Abstract
Background
Alcohol exposure during the period of rapid brain development produces structural damage in different brain regions, including the suprachiasmatic nucleus (SCN), that may have permanent neurobehavioral consequences. Thus, this study examined the long-term effects of neonatal alcohol exposure on circadian behavioral activity in adult rats.
Methods
Artificially reared Sprague-Dawley rat pups were exposed to alcohol (EtOH; 4.5 g/kg/day) or isocaloric milk formula (gastrostomy control; GC) on postnatal days 4–9. At 2 months of age, rats from the EtOH, GC, and suckle control (SC) groups were housed individually, and properties of the circadian rhythm in wheel-running behavior were continuously analyzed during exposure to a 12-hr light:12-hr dark photoperiod (LD 12:12) or constant darkness (DD).
Results
Neonatal alcohol exposure had distinctive effects on the rhythmic properties and quantitative parameters of adult wheel-running behavior. EtOH-treated animals were distinguished by unstable and altered entrainment to LD 12:12 such that their daily onsets of activity were highly variable and occurred at earlier times relative to control animals. In DD, circadian regulation of wheel-running behavior was altered by neonatal alcohol exposure such that the free-running period of the activity rhythm was shorter in EtOH-exposed rats than in control animals. Total amount of daily wheel-running activity in EtOH-treated rats was greater than that observed in the SC group. In addition, the circadian activity patterns of EtOH-exposed rats were fragmented such that the duration of the active phase and the number of activity bouts per day were increased.
Conclusions
These data indicate that neonatal alcohol exposure produces permanent changes in the circadian regulation of the rat activity rhythm and its entrainment to LD cycles. These long-term alterations in circadian behavior, along with the developmental alcohol-induced changes in SCN endogenous rhythmicity, may have important implications in clinical sleep-wake disturbances observed in neonates, children, and adults exposed to alcohol in utero.
Keywords: Circadian Rhythms, Clock, Ethanol, Wheel-Running Behavior, Suprachiasmatic Nucleus
Alcohol exposure during critical stages of development induces structural and cellular damage within the central nervous system. Detailed studies using a rat model indicate that alcohol exposure during the brain growth spurt (early postnatal period), which is equivalent to the period of human brain development during the third trimester (Dobbing and Sands, 1979), produces a wide range of damaging effects, including reductions in brain size and weight (Maier et al., 1996), cell loss in discrete brain regions such as the cerebellum, hippocampus, and olfactory bulb (Bonthius et al., 1992; Bonthius and West 1990; Chen et al., 1998; Goodlett et al., 1998), altered neuronal circuitry (West et al., 1981), and decreased expression of specific neurochemical signals (Manteuffel, 1996). In many instances, this alcohol-induced injury to specific brain regions is permanent and thus may have long-term consequences in the neural regulation of behavior. In rats exposed to alcohol during the early postnatal period, the cerebellum and hippocampus provide notable examples where alcohol-induced cell loss is, respectively, coupled with behavioral deficits on motor performance and learning/memory tasks (Goodlett et al., 1987, 1988; Kelly et al., 1988; Thomas et al., 1996, 1998). However, further analysis is necessary to delineate the full extent of the long-term neurobehavioral disturbances associated with the damaging effects of developmental alcohol on specific brain regions.
The neural center responsible for the regulation of circadian or 24-hr rhythms has been recently identified as another target for alcohol-induced insult during central nervous system development (Earnest et al., 2001). In mammals, the hypothalamic suprachiasmatic nucleus (SCN) functions as a biological clock that governs the generation of circadian rhythms in a variety of biological processes and their synchronization or entrainment to light-dark cycles (Moore, 1983). Exposure to alcohol during the early postnatal period has been shown to disrupt the circadian rhythm in SCN content of brain-derived neurotrophic factor in adult rats (Allen et al., 2004). Consistent with the effect of alcohol on this SCN output signal, preliminary studies suggest that developmental alcohol exposure also induces permanent alterations in SCN molecular oscillations that comprise the circadian clock mechanism (Farnell et al., 2004b). Because disruption of endogenous SCN rhythmicity can produce tangible changes in the circadian regulation of behavior (Reppert and Weaver, 2001), it is possible that alterations in circadian behavior may accompany developmental alcohol-induced effects on the SCN clockworks and its circadian output signals. On the basis of clinical evidence linking maternal alcohol consumption during pregnancy to sleep-wake disturbances in offspring (Rosett et al., 1979; Steinhausen and Spohr, 1998; Steinhausen et al., 1993), the present study used a rat model to determine whether alcohol exposure during the brain growth spurt also causes long-term changes in the circadian rhythm of wheel-running behavior. Specifically, we examined the effects of neonatal alcohol exposure on the quantitative parameters, light-dark entrainment and circadian regulation of wheel-running activity, in adult rats.
METHODS
Subjects
The subjects were 60 male Sprague-Dawley rat pups derived from 15 time-mated litters. Male subjects were used exclusively in these studies because female rats show considerable day-to-day variability in the circadian regulation of activity behavior (relative to that in males) that would present a confounding factor in the analysis of the effects of neonatal alcohol treatment. In this regard, female rodents are distinguished by daily irregularity in the period of the activity rhythm in constant conditions, the timing of their activity onsets during entrainment to a light-dark cycle, and the total amount of activity due to changing levels of estrogen over the course of the estrous cycle (Morin et al., 1977; Albers, 1981; Albers et al., 1981). The animals were born and reared in the vivarium at the Texas A&M University System Health Science Center under a standard 12-hr light:12-hr dark photoperiod (LD 12:12; lights-on at 0600 hr). On postnatal day (PD) 1 (date of birth designated as PD 0), the newborns within each litter were culled to 8–10 pups per litter, utilizing cross-fostering procedures as necessary. On PD 4, the rat pups were randomly assigned to one of three treatment groups: a suckle control group (SC; n = 20) and two artificial-rearing treatment groups. The artificial-rearing groups received either alcohol (EtOH; n = 20) or maltose-dextrin [gastrostomy control (GC); n = 20] from PD 4 through 9. The procedures used in this study were approved by the University Laboratory Animal Care Committee at Texas A&M University.
