Abstract
Asymmetric cell division is a mechanism for generating cell diversity as well as maintaining stem cell homeostasis in both Drosophila and mammals. In Drosophila, larval neuroblasts are stem cell-like progenitors that divide asymmetrically to generate neurons of the adult brain. Mitotic neuroblasts localize atypical protein kinase C (aPKC) to their apical cortex. Cortical aPKC excludes cortical localization of Miranda and its cargo proteins Prospero and Brain tumor, resulting in their partitioning into the differentiating, smaller ganglion mother cell (GMC) where they are required for neuronal differentiation. In addition to aPKC, the kinases Aurora-A and Polo also regulate neuroblast self-renewal, but the phosphatases involved in neuroblast self-renewal have not been identified. Here we report that aPKC is in a protein complex in vivo with Twins, a Drosophila B-type protein phosphatase 2A (PP2A) subunit, and that Twins and the catalytic subunit of PP2A, called Microtubule star (Mts), are detected in larval neuroblasts. Both Twins and Mts are required to exclude aPKC from the basal neuroblast cortex: twins mutant brains, twins mutant single neuroblast mutant clones, or mts dominant negative single neuroblast clones all show ectopic basal cortical localization of aPKC. Consistent with ectopic basal aPKC is the appearance of supernumerary neuroblasts in twins mutant brains or twins mutant clones. We conclude that Twins/PP2A is required to maintain aPKC at the apical cortex of mitotic neuroblasts, keeping it out of the differentiating GMC, and thereby maintaining neuroblast homeostasis.
Introduction
Asymmetric cell division is a mechanism for generating cell diversity as well as maintaining stem cell homeostasis (reviewed in Doe, 2008). Asymmetric cell division typically starts with the establishment of a cell polarity axis, followed by orientation of the mitotic spindle along the polarity axis, resulting in the production of molecularly distinct sibling cells. Drosophila neuroblasts (NBs) are a model system for studying the establishment of cell polarity, spindle orientation, and asymmetric cell division. Neuroblasts show polarization of cortical (membrane-associated) proteins during mitosis. At the apical cortex are the Par complex proteins Par-3 (Bazooka; Baz), Par-6, and atypical protein kinase C (aPKC), which are all partitioned into the self-renewing neuroblast during mitosis (Petronczki and Knoblich, 2001; Wodarz et al., 2000; Wodarz et al., 1999). At the basal cortex are the differentiation factors Miranda, Prospero, Brain tumor, and Numb which are all segregated into the smaller ganglion mother cell (GMC) during mitosis (Bello et al., 2006; Betschinger et al., 2006; Ikeshima-Kataoka et al., 1997; Knoblich et al., 1995; Lee et al., 2006c; Rhyu et al., 1994). There are two types of central brain larval neuroblasts: ∼90 "type 1" neuroblasts that divide asymmetrically to generate a series of smaller GMCs which divide just once to produce two neurons, and ∼8 "type 2" neuroblasts that generate smaller transit amplifying GMCs (TA-GMCs) that generate multiple neurons (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). All aspects of asymmetric cell division are similar in type 1 and type 2 neuroblasts, with the exception that type 2 neuroblasts have little or no cortical Prospero protein (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). In addition, there are optic lobe neuroblasts that develop from the margins of the neuroecctoderm of the outer optic lobe (Egger et al., 2007; Yasugi et al., 2008).
aPKC is required to exclude basal proteins from the apical cortex, ensuring that these differentiation factors are partitioned exclusively into the GMC. In addition, the mitotic kinases Polo and Aurora regulate neuroblast cell polarity and self-renewal (Lee et al., 2006a; Wang et al., 2007; Wang et al., 2006). However, the identity of the phosphatases that counteract the activity of aPKC, Polo, or Aurora-A in regulating neuroblast cell polarity and self-renewal have remained elusive.
