Abstract
Topoisomerase I (Top1) is a proven target for cancer therapeutics, and the level of Top1 in tumors has been employed as a biomarker for chemotherapeutic efficacy. In this study, we report the development and validation of a two-site enzyme chemiluminescent immunoassay for Top1, which we used to measure Top1 levels in the NCI-60 cancer cell line panel. Top1 levels ranged from 0.9 to 9.8 ng/mL/μg protein extract in these cell lines. Levels varied both within and between cancer types but were generally highest in colon cancer and leukemia cell lines and lowest in central nervous system and renal cancer cell lines. Top1 mRNA levels in the NCI-60 cell lines were also measured by microarray; mRNA values generally demonstrated a good correlation with protein levels (Pearson correlation = 0.8). When these expression levels were compared with the activity of the indenoisoquinoline class of Top1 inhibitors across the NCI-60 cell panel, low levels of Top1 were associated with increased resistance to these drugs. The results of our studies indicate that our Top1 assay can be used to quantify Top1 levels in untreated cells as well as cells treated with Top1 inhibitors, and that the assay has the potential to be adapted for use in predicting clinical response to Top1-active antineoplastic agents.
Keywords: Topoisomerase I, immunoassay, ELISA, NCI-60, pharmacodynamic assays
Introduction
Topoisomerase I (Top1) catalyzes the unwinding of DNA by creating single-strand breaks. Numerous biological processes require this activity, including DNA replication, transcription, recombination, chromatin assembly, chromosome segregation, and DNA repair (1). This expansive repertoire of vital functions makes topoisomerases an attractive target for drug treatment in cancer and other diseases. Expression of Top1 is associated with tumor growth, tumor differentiation, and poor prognosis for survival in colorectal cancers (2, 3). High levels of Top1 also correlate with response to the Top1 inhibitor irinotecan (CPT-11) (3, 4), whereas reduced expression of Top1 is linked to resistance to Top1 inhibitors (5–7). However, to date, a highly quantitative assay for measuring Top1 levels in a (semi) high throughput manner has been lacking.
Although Top1 mRNA levels can be measured easily by reverse transcriptase-PCR (RT-PCR) methods (8) and microarray, mRNA levels do not always directly correspond to protein expression (9, 10). Qualitative analysis of immunostained human sarcomas has shown that Top1 protein levels vary widely in these tumors (11). Colon cancer patients who benefit from treatment with irinotecan have been identified using Top1 immunohistochemistry (3). Another immunohistochemical analysis found that Top1 is elevated in 42% of malignant melanomas and that p53 status may also influence the efficacy of Top1 inhibitors (12). Top1 protein levels can also be measured by Western blotting in a semi-quantitative manner; by Western analysis, gastric adenocarcinomas have 1.51-fold higher and lung adenocarcinomas 1.84-fold higher levels of Top1 than their corresponding normal tissues (13). Western blot analysis has also been used to demonstrate that Top1 levels decrease in response to drug treatment by way of the ubiquitin-proteasome pathway (14, 15).
Top1 inhibitors (such as topotecan and irinotecan) are currently among the most effective anti-cancer drugs for colorectal, small-cell lung, and ovarian cancer (16). However, they are chemically unstable, and thus, substantial effort has been expended to improve upon these compounds, leading to the development of the indenoisoquinoline class of topoisomerase I inhibitors (17, 18). As the indenoisoquinolines progress toward clinical evaluation, quantitative methods for measuring topoisomerase levels would be of substantial utility for evaluating the potential to select patients for indenoisoquinolines therapy based on pre-treatment Top1 levels.
In this report, we describe a novel, quantitative, Top1 immunoassay that was developed and validated using cell culture extracts and purified recombinant Top1. The assay was used to measure Top1 levels as a pharmacodynamic biomarker in topotecan-treated A375 cells. The immunoassay was also used to measure Top1 levels in the NCI-60 cell line panel, and protein levels were correlated with mRNA levels measured by microarray analysis. Selected cells lines were subjected to further study by Western blotting. Top1 levels were also compared against efficacy data for topotecan and the indenoisoquinolines in the NCI-60 cell line panel.
Materials and Methods
Cells and Cell Culture
A375 cells and media were obtained from ATCC. NCI-60 cell lines were obtained from the Animal Testing Branch at NCI-Frederick (Frederick, MD). (A complete list of cells in the NCI-60 panel and additional details are available in the supplementary materials.) Cells were grown in T75 (75 cm2) flasks or 6-well plates from Corning in RPMI 1640 media supplemented with 10% fetal bovine serum (ATCC) and 50 mg/L gentamicin (Biowhittaker) in an incubator at 37°C with 5% CO2. Cells were harvested while in exponential growth.
Drug Treatment
A375 cells were grown to approximately 75% confluent in T75 flasks. Cells were treated with 0.1, 1, 10 or 100 μM topotecan (Hycamtin, GlaxoSmithKline) or vehicle (water) in 5 mL of media for 40, 75, and 165 minutes. The cells were rapidly washed with 10 mL PBS and immediately lysed in the flask and processed as described below.
