Abstract
Cell therapy is a promising option for treating ischemic diseases and heart failure. Adult stem and progenitor cells from various sources have experimentally been shown to augment the functional recovery after ischemia and clinical trials confirmed that autologous cell therapy using bone marrow-derived or circulating blood-derived progenitor cells is safe and provides beneficial effects. However, aging and risk factors for coronary artery disease affect the functional activity of the endogenous stem/progenitor cell pools, thereby, at least partially limiting the therapeutic potential of the applied cells. In addition, age and disease affect the tissue environment, in which the cells are infused or injected. The present review article will summarize current evidence for cell impairment during aging and disease but also discuss novel approaches how to reverse the dysfunction of cells or to refresh the target tissue. Pre-treatment of cells or the target tissue by small molecules, polymers, growth factors or combination thereof may provide useful approaches for enhancement of cell therapy for cardiovascular diseases.
Keywords: Angiogenesis, Diabetes, Stem cells
1. Introduction
Cell therapy using stem or progenitor cells is a promising option to improve the functional recovery after ischemia and restoration of heart function in patients with heart failure. Stem cells were considered to possess unlimited self-renewal capacity and to be able to replace themselves throughout the lifespan of the organism 1. However, it has recently been shown that a decrease in stem cell function plays a primary role in the pathogenesis of multiple diseases and tissue aging 2. Organ aging typically results in a marked decrease in the number of functionally competent stem cells dictated by forced entry of these cells in an irreversible quiescent state 3–5. DNA damage and apoptosis increase in aging stem cells and these defects reduce further the pool of undifferentiated cells 6. In addition, risk factors for cardiovascular diseases such as diabetes and heart failure itself affect endogenous progenitor cells 7–10, thereby, impairing endogenous repair and reducing the efficacy of patient-derived cells for therapeutic purposes. This review will summarize the impact of age and disease on bone marrow-derived and tissue-resident progenitor cells.
2. Impact of disease and aging on bone marrow-derived and circulating progenitor cells
Cell therapy with bone marrow-derived stem/progenitor cells is a novel option for improving neovascularization and cardiac function in ischemic heart disease. The bone marrow contains different types of stem cells. Hematopoietic stem / progenitor cells (HPC/HSC), defined as CD34+ cells in humans or c-kit+ Sca-1+ lin− cells in mice, and mesenchymal stem cells (MSC) have been successfully used to improve neovascularization and functional recovery in ischemic models. In addition, circulating hematopoietic or endothelial progenitor cells (EPC) (including pro-angiogenic cells), which can be mobilized from the bone marrow, were shown to give rise to new blood vessels and provide beneficial effects in vivo 11, 12. Clinically, most studies so far used bone marrow-derived mononuclear cell preparation (BMC), which contain HSC, EPC and - although to a very low extent – MSC (for review see 13). In addition, ex vivo cultured EPC, G-CSF-mobilized and purified circulating CD34+ and cultured MSC were clinically applied. Although the mechanism of action may differ between HPC, MSC, and EPC, the beneficial effects regarding functional recovery after ischemia were comparable in most if not all experimental studies. The initial pre-clinical studies have used human cells isolated from healthy, young humans or young mice. When investigators started to isolate patient-derived cells, it was obvious that the number and functional activity of the cells are significantly impaired in comparison to healthy control 7, 9. In addition to age, which is known to affect stem cells functions, risk factors for coronary artery disease (CAD) and heart failure diminish the capacity of the bone marrow-derived cell to contribute to functional repair (Figure 1). Although patients usually are exposed to more than one risk factor, the next paragraph will discuss the impact of the individual risk factors and disease entities on endogenous bone marrow-derived and circulating cells.
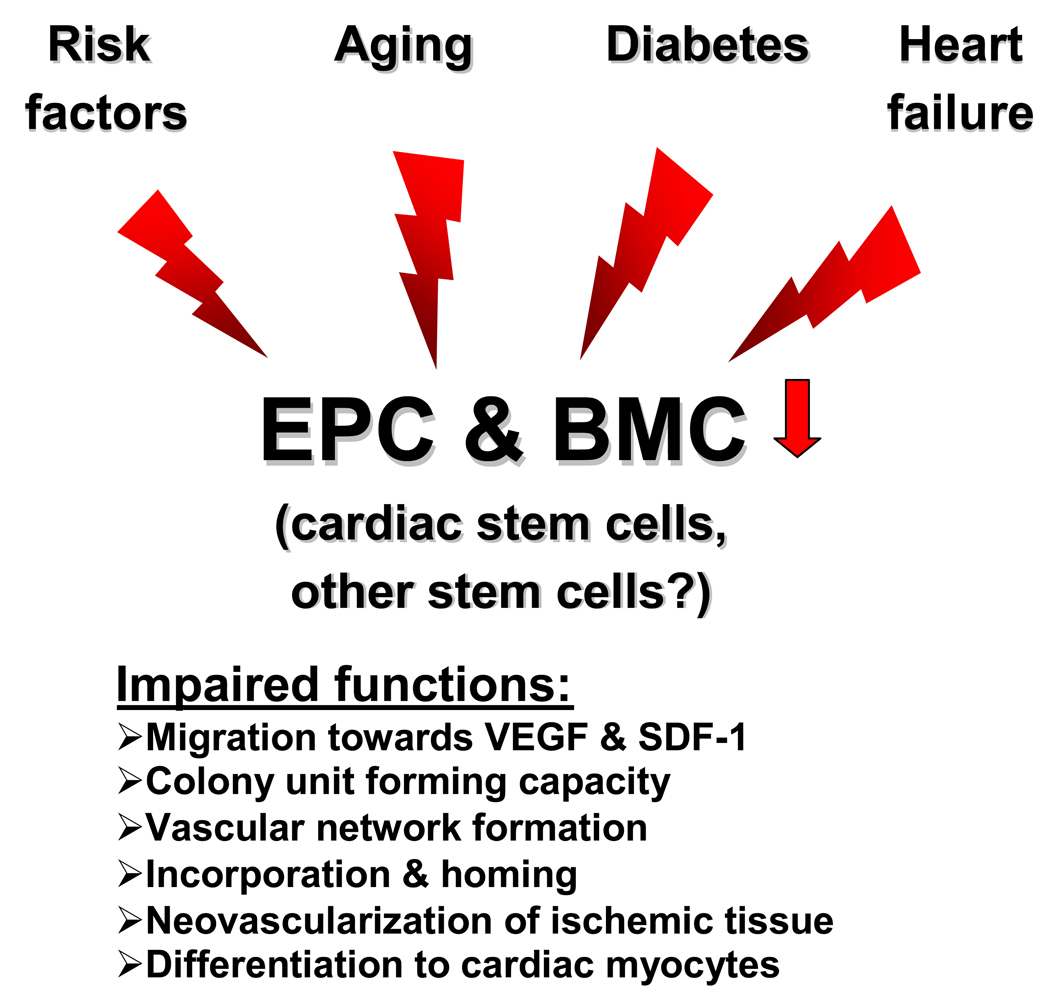
Figure 1. Effects of disease and aging.
Risk factors for coronary artery disease, diabetes, aging and heart failure affect the functional activity of endothelial progenitor cells (including pro-angiogenic cells) and bone marrow-derived mononuclear cells in experimental and clinical studies. The effect on cardiac stem cells has only been studied in animal models and the impact on other stem cell populations is unclear.
2.1. Impact of cardiovascular risk factors
Diabetes is one of the key risk factors for CAD and its prevalence is increasing over the last years. The analysis of cultured EPC and CD34+KDR+ cells and their functions in clinical studies, identified type II diabetes as a major determinant of impairment. Patients with type I and type II diabetes exhibit lower number of CD34+KDR+ EPC or cultured EPC 14–16 and the reduced number of CD34+KDR+ cells was associated with the severity of diabetic vasculopathy 17.
These results were consistent with animal experiments with obese diabetic mice (Lerdb), in which the function of progenitor and pro-angiogenic cells were impaired 18, 19. Of note, diabetes was not only associated with a reduction of cell numbers but lead to a profound impairment of cell functionality such as reduced migration towards cytokines, reduced proliferation and reduced ability of the cells to integrate into vascular networks in vitro 9, 15. The impaired migratory response resembled the previously shown diminished response of diabetic monocytes to vascular endothelial growth factor (VEGF) 20. Additionally, an increased sensitivity towards apoptosis or stress response of diabetic cells was described in some studies 9, 18 and high glucose concentrations induce senescence in cultured EPC 21. If cells isolated from patients with diabetes were used to therapeutically enhance blood flow recovery in ischemia models, transplanted cells were significantly less effective compared to healthy control-derived cells 22, 23. Likewise, the capacity of diabetic patient-derived cells to reendothelialize denuded arteries was impaired 24. In a skin wound assays, bone marrow-derived cells enriched for progenitor cells (lineage− fraction) isolated from obese diabetic mice even decreased vascularization 25. Such an inhibitory effect might be explained by the release of anti-angiogenic factors (such as thrombospondin), which are increased in diabetic EPC 19.
Similar to the impaired function of circulating or bone marrow-derived cells by diabetes, other risk factors such as hypercholesterolemia 26 and hypertension 26, 27 also were associated with reduced and dysfunctional circulating EPC. In addition, circulating CD34+KDR+ or CD34+CD133+KDR+CD45low cells were inversely correlated with smoking 7, 28 and this reduction was reversed by smoking cessation 28.
What are the mechanisms by which risk factors mediate their negative effect on progenitor cells? It appears that the signaling pathways mediating progenitor cell impairment are similar to the previously identified regulators of endothelial cell function and atherosclerosis and include a dysregulation of nitric oxide (NO) and reactive oxygen species (ROS). A diabetic environment or high glucose exposure in vitro is associated with reduced nitric oxide (NO) bioavailability in cultured EPC and plasma levels of endogenous NO-synthase inhibitors (asymmetric dimethyl-L-arginine) are associated with clinically reduced EPC numbers 29. In experimental studies, endothelial NO-synthase (eNOS)-derived NO was shown to be essential for basal, VEGF-, and SDF-1-induced migration of EPC or bone marrow-derived progenitor cells and eNOS−/− progenitor cells showed a reduced homing capacity in ischemia models in vivo 30, 31. The underlying mechanisms mediating the NO effects, e.g. on chemokines or integrin expression or signaling need to be further defined. Despite various studies supporting an important role of eNOS for progenitor cell mobilization and function 30, 32, 33, under certain conditions, eNOS uncoupling may lead to an increased ROS production 16. To what extent the redox balance in stem cells (as opposed to cultured EPC or crude bone marrow homogenates used in the study 16) favors such uncoupling processes is unclear and deserves further studies.