In an effort to identify aPKC-interacting proteins and thus potential aPKC regulators, including phosphatases, we performed a biochemical screen for proteins that could be immunoprecipitated together with aPKC (Chabu and Doe, 2008), and identified a subunit of protein phosphatase 2A (PP2A). PP2A is a serine/threonine phosphatase composed of a catalytic (C) subunit, a structural (A) subunit, and a variable regulatory (B) subunit (Janssens and Goris, 2001). The A and C subunits make up the enzymatic core of PP2A, while the variable B subunits competitively associate with the core unit and provide substrate specificity (Janssens et al., 2008). PP2A has been the focus of intense studies, particularly for its role in vertebrate oncogenesis (Schonthal, 2001) and tight junction assembly (Nunbhakdi-Craig et al., 2002). In Drosophila epithelia, PP2A antagonizes aPKC by dephosphorylating the polarity protein Par-1, a known aPKC substrate (Choi et al., 2007; Nam et al., 2007). Here we report that Twins, a Drosophila B-type PP2A subunit, is part of a multi-protein complex with aPKC in vivo, is present together with aPKC in mitotic neuroblasts, and is required to maintain aPKC at the apical cortex of mitotic neuroblasts. In the absence of Twins, aPKC is localized to the basal cortex, where it promotes a GMC-to-neuroblast cell fate transformation leading to expansion of larval neuroblast numbers.
Materials and Methods
Fly stocks and MARCM clones
The wild type fly stock was yellow white (y w). The twinsP FRT82B stock was a gift from Ken Irvine (Rutgers). tws02414 and a deficiency chromosome removing twins, Df(3)7732, were obtained from Bloomington stock center (Indiana). The UAS-mts-DN-HA was a gift from A. Seghal (University of Pennsylvania). Zygotic twins phenotypes were analyzed in twinsP FRT82B /Df(3)7732 trans-heterozygotes. To generate positively marked MARCM wild type or twins clones or twinsP clones in aPKC heterozygote background, FRT82B (Bloomington stock center, Indiana) or twinsP FRT82B/TM6, Tb, Hu or aPKCk06403/ CyO, ActGFP; twinsP FRT82B/TM3, Ser, ActGFP were mated to y w hsFLP; tubP-GAL4 UAS-mCD8::GFP; FRT82B, tubP-GAL80/TM6,Tb, Hu. To misexpress a dominant negative Microtubule star (Mts) PP2A catalytic subunit in neuroblasts we crossed y w hsFLP; Act25 FRT-STOP-FRT; UAS-lacZ females to UAS-Mts-DN-HA males. Clone induction was done by heat-shocking 24–28 hours larvae for 1 hour at 37°C. These larvae were aged for 48 or 72 hours at 25°C and then fixed and stained.
Immuoprecipitations
For immuoprecipitation experiments, a 0–12 hour collection of y w embryos were homogenized in lysis buffer (50mM HEPES pH7.5, 150mMNaCl, .1% Tween-20, 1 mM EDTA, 2.5 mM EGTA, 10% Glycerol, supplemented with protease inhibitor tablets; Roche). For larval CNS lysate, wild type or twinsP/Df7732 third-instar larval brains were dissected and homogenized. Lysates were pre-cleared with protein agarose-A beads for 1 hour at 4°C and incubated with 2 uL of either anti-GFP, anti-Twins antibodies (a gift from Tadashi Uemura, Japan), or anti-aPKC antibodies (Santa-Cruz) for 4 hours at 4°C. Lysates were then incubated with protein agarose-A beads for 1 hour at room temperature. For pulldowns, beads were precipitated and washed 3 times in modified lysis buffer containing 500mM NaCl, bound proteins were separated by SDS-PAGE, transferred onto nitrocellulose and probed for aPKC. Immunoprecipitant autorad band intensities were quantified with ImageJ.
Antibodies and immunostaining
The following primary antibodies were used: rabbit anti-aPKC, 1:1000 (Sigma), guinea pig or Rat anti-Miranda, 1:500 (Doe lab), rabbit anti-phosphohistone-H3, 1:1000 (Sigma, St. Louis, MO), rabbit anti-Scrib, 1:1000 (Doe lab), rat monoclonal anti-Deadpan, straight (Doe lab), rabbit anti-GFP, 1:1000 (Sigma, St. Louis, MO), rat anti-Twins, 1:1500 (Tadashi Uemura, Japan), rat anti-Par-6, 1:1000 (Doe lab), guinea-pig anti-Bazooka, 1:1000 (Doe lab), mouse anti-PP2A, 1: 2500 (BD transduction), rabbit anti-P(410)-PKC, 1:250 (Cell Signaling), mouse anti α-Tubulin, 1:2000 (sigma). Species-specific secondary antibodies (Invitrogen, Eugene, OR) were used at 1:400. Larval brains were dissected, fixed, and stained as described previously (Siller et al., 2005), and analyzed with a Bio-Rad Radiance 2000 or Leica TCS SP laser scanning confocal microscope using a 60×1.4 NA oil immersion objective. Images were processed with Illustrator software (Adobe).