Artificial-Rearing Procedure
The artificial-rearing procedures and diet have been described previously in detail (Diaz, 1991; West, 1993). Briefly, on PD 4, pups were anesthetized with isoflurane (VEDCO, Inc., St. Joseph, MO), and gastrostomy tubes were inserted down the esophagus and surgically implanted into the stomach (Diaz, 1991; West et al., 1984). From PD 4 to 9, pups were maintained in the artificial-rearing apparatus and received their daily nutritional requirements through formula (diet)–filled syringes that were operated by a timer-activated infusion pump (Model 935, Harvard Apparatus, Holliston, MA). The formula was provided daily in 12 20-min fractions (i.e., every 2 hr). Gastrostomized pups received either alcohol treatment or isocaloric maltose-dextrin solution from PD 4 to 9. After this treatment regimen, pups were maintained on the artificial-rearing apparatus for an additional 3 days with regular diet to allow alcohol withdrawal in the EtOH group. On PD 12, artificially reared pups were fostered to a lactating dam after their gastrostomy tubes had been cut and sealed. The SC group was included to control for any effect produced by artificial-rearing methods. To minimize the potential confound of litter effects, no more than two pups from the same litter were assigned to the same treatment condition. All pups were coded by injecting India ink to their paws for future identification (Geller and Geller, 1966). Animals were weaned on PD 21 and housed two to three per cage. Access to food and water was provided ad libitum for the remainder of the experiment.
Drug Administration
Beginning around midday of the LD 12:12 photoperiod (1200 hr) from PD 4 to 9, alcohol (10.2%, v/v) was administered to the EtOH group through 2 of the daily 12 feedings at the dosage of 4.5 g/kg/day. This alcohol dosage and treatment regimen have been shown to consistently produce structural damage in several different brain regions (Bonthius et al., 1992; Bonthius and West, 1990; Chen et al., 1998, 1999). The GC group (0 g/kg/day alcohol) was given the appropriate concentration of isocaloric maltose-dextrin solution in place of alcohol.
Blood Alcohol Concentration
Blood alcohol concentrations (BACs) for all alcohol-treated pups were determined using a gas chromatograph (Model 3400, Varian, Palo Alto, CA). Blood samples (20 μl) were collected from pup tails 90 min after the beginning of the second alcohol feeding on PD 6 (peak BAC) (Bonthius et al., 1988; Kelly et al., 1987a). Blood samples were stored in glass vials containing a 200-μl cocktail composed of 0.6 M perchloric acid and 4 mM n-propanol in double-distilled water until BAC analysis.
Analysis of Wheel-Running Activity
At 2 months of age, animals (n = 60) were housed individually in cages equipped with running wheels so that the circadian rhythm of wheel-running activity could be continuously recorded. All animals were initially maintained under a LD 12:12 cycle, and baseline activity behavior was recorded for 10–14 days. For 36 animals, these light-dark conditions were sustained, and the pattern of wheel-running activity was analyzed for 30–40 days during entrainment to LD 12:12. The remaining animals (n = 24) were exposed to constant darkness (DD) beginning at the offset of the light-dark cycle (1800 hr), and circadian properties of the activity rhythm were examined under free-running conditions for 50–60 days. Periodic animal care and equipment maintenance were performed at random times and accomplished in DD using an infrared viewer (FJW Optical Systems, Palatine, IL).
Wheel-running activity was continuously recorded, summed, and stored in 10-min bins using a computer running Dataquest IV data acquisition software (Data Sciences, Inc., St. Paul, MN). Graphical records of circadian activity rhythms were generated and analyzed using ClockLab data analysis software (ActiMetrics, Evanston, IL). Quantitative parameters and circadian properties of wheel-running behavior were measured over the same 30-day interval for every animal (days 1–30 in LD and days 25–54 in DD). The phase angle of entrainment (Ψ) was the only behavioral property assessed under LD conditions. During entrainment to LD 12:12, the onset of activity for a given cycle was identified as the first bin during which an animal attained 10% of peak running-wheel revolutions (i.e., intensity). To measure Ψ during entrainment to LD 12:12, least squares analyses was used to establish a regression line through the daily onsets of activity during the period of entrainment (30 days), and then the number of minutes before (positive) or after (negative) the time of lights-off in the LD cycle (1800 hr) was determined for each animal. For each animal, the steady-state period (τ) of the activity rhythm in DD was determined by χ2 periodogram and fast Fourier transform. The following parameters of wheel-running behavior were also evaluated during exposure to DD: total wheel revolutions per day, duration of the active period, number of activity bouts per day, bout duration, and number of wheel revolutions per bout (size). Total daily activity was calculated by averaging the number of wheel revolutions per 24 hr over the 30-day interval of analysis. For a given cycle in DD, the daily onset of activity was determined as described above, and the activity offset was defined as the last bin during which 5% maximal intensity was achieved. Activity duration (α) was then determined by measuring the time interval between the daily activity onsets and offsets. An activity bout was defined as a period during which wheel-running activity never dropped to <10 counts/bin for >20 min. These criteria for bout threshold and maximum intrabout interval were used to determine the number of activity bouts per day as well as bout duration and size. Statistical analyses were performed on the raw data using a one-way ANOVA to determine the significance of treatment effects on circadian properties and quantitative parameters of the activity rhythm, and Newman-Keuls post hoc analyses were applied if necessary. In addition, the relation between the BACs of individual animals and alcohol-induced changes in circadian properties and quantitative parameters of wheel-running activity in the group were assessed via Pearson correlational analysis.