The impact of reactive oxygen species (ROS) appears more complex. Several studies demonstrated that ROS production is increased in patient-derived cells and that ROS production is associated with functional impairment of progenitor cells 34, 35. A putative role of ROS for stem/progenitor cell dysfunction is supported by studies demonstrating that chemically-induced oxidative stress limits the life span of HSCs 36. However, other studies failed to reverse glucose-induced early and outgrowing EPC dysfunction by antioxidant treatment 37. It might be that the differential response is caused by the use of cells equipped with a different anti-oxidative defense system. For example cultured early EPC were shown to express high levels of Mn-superoxide dismutase and catalase 38, 39, which might render this specific subtype of cultured cell less prone to ROS-induced damage or dysfunction. Likewise, the analysis of the transcriptome of stem cells showed an enrichment of genes promoting resistance to environmental stresses 40. Moreover, physiological concentrations of ROS might be critically involved in migration and mobilization of progenitor cells, thereby, ROS might interfere with stem cell homeostasis on different levels resulting in opposing effects. Depending on the conditions (e.g. diminished antioxidative defense, excessive prolonged oxidative stress) the positive effect of endogenous ROS may be overridden by the harmful effects 41.
Risk factors and particularly diabetes additionally activate pro-inflammatory protein kinases such as the mitogen activated protein (MAP) kinase p38, thereby, reducing proliferation and differentiation of cultured EPC 22. Activation of p38 might also be a consequence of ROS production 36, although this link has not been experimentally demonstrated under diabetic conditions.
Finally, risk factors might profoundly affect the therapeutic benefit of cells by interfering with homing functions of progenitor cells. Only a few studies so far directly addressed the impact of risk factors on the complex set of processes mediating progenitor cell homing, which involve chemokine-induced attraction of cells and protease-mediated invasion and matrix degradation. Of the multiple steps involved in homing and recruitment, the interaction of the ischemia-induced cytokine stromal-derived factor-1 (SDF-1) with its receptor CXCR4, which is expressed on HSC and EPC, is crucial for cell-recruitment 42, 43. This critical pathway appears to be impaired in EPC cultured from coronary artery disease patients, which do not adequately responds to activation of the CXCR4 receptor 44. Reduction of CXCR4 expression in heterozygote mice to 50 % reduced basal and SDF-1-induced migration and abolished in vivo homing 44. Therefore, one may speculate that the defective CXCR4 signaling in patient-derived cells contributes to the migration and homing defect, which is associated with a reduced clinical benefit of cell therapy in pilot trials 45.
In summary, ample experimental studies and clinical observations provide evidence that cardiovascular risk factors interfere with circulating progenitor and pro-angiogenic cells. An important question is whether the impairment of circulating progenitor cells and pro-angiogenic cells in patients with high risk factor load is caused by a depletion of stem cell reserves and stem cell exhaustion in the bone marrow or might be due to signaling defects and increased apoptosis of circulating cells. Limited studies are available to answer this question particularly because in the clinical setting bone marrow-derived cells are not as easily accessible as circulating cells and classical stem cell assays (e.g. repopulation activity) with cells isolated from high risk patients have not been performed so far.
2.2. Impact of age and telomere shortening
In healthy individuals and patients with coronary artery disease, age is associated with a reduced number and function of cultured EPC, circulating CD34+KDR+ cells or CD133+ cells and of granulocyte macrophage colony forming units (GM-CFUs) in the bone marrow 7, 46, 47. First evidence that age directly affects cell-mediated improvement of neovascularization was provided by Edelberg et al, who demonstrated that young, but not old, bone marrow cells incorporated into the neovasculature and restored cardiac angiogenic functions 48. On a molecular level aging is linked to a reduction of telomere length. The proliferative history of a cell is written on telomeres: telomere erosion reflects the number of past divisions experienced by a cell and its proliferative potential 49. In addition, telomere erosion may contribute to telomere shortening. When long telomeres protect chromosomal ends, cells can undergo repeated cell divisions. Conversely, telomere shortening beyond a critical length leads to genomic instability, DNA damage, p53 activation and ultimately cell cycle arrest 50. Telomere shortening as it occurs with aging is associated with replicative exhaustion and reduced repopulation of HSC 51. Aging also is associated with a reduction of telomere length in circulating but also in bone marrow-derived cells 52. Interestingly, telomere length in circulating blood leukocytes and bone marrow-derived cells was not only associated with age but also reduced in patients with coronary artery disease or heart failure indicating that cardiovascular disease promotes telomere shortening 53, 54. While the limited number of cells available in the patient study precludes any information on the impact of age on telomere length of freshly isolated specific subpopulations such as CD34+ cells (or other stem cell populations), the reduction of telomere length in patient-derived cells was associated with a reduced ex vivo migratory response of the total BMC towards the chemokines VEGF and SDF-1 indicating that age-associated telomere reduction may contribute to impaired functionality of the cells 52. In addition, a reduced telomere length was shown in cultured EPC from coronary artery disease patients with metabolic syndrome 55. Aging also was associated with a reduction of CD34+CD133+ mobilization in patients undergoing coronary artery bypass surgery 56 indicating that aging might either affect the cytokine mileu and/or the signaling in the bone marrow stem cell niche to release the progenitor cells. In summary, experimental and clinical studies demonstrated that aging interferes with progenitor cell functions. To what extent the dysfunction is exclusively related to age-associated telomere shortening and intrinsic cell dysfunction (e.g. senescence) or might also involve age-dependent changes in paracrine activities remains to be defined.
2.3. Impact of heart failure
The ultimate goal of cardiac cell therapy is to treat patients with heart failure, which remains one of the major causes of mortality in the industrialized world despite optimized pharmacological and interventional technologies. Clinical trials successfully used autologous bone marrow-derived cells and demonstrated that bone marrow-derived mononuclear cells significantly increased left ventricular ejection fraction and reduced the levels of NT-pro-BNP, a prognostically relevant biomarker 57–60. However, several studies indicate that heart failure affects progenitor cell functions. A side-by-side comparison of bone marrow-derived and circulating blood derived cells in acute versus chronic heart failure showed a significantly diminished response of heart failure patients towards circulating progenitor cells 59, while in patients with acute myocardial infarction similar effects were detected 61. Of course, such differences in early clinical trials should be discussed with caution, however, one may speculate that the number and functional activity of the circulating progenitor cells (without mobilization) is not sufficient in heart failure patients to provide a beneficial effect. Mobilization of additional cells by cytokines may be a way to overcome these hurdles 62. However, when analyzing the number and functionality of bone marrow-derived cells as one of the reservoirs of endogenous stem cells, heart failure affected these cells as well. BMC isolated from bone marrow aspirates of patients with ischemic heart failure were less effective in improving recovery of blood flow after hind limb ischemia compared to healthy control 8. Detailed comparison of the composition and function of BMC demonstrated that the number of GM-CFUs was significantly lower in patients with ischemic heart failure compared to healthy controls 8, 47. One may argue that the observed dysfunction might be simply the consequence of CAD and the risk factor load discussed above. Indeed, the number of GM-CFU in the bone marrow was slightly higher in dilative compared to ischemic heart failure patients 47. However, statistical analysis revealed that chronic heart failure was an independent predictor of bone marrow cell impairment in this patient population, while the cardiovascular risk factors were statistically not predictive. These findings need to be confirmed in larger scale prospective studies and the molecular basis has to be further investigated.
3. Impact of disease and aging on tissue-resident stem cells
Various experimental studies document that tissue-resident stem or progenitor cells improve recovery after ischemia. The recognition that the adult heart possesses a pool of resident cardiac progenitor cells (CPCs), which are self-renewing, clonogenic and multipotent 63–65, has dictated a new area of research offering the potential to harvest cells, which are primed to acquire a cardiac phenotype and, therefore, might be optimally suited for cardiac repair. Several different populations have been identified and characterised: c-Kit+ cells 63, Sca-1+ cells 64, side population cells 66 and cells expressing the protein Islet-1 67. Whereas c-Kit+ cells, Sca-1+ cells and cardiac SP cells are isolated from adult hearts, cells expressing Islet-1 so far only have been detected in the post-natal stage. Whether c-Kit+, Sca-1+ and cardiac SP cells comprise three different cell populations is not entirely resolved. At least Sca-1+ cells contain a fraction of SP cells and among cardiac SP cells, the greatest potential for cardiomyogenic differentiation is restricted to cells positive for Sca-1 expression (but negative for CD31) 65 indicating that the two cell populations may share some common features. Cardiac stem cells also were obtained by growing self-adherent clusters (termed “cardiospheres”) from subcultures of murine or human biopsy specimens. Others have generated cardiac SP-cell derived cardiospheres by adapting a method used for creating neurospheres and claimed that cardiac neural crest cells contribute to cardiac SP cells 68. Cardiosphere-derived cardiac stem cells as well as c-Kit+ cardiac stem cells are capable of long-term self-renewal and can differentiate into the major specialized cell types of the heart: myocytes and vascular cells (i.e., cells with endothelial or smooth muscle markers). So far, the origin and the mechanisms maintaining the cardiac stem cell pool are unclear. Whereas two recent studies suggested that c-Kit+ and cardiac SP cells may derive from the bone marrow 69, 70, these studies cannot exclude that specific subpopulations of cardiac stem cells origin from distinct sources and may represent remnants from embryonic development in selected niches within the heart.
Adipose tissue is an additional source of distinct subsets of stem/progenitor cells potentially useful for cardiac repair and neovascularization improvement 71, 72. Both, mesenchymal stem cells and endothelial progenitor cells were isolated after enzymatic digestion of adipose tissue and showed beneficial effects in experimental studies. The therapeutic benefit of adipose-tissue derived cells is currently studied in two clinical trials.
While the relatively easy accessibility of circulating progenitor cells and the use of bone marrow-derived cells in clinical studies allowed getting first insights into the number and function of cells in patients with CAD or heart failure, the knowledge on the influence of disease and aging on CPC in humans is limited and the regulation of adipose tissue-derived cells during disease states is unknown. Therefore, this chapter will summarize the influence of heart failure, diabetes and aging on CPC homeostasis and function.
3.1. Impact of disease
Experimental studies demonstrated that the CPC pool size is expanded acutely after infarction. However, this growth response is attenuated in chronic heart failure 73. Myocyte division is markedly reduced in prolonged ischemic and dilated cardiomyopathy 73, 74 suggesting that a decrease in number and growth of CPCs underlies the attenuation in cell multiplication in end-stage heart failure. Telomerase activity in CPCs is decreased and cell proliferation is impaired by severe telomeric shortening and irreversible growth arrest 73. This replicative defect coupled with increased CPC apoptosis results in a decline of the pool of functional CPCs so that the formation of myocytes and coronary vessels can no longer counteract the chronic loss of parenchymal cells and vascular structures. This negative balance between myocardial regeneration and death may ultimately lead to progressive chamber dilation and deterioration of ventricular performance.