Statistical analysis
We used a t -test to test the significance of variations in neuroblast numbers per clone between wild type, twins mutants, and twins, apkc+/- double mutants.
Results
Twins and aPKC are in a common protein complex
In an earlier biochemical screen aimed at identifying aPKC-interacting proteins (Chabu and Doe, 2008), we identified B-type subunits of protein phosphatase 2A (PP2A) and focused on Twins. We tested the reproducibility of the interaction between aPKC and Twins by performing anti-GFP (negative control) or anti-Twins pull-down experiments using embryonic lysate and probed for the presence of aPKC. We found that aPKC specifically co-immunoprecipitated with Twins (Figure 1A), confirming that Twins is in a common protein complex with aPKC. Using a similar approach, we next tested whether the PP2A catalytic subunit Mts is also in a protein complex with aPKC. We performed anti-GFP or anti-aPKC pull-down experiments and found that Mts is specifically immunoprecipitated with anti-aPKC (Figure 1B), suggesting that aPKC associates with a Twins/PP2A protein phosphatase complex in vivo.
Figure 1. aPKC forms a protein complex with twins and PP2A.
(A) Immunoprecipitations from embryonic lysate using a Twins antibody and a GFP control antibody (labeled at top). Immunoprecipitates were probed for aPKC (labeled at right), which reveals that only Twins but not GFP can immunoprecipitate aPKC protein.
(B) Immunoprecipitations from embryonic lysate using a Twins antibody and a GFP control antibody (labeled at top). Immunoprecipitates were probed for vertebrate anti-PP2A C-subunit antibody that cross-react with Drosophila Mts (labeled at right), which reveals that only Twins but not GFP can immunoprecipitate Mts protein.
(C) Immunoprecipitations using an aPKC antibody from wild type or twins mutant larval CNS (labeled at top, second row). Top panel: probed with an antibody specific for phospho-T410 aPKC (P-aPKC, labeled at right) showing that twins mutant do not have reduced levels of P-aPKC(T410). Middle panel: probed with an aPKC antibody to show the total amount of aPKC protein in each lane. Bottom panel: probed with a Tubulin antibody to show total protein input for each lane. The P-aPKC(T410)/Tubulin band intensity ratio for wild type or twins mutant is 0.38 and 0.48, respectively.
(D) twins-lacZ larval brain lobe stained for the membrane marker Scribble (Scrib; red) and lacZ expressed from the twins locus (green) showing that twins-lacZ is expressed in central brain neuroblast (arrows) and in the optic lobe (circled).
(E–G) Mitotic larval neuroblast stained for Mts (green), Twins (Tws, red), cortical aPKC and the mitotic DNA marker phosphohistone H3 (blue) showing that Mts and Twins are cytoplasmic and have the potential to interact with cortical aPKC.
The interaction of aPKC with Twins and Mts raises the possibility that the PP2A complex may deactivate aPKC by dephosphorylating the self-activating T410 phosphorylation on aPKC, similar to the role of PP2A in vertebrates (Nunbhakdi-Craig et al., 2002). To test this, we performed anti-aPKC pull down experiments using wild type or twins larval central nervous system (CNS) lysates and compared the level of active aPKC with an antibody that specifically recognizes T410-phosphorylated aPKC. We found that twins mutant showed no decrease in active aPKC levels compared to wild type (Figure 1C), suggesting that Twins does not directly inhibit aPKC by dephosphorylating the T410 residue; however, Twins/PP2A may dephosphorylate T410 only transiently during the cell cycle, or dephosphorylate other sites on aPKC. We conclude that aPKC forms a protein complex with PP2A in vivo, but that PP2A does not inhibit aPKC via dephosphorylation of T410.
