen toxicity in 3 ATA O2. WT, eNOS−/−, nNOS−/−, iNOS−/−, SOD3−/−, and GPx1−/− mice (n = 10, 10, 10, 8, 8, and 8, respectively) exposed to oxygen at 3 ATA for 6 h experienced severe lung damage across all 3 indices of injury compared with WT mice (n = 3) breathing air (*P < 0.05 vs. WT in air). Although the nNOS mutants had significant increases in total cells, they showed no differences in protein and LDH compared with WT mice breathing room air. Mice deficient in eNOS had significantly increased LDH activity and total protein concentration in BALF after exposure to 3 ATA O2 compared with WT mice [#P < 0.05 vs. WT in hyperbaric hyperoxia (HBO2)], whereas nNOS−/− mice showed less LDH activity and total protein in BALF than WT in HBO2 (§P < 0.05 vs. WT in HBO2). The remaining knockouts (iNOS−/−, SOD3−/−, and GPx1−/−) showed lung injury similar to that of WT mice after 3 ATA O2.
HBO2 convulsions and lung injury
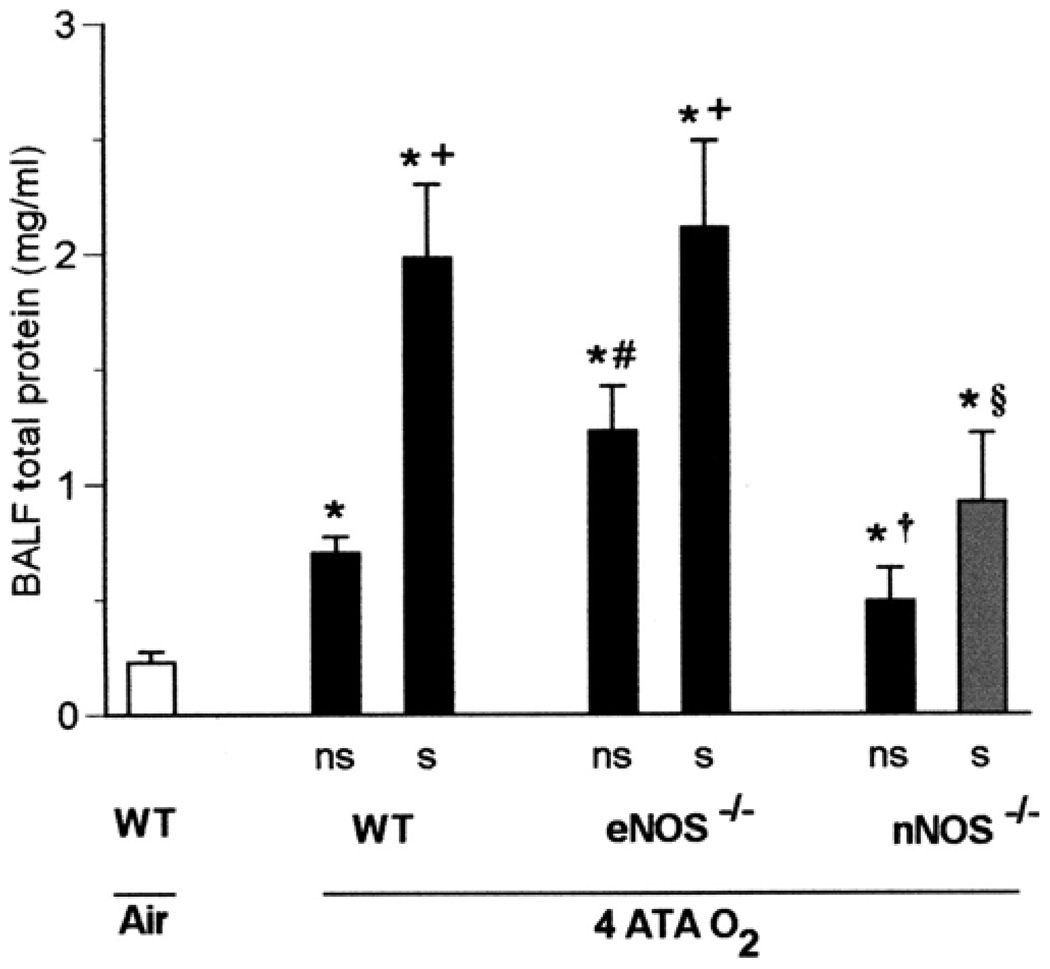
At 4 ATA, 53% of the mice convulsed between 1 and 2 h of exposure, including 69% of WT mice, 45% of nNOS−/− mice, and 42% of eNOS−/−mice. The latency period to the first motor convulsions was 79 ± 10 min in WT mice, 96 ± 11 min in nNOS−/− mice, and 107 ± 9 min in eNOS−/− mice. Among these three groups of mice, differences in seizure latency were significant only between WT and eNOS−/− animals. Seizures at 4 ATA were correlated with more severe lung injury in all strains, as indicated by increased total protein in BALF (Fig. 3). However, among the various groups, WT and eNOS−/− mice with seizures were more susceptible to pulmonary injury than were nNOS−/− mice with seizures. The differences seen in BALF total protein between nNOS−/− mutants with or without convulsions were not statistically significant. As for the seizure-free animals, eNOS−/− mice were more susceptible to pulmonary injury than WT mice.