However, even under extreme conditions of depressed ventricular function, a pool of cycling and telomerase-competent CPCs can be found 73 and two independent laboratories have demonstrated that CPCs can be isolated from small samples of human myocardium and expanded in vitro with the purpose to be administrated back to the same patient 75, 76. Although the growth curves of cardiospheres isolated from right ventricular endomyocardial biopsies of 59 transplanted (age: 53.6 years with left ventricular ejection fraction (EF) 63.9 %) and 11 non-transplanted patients (age: 47; EF: 36.9 %) showed a considerable heterogeneity, this study did not identify a major independent determinant of cell yield after culture 75. These data clearly indicates that cardiospheres can be obtained from patients with reduced ejection fraction, however, a detailed comparison of the functional capacities of the isolated cells and a comparison with cardiospheres derived from healthy controls has not yet been published.
In a manner similar to ischemic cardiomyopathy, diabetes is coupled with a marked reduction of the pool size of CPCs. A typical feature of the diabetic heart is the development of a myopathy that deteriorates with time, independently from intercurrent vascular manifestations 77. This myopathy presents itself as ventricular dilation, relative wall thinning, and impaired diastolic and systolic function. The imbalance between cardiac cell death and cell regeneration characterizes the diabetic heart. However, the death of mature myocytes, SMCs and ECs is only a secondary phenomenon; the premature aging and death of CPCs precedes the progression of the diabetic myopathy 10. The accumulation of old myocytes is mediated by a dramatic loss of CPCs that markedly attenuates the formation and efficient turnover of parenchymal cells. Importantly, the adaptor protein p66shc plays a major role in the effects of diabetes on the heart. In fact, ablation of p66shc has remarkable beneficial consequences on the viability and function of CPCs, positively interfering with death stimuli and inhibition of CPC growth and differentiation. CPC division and myocyte generation are potentiated by the absence of p66shc, which opposes the development of a decompensated diabetic heart. Expansion of the CPC pool and myocyte progenitor precursors is combined with the preservation of cardiac performance, suggesting that an intact stem cell compartment can counteract the impact of uncontrolled diabetes on the myocardium. This observation may have important implications for the treatment of patients affected by diabetes and cardiac diseases. The deletion of p66shc may offer an early cardiac protection that could be exploited clinically. Diabetes, thus, negatively affects the growth reserve of the heart so that the enhanced cell death cannot be opposed by repopulating cells and preservation of the architecture and function of the myocardium.
3.2. Impact of aging
In old rats, chronological age leads to telomeric shortening in CPCs, which by necessity generate a differentiated progeny that rapidly acquires the senescent phenotype 5. The daughter cells inherit the shortened telomeres of the maternal CPCs and, after a few rounds of division, express the senescence-associated protein p16INK4a. The pool of old cardiomyocytes progressively decreases and ventricular function is impaired. However, telomerase competent CPCs with long telomeres are present in the regions of storage in the atria and apex and these cells, following activation by growth factors, migrate to areas of damage where they create a population of young myocytes reversing to some extent the aging myopathy structurally and functionally. The senescent heart phenotype is partially corrected and the improvement in cardiac hemodynamics results in prolongation of maximum lifespan in the rat model 5.
3.3. Mechanism of CPC impairment during disease and aging
The loss of CPC function with aging is mediated partly by an imbalance between factors promoting growth, migration and survival, and factors enhancing oxidative stress, telomere attrition and death. Three growth-factor receptor systems appear to play a major role in the development of CPC senescence and myocardial aging: IGF-1-IGF-1R, HGF-c-Met and the rennin angiotensin system (RAS). In the heart, the IGF-1-IGF-1R induces CPC division, upregulates telomerase activity, hinders replicative senescence and preserves the pool of functionally-competent CPCs 78–80. The expression of IGF-1R and the synthesis of IGF-1 are attenuated in aging CPCs and these negative variables diminish the ability of IGF-1 to activate cell growth and interfere with oxidative damage and telomeric shortening 81. Additionally, the expression and secretion of HGF in CPCs decreases as a function of age and this modification has a major impact on the migration of CPCs 73, 82–84 and thereby on the ability of these cells to translocate spontaneously to areas of damage and promote cardiac repair. Defects in these two autocrine-paracrine effector pathways of CPCs may have profound physiological consequences and may account for the chronological increase in myocyte death, myocardial scarring and depressed performance of the aging heart.
The documentation that the various components of RAS are present in CPCs and the formation of Ang II is enhanced in old cells provides evidence in favor of the role of this octapeptide in CPC senescence and death. Ang II may be a significant contributor of the age-dependent accumulation of oxidative damage in the heart 78, 85. Inhibition of Ang II function positively interferes with heart failure and prolongs life in humans 86. Ang II generates reactive oxygen species (ROS) and sustained oxidative stress triggers telomeric shortening and uncapping 87. Conversely, IGF-1 interferes with the generation of ROS 78, decreases oxidative damage in the myocardium with age 79, and can repair oxidative DNA damage by homologous recombination 88. Collectively, these findings suggest that cardiac aging is associated with a dysfunction of the endogenous stem cell pool that is dictated partly by the imbalance between RAS and IGF-1/HGF.
Effort has been made to identify the etiology of CPC death with diabetes. Diabetes promotes the generation of reactive oxygen species (ROS) and DNA damage 89. Oxidative stress and p53 activation have more dramatic effects on cells capable of reentering the cell cycle and dividing than cells which are permanently withdrawn from the cell cycle 10, 85, 90. The DNA damage mediated by oxidative stress rapidly initiates the apoptotic cell death pathway in CPCs while it accumulates with time in myocytes. CPCs undergo apoptosis and experience cell necrosis only occasionally. Conversely, myocyte apoptosis is followed by cell necrosis and foci of myocardial scarring. The differential response of CPCs and myocytes to diabetes results over a short period in a marked reduction of the pool size of CPCs that is several fold higher than that of myocytes. As discussed above, the adaptor protein p66shc plays a major role in the effects of diabetes on the heart 10. Ablation of p66shc has remarkable beneficial consequences on the viability and function of CPCs positively interfering with death stimuli and inhibition of CPC growth and differentiation. CPC division and myocyte generation are potentiated by the absence of p66shc opposing the development of the diabetic myopathy. Expansion of the CPC pool and myocyte progenitors-precursors is combined with the preservation of cardiac performance, further suggesting that an intact stem cell compartment can counteract the impact of uncontrolled diabetes on the myocardium.
It is likely that during aging and chronic diseases, stem cells tend to become quiescent. While quiescence in young active progenitor cells is modulated by p21Cip1 91 in old/diseased stem cells irreversible growth arrest is regulated by p16INK4a 92. Loss of telomerase, critical telomere shortening and increased nuclear expression of p53 and p16INK4a may all occur resulting in loss of growth reserve 2, 50. Early stem cell depletion may induce premature aging while replenishment of stem cells in depleted organs may reverse aging and disease promoting positive remodeling and recovery of function.
4. Influence of disease and aging on the target tissue
When transplanting cells in patients, one also has to take into account that the environment in which the cells are infused or injected might be affected, thereby, modulating homing, incorporation and differentiation cues. Little is known about the influence of disease and aging on homing and functional integration requiring the complex interaction of the injected cells with the environment. Such environmental changes could reduce the ability of transplanted cells to contribute to functional repair on various levels. Several studies suggested the homing signals might be impaired in disease. As discussed above, the cytokine SDF-1 is essential for the homing of endogenous and intravascularly infused progenitor cells. However, SDF-1 mRNA and SDF-1 positive cells were significantly reduced in wound tissue of diabetic mice 93. These experimental studies are supported by findings in patients with peripheral artery disease, where the expression of VEGF, SDF-1 and CXCR4 in human limb muscle was significantly suppressed even below the levels detected in controls suggesting a lack of recruitment signals in these patients 94.
Age per se also interferes with the environment and also decreased hypoxia-inducible factor 1a leading to diminished expression SDF-1 95. Interestingly, a reduction of telomere length in Terc-deficient mice was associated with a dysfunction of the bone marrow stem cell niche as evidenced by limited function and engraftment of transplanted wild type HSC to the bone marrow of Terc-deficient mice 96. A change in the cytokine milieu (increased G-GSF levels) was observed in the mutant mice indicating that a disturbed cytokine milieu might have contributed to the dysfunction. Whether a similar effect can be seen in other organs is unknown, however, it is known that aging and disease influence the cytokine profile in the heart. Despite the limited knowledge with this respect, it is conceivable that the composition of an established healed scar or fibrotic tissue in an old and chronically ill patient provides an entirely different milieu for the transplanted cells compared to the acute injury models (in young and healthy animals) usually used in the experimental studies.
5. Therapeutic strategies to interfere with disease and age related dysfunction
The demonstration of impaired cell function and environment in disease and aging demands the development of strategies to overcome these limitations. The use of heterologous cells might be an option to compensate for the reduction in cell functions; however, this strategy also comprises additional risks. Particularly, when heterologous cells are expected to functionally differentiate in cardiovascular cells, activation of the immune response or even cell rejection might occur proposing a high risk for the treated patients. The pre-treatment of the patients own cells to re-activate their functions and/or direct their differentiation might be an alternative to improve the efficiency of cell therapy in the chronically ill. Second, the treatment of the target tissue in order to provide the cytokines and chemoattractant factors to stimulate incorporation of the transplanted cells and / or to re-activate endogenous repair might be considered.
5.1. Pre-activation of cells
To compensate for the risk factor-induced cell impairment, several studies used pharmacological approaches to interfere with the elicitor. Of note, the need to improve survival and incorporation is not limited to the use of patient-derived cells and various approaches have been experimentally used to increase survival of cells derived from healthy controls, murine cells and embryonic stem cells (for review see 97). For example, overexpression of pro-survival kinases such as Akt or the telomerase subunit TERT, to compensate for age-associated telomere length reduction, significantly improved the functional recovery after AMI mediated by transplantation of MSC and EPC-mediated limb perfusion, respectively 98, 99.