Twins, Mts, and aPKC have overlapping expression in larval neuroblasts
We next wanted to determine if Twins, Mts, and aPKC were co-expressed in larval brain neuroblasts. To ensure consistency in our analysis, we restricted our experiments to the dorsoanterior lateral (DAL) brain region, which contains only type 1 neuroblasts (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). We determined whether Twins is expressed in larval brain neuroblasts using two independent means: a twins-lacZ reporter gene (Uemura et al., 1993) and staining for endogenous Twins protein using a previously characterized anti-Twins antibody (Uemura et al., 1993). Both the twins-lacZ reporter line and Twins protein were detected in larval neuroblasts, optic lobe epithelial cells, and glia (Figure 1D, E). At the protein level, aPKC is enriched at the apical cortex of mitotic neuroblasts, while Mts is cytoplasmic and Twins is nuclear and cytoplasmic. Thus, Twins and Mts have the ability to interact with cortical proteins such as aPKC (Figure 1E–G;). We conclude that Twins/PP2A and aPKC have the ability to interact in mitotic larval brain neuroblasts.
Twins is required to restrict aPKC to the apical cortex of mitotic neuroblasts
aPKC regulates neuroblast cell polarity (Rolls et al., 2003), so we next examined the functional relevance of Twins/aPKC interaction in the specific context of neuroblast cell polarity. To test a role for Twins in regulating aPKC localization we generated wild type or twins mutant clones using the MARCM technique (Lee et al., 1999) and assayed aPKC localization in metaphase neuroblasts. Wild type clones showed aPKC apical crescents; aPKC was clearly absent from the basal cortex (100%, n=23; Figure 2C). In contrast, twins mutant clones showed cortical patches of aPKC at the basal cortex in metaphase neuroblasts and increased cytoplasmic aPKC (71%, n=17; Figure 2H), in addition to an apical crescent. We extended our analysis to the other Par complex proteins, Bazooka/Par-3 and Par-6. We found that they were still restricted to the apical cortex of metaphase neuroblasts, but showed smaller apical crescents (Figure 2F,G; quantified in Figure 2L). Thus, Twins is required to maintain the normal level of the Par complex proteins Baz, Par-6, and aPKC at the apical cortex, as well as preventing ectopic aPKC at the basal cortex.
Figure 2. Twins function is required for aPKC and Miranda neuroblast cortical polarity.
(A–E) Wild type neuroblast clones showing the GFP clone marker (green, second row) and the polarity protein (red or blue, second row; white single label, top row). Bazooka, Par-6, and aPKC, form apical crescents; Miranda and Numb form basal crescents. Apical, up.
(F–J) twins mutant neuroblast clones labeled as in A-E. Mutant neuroblasts show apical crescents of Bazooka and Par-6, but ectopic cortical localization of aPKC (arrowheads), Miranda is cytoplasmic, and Numb is ectopic cortical.
(K) twins mutant clones in heterozygote aPKC larval brains labeled as in A–J show rescue of Miranda basal cortical localization.
(L) Quantification of aPKC and Miranda cortical polarity defects. Numbers in the bars indicate the number of clones analyzed for the specified marker.
Active aPKC displaces Miranda and Numb from the basal cortex, while inactive aPKC does not (Atwood et al., 2007; Chabu and Doe, 2008; Lee et al., 2006b; Rolls et al., 2003), thus Miranda or Numb localization can be diagnostic for determining the activity of ectopic aPKC. To address whether the ectopic pool of aPKC seen at the basal cortex of twins mutant neuroblasts is active (as suggested by the biochemical data, see above), we analyzed Miranda localization in wild type or twins mutant neuroblasts. Wild type neuroblasts showed strong basal Miranda (100%, n=17; Figure 2D,L) and Numb (100%, n=12; Figure 2E,L) whereas twins mutant neuroblasts showed cytoplasmic Miranda (50%, n=18; Figure 2I,L) and weaker Numb crescents (Figure 2J,L), indicating that the basal pool of aPKC seen in twins mutant neuroblast is active. We conclude that Twins is not required to activate aPKC, but rather to restrict active aPKC to the neuroblast apical cortex.
Reduced Twins activity leads to an increase in larval neuroblast numbers
Ectopic basal localization of membrane-tethered aPKC leads to supernumerary larval brain neuroblasts (Lee et al., 2006b), so we tested whether the ectopic aPKC observed in twins mutants was sufficient to generate a similar GMC-to-neuroblast transformation. We first asked whether twinsP / Df(3)7732 trans-heterozygote mutants brains have more neuroblasts than wild type. We used the pan-neural transcription factor Deadpan (Dpn) to identify neuroblasts, nuclear Prospero (Pros) to mark differentiating GMCs and neurons (Lee et al., 2006b), and Scribble to mark cell membranes (Albertson and Doe, 2003). We found that twins mutant brains superficially contained more Dpn-positive, nuclear Pros-negative cells than wild type, although these cells were smaller than wild type in size (Figure 3A, B). A different trans-heteroallelic combination (twsP/tws02414) gave similar results (data not shown). We conclude that reduced Twins activity leads to an increase in larval neuroblast numbers.