Fig. 3.
Seizures and pulmonary oxygen toxicity in 4 ATA O2. Total protein was measured in BALF as a marker of lung injury in WT, eNOS−/−, and nNOS−/− mice (n = 13, 12, and 11, respectively) exposed to 4 ATA O2 for 2 h. Protein content was significantly higher in all animals that exhibited seizures (s) (8 WT, 5 eNOS−/−, and 5 nNOS−/−) as well as in those without seizures (ns) than in WT mice (n = 4) breathing air (*P < 0.05 vs. WT in air). However, differences in total protein between ns and s mice of the same genotype were significant only in WT and eNOS−/− mice (+P < 0.05 vs. ns). Among animals without seizures, eNOS−/− mice had significantly greater BALF total protein than did WT animals (#P < 0.05 vs. WT ns), whereas decreases in total protein in nNOS−/− mice had borderline significance (†P = 0.05 vs. WT ns). The nNOS−/− mice with seizures were significantly protected compared with WT or eNOS−/− individuals, as indicated by total protein in BALF (§P < 0.05 vs. WT s or eNOS−/− s).
Role of eNOS in lung injury in HBO2
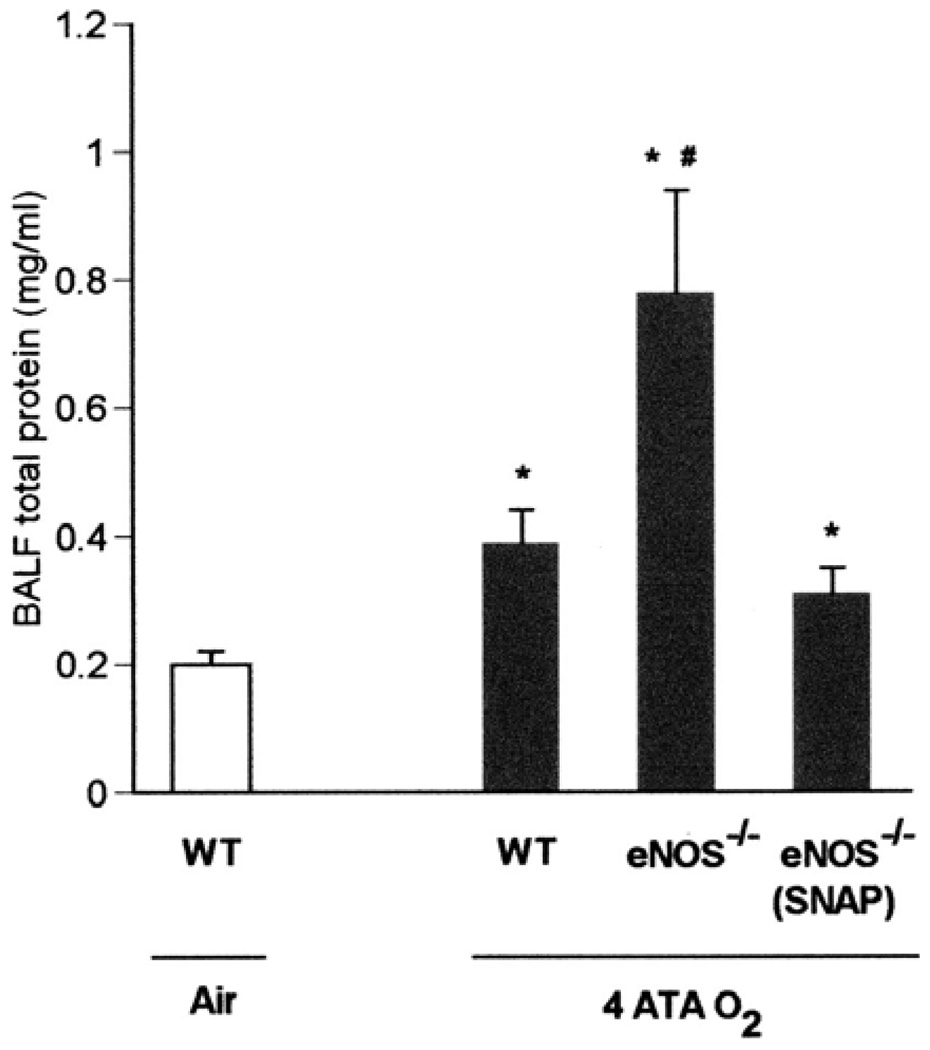
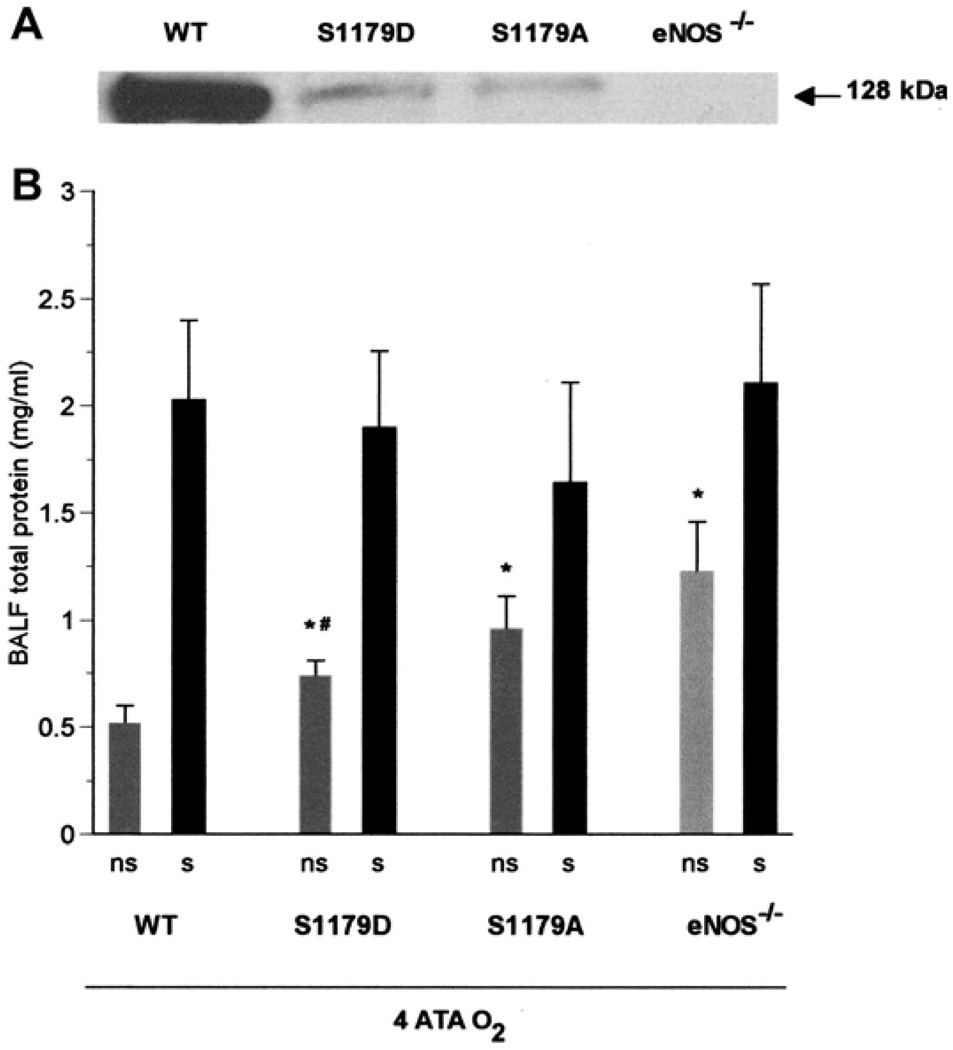
Lung injury, as indicated by BALF total protein, was significantly attenuated in eNOS−/− mice treated with the NO donor SNAP before exposure to HBO2 at 4 ATA for 2 h (Fig. 4). We also found that in the absence of seizures, the severity of lung injury in the four types of mice (WT, S1179D, S1179A, and eNOS−/−) apparently varied inversely with the level of WT eNOS protein (as indicated by Western blot analysis using antibody specific to WT eNOS). There was no statistical difference in BALF protein between S1179A mice and eNOS−/− mice (Fig. 5).
Fig. 4.