RESULTS
Blood Alcohol Concentration
The administration of 4.5 g/kg/day alcohol in 2 consecutive feedings of the 12 daily feedings resulted in peak BACs on PD 6 ranging from 268.7 to 387.5 mg/dl (or 0.269–0.388%). The mean peak BAC (± SEM) was 308.1 ± 13.4 mg/dl. These BACs are similar to those established in previous studies using the same alcohol dosage (Napper and West, 1995).
Entrainment and Circadian Properties of Wheel-Running Behavior
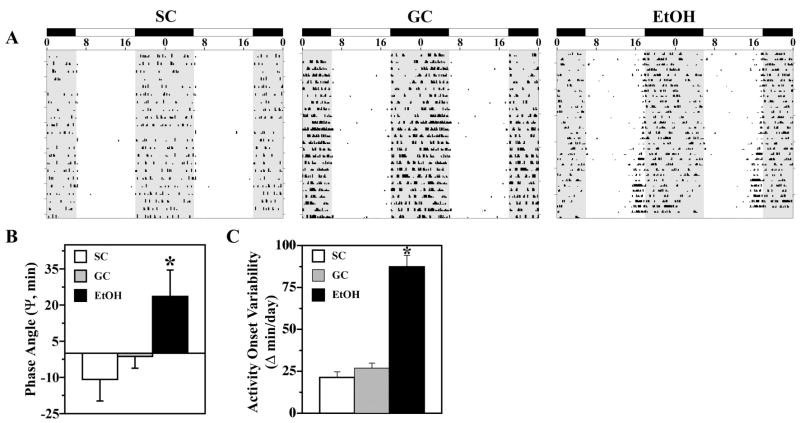
During exposure to LD 12:12, entrainment of the activity rhythm was observed in all control and EtOH animals. Representative actograms of animals from all three treatment groups are shown in Fig. 1A. SC and GC rats showed similar patterns of entrainment in which their daily onsets of activity occurred shortly after lights-off (Fig. 1A). In the SC and GC groups, the average phase angle (Ψ) between the daily onset of activity and the offset of the photoperiod was −10.90 ± 8.88 and −1.30 ± 4.90 min, respectively (Fig. 1B). In contrast, the activity rhythms of EtOH-treated rats were distinguished by an altered phase angle of entrainment to LD 12:12 such that their daily onsets of activity were advanced and occurred at earlier times relative to control animals, commencing up to 80–120 min before lights-off for some animals. In the EtOH group, the average Ψ between activity onsets and lights-off was +23.6 ± 10.9 min and was significantly different [F(2,33) = 4.28; p < 0.05] from that observed in control animals. Moreover, the degree to which the phase angle of entrainment was advanced in individual EtOH-exposed animals was positively correlated with their BAC (r = +0.954). In addition to the earlier onset of daily activity, EtOH-exposed animals showed unstable patterns of entrainment to LD 12:12 as indicated by high variability in the timing of their activity onsets between successive days. During exposure to LD 12:12, the activity onsets in individual EtOH-treated rats occurred at (earlier or later) times that differed on average by 87 min from the preceding day, whereas the average day-to-day variability in activity onset times of control animals was only 21–26 min (Fig. 1C). The absolute day-to-day variation in the onsets of activity in EtOH-exposed animals was significantly greater [F(2,21) = 60.41; p < 0.01] than that observed in both control groups.
Fig. 1.
Effects of early postnatal alcohol exposure on the light-dark entrainment of the rhythm in wheel-running activity. (A) Representative activity records of three adult male rats from the SC, GC, and EtOH groups during entrainment to LD 12:12. Actograms are double-plotted over a 48-hr period. The closed bars at the top and shading on the records signify the timing of the dark phase in the LD 12:12 cycle. (B) During LD 12:12 entrainment, the phase angle between activity onsets and lights-off in EtOH rats (n = 12) was significantly different (*p < 0.05) from that observed in the SC and GC groups (@ n = 12). Bars represent the mean (±SEM) phase angle of entrainment (ψ) to LD 12:12 in minutes. Negative values indicate that daily onsets of activity occur after lights-off, whereas positive values denote that activity onsets precede the end of the light phase. (C) The absolute day-to-day variability in onsets of activity was significantly greater in EtOH rats (*p < 0.01) than in SC and GC animals. Absolute differences in the timing of activity onsets on successive days were analyzed in individual animals, and bars represent mean (± SEM) determinations in minutes.
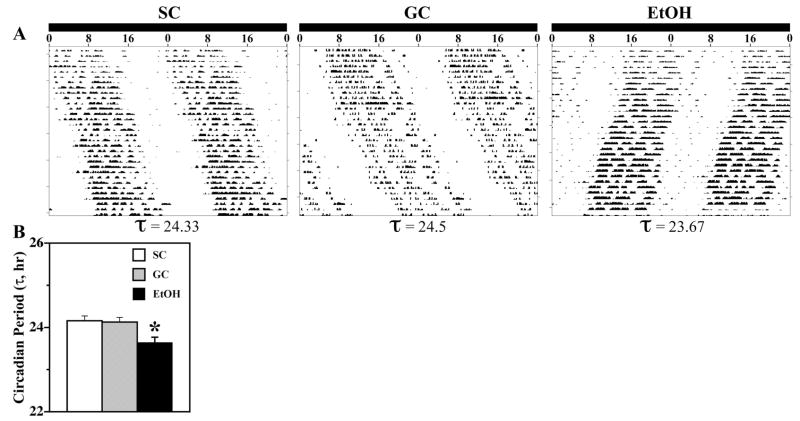
During exposure to DD, all animals showed free-running rhythms of wheel-running behavior (Fig. 2A). The circadian period of the activity rhythm in EtOH-exposed rats (mean τ = 23.64 ± 0.13 hr) was significantly shorter than that observed in SC and GC controls [mean τ = 24.16 ± 0.11 and 24.13 ± 0.11 hr, respectively; (F[2,21] = 6.29; p < 0.05)] (Fig. 2B). This alcohol-induced decrease in the circadian period of individual EtOH rats was strongly correlated with their BACs (r = −0.982) such that the periodicity of the activity rhythm was shorter in those exposed to higher alcohol levels. In conjunction with this decrease in the period of circadian behavior, EtOH treatment had a stimulatory effect on overall levels of wheel-running activity. Total wheel revolutions per day were significantly greater in EtOH rats than in SC animals [F(2,21) = 3.63; p < 0.05] (Fig. 3A). Although neonatal alcohol exposure has been shown to disrupt general motor coordination in rats (Thomas et al., 1998, 2003), the elevated wheel-running activity in EtOH-treated rats indicates that this treatment did not produce any major motor deficits that compromised their ability to run on the wheels.