Lipid lowering HMG-CoA reductase inhibitors (statins) were first reported to increase the number of EPC in patients and in experimental models 26, 33, 100, 101. In addition to the systemic effects, statins directly augmented the migratory capacity of EPC in vitro and pre-stimulation of EPC with statins increased their neovascularization-promoting effect. The mechanisms by which statins affect the in vitro functions of EPC involve the activation of the PI3-kinase and Akt and subsequent activation of the endothelial nitric oxide synthase (eNOS) 33, 100, 101. NO plays a crucial role in progenitor cell function and the expression of eNOS is required for bone marrow-derived mobilization of EPC 30, 32, 33, 102. Since NO bioavailability is systemically deteriorated in patients with coronary artery disease, an increased NO synthesis by statins may rescue the function of NO-deprived stem/progenitor cells. The nitric oxide system also was activated by compounds, which transcriptionally enhance eNOS expression in hematopoietic and endothelial progenitor cells 31. Specifically, the pre-treatment of patient-derived EPC or BMC with eNOS enhancers for 24 hours significantly enhanced the capacity of the intravenously infused cells to restored neovascularization, augmented the exercise capacity in a hind limb ischemia model and improved recovery after acute myocardial infarction 31. Other possibilities to augment eNOS expression and activity include the use of PPARγ agonists or systemic application of estrogen 102–104. Verma et al demonstrated that PPARγ agonists prevented the suppression of EPC function by C-reactive protein in a NO-dependent manner in vitro 103. Consistently, the PPARγ agonist rosiglitazone recently was shown to restore dysfunctional EPC from diabetic patients and improve the endothelial regenerating activities of infused progenitor cells 24. The diabetic impairment of NO-mediated progenitor cell functions leading to wound healing defects was rescued by hyperoxia and SDF-1 application 93. In diabetic patients-derived cells the blockade of kinases, which are up-regulated during disease such as the MAP-kinase p38, also might be an option to augment cellular functions. Pharmacological inhibitors of p38 indeed improved peripheral blood-derived cells isolated from patients with diabetes and cardiovascular diseases 22.
In addition to counteracting risk factor induced impairment and augment survival of injected cells, short term activation of receptors and signals involved in homing might be considered to improve the modest engraftment of cells. Since the SDF-1/CXCR4 axis is playing a crucial role in progenitor cell homing, several groups attempt to enhance this signaling cascade. In cells, which are characterized by low to absent expression of CXCR4 such as mesenchymal stem cells, overexpression of CXCR4 enhanced the in vivo engraftment to ischemic tissue 105. Activation of CXCR4 was used as an alternative approach in several cell types. CXCR4 was transactivated by sphingosin-1- phosphate (S1P) leading to enhanced EPC homing of patient-derived pro-angiogenic cells and improved recovery of blood flow in hind limb ischemia 23. Surprisingly, also pre-stimulation of the CXCR4 receptor directly by SDF-1 increased trans-endothelial migration of mesoangioblasts and stimulated the colonization and muscle fiber repair 106. Finally, integrin-dependent homing functions can be modulated by inflammatory cytokines such as the high mobility group box protein 1 (HMGB-1) 107 or by intracellular activation of integrin signaling 108. For example, pharmacologic activation of Epac1, a nucleotide exchange protein for Rap1, induce integrin polarization and activity and improves the adhesive and migratory capacity of distinct progenitor cell populations including human EPCs, CD34+ hematopoietic progenitor cells, and mesenchymal stem cells (MSCs) 108. Others identified an additional interesting ischemia-induced player, soluble E-selectin (s-E-selectin), which stimulates the homing of progenitor cells 109, while overexpression of the membrane form of E-selectin was shown to enhance the functional activity of endothelial progenitor cells 110. In summary, various strategies might be considered to enhance the engraftment and survival of cells applied to the heart leading to a second generation of cell therapy products. Which of the strategies will be chosen and will provide the best benefit, likely depends on the cell type and the delivery mode of the cells (Figure 2).
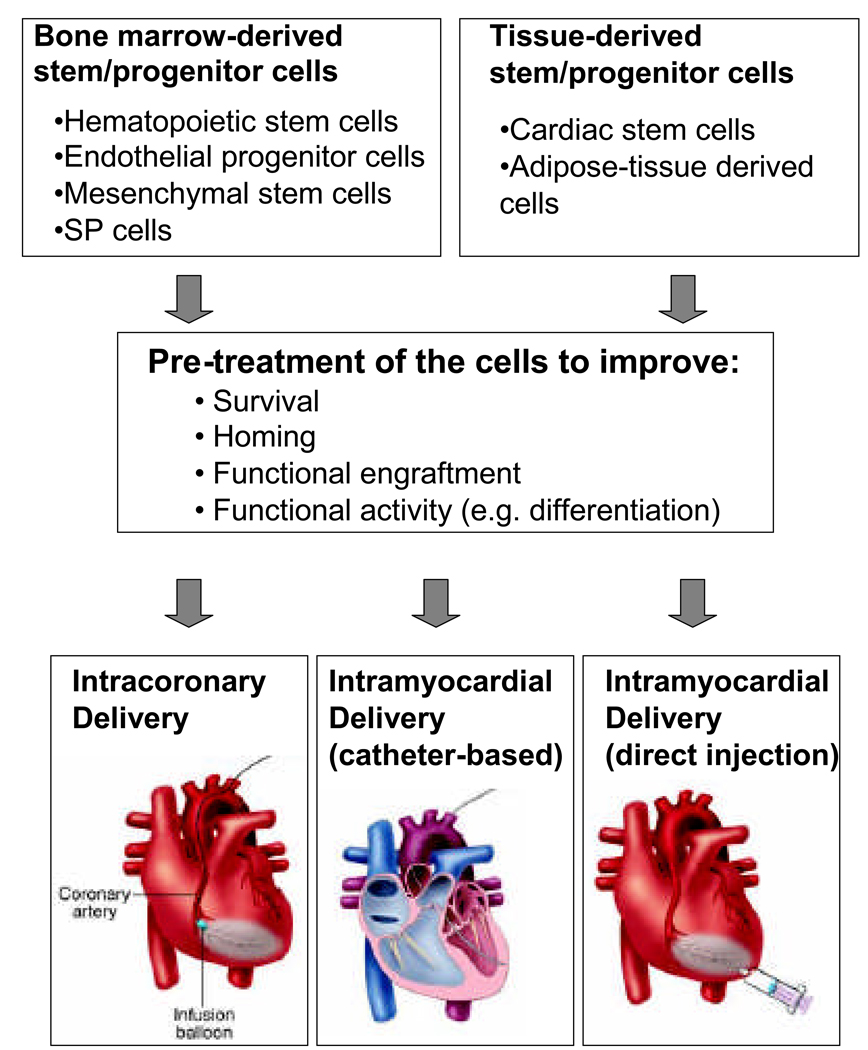
Figure 2. Cell enhancement strategy.
Stem/progenitor cells isolated from various sources can be pre-activated or modified to enhance incorporation and functional activity before being infused or injected in patients with acute or chronic heart disease. The optimal chose of the pre-treatment likely depends on the cell type and the mode of delivery. While cell survival appears a major limitation for intramyocardial delivery, homing functions are essential if cells are infused via the coronary arteries.
5.2. Treatment of the target tissue to improve homing and engraftment
The “refreshment” of the target tissue might become an important add-on to enhance recruitment and functional incorporation of any transplanted cell to the diseased, aged and chronically ill patient. Injection of cytokines might be used to attract progenitor cells in the absence of necrosis or acute ischemia (Table). Indeed, transplantation of syngeneic cardiac fibroblasts stably transfected to express SDF-1 induced homing of c-kit+ cells to the myocardium 43. An elegant approach to further enhance SDF-1 effects was used by Seger et al who generated a stabilized SDF-1 mutant 111. SDF-1 can be cleaved by exopeptidases and matrix metalloproteinase-2, thereby, generating an inactive and even harmful protein. By mutating the SDF-1 cleavage motif, the authors generated a cleavage resistant SDF-1 molecule and demonstrated that nanofiber delivery of the protease-resistant SDF-1 more efficiently improved cell recruitment and functional recovery after acute myocardial infarction compared to wild type SDF-1. Similarly, local myocardial delivery of insulin-like growth factor 1 (IGF-1) with biotinylated peptide nanofibers improved the effect of cell therapy with intramuscularly injected neonatal rat cardiomyocytes after myocardial infarction 80. Likewise, the IGF binding protein 3 either locally injected or when overexpressed in the injected cells, promotes proper vascular repair after hyperoxic insult mediated by hematopoietic and endothelial progenitor cells 112. Moreover, local injection of HMGB-1 enhanced the regeneration of infarcted myocardium by endogenous cardiac stem cells 113 and attracted systemically injected cells to muscle tissue 114.
Table 1.
Strategies to increase progenitor cell function, survival and homing
| Genes | Growth factors/Cytokines | Small molecules |
|---|---|---|
| Akt* | HMGB-1*,+ | Statins*;# |
| TERT* | Growth hormone # | PPARγ agonists *;# |
| Integrin-linked kinase* | PDGF* | Estrogen# |
| eNOS* | HGF+ | Nitric oxide enhancers* |
| GSK3-inhibitor* | IGF-1 (plus nanofibers)+ | p38 inhibitors* |
| IGF-1+ | SDF-1*,+ | EPAC activators* |
| IGF binding protein-3*;+ | Soluble E-selectin*;+ | Sphingosin-1-phosphate* |
| VEGF* | ||
treatment of the cells
systemic treatment
treatment of the target tissue
In addition to direct delivery of growth factors, it might be conceivable to activate the tissue by inducing the endogenous expression of cytokines. Indeed, activation of the tissue by low energy shock wave application stimulated the expression of SDF-1 and VEGF within the target tissue and promoted homing of intravenously infused EPC in uninjured and chronically ischemic rats 115.
5.3. Modulation of common endogenous modifiers of stem cell exhaustion and organism aging
Changing the environment may not only be useful to attract transplanted cells, but also might be an attractive approach to increase endogenous repair capacity. In fact, proliferative defects of old or diseased progenitor cells depend not only on cell-intrinsic mechanisms but also on structural alterations of the niches that host the undifferentiated cell compartment 96, 116, 117. This aspect has been studied in the skeletal muscle in which aged niches oppose the regenerative response of endogenous satellite cells and transplanted embryonic stem cells 116, 118. It is reasonable to assume that similar mechanisms are operative in the myocardium. Cardiac aging and diseases may have inhibitory effects on stem cell activation, migration and growth. Prevention of cell death and induction of cell replication are the ultimate goal of cell therapy; the former attenuates the extent of injury and the latter determines the degree of structural and functional recovery. This goal may be achieved not only by correcting the intrinsic defects of stem cells but also modifying the environmental niche.
Attempts have been made experimentally to document that changes of the cardiac milieu have critical effects on the regenerative ability of resident CPCs. Myocyte-restricted overexpression of IGF-1 is associated with delayed aging of CPCs and myocytes and retarded onset of ventricular dysfunction 79. IGF-1 promotes myocyte formation and positively interferes with the effects of myocardial infarction, chronic coronary artery constriction and diabetes on ventricular remodeling and heart failure. Similarly, a skeletal muscle specific IGF-1 isoform counteracts the decline in mass and functional performance in senescent IGF-1 transgenic mice and mdx mice suffering from Duchenne muscular dystrophy 119, 120. Growth hormone-induced increase in IGF-1 additionally improved age-associated dysfunction of EPC in experimental models and humans 121. Paradoxically, studies in nematodes or fruitflies demonstrated opposing effects of IGF-1 on aging and life span 122. The mechanisms underlying these controversial IGF-1 activities have not been clarified.