Exogenous nitric oxide overcomes eNOS deficiency. Seizure-free WT (n = 4) and 2 groups of eNOS−/− mice (n = 7 and 6) after exposure to 4 ATA for 2 h were used to determine if the pulmonary injury in HBO2 associated with eNOS deficiency could be prevented by administering the NO donor SNAP (S-nitroso-N-acetylpenicillamine, 0.1 mg/kg ip) before HBO2 exposure. Total protein levels in all groups of mice were significantly higher than in WT mice (n = 4) breathing air (*P < 0.05 vs. WT in air). A significant increase in total protein in BALF was observed in untreated eNOS−/− mice after HBO2 compared with both other groups (#P < 0.05 vs. WT or eNOS−/−+SNAP). SNAP decreased lung protein leak in eNOS−/− mice to levels seen in WT mice after exposure to HBO2.
Fig. 5.
Tolerance to pulmonary oxygen toxicity varies with eNOS activity. A: Western blot analysis of lung protein, using antibody specific to WT eNOS, shows decreasing order of eNOS protein expression from WT through S1179D and S1179A to eNOS−/− mice. B: after exposure to 4 ATA seizure-free (ns) eNOS−/− mice (n = 8), and both groups of transgenic mice had the most severe pulmonary damage in HBO2, as indicated by BALF total protein compared with WT mice (*P < 0.05 vs. WT, ns). Seizure-free S1179A transgenic mice (n = 6), in which eNOS could not be activated by phosphorylation, had less injury than seizure-free eNOS knockouts, but this difference was not statistically significant. By contrast, seizure-free S1179D transgenic mice with phosphomimetic eNOS (n = 5) had significantly less injury than eNOS−/− mice. #P < 0.05 vs. eNOS−/−, ns. All mice with seizures (s), including WT and both transgenic strains (7 WT, 5 eNOS−/−, 6 S1179A, and 7 S1179D), show similarly large increases in total protein, suggesting that CNS-mediated convulsions exacerbate pulmonary damage in HBO2.