Fig. 2.
Effects of early postnatal alcohol exposure on the circadian period (τ) of rat rhythm in wheel-running activity. (A) Representative activity records of three adult male rats from the SC, GC, and EtOH groups that were maintained under free-running conditions in DD. Actograms are double-plotted over a 48-hr period, and determinations of circadian period by χ2 periodogram and fast Fourier transform are indicated at the bottom of each record. (B) The circadian period of the activity rhythm in the EtOH group (n = 8) was significantly shorter (*p < 0.05) than that found in SC and GC animals (@ n = 8). Bars represent the mean (±SEM) circadian period (τ) of the activity rhythm in hours.
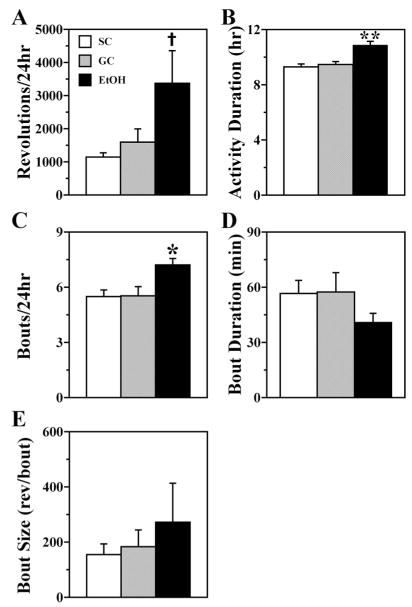
Fig. 3.
Quantitative parameters of wheel-running activity in SC (open bar), GC (shaded bar), and EtOH (solid bar) rats during exposure to DD. Bars represent the mean (± SEM) values for (A) total wheel revolutions per day, (B) duration of the active phase, (C) number of activity bouts per day, (D) duration of activity bouts, and (E) bout size expressed as the number of wheel revolutions per bout. Total daily activity was significantly increased (†p < 0.05) relative to that found only in the SC group. The duration of the active phase and the number of activity bouts per day were significantly greater (*p < 0.05; **p < 0.01) in EtOH rats (n = 8) than in both SC and GC animals (@ n = 8).
Neonatal alcohol exposure also had distinctive effects on the temporal distribution of wheel-running activity. The duration of the active phase (α) was significantly greater in the EtOH group than in SC and GC groups [F(2,21) = 13.54; p < 0.01]. EtOH-exposed rats were marked by an increase of ≈1 hr in the duration of α (mean: 10.87 ± 0.27 hr compared with 9.29 ± 0.22 hr for SC animals and 9.47 ± 0.21 hr for GC animals) (Fig. 3B). Early postnatal alcohol treatment also had a significant effect [F(2,21) = 5.82; p < 0.05] in increasing the number of bouts per day relative to that observed in both control groups (Fig. 3C). However, these changes in activity duration and the number of bouts per day were not correlated with the BACs observed in individual animals (r = −0.018 and r = +0.142, respectively). Other comparisons of activity bout parameters revealed trends in which bout duration was decreased, whereas the number of activity counts per bout or bout size was elevated and more variable in EtOH animals relative to controls. As a collective result of the increased activity duration, increased number of activity bouts, and decreased bout duration, wheel-running activity in EtOH-treated animals was differentially fragmented across the course of the circadian cycle with more frequent intervening “rest” periods.
DISCUSSION
The present findings demonstrate that alcohol exposure during a critical period of central nervous system development has long-term neurobehavioral consequences with regard to the regulation of rat wheel-running activity. After early postnatal exposure to alcohol, adult rats displayed permanent alterations in the total amount, temporal distribution, light-dark entrainment, and circadian regulation of wheel-running behavior. In EtOH-exposed rats, entrainment of the rhythm in wheel-running activity to LD 12:12 was altered such that the timing of their daily onsets of nocturnal activity was highly variable from cycle to cycle and earlier in comparison with control animals. Because steady-state entrainment to light-dark cycles is determined by both the circadian period and phase-shifting properties of the underlying clock mechanism (for review, see Johnson et al., 2003; Pittendrigh and Daan, 1976a), the positive phase angle or advanced activity onsets in the EtOH group during photoentrainment is probably related to the shortened free-running period of the activity rhythm observed in these animals. Given that the endogenous period of the clock mechanism in the EtOH group is shorter than the 24-hr periodicity of the light-dark cycle, circadian photoentrainment requires the daily induction of small phase delays to “correct” this difference between intrinsic and environmental cycles. In contrast to EtOH-exposed rats, daily phase advances are needed for entrainment in both control groups because the circadian period of their free-running rhythms is longer than that of the LD 12:12 cycle. Consistent with the phase response curves to light (Pittendrigh and Daan, 1976a; Summer et al., 1984), the appropriate phase adjustments in each cycle are differentially obtained in EtOH animals by aligning the end of the light phase with the early subjective night (i.e., interval immediately after the onset of activity) and in both control groups by situating the onset of the light phase so as to coincide with the late subjective night. EtOH-induced changes in the phase-shifting responses of the clock mechanism to light may similarly contribute to the altered patterns of photic entrainment in these animals. This possibility is supported by recent observations indicating that exposure to alcohol in utero or during the early postnatal period produces long-term alterations in the phase-shifting effects of light on rat circadian rhythms (Farnell et al., 2004a; Sei et al., 2003). Collectively, these findings provide evidence that alcohol exposure during early brain development induces long-term perturbations in fundamental properties of the rat circadian system, including its free-running period, photic entrainment, and phase-shifting responses to light.