Another cytokine linked to cardiac repair and aging is the platelet-derived growth factor (PDGF). PDGF is down-regulated during aging and its supplementation reversed the senescent predisposition to increased cardiac injury 123. Interestingly, a reduced expression of PDGF was detected in aged Oct3/4+ bone marrow-derived stem cells and the age-dependent down-regulation of PDGF was associated with an impaired cardiac differentiation capacity of the cells 124.
In addition to insulin/IGF-1 and PDGF, an important modulator of lifespan and aging is the transmembrane protein Klotho 125. The extracellular domain of Klotho circulates in the blood 126 suggesting that this protein may function as an anti-aging hormone possibly influencing the regenerative response of tissue-resident stem cells in multiple organs. Klotho mutant mice have a very short lifespan and display a variety of aging-related phenotypes in multiple organs, including atherosclerosis, decreased fertility, impaired angiogenesis and skin atrophy 127. In contrast, the overexpression of Klotho in mice extends lifespan 125. This effect was initially linked to the attenuation of insulin/IGF-1-signaling 128 but the consequences of Klotho overexpression are more complex. Klotho increases the cellular resistance to oxidative stress by upregulation of manganese superoxide dismutase and is an essential cofactor of fibroblast growth factor signaling 128. It is difficult to establish which one of these effects is the determining variable of prolonged lifespan. A human homologue of Klotho has been identified and a functional variant of Klotho, termed KL-VS, is associated with human aging, reduced longevity and early-onset of coronary artery disease 129. In a recent study, Klotho deficiency was found to be coupled with enhanced Wnt signaling 130. Klotho binds to several members of the Wnt family of proteins and is a powerful repressor the Wnt pathway. This work has revealed a critical interaction among secreted factors, intracellular signaling pathways and stem cell function. In fact, chronic Wnt stimulation dictated by the absence of Klotho results in precocious senescence of skin stem cells and exhaustion of the long-term repopulating pool of hematopoietic stem cells in the bone marrow 130, 131. Of note, the effects of Wnts are complex and short term activation might be useful stimulate proliferation or modulate differentiation cues 132.
In summary, the mechanisms underlying organ and organism aging and the impact of disease on organ functions are complex and only in part understood. Caution has to be exercised in the translation of results in simple post-mitotic organisms to large mammals and particularly humans. This is because the life and death of most somatic organs in mammals is regulated by a stem cell compartment, which plays a critical role in aging and in the response of organs to disease. The complex relationship between stem cells and their surrounding tissue has to be considered as a critical determinant for the success of cell therapy. As such the impact of disease and age on endogenous stem and progenitor cells and on the environment may limit the benefit of cell therapy of the chronically ill patients; however, this also opens a new horizon for therapeutic strategies to counteract the dysregulated cell intrinsic and extrinsic signaling pathways.
Acknowledgments
Sources of Funding
S.D. is supported by the Leducq Foundation and the Deutsche Forschungsgemeinschaft (Exc 147/1 and Di600/6-3)
Footnotes
Disclosures: None.
References
- 1.Geiger H, Van Zant G. The aging of lympho-hematopoietic stem cells. Nat Immunol. 2002;3:329–333. doi: 10.1038/ni0402-329. [DOI] [PubMed] [Google Scholar]
- 2.Rossi DJ, Jamieson CH, Weissman IL. Stems cells and the pathways to aging and cancer. Cell. 2008;132:681–696. doi: 10.1016/j.cell.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 3.Sharpless NE, DePinho RA. Telomeres, stem cells, senescence, and cancer. J Clin Invest. 2004;113:160–168. doi: 10.1172/JCI20761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serrano M, Blasco MA. Cancer and ageing: convergent and divergent mechanisms. Nat Rev Mol Cell Biol. 2007;8:715–722. doi: 10.1038/nrm2242. [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Math S, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, Leri A. Activation of Cardiac Progenitor Cells Reverses the Failing Heart Senescent Phenotype and Prolongs Lifespan. Circ Res. 2008 doi: 10.1161/CIRCRESAHA.107.165464. [DOI] [PubMed] [Google Scholar]
- 6.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J, Weissman IL. Deficiencies in DNA damage repair limit the function of haematopoietic stem cells with age. Nature. 2007;447:725–729. doi: 10.1038/nature05862. [DOI] [PubMed] [Google Scholar]
- 7.Vasa M, Fichtlscherer S, Aicher A, Adler K, Urbich C, Martin H, Zeiher AM, Dimmeler S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ Res. 2001;89:E1–E7. doi: 10.1161/hh1301.093953. [DOI] [PubMed] [Google Scholar]
- 8.Heeschen C, Lehmann R, Honold J, Assmus B, Aicher A, Walter DH, Martin H, Zeiher AM, Dimmeler S. Profoundly Reduced Neovascularization Capacity of Bone Marrow Mononuclear Cells Derived From Patients with Chronic Ischemic Heart Disease. Circulation. 2004;109:1615–1622. doi: 10.1161/01.CIR.0000124476.32871.E3. [DOI] [PubMed] [Google Scholar]
- 9.Tepper OM, Galiano RD, Capla JM, Kalka C, Gagne PJ, Jacobowitz GR, Levine JP, Gurtner GC. Human endothelial progenitor cells from type II diabetics exhibit impaired proliferation, adhesion, and incorporation into vascular structures. Circulation. 2002;106:2781–2786. doi: 10.1161/01.cir.0000039526.42991.93. [DOI] [PubMed] [Google Scholar]
- 10.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res. 2006;99:42–52. doi: 10.1161/01.RES.0000231289.63468.08. [DOI] [PubMed] [Google Scholar]
- 11.Asahara T, Murohara T, Sullivan A Silver M, van der Zee R LiT, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- 12.Urbich C, Heeschen C, Aicher A, Sasaki KI, Bruhl T, Farhadi MR, Vajkoczy P, Hofmann WK, Peters C, Pennacchio LA, Abolmaali ND, Chavakis E, Reinheckel T, Zeiher AM, Dimmeler S. Cathepsin L is required for endothelial progenitor cell-induced neovascularization. Nat Med. 2005;11:206–213. doi: 10.1038/nm1182. [DOI] [PubMed] [Google Scholar]
- 13.Dimmeler S, Burchfield J, Zeiher AM. Cell-Based Therapy of Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2007 doi: 10.1161/ATVBAHA.107.155317. [DOI] [PubMed] [Google Scholar]
- 14.Fadini GP, Miorin M, Facco M, Bonamico S, Baesso I, Grego F, Menegolo M, de Kreutzenberg SV, Tiengo A, Agostini C, Avogaro A. Circulating endothelial progenitor cells are reduced in peripheral vascular complications of type 2 diabetes mellitus. J Am Coll Cardiol. 2005;45:1449–1457. doi: 10.1016/j.jacc.2004.11.067. [DOI] [PubMed] [Google Scholar]
- 15.Loomans CJ, de Koning EJ, Staal FJ, Rookmaaker MB, Verseyden C, de Boer HC, Verhaar MC, Braam B, Rabelink TJ, van Zonneveld AJ. Endothelial progenitor cell dysfunction: a novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes. 2004;53:195–199. doi: 10.2337/diabetes.53.1.195. [DOI] [PubMed] [Google Scholar]
- 16.Thum T, Fraccarollo D, Schultheiss M, Froese S, Galuppo P, Widder JD, Tsikas D, Ertl G, Bauersachs J. Endothelial nitric oxide synthase uncoupling impairs endothelial progenitor cell mobilization and function in diabetes. Diabetes. 2007;56:666–674. doi: 10.2337/db06-0699. [DOI] [PubMed] [Google Scholar]
- 17.Fadini GP, Sartore S, Albiero M, Baesso I, Murphy E, Menegolo M, Grego F, Vigili de Kreutzenberg S, Tiengo A, Agostini C, Avogaro A. Number and function of endothelial progenitor cells as a marker of severity for diabetic vasculopathy. Arterioscler Thromb Vasc Biol. 2006;26:2140–2146. doi: 10.1161/01.ATV.0000237750.44469.88. [DOI] [PubMed] [Google Scholar]
- 18.Awad O, Jiao C, Ma N, Dunnwald M, Schatteman GC. Obese diabetic mouse environment differentially affects primitive and monocytic endothelial cell progenitors. Stem Cells. 2005;23:575–583. doi: 10.1634/stemcells.2004-0185. [DOI] [PubMed] [Google Scholar]
- 19.Ii M, Takenaka H, Asai J, Ibusuki K, Mizukami Y, Maruyama K, Yoon YS, Wecker A, Luedemann C, Eaton E, Silver M, Thorne T, Losordo DW. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in reendothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 20.Waltenberger J, Lange J, Kranz A. Vascular endothelial growth factor-A-induced chemotaxis of monocytes is attenuated in patients with diabetes mellitus: A potential predictor for the individual capacity to develop collaterals. Circulation. 2000;102:185–190. doi: 10.1161/01.cir.102.2.185. [DOI] [PubMed] [Google Scholar]
- 21.Rosso A, Balsamo A, Gambino R, Dentelli P, Falcioni R, Cassader M, Pegoraro L, Pagano G, Brizzi MF. p53 Mediates the accelerated onset of senescence of endothelial progenitor cells in diabetes. J Biol Chem. 2006;281:4339–4347. doi: 10.1074/jbc.M509293200. [DOI] [PubMed] [Google Scholar]
- 22.Seeger FH, Haendeler J, Walter DH, Rochwalsky U, Reinhold J, Urbich C, Rossig L, Corbaz A, Chvatchko Y, Zeiher AM, Dimmeler S. p38 mitogen-activated protein kinase downregulates endothelial progenitor cells. Circulation. 2005;111:1184–1191. doi: 10.1161/01.CIR.0000157156.85397.A1. [DOI] [PubMed] [Google Scholar]