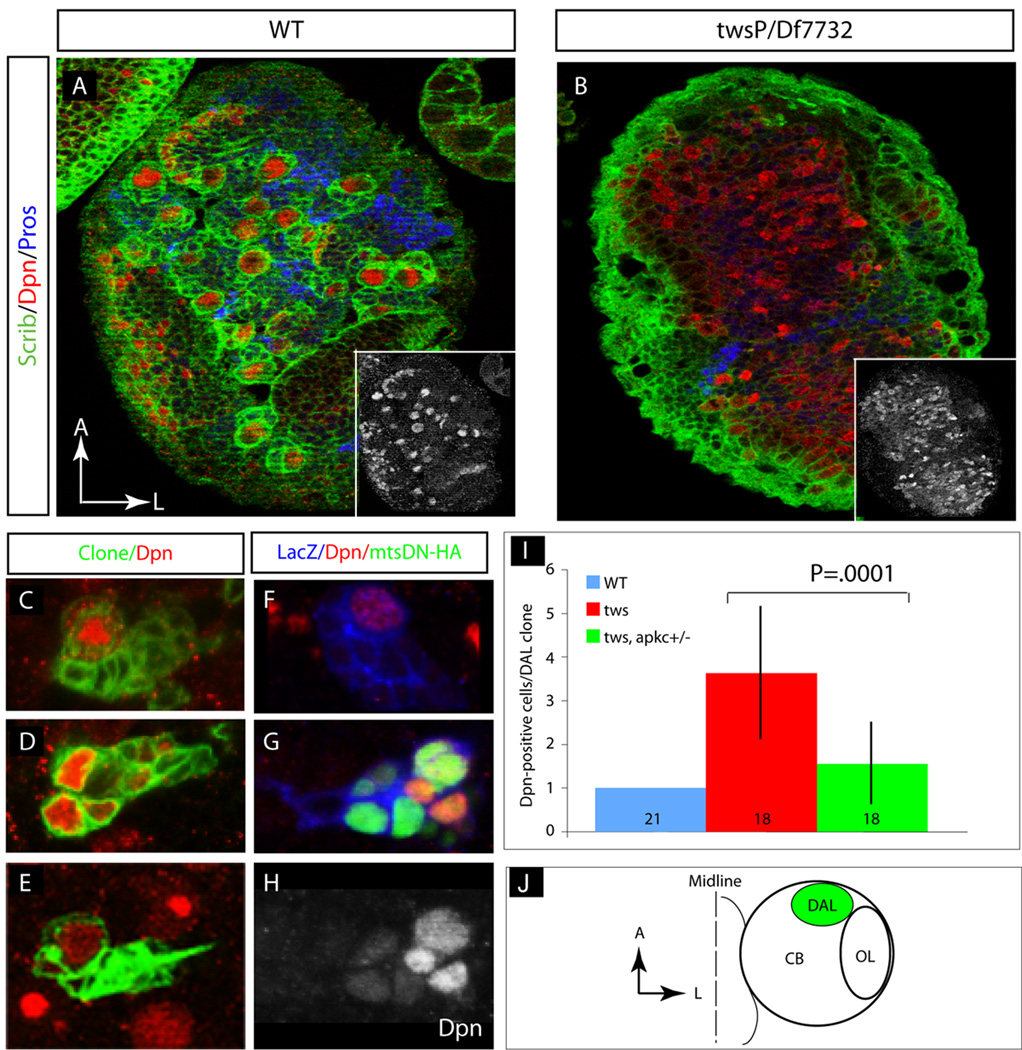
Figure 3. Loss of twins/PP2A function leads to ectopic neuroblasts in the larval brain.
(A–B) twins mutants have more neuroblasts per brain lobe. (A) Wild type or (B) twins mutant third instar brains stained with the membrane marker Scribble (Scrib, green), the neuroblast marker Deadpan (Dpn, red), and the GMC/neuron marker Prospero (Pros, blue). twins mutant brains have an increased number of Dpn-positive neuroblasts. Insets: Dpn single label from each panel. Quantified in panel I.