The precise mechanism by which developmental alcohol exposure alters the photic entrainment and circadian period of the rat activity rhythm is currently unknown. However, these long-term perturbations in the photoentrainment and circadian regulation of rat wheel-running behavior presumably reflect EtOH-induced damage to the circadian clock in the SCN and/or the neural pathway responsible for the communication of entraining light signals to the SCN. Similar to the deficits in motor coordination that have been linked to cerebellar Purkinje cell loss after neonatal alcohol exposure (Thomas et al., 1996, 1998), alcohol-induced cell loss in the SCN may contribute to the observed alterations in the rhythm of wheel-running activity. In this regard, it is noteworthy that the effects of neonatal alcohol treatment in shortening the free-running period of the clock mechanism and in shifting the daily onsets of activity to earlier times than normal during entrainment to light-dark cycles are similar to those observed after partial destruction of the SCN (Pickard and Turek, 1985). Indeed, the possible role of EtOH-induced cell loss in the observed changes in the photic and circadian regulation of the rat activity rhythm is supported by our previous finding that early postnatal alcohol exposure causes a significant reduction in SCN neuronal density relative to SCs (Farnell et al., 2004a). The functional effects of EtOH-related structural damage may occur as a consequence of changes in intranuclear synaptic connections that serve to couple multiple oscillators within the SCN because the free-running period of circadian rhythms and their phase-shifting responses to light are dependent on this fundamental property of clocks derived from populations of cell-autonomous oscillators (for review, see Michel and Colwell, 2001; Pittendrigh and Daan, 1976b).
Alternatively, the changes in the circadian properties of the activity rhythm in EtOH-treated animals may stem from damage to the circadian timekeeping mechanism and/or critical output signals of the SCN clock that regulate overt rhythmicity. The possible impact of developmental alcohol exposure on molecular components of the SCN clock mechanism is supported by our preliminary findings that the circadian rhythms of Per2 and Cry1 mRNA expression in the rat SCN are altered in adult rats exposed to alcohol during the early postnatal period (Farnell et al., 2004b). SCN output signals have been identified as potential targets for the long-term effects of alcohol exposure during early brain development in a recent study demonstrating that the rhythm in SCN content of brain-derived neurotrophic factor is abolished in adult rats subjected to neonatal alcohol treatment (Allen et al., 2004). Neonatal alcohol exposure may also affect the photoentrainment properties of circadian rhythms by permanently altering key elements of pathways that mediate the communication of light input to the SCN such as the presumptive photopigment, melanopsin, and the neurochemical signals glutamate and pituitary adenylate cyclase–activating polypeptide (De Vries et al., 1993; Ding et al., 1994; Hannibal, 2002; Harrington et al., 1999; Hattar et al., 2002; Meijer et al., 1988; Ruby et al., 2002; van den Pol, 1993). Further analysis is necessary to fully identify the long-term effects of developmental alcohol exposure on circadian timekeeping and elucidate the basic mechanisms underlying this alcohol-induced brain injury.
Detailed analysis of quantitative parameters of wheel-running behavior in DD revealed that the duration of the active phase, fragmentation of activity across the circadian cycle, and total amount of daily activity were increased in EtOH-treated rats. This EtOH-induced elevation in levels of wheel-running activity is consistent with previous observations demonstrating that rats exposed to alcohol during the neonatal period exhibit heightened ambulatory activity (i.e., hyperactivity) in open-field behavioral tests (Kelly et al., 1987b; Thomas et al., 2003). It is also noteworthy that the inverse relationship between this increase in daily wheel-running activity and decrease in circadian period observed in EtOH-exposed animals concurs with evidence from other studies indicating that fundamental clock properties such as period and phase-shifting responses are regulated by the state of behavioral arousal through feedback mechanisms (Turek, 1989). The biochemical identity of the signal mediating this activity-dependent feedback on the clock mechanism is presently unknown. However, serotonergic (Mistlberger et al., 1998; Shiori et al., 1991) and dopaminergic (Isobe and Nishino, 2001) inputs to the SCN have been implicated in the regulation of activity-dependent changes in free-running period. The SCN is innervated by a dense plexus of serotonin and dopamine fibers that arise, respectively, from the midbrain raphe nucleus and ventral tegmental area (Meyer–Bernstein and Morin, 1996; van den Pol and Tsujimoto, 1985). Because activation and/or metabolism of these neurotransmitter systems in the brain are vulnerable to EtOH-induced insults during development (Choong and Shen, 2004; Sari and Zhou, 2004), impairment of serotonin and/or dopamine input to the SCN may contribute to the effects of early postnatal alcohol exposure on behavioral activity and its feedback regulation of circadian clock properties.