Sample Processing and Protein Determination
Cells were lysed directly in the flask or plate with lysis buffer consisting of 10 mM Tris-HCl buffer pH 7.5, 1% SDS, 1% Triton-X-100, 1 mM phenylmethylsulfonyl fluoride, and EDTA-free Complete Protease Inhibitor Cocktail (Roche). Lysates were sonicated 3 times, 10 seconds each, on ice, with a sonic dismembranator (Fisher). Protein levels were determined with the bicinchoninic acid (BCA) kit (Thermo Scientific) using bovine serum albumin as the standard.
ELISA Assay
Optimal antibody dilutions, times and temperatures were determined for each step. White 96-well Reactibind (Thermo Scientific Pierce) plates were coated with a mouse anti-Top1 monoclonal antibody clone C21.1 (BD Biosciences Pharmingen), diluted 1:1000 in carbonate buffer pH 9.6, and incubated for 2 hours at 37°C. The plates were then washed once with PBS with 0.1% Tween-20 (PBS-T) on a BioTek ELx405 plate washer (BioTek Instruments, Inc.). The plates were blocked at least 2 hours at 37°C with PBS-casein (BioFx Laboratories) and washed once with PBS-T. Samples and standards were then loaded and incubated overnight at 4°C. Recombinant Top1 (rTop1; EMD Biosciences, Inc.) was used as the standard. The plates were then washed four times with PBS-T. Rabbit anti-Top1 polyclonal antibody (pAb) Ab28432 (Abcam), diluted 1:500 in PBS-casein, was used as the probe and incubated for 2 hours at 25°C. The plates were washed four times with PBS-T, followed by the addition of extra serum-absorbed goat-anti-rabbit horseradish peroxidase conjugate diluted 1:1000 in PBS-casein for 1 hour at 25°C. The plates were washed again four times with PBS-T. Finally, Pico-ELISA substrate (Thermo Scientific Pierce) was added and chemiluminescence was measured on an Infinite 200M (Tecan Group Ltd.).
ELISA Assay Validation
The Top1 immunoassay was subject to a validation protocol for analytical performance (see Supplementary Materials).
Quantitation of Transcript Expression from Three Microarray Platforms
Processing and normalization of transcript expression data from the NCI-60 has previously been described for the 60,000-feature Affymetrix Human Genome U95 Set (HG_U95) and 44,000-feature Affymetrix Human Genome U133 (HG_U133) microarrays (10). Transcript expression quantification for the Human Genome U133 Plus 2.0 Array (HG_U133 Plus 2.0) was obtained using Robust Multichip Analysis. Inclusion of probes was based on their having a minimum range of 1.2 (log2) (19), in addition to a correlation to all other probes used of 0.52. Probes used in this analysis are listed in Supplementary Table S1. Data from the HG_U95 and HG_U133 microarrays can be accessed at our queryable relational database CellMiner at http://discover.nci.nih.gov/cellminer/.
Comparison of Top1 Enzyme and mRNA levels in the NCI-60 Cell Line Panel
Top1 enzyme levels were plotted against Top1 mRNA levels determined using six separate probes and the average intensity of the four probes. Correlations were determined from least square linear regression fit.
Western Blotting
Cell lysates from HT-29, HCT-116, A498, SKMEL28, SR, and H322M cell lines were loaded onto 7.5% precast gels (Bio-Rad Laboratories) for SDS-PAGE run at 90V for 90 minutes. Protein concentrations were determined by BCA assay (Thermo Scientific) and 50 μg was loaded per well. Proteins were then transferred to PVDF membranes at 100V for 2 hours. Membranes were blocked overnight with blocking buffer (LICOR). Separate blots were probed with either mouse anti-Top1 monoclonal antibody C21.1 (diluted 1:1000) or rabbit anti-Top1 polyclonal antibody Ab28432 (diluted 1:1000). Anti-mouse IR-680 (LICOR; diluted 1:10,000) or anti-rabbit IR-800 (LICOR; diluted 1:10,000) were used as secondary antibodies. Blots were scanned on the Odyssey IR imager (LICOR). Quantification was performed using LICOR software v.3.0.
Drug Activity
Drug activities (GI50, the concentration that causes 50% growth inhibition (IC50) with a correction for the cell count at time zero) in the NCI-60 panel were obtained from NCI’s Developmental Therapeutics Program, NIH. The drug activity assay involves a 48 h exposure of cells in exponential growth to a 5 log concentration range of drug followed by a colorimetic detection of viable cells with sulforhodamine B dye at 515nm. Details on the methodology of the in vitro cancer screen be found at http://www.dtp.nci.nih.gov/branches/btb/ivclsp.html. The data for topotecan (NSC 609699), and the indenoisoquinoline compounds (NSC 724998 and NSC 725776) can be accessed by NSC number at http://dtp.nci.nih.gov/dtpstandard/cancerscreeningdata/index.jsp.