Role of autonomic pathways in HBO2
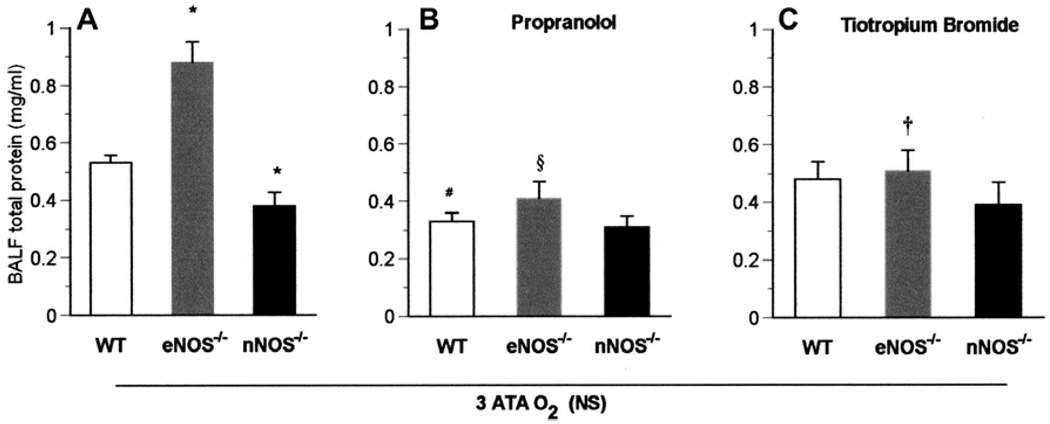
All mice exposed to 3 ATA HBO2, with or without propranolol or tiotropium bromide, were seizure free. Also, lung injury in HBO2 was less severe in nNOS−/− than in WT mice, but was more severe in eNOS−/− mice (Fig. 6A). Lung injury was significantly attenuated in WT and eNOS−/− mice exposed to 3 ATA HBO2 that were pretreated with either the β-adrenergic blocker propranolol (Fig. 6B) or in eNOS−/− mice pretreated with cholinergic blocker tiotropium bromide, which disrupts parasympathetic pathways (Fig. 6C). However, nNOS−/− mice were not significantly affected, suggesting that nNOS is required for the toxicity modulated by these inhibitable autonomic pathways (Fig. 6). These results are consistent with the ideas that the injury associated with nNOS activity is mediated by the autonomic nervous system and that eNOS activity is protective. Thus, when eNOS is absent and both nNOS and autonomic activity are present, the most severe injury occurs (Fig. 6A).
Fig. 6.
Autonomic pathways to pulmonary oxygen toxicity at 3 ATA O2. A: after exposure to 3 ATA HBO2, lung protein leak was significantly higher in eNOS−/− and significantly less in nNOS−/− animals (*P < 0.05 vs. WT; data taken from Fig. 2). B: WT (n = 7) and eNOS−/− (n = 7) mice pretreated with the β-adrenergic blocker propranolol showed significantly less lung injury compared with corresponding untreated mice (#P < 0.05 vs. WT in A; §P < 0.05 vs. eNOS−/− in A), but not nNOS−/− mutants. There were no significant differences among the 3 groups of animals treated with propranolol. C: eNOS−/− mice (n = 7) pretreated with the cholinergic receptor blocker tiotropium bromide showed lower protein levels compared with untreated eNOS−/− animals. †P < 0.05 vs. eNOS−/− in A. However, no difference in lung protein leak was observed after 3 ATA HBO2 among the 3 pretreated groups, and tiotropium bromide was not protective for WT mice (n = 8) and nNOS−/− mice (n = 7).
DISCUSSION
This study elucidates three mechanisms underlying our previous findings that there is more than one distinct pattern of pulmonary oxygen toxicity (13) reflecting shifting contributions of multiple pathological processes. First, iNOS plays a major role in lung injury induced by NBO2, and SOD3 is important in protecting the lungs under these conditions. Second, eNOS plays a protective role in the lung in HBO2. Third, nNOS contributes to lung injury induced by HBO2, perhaps through a neurogenic mechanism that utilizes adrenergic/cholinergic pathways, particularly if seizures occur. No previous account in the literature has shown these differential effects of the three NOS isoforms on pulmonary oxygen toxicity.