On the basis of the collective observations derived from this and other studies, there is now substantial evidence indicating that developmental alcohol exposure permanently alters the circadian clock in the SCN and its regulation of overt behavioral and physiological rhythms. Exposure to alcohol during the prenatal or early postnatal period has been shown to induce long-term changes in endogenous oscillations in the SCN (Allen et al., 2004; Farnell et al., 2004b; Lund et al., 2004), circadian entrainment to light-dark cycles (Sei et al., 2003), and fundamental properties of the circadian clock mechanism such as its free-running period and phase-shifting responses to light (Farnell et al., 2004a). Although information on the long-term behavioral consequences of developmental alcohol exposure is limited, these permanent alcohol-induced alterations in circadian timekeeping function pose a striking parallel to some of the disturbances associated with fetal alcohol syndrome. Circadian rhythm abnormalities like those observed after developmental alcohol exposure have been associated with bipolar affective disorder (i.e., manic-depressive illness) (Moore, 1991; Schwartz, 1993) and depression, both of which are behavioral manifestations of fetal alcohol syndrome (Sher, 2004). Although sleep was not examined in this study, changes in the activity patterns of EtOH-exposed rats with regard to their phase angle during photic entrainment and fragmentation over the circadian cycle also mirror clinical sleep-wake disturbances observed in human neonates, children, and adolescents after prenatal exposure to alcohol (Rosett et al., 1979; Smith and Eckardt, 1991; Steinhausen and Spohr, 1998; Steinhausen et al., 1993). Consequently, these permanent alcohol-related alterations in circadian timekeeping may provide a useful index or marker for the long-term neurobehavioral effects of fetal alcohol syndrome and fetal alcohol spectrum disorders (Chudley et al., 2005; Hoyme et al., 2005). Finally, research on how the SCN and its regulation of circadian behavior are affected by developmental alcohol exposure could yield important insight into the basic mechanisms by which alcohol-induced injury during rapid brain growth produces permanent behavioral deficits.
Acknowledgments
Supported by NIH Grants AA13242 and MH60147 (D.J.E) and NIH Grant AA05523 (J.R.W.).
The authors thank Jo Mahoney and Rodney Walline for excellent technical assistance.
References
- Albers HE. Gonadal hormones organize and modulate the circadian system of the rat. Am J Physiol. 1981;241:R62–R66. doi: 10.1152/ajpregu.1981.241.1.R62. [DOI] [PubMed] [Google Scholar]
- Albers HE, Gerall AA, Axelson JF. Effect of reproductive state on circadian periodicity in the rat. Physiol Behav. 1981;26:21–25. doi: 10.1016/0031-9384(81)90073-1. [DOI] [PubMed] [Google Scholar]
- Allen GC, West JR, Chen W-JA, Earnest DJ. Developmental alcohol exposure disrupts circadian regulation of BDNF in the rat suprachiasmatic nucleus. Neurotoxicol Teratol. 2004;26:353–358. doi: 10.1016/j.ntt.2004.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonthius DJ, Bonthius NE, Napper RM, West JR. Early postnatal alcohol exposure acutely and permanently reduces the number of granule cells and mitral cells in the rat olfactory bulb: a stereological study. J Comp Neurol. 1992;324:557–566. doi: 10.1002/cne.903240408. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, Goodlett CR, West JR. Blood alcohol concentration and severity of microencephaly in neonatal rats depend on the pattern of alcohol administration. Alcohol. 1988;5:209–214. doi: 10.1016/0741-8329(88)90054-7. [DOI] [PubMed] [Google Scholar]
- Bonthius DJ, West JR. Alcohol-induced neuronal loss in developing rats: increased brain damage with binge exposure. Alcohol Clin Exp Res. 1990;14:107–118. doi: 10.1111/j.1530-0277.1990.tb00455.x. [DOI] [PubMed] [Google Scholar]
- Chen W-JA, Parnell SE, West JR. Neonatal alcohol and nicotine exposure limits brain growth and depletes cerebellar Purkinje cells. Alcohol. 1998;15:33–41. doi: 10.1016/s0741-8329(97)00084-0. [DOI] [PubMed] [Google Scholar]
- Chen W-JA, Parnell SE, West JR. The effects of alcohol and nicotine on the developing olfactory bulb: loss of mitral cells and changes in neurotransmitter levels. Alcohol Clin Exp Res. 1999;23:18–25. [PubMed] [Google Scholar]
- Choong K, Shen R. Prenatal ethanol exposure alters the postnatal development of the spontaneous electrical activity of dopamine neurons in the ventral tegmental area. Neuroscience. 2004;126:1083–1091. doi: 10.1016/j.neuroscience.2004.04.041. [DOI] [PubMed] [Google Scholar]
- Chudley AE, Conry J, Cook JL, Loock C, Rosales T, LeBlanc N. Fetal alcohol spectrum disorder: Canadian guidelines for diagnosis. CMAJ. 2005;172(Suppl 5):S1–S21. doi: 10.1503/cmaj.1040302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vries MJ, Cardazo BN, van der Want J, de Wolf A, Meijer JH. Glutamate immunoreactivity in terminals of the retinohypothalamic tract of the brown Norwegian rat. Brain Res. 1993;612:231–237. doi: 10.1016/0006-8993(93)91665-f. [DOI] [PubMed] [Google Scholar]
- Diaz J. Experimental rearing of rat pups using chronic gastric fistulas. In: Shair HN, Barr GA, Hofer MA, editors. Developmental Psychobiology: New Methods and Changing Concepts. Oxford University Press; New York: 1991. pp. 272–284. [Google Scholar]