Statistical Analysis
Pearson’s correlation coefficients for the drug activity versus mRNA expression comparisons (Table 1) were done using Microsoft Excel 2004 for Mac, Version 11.2.5.
Table 1.
Top1 protein levels by cancer type in the NCI-60 cell line panel.
| Cancer type | Top1 protein level (ng/mL/μg; Mean ± SD) | |
|---|---|---|
| Colon | (n=7) | 5.69 ± 2.36 |
| Leukemia | (n=6) | 4.87 ± 3.23 |
| Prostate | (n=2) | 3.68 ± 1.25 |
| Ovarian | (n=7) | 3.08 ± 1.13 |
| Lung | (n=9) | 2.71 ± 1.43 |
| Breast | (n=5) | 2.59 ± 1.98 |
| Melanoma | (n=9) | 2.39 ± 1.01 |
| Central Nervous System | (n=6) | 1.94 ± 0.44 |
| Renal | (n=8) | 1.77 ± 0.47 |
| NCI-60 | (n=59) | 3.09 ± 2.01 |
Results
Here we describe a novel assay based on the enzyme-linked immunosorbent assay (ELISA) developed to provide quantitative determination of Top1 levels. The development and analytical validation of the immunoassay are described in the supplementary materials. For all samples, quantification was achieved using rTop1 to generate a standard curve. Functional validation was done using cell extracts from cells treated with and without topotecan as described below. Top1 levels were determined for the NCI-60 cell line panel using this ELISA-based assay, and were compared with Top1 mRNA levels determined by microarray, Top1 levels determined by Western blot analysis, and with the known efficacy of Top1 inhibitors in the NCI-60 panel.
Top1 Levels in Topotecan-Treated A375 Cells
Top1 levels in topotecan-treated A375 human melanoma cells were measured as a functional validation of the immunoassay to demonstrate that drug-dependent changes in Top1 levels could be detected. Top1 protein levels in A375 cells increased to approximately 110% of the vehicle control (water) at all three time points (40, 75, 165 minutes) following treatment with 0.1 μM topotecan (Fig. 1). Top1 levels decreased to 65% of controls in response to 1 μM topotecan treatment for 165 minutes, while the 40 and 75 minute time points were essentially unchanged from the vehicle control. At the 10 μM and 100 μM concentrations, Top1 levels began to decrease at the 40 and 75 minute time points. Top1 protein levels at the 165 minute time point leveled out at approximately 55% of the vehicle control for both 10 μM and 100 μM concentrations.
Figure 1.
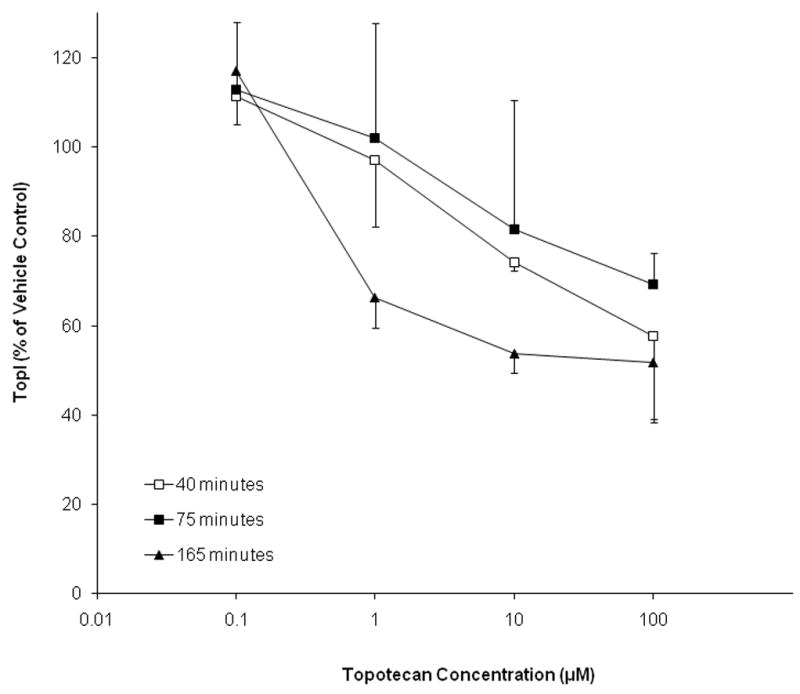
Top1 levels decrease in a dose-dependent manner in topotecan-treated A375 cells. Cells were treated with 0.1 to 100 μM topotecan for 40, 75, or 165 minutes. Data are expressed relative to vehicle control (water).