(C–E) twins mutant clones induced in single neuroblasts result in a lineage-autonomous increase in neuroblast numbers. (C) Wild type, (D) twins mutant, and (E) twins mutant in aPKC/+ single neuroblast clones stained with the clone marker GFP (clone, green) and the neuroblast marker Deadpan (Dpn, red). Wild type single neuroblast clones invariably contain a single Deadpan-positive neuroblast (C), whereas twins mutant clones often show multiple neuroblasts, and twins mutants in an aPKC/+ brain show intermediate numbers of neuroblasts per clone (see text for quantification).
(F–H) Clonal misexpression of a Mts dominant negative protein (Mts-DN-HA) in a single neuroblasts and its progeny result in a lineage-autonomous increase in neuroblast numbers. (F) Wild type or (G,H) Mts-DN-HA misexpression; βgal staining marks the clone (LacZ, blue), HA marks the Mts-DN-HA protein (green), and Dpn marks neuroblasts (Dpn, red). The single label Dpn channel is shown in (H). See taxt for quantification of neuroblast numbers in each genotype.
(I) Quantification of neuroblast number per clone for the indicated genotypes. Numbers in the bars denote the number of single clones analyzed.
(J) Schematic depicting the dorso-anterior lateral (DAL; green) brain region where all clones were analyzed; this region of the brain contains only type I / canonical neuroblast lineages that normally have only one Deadpan-positive neuroblast per lineage (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008).
In addition to neuroblasts, Twins is detected in many other cell types within the larval brain (Figure 1D), raising the possibility that the twins mutant phenotype in neuroblasts may be due to loss of Twins in surrounding or multiple cell types. To address this possibility, we induced wild type or twins mutant MARCM clones in single neuroblasts and scored the number of Dpn-positive or Asense-positive cells per clone. We focused on clones positioned in the dorsal-anterior-lateral (DAL) brain region, since wild type neuroblasts in this region all follow canonical neuroblast lineages to maintain a single Dpn-positive, Asense-positive neuroblast (Bello et al., 2008; Boone and Doe, 2008; Bowman et al., 2008). As expected, we found that single wild type neuroblast clones invariably contained a single Dpn-positive or Asense-positive neuroblast and many Dpn-negative progeny (Figure 3C; Supplemental Figure 1G), whereas twins mutant clones contained 3.6 (n=18) Dpn-positive or Asense-positive neuroblasts per clone (Figure 3D,I; Supplemental Figure 1H). We conclude that Twins acts lineage-autonomously, most likely within the neuroblast, to maintain a single neuroblast per lineage and thus maintain ∼100 neuroblasts per larval brain lobe.
Because Twins can control substrate specificity of PP2A catalytic subunit Mts, we next tested whether reduced levels of Mts can mimic twins ectopic neuroblast self-renewal phenotype. Drosophila mts mutants are embryonic lethal (Nam et al., 2007; Snaith et al., 1996), so we used a HA-tagged dominant-negative form of Mts (Mts-DN-HA) that is known to attenuate endogenous PP2A activity (Hannus et al., 2002). We generated wild type or Mts-DN-HA over-expression clones and scored the number of Dpn-positive cells/clone in the DAL region of the brain. We found that wild type clones had a single Dpn-positive cell per clone (100%, n=8; Figure 3F,I), while Mts-DN-HA over-expressing clones contained an average of 3.2 Dpn-positive cells per clone (n= 6; Figure 3F–I). We conclude that Twins acts via PP2A phosphatase activity to maintain a single neuroblast per lineage.
The twins ectopic neuroblast phenotype is aPKC-dependent
Neuroblast lineages with reduced PP2A activity show ectopic neuroblast self-renewal and ectopic active aPKC localization (Figure 3G,H; Supplemental Figure 1D,F). To test whether this phenotype is due to elevated or ectopic aPKC activity, we assayed the twins mutant phenotype in flies heterozygous for aPKC. Whereas twins mutant clones have 3.6 neuroblasts per clone (n=18), the twins mutant clones in an aPKC+/− background contain only to 1.5 neuroblasts per clone (n=18; Figure 3I). Next we wondered whether this reduction in aPKC dosage could also rescue Miranda cortical localization in twins mutant neuroblasts. Indeed, reducing aPKC levels restored Miranda cortical polarity in twins mutant neuroblasts (Figure 2K,L). We conclude that Twins normally suppresses neuroblast self-renewal and promotes basal Miranda localization by reducing the activity or basal targeting of aPKC in mitotic neuroblasts.