In our earlier study, using genetically identical rats, we found that exposure to NBO2 for 56 h caused diffuse lung damage, principally manifested by widespread injury to the alveolar-capillary region (13). Here, we similarly demonstrate significant lung injury in mice after 72 h in NBO2, as indicated by elevations of total protein concentration and LDH activity in BALF, corroborating the work of other investigators who have demonstrated pulmonary injury when mice are exposed to prolonged NBO2 (10, 26). The mechanism for this injury is thought to involve activation of inflammatory cells, resulting in increased generation of ROS and inflammatory cytokines leading to endothelial dysfunction, edema, and inactivation of surfactant (11, 15, 32). Thus, we find that the proinflammatory isoform of NOS (iNOS) plays a major role in the pulmonary pathophysiology of NBO2. Although the pulmonary injuries sustained in NBO2 by WT, nNOS−/−, and eNOS−/− mice are not significantly different, lung injury is significantly reduced in iNOS KO. This is also consistent with other studies showing that oxidative injury due to nitrosylation of proteins and peroxidation of lipids persists even when iNOS activity is attenuated (1, 32, 43), since iNOS deficiency reduces but does not prevent molecular oxidation, nitration, and cytotoxicity in acute hyperoxic exposure. To counter this direct inflammatory effect of NBO2 on the lung, pulmonary antioxidant enzymes come into play. For example, it is known that the extracellular antioxidant enzyme SOD3 helps protect the lung against the direct toxic effect of prolonged NBO2 (11, 18) and that mice deficient in this enzyme are more susceptible to hyperoxia (7), findings confirmed here in mice having altered expression of SOD3. However, we also confirm that deletion of cytosolic GPx (GPx1−/−), which is widely distributed in most normal cells, has no significant effect on pulmonary sensitivity to oxygen (17). Thus, earlier studies, along with our present data, suggest that SOD3 is a major contributor to the pulmonary defenses against oxygen toxicity, whereas GPx1 is not. This picture changes in HBO2, as a different pattern of pulmonary injury emerges, and a second pair of enzymes comes into play.
Previously, we found that pharmacological inhibition of nNOS in rats exposed to 3 ATA O2 protected against lung injury and prevented seizures, suggesting a special role for this NOS isoform as well as a link between the development of CNS O2 toxicity and pulmonary O2 toxicity. The present study substantiates this finding using mutant animals instead of pharmacology and also yields the novel finding that NO derived from eNOS protects the lung in hyperbaric oxygen, since eNOS KO animals were more sensitive to pulmonary injury in hyperoxia than were WT and nNOS null animals, and iNOS does not appear to have a significant role in lung injury induced by HBO2.
It is our hypothesis that the pulmonary injury that rapidly appears in HBO2 above 2 ATA results from acute pulmonary hypertension leading to microvascular damage with focal hemorrhage (13) and that eNOS protects the lung by attenuating pulmonary hypertension. This is consistent with the findings of reduced eNOS in lungs of human patients with pulmonary hypertension (16) and with similar results reported in rats (47). Because local NO production by eNOS maintains basal vasodilation in the lungs, its normal levels or increased production in HBO2 could attenuate pulmonary hypertension and the injury resulting from it. The results of our experiments with four strains of mice having different levels of eNOS activity (eNOS−/−, S1179D, S1179A, and WT) further support this hypothesis, since lung protection occurred in direct proportion to levels of eNOS protein (Fig. 5). However, because the expression of the S1179A and S1179D transgenes in specific pulmonary cells in these mice is unknown, we draw no definite conclusions from the lack of statistical difference in BALF protein between S1179A mice and eNOS−/− mice. However, both WT and eNOS−/− mice that experienced seizures had similarly severe pulmonary injury, suggesting that the toxic effect of NO from nNOS masks the beneficial effect of NO derived from eNOS.
We also surmise that nNOS is involved in CNS-mediated autonomic control of cardio-respiratory function leading to this distinct pattern of lung injury; this is because the β-adrenergic antagonist propranolol diminished lung injury in WT and eNOS−/− mice but not in nNOS−/− mice. Tiotropium bromide was protective for eNOS−/− mice but not for nNOS−/− or WT mice. Propranolol or tiotropium bromide, however, do not lower BALF protein in the WT or mutant mice exposed to 3 ATA O2 to protein levels found in WT mice breathing air.
There are cogent physiological reasons to suggest that neu-ronally derived NO is part of a neurogenic mechanism of pulmonary injury in HBO2. First, the lung’s innervation includes nonadrenergic and non-cholinergic systems using neurotransmitters that include NO, and immunohistochemical studies have revealed the presence of nNOS in nerve terminals supplying lung vessels and the lower airways (27, 28).
Second, NO is an important mediator in cardiac and pulmonary autonomic control, since numerous discrete neuronal populations possessing nNOS have been found within cardiorespiratory autonomic pathways (56, 57, 68). NO also stimulates neuronal activity within the nucleus tractus solitarius (63), activates central vagal motoneurons (14), and potentiates cardiac responses to vagal stimulation (64). NO released along the hypothalamic-medulla oblongata-sympathetic axis may also affect peripheral neuronal activity, since intracerebral administration of NO donors elicits alterations in blood pressure and heart rate via sympathetic neurons (17).