- Ding JM, Chen D, Weber ET, Faiman LE, Rea MA, Gillette MU. Resetting the biological clock: mediation of nocturnal circadian shifts by glutamate and NO. Science. 1994;266:1713–1717. doi: 10.1126/science.7527589. [DOI] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early Hum Dev. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Earnest DJ, Chen W-JA, West JR. Developmental alcohol and circadian clock function. Alcohol Res Health. 2001;25:136–140. [PMC free article] [PubMed] [Google Scholar]
- Farnell YZ, West JR, Chen W-JA, Allen GC, Earnest DJ. Developmental alcohol exposure alters light-induced phase shifts of the circadian activity rhythm in rats. Alcohol Clin Exp Res. 2004a;28:1020–1027. doi: 10.1097/01.alc.0000130807.21020.1b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farnell YZ, West JR, Chen W-JA, Allen GC, Marchette LD, Earnest DJ. Developmental alcohol exposure disrupts clock gene rhythms in the rat SCN and cerebellum (abstract) Alcohol Clin Exp Res. 2004b;28(Suppl 5):164A. [Google Scholar]
- Geller LM, Geller EH. A simple technique for the permanent marking of newborn albino rats. Psychol Rep. 1966;18:221–222. doi: 10.2466/pr0.1966.18.1.221. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Kelly SJ, West JR. Early postnatal alcohol exposure that produces high blood alcohol levels impairs development of spatial navigation learning. Psychobiology. 1987;15:64–74. [Google Scholar]
- Goodlett CR, Nonneman AJ, Valentino ML, West JR. Constraints on water maze spatial learning in rats: implications for behavioral studies of brain damage and recovery of function. Behav Brain Res. 1988;28:275–286. doi: 10.1016/0166-4328(88)90130-1. [DOI] [PubMed] [Google Scholar]
- Goodlett CR, Pearlman AD, Lundahl KR. Binge neonatal alcohol intubations induce dose-dependent loss of Purkinje cells. Neurotoxicol Teratol. 1998;20:285–292. doi: 10.1016/s0892-0362(97)00102-5. [DOI] [PubMed] [Google Scholar]
- Hannibal J. Neurotransmitters of the retino-hypothalamic tract. Cell Tissue Res. 2002;309:73–88. doi: 10.1007/s00441-002-0574-3. [DOI] [PubMed] [Google Scholar]
- Harrington ME, Hoque S, Hall A, Golombek D, Biello S. Pituitary adenylate cyclase activating peptide phase shifts circadian rhythms in a manner similar to light. J Neurosci. 1999;19:6637–6642. doi: 10.1523/JNEUROSCI.19-15-06637.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Liao H-W, Takao M, Berson DM, Yau K-W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyme HE, May PA, Kalberg WO, Kodituwakku P, Gossage JP, Trujillo PM, Buckley DG, Miller JH, Aragon AS, Khaole N, Viljoen DL, Jones KL, Robinson LK. A practical clinical approach to diagnosis of fetal alcohol spectrum disorders: clarification of the 1996 Institute of Medicine criteria. Pediatrics. 2005;115:39–47. doi: 10.1542/peds.2004-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isobe Y, Nishino H. Circadian rhythm of drinking and running-wheel activity in rats with 6-hydroxydopamine lesions of the ventral tegmental area. Brain Res. 2001;899:187–192. doi: 10.1016/s0006-8993(01)02223-5. [DOI] [PubMed] [Google Scholar]
- Johnson CH, Elliott JA, Foster R. Entrainment of circadian programs. Chronobiol Int. 2003;20:741–774. doi: 10.1081/cbi-120024211. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Bonthius DJ, West JR. Developmental changes in alcohol pharmacokinetics in rats. Alcohol Clin Exp Res. 1987a;11:281–286. doi: 10.1111/j.1530-0277.1987.tb01308.x. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Goodlett CR, Hulsether SA, West JR. Impaired spatial navigation in adult female but not adult male rats exposed to alcohol during the brain growth spurt. Behav Brain Res. 1988;27:247–257. doi: 10.1016/0166-4328(88)90121-0. [DOI] [PubMed] [Google Scholar]
- Kelly SJ, Pierce DR, West JR. Microencephaly and hyperactivity in adult rats can be induced by neonatal exposure to high blood alcohol concentrations. Exp Neurol. 1987b;96:580–593. doi: 10.1016/0014-4886(87)90220-2. [DOI] [PubMed] [Google Scholar]
- Lund TD, McGivern RF, Handa RJ. Prenatal exposure to ethanol alters biological rhythms of adult male rats (abstract) Alcohol Clin Exp Res. 2004;28(Suppl 5):136A. [Google Scholar]
- Maier SE, Chen W-JA, West JR. The effect of timing and duration of alcohol exposure on development of the fetal brain. In: Abel EL, et al., editors. Fetal Alcohol Syndrome. From Mechanism to Prevention. CRC Press; Boca Raton, FL: 1996. pp. 27–50. [Google Scholar]
- Manteuffel MD. Neurotransmitter function: changes associated with in utero alcohol exposure. In: Abel EL, editor. Fetal Alcohol Syndrome. From Mechanisms to Prevention. CRC Press; Boca Raton, FL: 1996. pp. 171–189. [Google Scholar]
- Meijer JH, van der Zee EA, Dietz M. Glutamate phase shifts circadian activity rhythms in hamsters. Neurosci Lett. 1988;86:177–183. doi: 10.1016/0304-3940(88)90567-8. [DOI] [PubMed] [Google Scholar]
- Meyer-Bernstein EL, Morin LP. Differential serotonergic innervation of the suprachiasmatic nucleus and the intergeniculate leaflet and its role in circadian rhythm modulation. J Neurosci. 1996;16:2097–2111. doi: 10.1523/JNEUROSCI.16-06-02097.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel S, Colwell CS. Cellular communication and coupling within the suprachiasmatic nucleus. Chronobiol Int. 2001;18:579–600. doi: 10.1081/cbi-100106074. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Bossert JM, Holmes MM, Marchant EG. Serotonin and feedback effects of behavioral activity on circadian rhythms in mice. Behav Brain Res. 1998;96:93–99. doi: 10.1016/s0166-4328(98)00007-2. [DOI] [PubMed] [Google Scholar]
- Moore RY. Organization and function of a central nervous system circadian oscillator: the suprachiasmatic nucleus. Fed Proc Fed Am Soc Exp Biol. 1983;42:2783–2789. [PubMed] [Google Scholar]