Top1 Protein Levels in the NCI-60 Panel Measured by ELISA
Top1 protein levels in the NCI-60 panel were measured by ELISA and were examined by cancer type (Fig. 2A). Top1 protein levels varied by 10.7-fold from the highest (9.8 ng/mL/μg extract) to the lowest (0.9 ng/mL/μg extract) in these cell lines. As a group, colon cancer cell lines had the highest mean levels of Top1 (5.7 ± 2.4 ng/mL/μg protein), whereas renal cancer cell lines had the lowest mean levels of Top1 (1.8 ± 0.5 ng/mL/μg protein; Table 2). The overall average Top1 protein level in the NCI-60 panel was 3.1 ± 2.0 ng/mL/μg protein.
Figure 2.
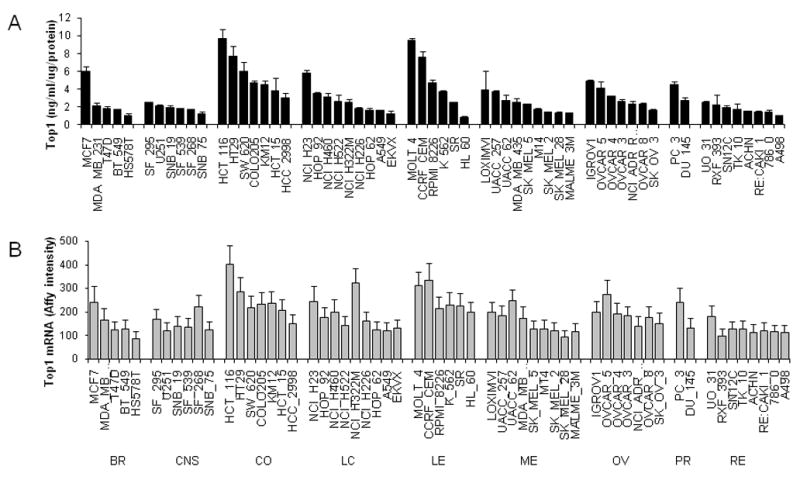
Top1 (A) enzyme and (B) mRNA levels in the NCI-60 cell line panel. Data are presented by cancer type in decreasing order of Top1 enzyme level. Top1 ELISA data have been normalized to 1 μg protein; data represent the mean ± SD for at least three cell extracts. Affymetrix microarray data is the mean ± SD for data from six probes each normalized using gcRMA method. BR-Breast, CNS-central nervous system, CO-colon, LC- lung cancer, LE-leukemia, ME- melanoma, OV-ovarian, PR-prostate, RE-renal.
Table 2.
Correlations of indenoisoquinoline activity* to Top1 mRNA and protein levels in the NCI-60 cell line panel.
| Indenoisoquinolines |
|||||
|---|---|---|---|---|---|
| NSC 724998† |
NSC 725776† |
NSC 609699†, Topotecan‡ | |||
| Experiment 1 | Experiment 2 | Experiment 1 | Experiment 2 | ||
| mRNA§ | 0.258** | 0.31** | 0.29** | 0.32** | 0.24 |
| Protein|| | 0.21 | 0.27** | 0.30** | 0.22 | 0.26 |
Values for GI50 drug activity from the Developmental Therapeutics Program, NCI Web site at http://dtp.nci.nih.gov/dtpstandard/cancerscreeningdata/index.jsp
Cancer Chemotherapy National Service Center (NSC) numbers.
Correlations given based on average of 23 independent experiments.
mRNA, z score derived from Top1 probes from Affymetrix HG_U95, HG_U133, and HG_U122 Plus2 microarrays.
Protein values from ELISA assay in Figure 2.
Statistically significant correlation at p < 0.05, without multiple comparisons correction.
Top1 mRNA Levels in the NCI-60 Panel Measured by Microarray
Top1 transcript levels in the NCI-60 were measured using three Affymetrix microarray platforms: HG_U95, HG_U133, and HG_U133 Plus 2.0. The average intensities for each cell line in the NCI-60 panel are shown in Figure 2B. Top1 mRNA levels in the NCI-60 cell line panel measured by microarray varied from 4.7 to15.6 fold (average 5.7 fold) for the six probes used in this study, which agrees with the levels determined by ELISA.
Comparison of Top1 Protein and mRNA Levels in the NCI-60 Panel
Top1 protein levels were plotted against Top1 mRNA levels determined using six separate probes and the average intensity of the six probes (Fig. 3). The correlation between the enzyme and mRNA level from the average intensities of the six probes was 0.83 (R2 = 0.6915). Correlations for each of the six individual probe/chip sets ranged from 0.57 to 0.80 (Supp. Table S1).
Figure 3.