The ability of NO to exert discrete effects on the lung, even opposing effects, derives not only from the properties of the NO molecule itself but from the anatomical localization of the particular isozyme of NOS that produces it. Because molecular NO has a brief half-life in vivo, its scope of action may be restricted to a very short distance from its source of production, which minimizes cross talk between the various NO signaling pathways and delimits their actions to specific cells, tissues, or systems. An excellent example of the specificity of action conferred by anatomical localization is the fact that eNOS in the brain appears to potentiate oxygen seizures due to a NO-mediated increase in cerebral blood flow (12), whereas eNOS in the lung is protective against pulmonary oxygen toxicity in HBO2, as shown in this study. But, in both cases, NO derived from eNOS affects cerebral or pulmonary vessels locally, where it is produced. NO derived from iNOS has the same local effect on the lung in NBO2, whereas nNOS in particular has the ability to interact with many other physiological systems, mediated by the nervous system. Thus, localization and interaction may account for many of the discrete effects on the lung in hyperoxia that are correlated with the expression or nonexpression of each of the three NOS isozymes.
In summary, evidence presented here supports the idea that NO can be key factor in the development of pulmonary oxygen toxicity and that among NOS isoforms, iNOS predominates in lung injury induced by normobaric oxygen, whereas nNOS assumes a major role in hyperbaric oxygen. We also observed that eNOS may protect the lung from hyperoxia. Further disentanglement of the discrete effects of NO derived from each NOS isoform may permit a more complete elucidation of the mechanisms by which the lung is injured by oxidative stress, or is protected, by mobilizing NO from some sites and suppressing it at others.
Acknowledgments
GRANTS
This work was sponsored by the Office of Naval Research (to C. A. Piantadosi), by PO-1-42444 (to C. A. Piantadosi), by Russian Foundation for Basic Research Grant 06-04-49697 (to I. T. Demchenko), and by National Institute of Neurological Disorders and Stroke Grants RO-1 NS-333335 and RO-1 NS-S48246 (to P. L. Huang).
REFERENCES
- 1.Arkovitz M, Szabo C, Garcia V, Wong H, Wispe J. Differential effects of hyperoxia on the inducible and constitutive isoforms of nitric oxide synthase in the lung. Shock. 1997;5:345–350. doi: 10.1097/00024382-199705000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Atochin DN, Wang A, Liu VWT, Critchlow JD, Dantas APV, Looft-Wilson R, Murata T, Salomone S, Shin HK, Ayata C, Moskowitz MA, Michel T, Sessa WC, Huang PL. The phosphorylation state of eNOS modulates vascular reactivity and outcome of cerebral ischemia in vivo. J Clin Invest. 2007;117:1961–1967. doi: 10.1172/JCI29877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balentine JD. Pathology of Oxygen Toxicity. New York: Academic Press; 1982. [Google Scholar]
- 4.Bean J, Zee D, Thom B. Pulmonary changes with convulsions induced by drugs and oxygen at high pressure. J Appl Physiol. 1966;21:865–872. doi: 10.1152/jappl.1966.21.3.865. [DOI] [PubMed] [Google Scholar]
- 5.Bean JW. Effects of oxygen at increased pressure. Physiol Rev. 1945;25:1–147. [Google Scholar]
- 6.Capellier G, Maupoli V, Boillot A, Kantelip JP, Rochette L, Regnard J, Barale F. l-NAME aggravates pulmonary oxygen toxicity in rats. Eur Respir J. 1996;9:2531–2536. doi: 10.1183/09031936.96.09122531. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson LM, Jonsson J, Edlund T, Marklund SL. Mice lacking extracellular superoxide dismutase are more sensitive to hyperoxia. PNAS. 1995;92:6264–6268. doi: 10.1073/pnas.92.14.6264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clark JM, Lambertsen CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971;23:37–133. [PubMed] [Google Scholar]
- 9.Clark JM, Thom SR. Oxygen under pressure. In: Brubakk AO, Newman TS, editors. Bennett and Elliott’s Physiology and Medicine of Diving. Flagstaff, AZ: Best Publishing Company; 2003. [Google Scholar]
- 10.Crapo JD. Morphologic changes in pulmonary oxygen toxicity. Ann Rev Physiol. 1986;48:721–731. doi: 10.1146/annurev.ph.48.030186.003445. [DOI] [PubMed] [Google Scholar]