- Moore RY. Disorders of circadian function and the human circadian timing system. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. Oxford University Press; New York: 1991. pp. 429–441. [Google Scholar]
- Morin LP, Fitzgerald KM, Zucker I. Estradiol shortens the period of hamster circadian rhythms. Science. 1977;196:305–307. doi: 10.1126/science.557840. [DOI] [PubMed] [Google Scholar]
- Napper RM, West JR. Permanent neuronal cell loss in the cerebellum of rats exposed to continuous low blood alcohol levels during the brain growth spurt: a stereological investigation. J Comp Neurol. 1995;362:283–292. doi: 10.1002/cne.903620210. [DOI] [PubMed] [Google Scholar]
- Pickard GE, Turek FW. Effects of partial destruction of the suprachiasmatic nuclei on two circadian parameters: wheel-running activity and short-day induced testicular regression. J Comp Physiol. 1985;156:803–815. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. IV. Entrainment: pacemaker as clock. J Comp Physiol. 1976a;106:291–331. [Google Scholar]
- Pittendrigh CS, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol. 1976b;106:333–355. [Google Scholar]
- Reppert SM, Weaver DR. Molecular analysis of mammalian circadian rhythms. Annu Rev Physiol. 2001;63:647–676. doi: 10.1146/annurev.physiol.63.1.647. [DOI] [PubMed] [Google Scholar]
- Rosett HL, Snyder P, Sander LW, Lee A, Cook P, Weiner L, Gould J. Effects of maternal drinking on neonatal state regulation. Dev Med Child Neurol. 1979;21:464–473. doi: 10.1111/j.1469-8749.1979.tb01650.x. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Sari Y, Zhou FC. Prenatal alcohol exposure causes long-term serotonin neuron deficit in mice. Alcohol Clin Exp Res. 2004;28:941–948. doi: 10.1097/01.alc.0000128228.08472.39. [DOI] [PubMed] [Google Scholar]
- Schwartz WJ. A clinician’s primer on the circadian clock: its localization, function and resetting. Adv Intern Med. 1993;38:81–106. [PubMed] [Google Scholar]
- Sei H, Sakata-Haga H, Ohta K, Sawada K, Morita Y, Fukui Y. Prenatal exposure to alcohol alters the light response in postnatal circadian rhythm. Brain Res. 2003;987:131–134. doi: 10.1016/s0006-8993(03)03329-8. [DOI] [PubMed] [Google Scholar]
- Sher L. Etiology, pathogenesis, and treatment of seasonal and non-seasonal mood disorders: possible role of circadian rhythm abnormalities related to developmental alcohol exposure. Med Hypotheses. 2004;62:797–801. doi: 10.1016/j.mehy.2003.12.021. [DOI] [PubMed] [Google Scholar]
- Shioiri T, Takahashi K, Yamada N, Takahashi S. Motor activity correlates negatively with free-running period, while positively with serotonin contents in SCN in free-running rats. Physiol Behav. 1991;49:779–786. doi: 10.1016/0031-9384(91)90318-i. [DOI] [PubMed] [Google Scholar]
- Smith KJ, Eckardt MJ. The effects of prenatal alcohol on the central nervous system. Recent Dev Alcohol. 1991;9:151–164. [PubMed] [Google Scholar]
- Steinhausen HC, Spohr H-L. Long-term outcome of children with fetal alcohol syndrome: psychopathology, behavior, and intelligence. Alcohol Clin Exp Res. 1998;22:334–338. doi: 10.1111/j.1530-0277.1998.tb03657.x. [DOI] [PubMed] [Google Scholar]
- Steinhausen HC, Willms J, Spohr H-L. Long-term psychopathological and cognitive outcome of children with fetal alcohol syndrome. J Am Acad Child Adolesc Psychiatry. 1993;32:990–994. doi: 10.1097/00004583-199309000-00016. [DOI] [PubMed] [Google Scholar]
- Summer TL, Ferraro JS, McCormack CE. Phase-response and Aschoff illuminance curves for locomotor activity of the rat. Am J Physiol. 1984;246:R299–R304. doi: 10.1152/ajpregu.1984.246.3.R299. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Goodlett CR, West JR. Alcohol-induced Purkinje cell loss depends on developmental timing of alcohol exposure and correlates with motor performance. Brain Res Dev Brain Res. 1998;105:159–166. doi: 10.1016/s0165-3806(97)00164-8. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Leany BD, Riley EP. Differential vulnerability to motor deficits in second replicate HAS and LAS rats following neonatal alcohol exposure. Pharmacol Biochem Behav. 2003;75:17–24. doi: 10.1016/s0091-3057(03)00031-5. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Wasserman EA, West JR, Goodlett CR. Behavioral deficits induced by bingelike exposure to alcohol in neonatal rats: importance of developmental timing and number of episodes. Dev Psychobiol. 1996;29:433–452. doi: 10.1002/(SICI)1098-2302(199607)29:5<433::AID-DEV3>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Turek FW. Effects of stimulated physical activity on the circadian pacemaker of vertebrates. J Biol Rhythms. 1989;4:135–147. [PubMed] [Google Scholar]
- van den Pol AN. Glutamate and GABA presence and action in the suprachiasmatic nucleus. J Biol Rhythms (Suppl) 1993;8:S11–S15. [PubMed] [Google Scholar]
- van den Pol AN, Tsujimoto KL. Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience. 1985;15:1049–1086. doi: 10.1016/0306-4522(85)90254-4. [DOI] [PubMed] [Google Scholar]
- West JR. Use of pup in a cup model to study brain development. J Nutr. 1993;123:382–385. doi: 10.1093/jn/123.suppl_2.382. [DOI] [PubMed] [Google Scholar]
- West JR, Hamre KM, Pierce DR. Delay in brain growth induced by alcohol in artificially reared rat pups. Alcohol. 1984;1:213–222. doi: 10.1016/0741-8329(84)90101-0. [DOI] [PubMed] [Google Scholar]
- West JR, Hidges CA, Black AC., Jr Prenatal exposure to alcohol alters the organization of hippocampal mossy fibers in rats. Science. 1981;211:957–959. doi: 10.1126/science.7466371. [DOI] [PubMed] [Google Scholar]