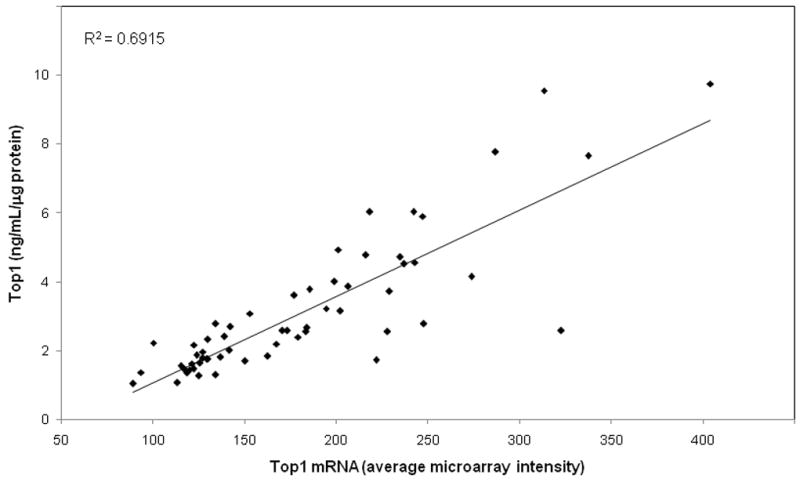
Correlation of Top1 enzyme and mRNA levels in the NCI-60 cell line panel. Cell lines are shown as solid diamonds. mRNA levels are the average intensities of six probes.
Top1 Western Blot Analysis of Select Cell Lines from the NCI-60 Panel
Western blot analysis with the rabbit pAb used as the probe in the ELISA assay was performed for select cell lines (Fig. 4A) with high or low Top1 measured by both ELISA and microarray as well as for three cell lines that had high mRNA Affychip intensity to enzyme ratios. HCT-116 and HT-29 had very intense bands at about 100 kDa, corresponding to full-length Top1, in the blot probed with the rabbit pAb Ab28432. H332M had a slightly less intense full-lengthTop1 band, while A498 and SKMEL28 cells had an even fainter band. Additional bands at lower molecular weights were present for most of the cell lines and were of varying molecular weight, possibly due to proteolytic turnover of the enzyme. HCT-116, HT-29, and SR cell lines had similar lower molecular weight bands of about 70 kDa and 40 kDa. SR and A498 appeared to have bands of slightly higher molecular weight at about 75 kDa. The Top1 band intensities at about 100 kDa were quantified and normalized to the HCT-116 values. Relative intensities of the Top1 band at 100 kDa were compared to the ELISA data (Fig. 4B).
Figure 4.
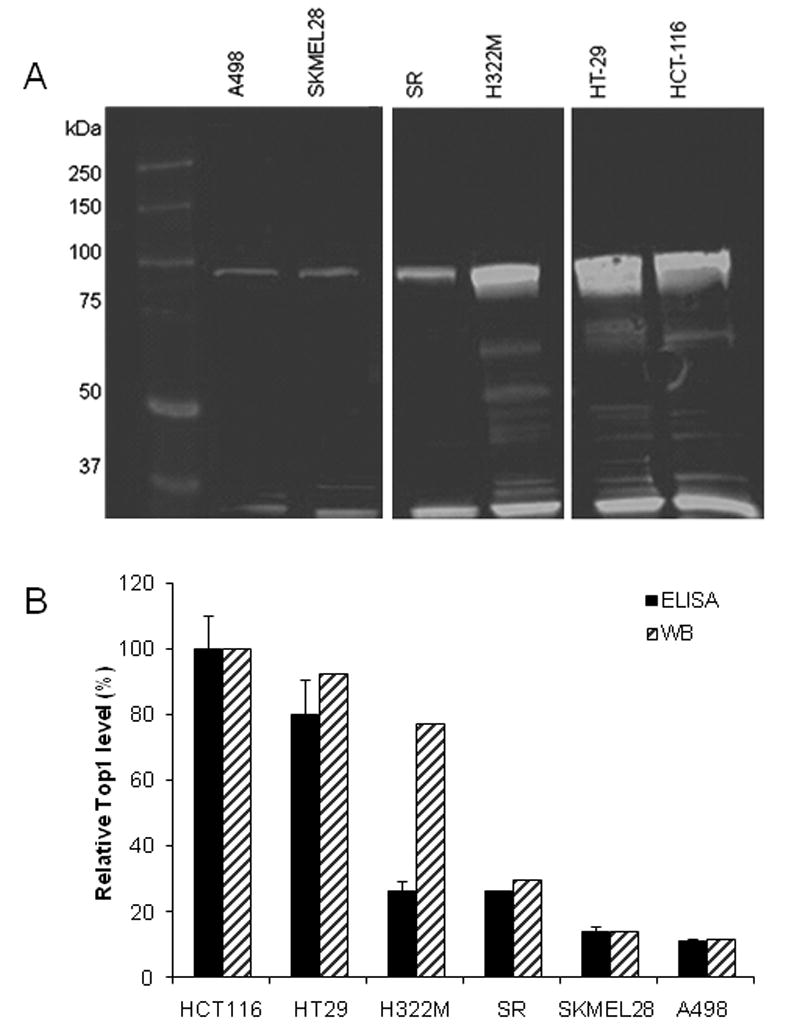
(A) Western blot analysis of 50 μg of whole cell lysate from select NCI-60 cells lines for Top1. Blot probed with rabbit anti-Top1 pAb followed by goat anti-rabbit-IR800. Full-length Top1 is approximately100 kDa. (B) Relative quantification of Top1 levels from 100 kDa band on Western blot in panel A (hashed bars) compared to ELISA (solid bars). Top1 ELISA data are mean ± SD for at least 3 cell extracts. All data sets were normalized to and expressed as a percent of HCT-116 levels.