- 11.Crapo JD. Oxidative stress as an initiator of cytokine release and cell damage. Eur Respir J. 2003;22:4–6. doi: 10.1183/09031936.03.00000203a. [DOI] [PubMed] [Google Scholar]
- 12.Demchenko IT, Boso AE, Whorton AR, Piantadosi CA. Nitric oxide production is enhanced in rat brain before oxygen-induced convulsions. Brain Res. 2001;917:253–261. doi: 10.1016/s0006-8993(01)03057-8. [DOI] [PubMed] [Google Scholar]
- 13.Demchenko IT, Welty-Wolf KE, Allen BW, Piantadosi CA. Similar but not the same: normobaric and hyperbaric pulmonary oxygen toxicity, the role of nitric oxide. Am J Physiol Lung Cell Mol Physiol. 2007;293:L229–L238. doi: 10.1152/ajplung.00450.2006. [DOI] [PubMed] [Google Scholar]
- 14.Fisher AB, Forman HJ, Glass M. Mechanisms of pulmonary oxygen toxicity. Lung. 1984;162:255–259. doi: 10.1007/BF02715655. [DOI] [PubMed] [Google Scholar]
- 15.Freeman BA, Crapo JD. Hyperoxia increases oxygen radical production in rat lungs and lung mitochondria. J Biol Chem. 1981;256:10986–10992. [PubMed] [Google Scholar]
- 16.Giaid A, Saleh D. Reduced expression of endothelial nitric oxide synthase in the lungs of patients with pulmonary hypertension. N Engl J Med. 1995;333:214–221. doi: 10.1056/NEJM199507273330403. [DOI] [PubMed] [Google Scholar]
- 17.Ho YS, Magnenat JL, Bronson RT, Cao J, Gargano M, Sugawara M, Funk CD. Mice deficient in cellular glutathione peroxidase develop normally and show no increased sensitivity to hyperoxia. J Biol Chem. 1997;272:16644–16651. doi: 10.1074/jbc.272.26.16644. [DOI] [PubMed] [Google Scholar]
- 18.Ho YS, Dey MS, Crapo JD. Antioxidant enzyme expression in rat lungs during hyperoxia. Am J Physiol Lung Cell Mol Physiol. 1996;270:L810–L818. doi: 10.1152/ajplung.1996.270.5.L810. [DOI] [PubMed] [Google Scholar]
- 19.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell. 1993;75:1273–1286. doi: 10.1016/0092-8674(93)90615-w. [DOI] [PubMed] [Google Scholar]
- 20.Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA, Fishman MC. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 21.Jamieson D, Cass N. CNS and pulmonary damage in anesthetized rats exposed to hyperbaric oxygen. J Appl Physiol. 1967;23:235–242. doi: 10.1152/jappl.1967.23.2.235. [DOI] [PubMed] [Google Scholar]
- 22.Jamieson D, Chance B, Cadenas E, Boveris A. The relation of free radical production to hyperoxia. Ann Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- 23.Jamieson D, Van Den, Brenk HA. Pulmonary damage due to high pressure oxygen breathing in rats. Aust J Exp Biol Med Sci. 1962;40:309–314. doi: 10.1038/icb.1962.34. [DOI] [PubMed] [Google Scholar]
- 24.Kelm M, Dahmann R, Wink D, Feelisch M. The nitric oxide/superoxide assay, insights into the biological chemistry of the interaction. J Biol Chem. 1997;272:9922–9932. doi: 10.1074/jbc.272.15.9922. [DOI] [PubMed] [Google Scholar]
- 25.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med. 2003;167:1600–1619. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 26.Klein J. Normobaric pulmonary oxygen toxicity. Anesth Analg. 1990;70:195–207. doi: 10.1213/00000539-199002000-00012. [DOI] [PubMed] [Google Scholar]
- 27.Kobzik L, Bredt DS, Lowenstein CJ, Drazen J, Gaston B, Sugarbaker D, Stamler JS. Nitric oxide synthase in human and rat lung: immunocy-tochemical and histochemical localization. Am J Respir Cell Mol Biol. 1993;9:1044–1549. doi: 10.1165/ajrcmb/9.4.371. [DOI] [PubMed] [Google Scholar]
- 28.Kubota EY, Hamasaki T, Sata T, Saga H, Said SI. Autonomic innervations of pulmonary artery: evidence for a nonadrenergic noncholinergic inhibitory system. Exp Lung Res. 1988;14:349–358. doi: 10.3109/01902148809087813. [DOI] [PubMed] [Google Scholar]
- 29.Laubach VE, Shesely EG, Smithies O, Sherman PA. Mice lacking inducible nitric oxide synthase are not resistant to lipopolysaccharide-induced death. Proc Natl Acad Sci. 1995;92:10688–10692. doi: 10.1073/pnas.92.23.10688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li YF, Jackson KL, Stern JE, Rabeler B, Patel KP. Interaction between glutamate and GABA systems in the integration of sympathetic outflow by the paraventricular nucleus of the hypothalamus. Am J Physiol Heart Circ Physiol. 2006;291:H2847–H2856. doi: 10.1152/ajpheart.00625.2005. [DOI] [PubMed] [Google Scholar]