Twins promotes timely neural epithelia-to-neuroblast transition in the larval optic lobe
Twins is expressed in the Drosophila outer optic lobe, which consists of a lateral sheet of neuroectodermal cells that differentiates into neuroblasts at its medial margin (Egger et al., 2007; Yasugi et al., 2008) (Figure 4A,B). Here we test whether Twins regulates the formation or self-renewal of the optic lobe neuroblasts. In the wild type third instar larval optic lobe, Dpn-positive neuroblasts were restricted to the medial edge of the optic lobe in a one-cell layer domain, while the remainder of the optic lobe contained Dpn-negative columnar neuroectodermal cells (100%, n=7; Figure 4B). In contrast, the twins mutant third instar optic lobe contained only neuroblasts and no recognizable neuroectoderm; the neuroblasts expanded up to four-cells layer deep into the optic lobe and showed no columnar cell morphology (63%, n=8; Figure 4E). There is no detectable phenotype in the inner optic lobe. The outer optic lobe phenotype is not due to a general mis-patterning defect of the optic lobe (e.g. from optic anlagen to a medial brain anlagen), because (a) the inner optic lobe appears normal, and (b) the twins mutant optic lobe neuroectoderm was readily identifiable and looked similar to wild type at second larval instar stages (Figure 4A,D). Analysis of intermediate stages showed that the ectopic Deadpan-positive neuroblasts could arise in a "salt-and-pepper" manner within the neuroectodermal layer, or by apparent rapid expansion of Deadpan-positive neuroblasts from medial to lateral regions of the optic lobe (Supplemental Figure 1A). This phenotype is aPKC-dependent, as reducing aPKC levels by 50% (apkc/+; twins/twins) partially reduced the number of outer optic lobe Deadpan-positive neuroblasts (44% partially rescued, n=9; Figure 4F, dotted lines and brackets, respectively). We conclude that Twins negatively regulates aPKC to maintain neuroectodermal fate in the optic lobe; loss of Twins function leads to a premature neuroectoderm-to-neuroblast transition.
Figure 4. Twins blocks precocious neuroectoderm-to-neuroblast transition in the outer optic lobe.
Brain lobes from wild type larvae (A, B), twins mutant larvae (D–E), or aPKC/+ twins/twins mutant larvae (F). The stage of the brain is shown in parenthesis (L2, second instar; L3, third instar), and see methods for exact twins alleles used. Brains were stained with the membrane marker Scribble (Scrib, green) and the neuroblast marker Deadpan (Dpn, red). Lateral region of neuroblast formation is marked by white brackets; medial region normally containing neuroectodermal cells is circled with a dashed line. Note the extra Dpn-positive neuroblasts (red) in the outer optic lobe neuroepithelium (dashed circle) of the third instar twins mutant brains (E) and to a slightly lesser extent in the outer optic lobe neuroepithelium (dashed circle) of the third instar aPKC/+ twins/twins mutant brains (F); second instar wild type and twins mutants show similar patterns of neuroblasts in the outer optic lobe neuroepithelium (compare A and D, (dashed circle).
(C) Cartoon summarizing the twins mutant premature differentiation defect.
Discussion
Drosophila aPKC regulates neuroblast cell polarity and neuroblast self-renewal (Lee et al., 2006b; Rolls et al., 2003), however our understanding of how aPKC is regulated is far from complete. Several kinases regulate neuroblast cell polarity and cell fate (Lee et al., 2006a; Lee et al., 2006b; Wang et al., 2007; Wang et al., 2006), but the identity of opposing phosphatases have remained elusive. Here we identify Twins as part of a protein complex containing aPKC. Twins is a regulatory subunit of PP2A, and we also show here that the catalytic subunit of PP2A, Mts, is immunoprecipitated by aPKC. Furthermore, mts and twins mutants have similar defects in neuroblast cell polarity and expansion in neuroblast numbers. This strongly suggests that the Twins/PP2A complex regulates neuroblast polarity and self-renewal.