- 23.Walter DH, Rochwalsky U, Reinhold J, Seeger F, Aicher A, Urbich C, Spyridopoulos I, Chun J, Brinkmann V, Keul P, Levkau B, Zeiher AM, Dimmeler S, Haendeler J. Sphingosine-1-phosphate stimulates the functional capacity of progenitor cells by activation of the CXCR4-dependent signaling pathway via the S1P3 receptor. Arterioscler Thromb Vasc Biol. 2007;27:275–282. doi: 10.1161/01.ATV.0000254669.12675.70. [DOI] [PubMed] [Google Scholar]
- 24.Sorrentino SA, Bahlmann FH, Besler C, Muller M, Schulz S, Kirchhoff N, Doerries C, Horvath T, Limbourg A, Limbourg F, Fliser D, Haller H, Drexler H, Landmesser U. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus: restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation. 2007;116:163–173. doi: 10.1161/CIRCULATIONAHA.106.684381. [DOI] [PubMed] [Google Scholar]
- 25.Stepanovic V, Awad O, Jiao C, Dunnwald M, Schatteman GC. Leprdb diabetic mouse bone marrow cells inhibit skin wound vascularization but promote wound healing. Circ Res. 2003;92:1247–1253. doi: 10.1161/01.RES.0000074906.98021.55. [DOI] [PubMed] [Google Scholar]
- 26.Vasa M, Fichtlscherer S, Adler K, Mildner-Rihm C, Aicher A, Martin H, Zeiher AM, Dimmeler S. Increase in circulating endothelial progenitor cells by statin therapy in patients with stable coronary artery disease. Circulation. 2001;103:2885–2890. doi: 10.1161/hc2401.092816. [DOI] [PubMed] [Google Scholar]
- 27.Pirro M, Schillaci G, Menecali C, Bagaglia F, Paltriccia R, Vaudo G, Mannarino MR, Mannarino E. Reduced number of circulating endothelial progenitors and HOXA9 expression in CD34+ cells of hypertensive patients. J Hypertens. 2007;25:2093–2099. doi: 10.1097/HJH.0b013e32828e506d. [DOI] [PubMed] [Google Scholar]
- 28.Kondo T, Hayashi M, Takeshita K, Numaguchi Y, Kobayashi K, Iino S, Inden Y, Murohara T. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 29.Thum T, Tsikas D, Stein S, Schultheiss M, Eigenthaler M, Anker SD, Poole-Wilson PA, Ertl G, Bauersachs J. Suppression of endothelial progenitor cells in human coronary artery disease by the endogenous nitric oxide synthase inhibitor asymmetric dimethylarginine. J Am Coll Cardiol. 2005;46:1693–1701. doi: 10.1016/j.jacc.2005.04.066. [DOI] [PubMed] [Google Scholar]
- 30.Aicher A, Heeschen C, Mildner-Rihm C, Urbich C, Ihling C, Technau-Ihling K, Zeiher AM, Dimmeler S. Essential role of endothelial nitric oxide synthase for mobilization of stem and progenitor cells. Nat Med. 2003;9:1370–1376. doi: 10.1038/nm948. [DOI] [PubMed] [Google Scholar]
- 31.Sasaki K, Heeschen C, Aicher A, Ziebart T, Honold J, Urbich C, Rossig L, Koehl U, Koyanagi M, Mohamed A, Brandes RP, Martin H, Zeiher AM, Dimmeler S. Ex vivo pretreatment of bone marrow mononuclear cells with endothelial NO synthase enhancer AVE9488 enhances their functional activity for cell therapy. Proc Natl Acad Sci U S A. 2006;103:14537–14541. doi: 10.1073/pnas.0604144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laufs U, Werner N, Link A, Endres M, Wassmann S, Jurgens K, Miche E, Bohm M, Nickenig G. Physical Training Increases Endothelial Progenitor Cells, Inhibits Neointima Formation, and Enhances Angiogenesis. Circulation. 2004;109:220–226. doi: 10.1161/01.CIR.0000109141.48980.37. [DOI] [PubMed] [Google Scholar]
- 33.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, Mueller M, Fuchs M, Hornig B, Haller H, Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 34.Thum T, Fraccarollo D, Galuppo P, Tsikas D, Frantz S, Ertl G, Bauersachs J. Bone marrow molecular alterations after myocardial infarction: Impact on endothelial progenitor cells. Cardiovasc Res. 2006;70:50–60. doi: 10.1016/j.cardiores.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Ceradini DJ, Yao D, Grogan RH, Callaghan MJ, Edelstein D, Brownlee M, Gurtner GC. Decreasing intracellular superoxide corrects defective ischemia-induced new vessel formation in diabetic mice. J Biol Chem. 2008 doi: 10.1074/jbc.M707451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 37.Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, Liu PL, Chen YL, Chen JW. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 38.Dernbach E, Urbich C, Brandes RP, Hofmann WK, Zeiher AM, Dimmeler S. Anti-oxidative stress-associated genes in circulating progenitor cells: evidence for enhanced resistance against oxidative stress. Blood. 2004;104:3591–3597. doi: 10.1182/blood-2003-12-4103. [DOI] [PubMed] [Google Scholar]
- 39.He T, Peterson TE, Holmuhamedov EL, Terzic A, Caplice NM, Oberley LW, Katusic ZS. Human endothelial progenitor cells tolerate oxidative stress due to intrinsically high expression of manganese superoxide dismutase. Arterioscler Thromb Vasc Biol. 2004;24:2021–2027. doi: 10.1161/01.ATV.0000142810.27849.8f. [DOI] [PubMed] [Google Scholar]
- 40.Moore KA, Lemischka IR. Stem cells and their niches. Science. 2006;311:1880–1885. doi: 10.1126/science.1110542. [DOI] [PubMed] [Google Scholar]
- 41.Kiyomoto H, Nishiyama A. Do vasculature reactive oxygen species play a role in the mobilization of bone marrow endothelial progenitor cells? J Hypertens. 2008;26:188–190. doi: 10.1097/HJH.0b013e3282f2851a. [DOI] [PubMed] [Google Scholar]
- 42.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 43.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 44.Walter DH, Haendeler J, Reinhold J, Rochwalsky U, Seeger F, Honold J, Hoffmann J, Urbich C, Lehmann R, Arenzana-Seisdesdos F, Aicher A, Heeschen C, Fichtlscherer S, Zeiher AM, Dimmeler S. Impaired CXCR4 signaling contributes to the reduced neovascularization capacity of endothelial progenitor cells from patients with coronary artery disease. Circ Res. 2005;97:1142–1151. doi: 10.1161/01.RES.0000193596.94936.2c. [DOI] [PubMed] [Google Scholar]
- 45.Britten MB, Abolmaali ND, Assmus B, Lehmann R, Honold J, Schmitt J, Vogl TJ, Martin H, Schachinger V, Dimmeler S, Zeiher AM. Infarct remodeling after intracoronary progenitor cell treatment in patients with acute myocardial infarction (TOPCARE-AMI): mechanistic insights from serial contrast-enhanced magnetic resonance imaging. Circulation. 2003;108:2212–2218. doi: 10.1161/01.CIR.0000095788.78169.AF. [DOI] [PubMed] [Google Scholar]
- 46.Werner N, Kosiol S, Schiegl T, Ahlers P, Walenta K, Link A, Bohm M, Nickenig G. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 47.Kissel CK, Lehmann R, Assmus B, Aicher A, Honold J, Fischer-Rasokat U, Heeschen C, Spyridopoulos I, Dimmeler S, Zeiher AM. Selective functional exhaustion of hematopoietic progenitor cells in the bone marrow of patients with postinfarction heart failure. J Am Coll Cardiol. 2007;49:2341–2349. doi: 10.1016/j.jacc.2007.01.095. [DOI] [PubMed] [Google Scholar]
- 48.Edelberg JM, Tang L, Hattori K, Lyden D, Rafii S. Young adult bone marrow-derived endothelial precursor cells restore aging-impaired cardiac angiogenic function. Circ Res. 2002;90:E89–E93. doi: 10.1161/01.res.0000020861.20064.7e. [DOI] [PubMed] [Google Scholar]
- 49.Blasco MA. Telomere length, stem cells and aging. Nat Chem Biol. 2007;3:640–649. doi: 10.1038/nchembio.2007.38. [DOI] [PubMed] [Google Scholar]
- 50.Sharpless NE, DePinho RA. How stem cells age and why this makes us grow old. Nat Rev Mol Cell Biol. 2007;8:703–713. doi: 10.1038/nrm2241. [DOI] [PubMed] [Google Scholar]
- 51.Allsopp RC, Weissman IL. Replicative senescence of hematopoietic stem cells during serial transplantation: does telomere shortening play a role? Oncogene. 2002;21:3270–3273. doi: 10.1038/sj.onc.1205314. [DOI] [PubMed] [Google Scholar]
- 52.Spyridopoulos I, Erben Y, Brummendorf TH, Haendeler J, Dietz K, Seeger F, Kissel C, Martin H, Hoffmann J, Assmus B, Zeiher AM, Dimmeler S. Telomere Gap Between Granulocytes and Lymphocytes Is a Determinant for Haematopoetic Progenitor Cell Impairment in Patients With Previous Myocardial Infarction. Arterioscler Thromb Vasc Biol. 2008 doi: 10.1161/ATVBAHA.107.160846. [DOI] [PubMed] [Google Scholar]
- 53.Brouilette SW, Moore JS, McMahon AD, Thompson JR, Ford I, Shepherd J, Packard CJ, Samani NJ. Telomere length, risk of coronary heart disease, and statin treatment in the West of Scotland Primary Prevention Study: a nested case-control study. Lancet. 2007;369:107–114. doi: 10.1016/S0140-6736(07)60071-3. [DOI] [PubMed] [Google Scholar]
- 54.van der Harst P, van der Steege G, de Boer RA, Voors AA, Hall AS, Mulder MJ, van Gilst WH, van Veldhuisen DJ. Telomere length of circulating leukocytes is decreased in patients with chronic heart failure. J Am Coll Cardiol. 2007;49:1459–1464. doi: 10.1016/j.jacc.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 55.Satoh M, Ishikawa Y, Takahashi Y, Itoh T, Minami Y, Nakamura M. Association between oxidative DNA damage and telomere shortening in circulating endothelial progenitor cells obtained from metabolic syndrome patients with coronary artery disease. Atherosclerosis. 2007 doi: 10.1016/j.atherosclerosis.2007.09.040. [DOI] [PubMed] [Google Scholar]
- 56.Scheubel RJ, Zorn H, Silber RE, Kuss O, Morawietz H, Holtz J, Simm A. Age-dependent depression in circulating endothelial progenitor cells in patients undergoing coronary artery bypass grafting. J Am Coll Cardiol. 2003;42:2073–2080. doi: 10.1016/j.jacc.2003.07.025. [DOI] [PubMed] [Google Scholar]