Top1 Expression Correlated With Top1 Inhibitor Activity
Indenoisoquinoline drug activity values (IC50) obtained for the NCI-60 were correlated to both the mRNA and protein expression levels of Top1 (Table 1). For the indenoisoquinolines NSC 724998 and NSC 725776, correlation of drug activity to mRNA levels was statistically significant in all cases (p < 0.05 in the absence of multiple comparisons correction). Indenoisoquinoline activity correlations to Top1 protein expression levels were statistically significant in one out of two cases for each experiment. Top1 expression correlations to topotecan activity were not found to be significant (Table 1).
Discussion
A sensitive and quantitative immunoassay for Top1 was developed, validated, and used to measure Top1 levels in the NCI-60 cell line panel and in cells treated with the Top1 inhibitor topotecan. Top1 mRNA levels in the NCI-60 panel were also determined and compared to Top1 enzyme levels determined by the immunoassay. Known efficacy of Top1 inhibitors was also compared to the Top1 levels across the NCI-60 panel.
Top1 protein levels in the NCI-60 cell line panel, as determined by ELISA, varied by about 11-fold in these cancer cell lines and generally had good correlation (R=0.83) with mRNA levels as measured using Affymetrix microarrays. Furthermore, the activity of the indenoisoquinoline Top1 inhibitors, NSC 724998 and NSC 725776, correlated with Top1 expression in the NCI-60 cell lines.
Top1 levels in topotecan-treated A375 cells determined using the ELISA assay showed a time-and dose-dependent decrease in response to topotecan treatment. This is consistent with previous reports demonstrating that Top1 is degraded via the ubiquitin-proteasome pathway in response to camptothecin (14, 15, 20–22), which shares the same ring structure and mechanism of inhibition as topotecan. In contrast, increases in expression of Top1 have been observed in peripheral blood mononuclear cells from patients undergoing chemotherapy (23). Our assay could, therefore, be used to monitor the pharmacodynamic effects of Top1 inhibitors such as topotecan as well as compounds that result in alteration of Top1 levels.
Colon cancer cell lines had the highest levels of Top1, consistent with the clinical efficacy of Top1 inhibitors in the treatment of this disease. The recent Fluorouracil, Oxaliplatin, CPT-11: Use and Sequencing (FOCUS) trial demonstrated a correlation between Top1 levels and the efficacy or irinotecan-based chemotherapy (3). The wide variation in Top1 levels among colon cancer cell lines may partially explain why Top1 inhibitors show better efficacy in some patients with colon cancer than others. Our immunoassay could potentially be adapted to provide a means of screening patients prior to treatment and monitoring patient response during treatment with Top1 inhibitors. It could also be used in conjunction with other biomarkers, such as p53 status, that may affect sensitivity to treatment (12, 24). BRCA1 status has also been shown to be involved in transcription induced degradation of Top1 (24, 25).
Leukemia, prostate, ovarian, lung, and breast cancer cell lines also had individual cell lines with high (>4 ng/mL/μg protein) Top1 levels. Besides colorectal cancer, Top1 inhibitors are approved for the treatment of ovarian and small cell lung carcinoma (SCLC) (16). Clinical trials have been conducted for Top1 inhibitors (both as monotherapy and in combination studies with other anticancer agents) for many types of cancer, including colorectal, lung (non-SCLC and SCLC), ovarian, breast, and chronic myelomonocytic leukemia, with varying response rates (26). Topotecan has been shown to be effective in inducing remission when given before standard induction therapy for childhood acute lymphoblastic leukemia in the first relapse (27). In comparison, central nervous system and renal cancer cell lines had lower levels of Top1. Overall, the results of this study show that there is substantial variation in Top1 levels both within a cancer cell type and between the histologies represented in the NCI-60 cell line panel (as shown in Fig. 2).
Western blot data was concordant with ELISA data for five of the seven cell lines examined. Western blot results for the H322M cell line were intermediate between the ELISA and microarray data. Like the ELISA, the Western blot data shows about a 10-fold difference in Top1 enzyme levels across the NCI-60 panel.
The activity of indenoisoquinolines NSC 724998 and NSC 725776 correlated with Top1 expression in the NCI-60 cell line panel. This supports the findings of the FOCUS trial, which found an association between Top1 levels and response to another Top1 inhibitor, irinotecan (3). We did not, however, find significant correlation between Top1 expression and topotecan activity. This difference in correlation with the efficacy of topotecan versus the indenoisoquinolines may be partially explained by differences in the efflux of these drugs by multi-drug resistance pumps and in the persistence of the covalent complex of Top1 with the anticancer agent (17). Our data also suggest that mRNA levels could be used with high confidence to aid in the prediction of response to Top1 inhibitors if Top1 enzyme levels were not available.