- 31.Lin MI, Fulton D, Babbitt R, Fleming I, Busse R, Pritchard KA, Jr, Sessa WC. Phosphorylation of threonine 497 in endothelial nitric-oxide synthase coordinates the coupling of L-arginine metabolism to efficient nitric oxide production. J Biol Chem. 2003;278:44719–44726. doi: 10.1074/jbc.M302836200. [DOI] [PubMed] [Google Scholar]
- 32.Mantell LL, Horowitz S, Davis JM, Kazzaz JA. Hyperoxia-induced cell death in the lung–the correlation of apoptosis, necrosis, and inflammation. Ann NY Acad Sci. 1999;887:171–180. doi: 10.1111/j.1749-6632.1999.tb07931.x. [DOI] [PubMed] [Google Scholar]
- 33.Mantell LL, Lee PJ. Signal transduction pathways in hyperoxia-induced lung cell death. Mol Genet Metab. 2000;71:359–370. doi: 10.1006/mgme.2000.3046. [DOI] [PubMed] [Google Scholar]
- 34.Mineo C, Yuhanna IS, Quon MJ, Shaul PW. High density lipoprotein-induced endothelial nitric-oxide synthase activation is mediated by Akt and MAP kinases. J Biol Chem. 2003;278:9142–9149. doi: 10.1074/jbc.M211394200. [DOI] [PubMed] [Google Scholar]
- 35.Patel KP, Li YF, Hirooka Y. Role of nitric oxide in central sympathetic outflow. Exp Biol Med. 2001;226:814–824. doi: 10.1177/153537020122600902. [DOI] [PubMed] [Google Scholar]
- 36.Patel YJ, Gowdey CW. Effects of single and repeated exposures to oxygen at high pressure on normal rats. Can J Physiol Pharmacol. 1964;42:245–264. doi: 10.1139/y64-028. [DOI] [PubMed] [Google Scholar]
- 37.Pepperl S, Dorger M, Ringel F, Kupatt C, Krombach F. Hyperoxia upregulates the NO pathway in alveolar macrophages in vitro: role of AP-1 and NF-κB. Am J Physiol Lung Cell Mol Physiol. 2001;280:L905–L913. doi: 10.1152/ajplung.2001.280.5.L905. [DOI] [PubMed] [Google Scholar]
- 38.Rhee SG, Kang SW, Netto LE, Seo MS, Stadtman ER. A family of novel peroxidases, peroxiredoxins. Biofactors. 1999;10:207–209. doi: 10.1002/biof.5520100218. [DOI] [PubMed] [Google Scholar]
- 39.Shapoval LN. Nitric oxide: involvement in the nervous control of cardiovascular function. Neurophysiology. 2004;36:418–431. [Google Scholar]
- 40.Shapoval LN, Sagach VF, Pobegailo LS. Nitric oxide influences ventrolateral medullary mechanisms of vasomotor control in the cat. Neurosci Lett. 1991;132:47–50. doi: 10.1016/0304-3940(91)90430-2. [DOI] [PubMed] [Google Scholar]
- 41.Simon A, Torbati D. Effect of hyperbaric oxygen on heart, brain and lung functions in rat. Undersea Biomed Res. 1982;9:263–274. [PubMed] [Google Scholar]
- 42.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 43.Steudel W, Watanabe M, Dikranian K, Jacobson M, Jones RC. Expression of nitric oxide synthase isoforms (NOS II and NOS III) in adult rat lung in hyperoxic pulmonary hypertension. Cell Tissue Res. 1999;295:317–329. doi: 10.1007/s004410051238. [DOI] [PubMed] [Google Scholar]
- 44.Swanson LW, Sawchenko PE. Hypothalamic integration. Organization of the paraventricular and supraoptic nuclei. Ann Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- 45.Travagli RA, Gillis RA. Nitric oxide-mediated excitatory effect on neurons of dorsal motor nucleus of vagus. Am J Physiol Gastrointest Liver Physiol. 1994;266:G154–G160. doi: 10.1152/ajpgi.1994.266.1.G154. [DOI] [PubMed] [Google Scholar]
- 46.Turanlahti M, Pesonen E, Lassus P, Andersson S. Nitric oxide and hyperoxia in oxidative lung injury. Acta Paediatr. 2000;89:966–970. doi: 10.1080/080352500750043440. [DOI] [PubMed] [Google Scholar]
- 47.Tyler RC, Muramatsu M, Abman SH, Stelzner TJ, Rodman DM, Bloch KD, McMurtry IF. Variable expression of endothelial NO synthase in three forms of rat pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 1999;276:L297–L303. doi: 10.1152/ajplung.1999.276.2.L297. [DOI] [PubMed] [Google Scholar]
- 48.Van Den Brenk, Jamieson D. Pulmonary damage due to high pressure O2 breathing in rats. Australian J Exptl Biol. 1962;40:37–50. doi: 10.1038/icb.1962.34. [DOI] [PubMed] [Google Scholar]
- 49.Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- 50.Wang Y, Phelan SA, Manevich Y, Feinstein SI, Fisher AB. Transgenic mice overexpressing peroxiredoxin 6 show increased resistance to lung injury in hyperoxia. Am J Respir Cell Mol Biol. 2006;34:481–486. doi: 10.1165/rcmb.2005-0333OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wood JD, Stacey NE, Watson WJ. Pulmonary and central nervous system damage in rats exposed to hyperbaric oxygen and protection therefrom by gamma-aminobutyric acid. Can J Physiol Pharmacol. 1965;43:405–410. doi: 10.1139/y65-040. [DOI] [PubMed] [Google Scholar]