- 57.Perin EC, Dohmann HF, Borojevic R, Silva SA, Sousa AL, Mesquita CT, Rossi MI, Carvalho AC, Dutra HS, Dohmann HJ, Silva GV, Belem L, Vivacqua R, Rangel FO, Esporcatte R, Geng YJ, Vaughn WK, Assad JA, Mesquita ET, Willerson JT. Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation. 2003;107:2294–2302. doi: 10.1161/01.CIR.0000070596.30552.8B. [DOI] [PubMed] [Google Scholar]
- 58.Assmus B, Fischer-Rasokat U, Honold J, Seeger FH, Fichtlscherer S, Tonn T, Seifried E, Schachinger V, Dimmeler S, Zeiher AM. Transcoronary transplantation of functionally competent BMCs is associated with a decrease in natriuretic peptide serum levels and improved survival of patients with chronic postinfarction heart failure: results of the TOPCARE-CHD Registry. Circ Res. 2007;100:1234–1241. doi: 10.1161/01.RES.0000264508.47717.6b. [DOI] [PubMed] [Google Scholar]
- 59.Assmus B, Honold J, Schachinger V, Britten MB, Fischer-Rasokat U, Lehmann R, Teupe C, Pistorius K, Martin H, Abolmaali ND, Tonn T, Dimmeler S, Zeiher AM. Transcoronary transplantation of progenitor cells after myocardial infarction. N Engl J Med. 2006;355:1222–1232. doi: 10.1056/NEJMoa051779. [DOI] [PubMed] [Google Scholar]
- 60.Strauer BE, Brehm M, Zeus T, Bartsch T, Schannwell C, Antke C, Sorg RV, Kogler G, Wernet P, Muller HW, Kostering M. Regeneration of human infarcted heart muscle by intracoronary autologous bone marrow cell transplantation in chronic coronary artery disease: the IACT Study. J Am Coll Cardiol. 2005;46:1651–1658. doi: 10.1016/j.jacc.2005.01.069. [DOI] [PubMed] [Google Scholar]
- 61.Assmus B, Schachinger V, Teupe C, Britten M, Lehmann R, Dobert N, Grunwald F, Aicher A, Urbich C, Martin H, Hoelzer D, Dimmeler S, Zeiher AM. Transplantation of Progenitor Cells and Regeneration Enhancement in Acute Myocardial Infarction (TOPCARE-AMI) Circulation. 2002;106:3009–3017. doi: 10.1161/01.cir.0000043246.74879.cd. [DOI] [PubMed] [Google Scholar]
- 62.Erbs S, Linke A, Schuler G, Hambrecht R. Intracoronary administration of circulating blood-derived progenitor cells after recanalization of chronic coronary artery occlusion improves endothelial function. Circ Res. 2006;98:e48. doi: 10.1161/01.RES.0000214407.58341.c8. [DOI] [PubMed] [Google Scholar]
- 63.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- 64.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, Schneider MD. Cardiac progenitor cells from adult myocardium: Homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A. 2003;100:12313–12318. doi: 10.1073/pnas.2132126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, Liao R. CD31- but Not CD31+ cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res. 2005;97:52–61. doi: 10.1161/01.RES.0000173297.53793.fa. [DOI] [PubMed] [Google Scholar]
- 66.Hierlihy AM, Seale P, Lobe CG, Rudnicki MA, Megeney LA. The post-natal heart contains a myocardial stem cell population. FEBS Lett. 2002;530:239–243. doi: 10.1016/s0014-5793(02)03477-4. [DOI] [PubMed] [Google Scholar]
- 67.Laugwitz KL, Moretti A, Lam J, Gruber P, Chen Y, Woodard S, Lin LZ, Cai CL, Lu MM, Reth M, Platoshyn O, Yuan JX, Evans S, Chien KR. Postnatal isl1+ cardioblasts enter fully differentiated cardiomyocyte lineages. Nature. 2005;433:647–653. doi: 10.1038/nature03215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tomita Y, Matsumura K, Wakamatsu Y, Matsuzaki Y, Shibuya I, Kawaguchi H, Ieda M, Kanakubo S, Shimazaki T, Ogawa S, Osumi N, Okano H, Fukuda K. Cardiac neural crest cells contribute to the dormant multipotent stem cell in the mammalian heart. J Cell Biol. 2005;170:1135–1146. doi: 10.1083/jcb.200504061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mouquet F, Pfister O, Jain M, Oikonomopoulos A, Ngoy S, Summer R, Fine A, Liao R. Restoration of cardiac progenitor cells after myocardial infarction by self-proliferation and selective homing of bone marrow-derived stem cells. Circ Res. 2005;97:1090–1092. doi: 10.1161/01.RES.0000194330.66545.f5. [DOI] [PubMed] [Google Scholar]
- 71.Fraser JK, Schreiber R, Strem B, Zhu M, Alfonso Z, Wulur I, Hedrick MH. Plasticity of human adipose stem cells toward endothelial cells and cardiomyocytes. Nat Clin Pract Cardiovasc Med. 2006;3 Suppl 1:S33–S37. doi: 10.1038/ncpcardio0444. [DOI] [PubMed] [Google Scholar]
- 72.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A. 2005;102:8692–8697. doi: 10.1073/pnas.0500169102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A. 1998;95:8801–8805. doi: 10.1073/pnas.95.15.8801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 76.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A. 2007;104:14068–14073. doi: 10.1073/pnas.0706760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bell DS. Diabetic cardiomyopathy. Diabetes Care. 2003;26:2949–2951. doi: 10.2337/diacare.26.10.2949. [DOI] [PubMed] [Google Scholar]
- 78.Kajstura J, Fiordaliso F, Andreoli AM, Li B, Chimenti S, Medow MS, Limana F, Nadal-Ginard B, Leri A, Anversa P. IGF-1 overexpression inhibits the development of diabetic cardiomyopathy and angiotensin II-mediated oxidative stress. Diabetes. 2001;50:1414–1424. doi: 10.2337/diabetes.50.6.1414. [DOI] [PubMed] [Google Scholar]
- 79.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res. 2004;94:514–524. doi: 10.1161/01.RES.0000117306.10142.50. [DOI] [PubMed] [Google Scholar]
- 80.Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Local myocardial insulin-like growth factor 1 (IGF-1) delivery with biotinylated peptide nanofibers improves cell therapy for myocardial infarction. Proc Natl Acad Sci U S A. 2006;103:8155–8160. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kenyon C. The plasticity of aging: insights from long-lived mutants. Cell. 2005;120:449–460. doi: 10.1016/j.cell.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 82.Kollet O, Shivtiel S, Chen YQ, Suriawinata J, Thung SN, Dabeva MD, Kahn J, Spiegel A, Dar A, Samira S, Goichberg P, Kalinkovich A, Arenzana-Seisdedos F, Nagler A, Hardan I, Revel M, Shafritz DA, Lapidot T. HGF, SDF-1, and MMP-9 are involved in stress-induced human CD34+ stem cell recruitment to the liver. J Clin Invest. 2003;112:160–169. doi: 10.1172/JCI17902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A. 2005;102:8966–8971. doi: 10.1073/pnas.0502678102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rosu-Myles M, Stewart E, Trowbridge J, Ito CY, Zandstra P, Bhatia M. A unique population of bone marrow cells migrates to skeletal muscle via hepatocyte growth factor/c-met axis. J Cell Sci. 2005;118:4343–4352. doi: 10.1242/jcs.02555. [DOI] [PubMed] [Google Scholar]
- 85.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes. 2001;50:2363–2375. doi: 10.2337/diabetes.50.10.2363. [DOI] [PubMed] [Google Scholar]
- 86.Pfeffer MA, McMurray JJ, Velazquez EJ, Rouleau JL, Kober L, Maggioni AP, Solomon SD, Swedberg K, Van de Werf F, White H, Leimberger JD, Henis M, Edwards S, Zelenkofske S, Sellers MA, Califf RM. Valsartan, captopril, or both in myocardial infarction complicated by heart failure, left ventricular dysfunction, or both. N Engl J Med. 2003;349:1893–1906. doi: 10.1056/NEJMoa032292. [DOI] [PubMed] [Google Scholar]
- 87.Kawanishi S, Oikawa S. Mechanism of telomere shortening by oxidative stress. Ann N Y Acad Sci. 2004;1019:278–284. doi: 10.1196/annals.1297.047. [DOI] [PubMed] [Google Scholar]
- 88.Yang S, Chintapalli J, Sodagum L, Baskin S, Malhotra A, Reiss K, Meggs LG. Activated IGF-1R inhibits hyperglycemia-induced DNA damage and promotes DNA repair by homologous recombination. Am J Physiol Renal Physiol. 2005;289:F1144–F1152. doi: 10.1152/ajprenal.00094.2005. [DOI] [PubMed] [Google Scholar]
- 89.Raha S, Robinson BH. Mitochondria, oxygen free radicals, disease and ageing. Trends Biochem Sci. 2000;25:502–508. doi: 10.1016/s0968-0004(00)01674-1. [DOI] [PubMed] [Google Scholar]
- 90.Martindale JL, Holbrook NJ. Cellular response to oxidative stress: signaling for suicide and survival. J Cell Physiol. 2002;192:1–15. doi: 10.1002/jcp.10119. [DOI] [PubMed] [Google Scholar]
- 91.Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT. Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science. 2000;287:1804–1808. doi: 10.1126/science.287.5459.1804. [DOI] [PubMed] [Google Scholar]
- 92.Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- 93.Gallagher KA, Liu ZJ, Xiao M, Chen H, Goldstein LJ, Buerk DG, Nedeau A, Thom SR, Velazquez OC. Diabetic impairments in NO-mediated endothelial progenitor cell mobilization and homing are reversed by hyperoxia and SDF-1 alpha. J Clin Invest. 2007;117:1249–1259. doi: 10.1172/JCI29710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.van Weel V, Seghers L, de Vries MR, Kuiper EJ, Schlingemann RO, Bajema IM, Lindeman JH, Delis-van Diemen PM, van Hinsbergh VW, van Bockel JH, Quax PH. Expression of vascular endothelial growth factor, stromal cell-derived factor-1, and CXCR4 in human limb muscle with acute and chronic ischemia. Arterioscler Thromb Vasc Biol. 2007;27:1426–1432. doi: 10.1161/ATVBAHA.107.139642. [DOI] [PubMed] [Google Scholar]
- 95.Chang EI, Loh SA, Ceradini DJ, Lin SE, Bastidas N, Aarabi S, Chan DA, Freedman ML, Giaccia AJ, Gurtner GC. Age decreases endothelial progenitor cell recruitment through decreases in hypoxia-inducible factor 1alpha stabilization during ischemia. Circulation. 2007;116:2818–2829. doi: 10.1161/CIRCULATIONAHA.107.715847. [DOI] [PubMed] [Google Scholar]