This newly developed Top1 assay is a sandwich-ELISA run in a 96-well format, using commercially available Top1 antibodies, making it available for use in most laboratory settings. We have validated that the assay can provide maximal sensitivity over a range of Top1 concentrations from 0.4 to 12.5 ng/mL. In addition, the assay had good precision and reproducibility, with low inter-plate coefficients of variance and high Top1 recoveries. These data indicate that this novel Top1 immunoassay should be useful for routine quantification of Top1 levels in cell extracts. Because Top1 levels have been implicated in the efficacy and resistance of Top1 inhibitors (3–6), the assay could potentially be adapted for use in predicting response to Top1 inhibitors in a clinical setting.
Supplementary Material
Acknowledgments
We would like to thank Dr. Yvonne A. Evrard and Ms. Gina Uhlenbrauck, SAIC-Frederick, Inc., for editorial assistance in the preparation of this manuscript.
Grant support: This project has been funded in whole or in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. This research was support [in part] by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The research was supported [in part] by the Developmental Therapeutics Program in the Division of Cancer Treatment and Diagnosis of the National Cancer Institute.
Abbreviations
- BCA
Bicinchoninic acid
- ELISA
Enzyme-linked immunosorbent assay
- pAb
polyclonal antibody
- FOCUS trial
Fluorouracil, Oxaliplatin, CPT-11: Use and Sequencing trial
- NCI
National Cancer Institute
- rTop1
recombinant Topoisomerase I
- RT-PCR
Reverse transcriptase-PCR
- SCLC
small cell lung carcinoma
- Top1
Topoisomerase I
Footnotes
Note: Supplementary material for this article is available at Molecular Cancer Therapeutics Online (http://mct.aacrjournals.org/)
References
- 1.Gupta M, Fujimori A, Pommier Y. Eukaryotic DNA Topoisomerases-I. Biochim Biophys Acta-Gene Struct Expression. 1995;1262:1–14. doi: 10.1016/0167-4781(95)00029-g. [DOI] [PubMed] [Google Scholar]
- 2.Ataka M, Ikeguchi M, Yamamoto M, et al. Topoisomerase I Protein Expression and Prognosis of Patients with Colorectal Cancer. Yonago Acta Medica. 2007;50:81–7. [Google Scholar]
- 3.Braun MS, Richman SD, Quirke P, et al. Predictive Biomarkers of Chemotherapy Efficacy in Colorectal Cancer: Results From the UK MRC FOCUS Trial. J Clin Oncol. 2008;26:2690–8. doi: 10.1200/JCO.2007.15.5580. [DOI] [PubMed] [Google Scholar]
- 4.Boyer J, McLean EG, Aroori S, et al. Characterization of p53 wild-type and null isogenic colorectal cancer cell lines resistant to 5-fluorouracil, oxaliplatin, and irinotecan. Clin Cancer Res. 2004;10:2158–67. doi: 10.1158/1078-0432.ccr-03-0362. [DOI] [PubMed] [Google Scholar]
- 5.Liao Z, Robey RW, Guirouilh-Barbat J, et al. Reduced expression of DNA topoisomerase I in SF295 human glioblastoma cells selected for resistance to homocamptothecin and diflomotecan. Mol Pharmacol. 2008;73:490–7. doi: 10.1124/mol.107.041178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sorensen M, Sehested M, Jensen PB. Characterization of a Human Small-Cell Lung-Cancer Cell-Line Resistant to the DNA Topoisomerase I-Directed Drug Topotecan. Br J Cancer. 1995;72:399–404. doi: 10.1038/bjc.1995.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darren JB, Jason D, Lars Z, et al. Topoisomerase levels determine chemotherapy response in vitro and in vivo. Proc Nat Acad Sci. 2008;105:9053–8. doi: 10.1073/pnas.0803513105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durand-Faucher K, Rabinovitch-Chable H, Dzugan N, et al. A quantitative RT-PCR method to determine topoisomerase I mRNA levels in human tissue samples. Clin Chem Lab Med. 2005;43:707–14. doi: 10.1515/CCLM.2005.120. [DOI] [PubMed] [Google Scholar]
- 9.Gygi SP, Rochon Y, Franza BR, Aebersold R. Correlation between Protein and mRNA Abundance in Yeast. Mol Cell Biol. 1999;19:1720–30. doi: 10.1128/mcb.19.3.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankavaram UT, Reinhold WC, Nishizuka S, et al. Transcript and protein expression profiles of the NCI-60 cancer cell panel: an integromic microarray study. Mol Cancer Ther. 2007;6:820–32. doi: 10.1158/1535-7163.MCT-06-0650. [DOI] [PubMed] [Google Scholar]