- 96.Ju Z, Jiang H, Jaworski M, Rathinam C, Gompf A, Klein C, Trumpp A, Rudolph KL. Telomere dysfunction induces environmental alterations limiting hematopoietic stem cell function and engraftment. Nat Med. 2007;13:742–747. doi: 10.1038/nm1578. [DOI] [PubMed] [Google Scholar]
- 97.Seeger FH, Zeiher AM, Dimmeler S. Cell-enhancement strategies for the treatment of ischemic heart disease. Nat Clin Pract Cardiovasc Med. 2007;4 Suppl 1:S110–S113. doi: 10.1038/ncpcardio0734. [DOI] [PubMed] [Google Scholar]
- 98.Mangi AA, Noiseux N, Kong D, He H, Rezvani M, Ingwall JS, Dzau VJ. Mesenchymal stem cells modified with Akt prevent remodeling and restore performance of infarcted hearts. Nat Med. 2003;9:1195–1201. doi: 10.1038/nm912. [DOI] [PubMed] [Google Scholar]
- 99.Murasawa S, Llevadot J, Silver M, Isner JM, Losordo DW, Asahara T. Constitutive human telomerase reverse transcriptase expression enhances regenerative properties of endothelial progenitor cells. Circulation. 2002;106:1133–1139. doi: 10.1161/01.cir.0000027584.85865.b4. [DOI] [PubMed] [Google Scholar]
- 100.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Llevadot J, Murasawa S, Kureishi Y, Uchida S, Masuda H, Kawamoto A, Walsh K, Isner JM, Asahara T. HMG-CoA reductase inhibitor mobilizes bone marrow--derived endothelial progenitor cells. J Clin Invest. 2001;108:399–405. doi: 10.1172/JCI13131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iwakura A, Luedemann C, Shastry S, Hanley A, Kearney M, Aikawa R, Isner JM, Asahara T, Losordo DW. Estrogen-mediated, endothelial nitric oxide synthase-dependent mobilization of bone marrow-derived endothelial progenitor cells contributes to reendothelialization after arterial injury. Circulation. 2003;108:3115–3121. doi: 10.1161/01.CIR.0000106906.56972.83. [DOI] [PubMed] [Google Scholar]
- 103.Verma S, Kuliszewski MA, Li SH, Szmitko PE, Zucco L, Wang CH, Badiwala MV, Mickle DA, Weisel RD, Fedak PW, Stewart DJ, Kutryk MJ. C-reactive protein attenuates endothelial progenitor cell survival, differentiation, and function: further evidence of a mechanistic link between C-reactive protein and cardiovascular disease. Circulation. 2004;109:2058–2067. doi: 10.1161/01.CIR.0000127577.63323.24. [DOI] [PubMed] [Google Scholar]
- 104.Strehlow K, Werner N, Berweiler J, Link A, Dirnagl U, Priller J, Laufs K, Ghaeni L, Milosevic M, Bohm M, Nickenig G. Estrogen increases bone marrow-derived endothelial progenitor cell production and diminishes neointima formation. Circulation. 2003;107:3059–3065. doi: 10.1161/01.CIR.0000077911.81151.30. [DOI] [PubMed] [Google Scholar]
- 105.Zhang D, Fan GC, Zhou X, Zhao T, Pasha Z, Xu M, Zhu Y, Ashraf M, Wang Y. Over-expression of CXCR4 on mesenchymal stem cells augments myoangiogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2008;44:281–292. doi: 10.1016/j.yjmcc.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galvez BG, Sampaolesi M, Brunelli S, Covarello D, Gavina M, Rossi B, Constantin G, Torrente Y, Cossu G. Complete repair of dystrophic skeletal muscle by mesoangioblasts with enhanced migration ability. J Cell Biol. 2006;174:231–243. doi: 10.1083/jcb.200512085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chavakis E, Hain A, Vinci M, Carmona G, Bianchi ME, Vajkoczy P, Zeiher AM, Chavakis T, Dimmeler S. High-mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ Res. 2007;100:204–212. doi: 10.1161/01.RES.0000257774.55970.f4. [DOI] [PubMed] [Google Scholar]
- 108.Carmona G, Chavakis E, Koehl U, Zeiher AM, Dimmeler S. Activation of Epac stimulates integrin-dependent homing of progenitor cells. Blood. 2008;111:2640–2646. doi: 10.1182/blood-2007-04-086231. [DOI] [PubMed] [Google Scholar]
- 109.Oh IY, Yoon CH, Hur J, Kim JH, Kim TY, Lee CS, Park KW, Chae IH, Oh BH, Park YB, Kim HS. Involvement of E-selectin in recruitment of endothelial progenitor cells and angiogenesis in ischemic muscle. Blood. 2007 doi: 10.1182/blood-2006-10-048991. [DOI] [PubMed] [Google Scholar]
- 110.Nishiwaki Y, Yoshida M, Iwaguro H, Masuda H, Nitta N, Asahara T, Isobe M. Endothelial E-selectin potentiates neovascularization via endothelial progenitor cell-dependent and -independent mechanisms. Arterioscler Thromb Vasc Biol. 2007;27:512–518. doi: 10.1161/01.ATV.0000254812.23238.2b. [DOI] [PubMed] [Google Scholar]
- 111.Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–1692. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- 112.Chang KH, Chan-Ling T, McFarland EL, Afzal A, Pan H, Baxter LC, Shaw LC, Caballero S, Sengupta N, Li Calzi S, Sullivan SM, Grant MB. IGF binding protein-3 regulates hematopoietic stem cell and endothelial precursor cell function during vascular development. Proc Natl Acad Sci U S A. 2007;104:10595–10600. doi: 10.1073/pnas.0702072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Limana F, Germani A, Zacheo A, Kajstura J, Di Carlo A, Borsellino G, Leoni O, Palumbo R, Battistini L, Rastaldo R, Muller S, Pompilio G, Anversa P, Bianchi ME, Capogrossi MC. Exogenous high-mobility group box 1 protein induces myocardial regeneration after infarction via enhanced cardiac C-kit+ cell proliferation and differentiation. Circ Res. 2005;97:e73–e83. doi: 10.1161/01.RES.0000186276.06104.04. [DOI] [PubMed] [Google Scholar]
- 114.Palumbo R, Bianchi ME. High mobility group box 1 protein, a cue for stem cell recruitment. Biochem Pharmacol. 2004;68:1165–1170. doi: 10.1016/j.bcp.2004.03.048. [DOI] [PubMed] [Google Scholar]
- 115.Aicher A, Heeschen C, Sasaki K, Urbich C, Zeiher AM, Dimmeler S. Low-energy shock wave for enhancing recruitment of endothelial progenitor cells: a new modality to increase efficacy of cell therapy in chronic hind limb ischemia. Circulation. 2006;114:2823–2830. doi: 10.1161/CIRCULATIONAHA.106.628623. [DOI] [PubMed] [Google Scholar]
- 116.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 117.Morrison SJ, Spradling AC. Stem cells and niches: mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Conboy IM, Conboy MJ, Smythe GM, Rando TA. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302:1575–1577. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 119.Musaro A, McCullagh K, Paul A, Houghton L, Dobrowolny G, Molinaro M, Barton ER, Sweeney HL, Rosenthal N. Localized Igf-1 transgene expression sustains hypertrophy and regeneration in senescent skeletal muscle. Nat Genet. 2001;27:195–200. doi: 10.1038/84839. [DOI] [PubMed] [Google Scholar]
- 120.Musaro A, Giacinti C, Borsellino G, Dobrowolny G, Pelosi L, Cairns L, Ottolenghi S, Cossu G, Bernardi G, Battistini L, Molinaro M, Rosenthal N. Stem cell-mediated muscle regeneration is enhanced by local isoform of insulin-like growth factor 1. Proc Natl Acad Sci U S A. 2004;101:1206–1210. doi: 10.1073/pnas.0303792101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Thum T, Hoeber S, Froese S, Klink I, Stichtenoth DO, Galuppo P, Jakob M, Tsikas D, Anker SD, Poole-Wilson PA, Borlak J, Ertl G, Bauersachs J. Age-dependent impairment of endothelial progenitor cells is corrected by growth-hormone-mediated increase of insulin-like growth-factor-1. Circ Res. 2007;100:434–443. doi: 10.1161/01.RES.0000257912.78915.af. [DOI] [PubMed] [Google Scholar]
- 122.Russell SJ, Kahn CR. Endocrine regulation of ageing. Nat Rev Mol Cell Biol. 2007;8:681–691. doi: 10.1038/nrm2234. [DOI] [PubMed] [Google Scholar]
- 123.Edelberg JM, Cai D, Xaymardan M. Translation of PDGF cardioprotective pathways. Cardiovasc Toxicol. 2003;3:27–35. doi: 10.1385/ct:3:1:27. [DOI] [PubMed] [Google Scholar]
- 124.Pallante BA, Duignan I, Okin D, Chin A, Bressan MC, Mikawa T, Edelberg JM. Bone marrow Oct3/4+ cells differentiate into cardiac myocytes via age-dependent paracrine mechanisms. Circ Res. 2007;100:e1–e11. doi: 10.1161/01.RES.0000253487.02398.85. [DOI] [PubMed] [Google Scholar]
- 125.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. doi: 10.1126/science.1112766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Imura A, Iwano A, Tohyama O, Tsuji Y, Nozaki K, Hashimoto N, Fujimori T, Nabeshima Y. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett. 2004;565:143–147. doi: 10.1016/j.febslet.2004.03.090. [DOI] [PubMed] [Google Scholar]
- 127.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;390:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- 128.Nabeshima Y. Toward a better understanding of Klotho. Sci Aging Knowledge Environ. 2006;2006:e11. doi: 10.1126/sageke.2006.8.pe11. [DOI] [PubMed] [Google Scholar]
- 129.Arking DE, Atzmon G, Arking A, Barzilai N, Dietz HC. Association between a functional variant of the KLOTHO gene and high-density lipoprotein cholesterol, blood pressure, stroke, and longevity. Circ Res. 2005;96:412–418. doi: 10.1161/01.RES.0000157171.04054.30. [DOI] [PubMed] [Google Scholar]
- 130.Liu H, Fergusson MM, Castilho RM, Liu J, Cao L, Chen J, Malide D, Rovira, Schimel D, Kuo CJ, Gutkind JS, Hwang PM, Finkel T. Augmented Wnt signaling in a mammalian model of accelerated aging. Science. 2007;317:803–806. doi: 10.1126/science.1143578. [DOI] [PubMed] [Google Scholar]
- 131.Scheller M, Huelsken J, Rosenbauer F, Taketo MM, Birchmeier W, Tenen DG, Leutz A. Hematopoietic stem cell and multilineage defects generated by constitutive beta-catenin activation. Nat Immunol. 2006;7:1037–1047. doi: 10.1038/ni1387. [DOI] [PubMed] [Google Scholar]
- 132.Tzahor E. Wnt/beta-catenin signaling and cardiogenesis: timing does matter. Dev Cell. 2007;13:10–13. doi: 10.1016/j.devcel.2007.06.006. [DOI] [PubMed] [Google Scholar]