- 11.Coleman LW, Rohr LR, Bronstein IB, Holden JA. Human DNA topoisomerase I: An anticancer drug target present in human sarcomas. Hum Pathol. 2002;33:599–607. doi: 10.1053/hupa.2002.124911. [DOI] [PubMed] [Google Scholar]
- 12.Lynch BJ, Komaromy-Hiller G, Bronstein IB, Holden JA. Expression of DNA topoisomerase I, DNA topoisomerase II-alpha, and p53 in metastatic malignant melanoma. Hum Pathol. 1998;29:1240–5. doi: 10.1016/s0046-8177(98)90251-9. [DOI] [PubMed] [Google Scholar]
- 13.Liebes L, Potmesil M, Kim T, et al. Pharmacodynamics of topoisomerase I inhibition: Western blot determination of topoisomerase I and cleavable complex in patients with upper gastrointestinal malignancies treated with topotecan. Clin Cancer Res. 1998;4:545–57. [PubMed] [Google Scholar]
- 14.Beidler DR, Cheng YC. Camptothecin induction of a time- and concentration-dependent decrease of topoisomerase I and its implication in camptothecin activity. Mol Pharmacol. 1995;47:907–14. [PubMed] [Google Scholar]
- 15.Desai SD, Li T-K, Rodriguez-Bauman A, Rubin EH, Liu LF. Ubiquitin/26S Proteasome-mediated Degradation of Topoisomerase I As a Resistance Mechanism to Camptothecin in Tumor Cells. Cancer Res. 2001;61:5926–32. [PubMed] [Google Scholar]
- 16.Pommier Y. Topoisomerase I inhibitors: camptothecins and beyond. Nat Rev Cancer. 2006;6:789–802. doi: 10.1038/nrc1977. [DOI] [PubMed] [Google Scholar]
- 17.Antony S, Agama KK, Miao ZH, et al. Novel indenoisoquinolines NSC 725776 and NSC 724998 produce persistent topolsomerase I cleavage complexes and overcome multidrug resistance. Cancer Res. 2007;67:10397–405. doi: 10.1158/0008-5472.CAN-07-0938. [DOI] [PubMed] [Google Scholar]
- 18.Teicher BA. Next generation topoisomerase I inhibitors: Rationale and biomarker strategies. Biochem Pharmacol. 2008;75:1262–71. doi: 10.1016/j.bcp.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 19.Irizarry RA, Bolstad BM, Collin F, Cope LM, Hobbs B, Speed TP. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 2003;31:e15. doi: 10.1093/nar/gng015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai SD, Mao Y, Sun MEI, Li T-K, Wu J, Liu LF. Ubiquitin, SUMO-1, and UCRP in Camptothecin Sensitivity and Resistance. Ann N Y Acad Sci. 2000;922:306–8. doi: 10.1111/j.1749-6632.2000.tb07050.x. [DOI] [PubMed] [Google Scholar]
- 21.Desai SD, Zhang H, Rodriguez-Bauman A, et al. Transcription-Dependent Degradation of Topoisomerase I-DNA Covalent Complexes. Mol Cell Biol. 2003;23:2341–50. doi: 10.1128/MCB.23.7.2341-2350.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Devy J, Wargnier R, Pluot M, Nabiev I, Sukhanova A. Topotecan-induced alterations in the amount and stability of human DNA topoisomerase I in solid tumor cell lines. Anticancer Res. 2004;24:1745–51. [PubMed] [Google Scholar]
- 23.Zustovich F, Cartei G, Trestin A, et al. Analysis of topoisomerase (TOP) expression in peripheral blood mononuclear cell (PBMCs) from patients (PTS) undergoing chemotherapy (CHT) for solid tumors( ST) J Clin Oncol. 2004;22:2124. (Meeting Abstracts) [Google Scholar]
- 24.Pommier Y, Barcelo JM, Rao VA, et al. Repair of Topoisomerase I-Mediated DNA Damage. Prog Nucleic Acid Res Mol Biol. 2006;81:179–229. doi: 10.1016/S0079-6603(06)81005-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sordet O, Larochelle S, Nicolas E, et al. Hyperphosphorylation of RNA Polymerase II in Response to Topoisomerase I Cleavage Complexes and Its Association with Transcription- and BRCA1-dependent Degradation of Topoisomerase I. J Mol Biol. 2008;381:540–9. doi: 10.1016/j.jmb.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Galindo C, Radomski K, Stewart CF, Furman W, Santana VM, Houghton PJ. Clinical use of topoisomerase I inhibitors in anticancer treatment. Med Pediatr Oncol. 2000;35:385–402. doi: 10.1002/1096-911x(20001001)35:4<385::aid-mpo1>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 27.Hijiya N, Stewart CF, Zhou YM, et al. Phase II study of topotecan in combination with dexamethasone, asparaginase, and vincristine in pediatric patients with acute lymphoblastic leukemia in first relapse. Cancer. 2008;112:1983–91. doi: 10.1002/cncr.23395. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