The primary defect in twins mutant neuroblasts is an expansion of aPKC from the apical cortex to the basal cortex, and this ectopic aPKC is active based on its ability to exclude Miranda from the basal cortex. Twins/PP2A may promote apical Baz localization, similar to the role of PP2A in promoting Baz/Par-3 apical localization in epithelia (Benton and St Johnston, 2003; Nam et al., 2007); a reduced level of apical Baz in neuroblasts may lead to failure to localize all cortical aPKC at the apical cortex and hence ectopic basal aPKC. Alternatively, PP2A may keep active aPKC from the basal cortex by directly dephosphorylating aPKC at its N-terminus, consistent with the role of mammalian PP2A in dephosphorylating aPKCλ/ζ(Nunbhakdi-Craig et al., 2002). In support of this model, overexpression of aPKC lacking its N-terminus (aPKCΔN) displaces Miranda from the basal cortex into the cytoplasm (Betschinger et al., 2003), similar to twins mutant neuroblasts.
How does Twins regulate neuroblast self-renewal? Ectopic active aPKC causes formation of supernumerary neuroblasts, as does reduced levels of the basal cortical protein Miranda (Lee et al., 2006b; Lee et al., 2006c). twins mutant neuroblasts have both ectopic basal cortical aPKC and a loss of basal cortical Miranda. It is likely that the primary defect causing supernumerary neuroblasts is ectopic aPKC, because reducing aPKC levels in twins mutants can rescue both basal Miranda targeting and the formation of supernumerary neuroblasts. This is in contrast to the role of another phosphatase, PP4, in regulating Miranda localization independent of aPKC (Sousa-Nunes et al., 2009).
We previously showed that Dap160, a protein related to mammalian Intersectin, is apically localized and required to anchor aPKC at the apical cortex (Chabu and Doe, 2008). Here we show that Twins is also required for tight apical localization of aPKC. A major difference, however, is that Dap160 directly stimulates the activity of aPKC, so that in dap160 mutant neuroblasts the ectopic basal aPKC is inactive and unable to exclude Miranda from the cortex. In contrast, twins mutants have ectopic basal aPKC that remains active and thus can drive Miranda off the cortex. This supports our conclusion from biochemical experiments that Twins does not stimulate aPKC activity. However, we can’t exclude the possibility that another regulatory subunit can target PP2A to aPKC in the absence of Twins.
Neuroectoderm cells of the optic lobe undergo a progressive differentiation to adopt a neuroblast fate (Egger et al., 2007; Yasugi et al., 2008). twins mutant optic lobes show a dramatic increase in optic lobe neuroblast numbers, suggesting that Twins normally function to inhibit precocious neuroblast fate in the optic lobe neuroectoderm cells. How does Twins normally suppress precocious neuroectodermal-to-neuroblast differentiation? We show that at least one pathway utilizes aPKC to regulate neuroectoderm differentiation; twins mutant optic lobe with reduced active aPKC have a less severe phenotype compared to their twins mutant counter parts. Another pathway that has been implicated in the differentiation of neuroectoderm cells to neuroblast is the Janus Kinase/Signal transducer and activation of transcription (JAK/STAT) pathway. JAK/STAT signaling functions in neuroectoderm cells inhibits expression of proneural genes, thereby blocking precocious neuroblast differentiation (Yasugi et al., 2008). Twins/PP2A could act positively at any point in the JAK/STAT-proneural pathway, or in an independent pathway in promoting the neuroectodermal-to-neuroblast transition in the optic lobe.
Supplementary Material
(A) Third instar larval twins mutant optic lobe showing ectopic Dpn-positive neuroblasts (red); cell membranes shown in green (Scrib).
(B–F) Third instar larval neuroblasts at mitosis stained for aPKC (top row) or Miranda (Mir) bottom row; the same neuroblast is shown in each column. Genotypes are indicated at top.
(G,H) Wild type clone (G) or twins mutant clone (H) stained for the neuroblast marker Asense. Wild type clone has a single Asense-positive neuroblast (white asterisk); twins mutant clone has multiple neuroblasts (red asterisks).
Acknowledgements
We would like to thank Ken Irvine and the Bloomington stock center for providing reagents. We also thank Jason Boone, Clemens Cabernard, and Ken Prehoda for comments on the manuscript. This work was supported by an AHA pre-doctoral fellowship to C.C. (0615592Z), and the Howard Hughes Medical Institute, where C.Q.D. is an Investigator.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- Albertson R, Doe CQ. Dlg, Scrib and Lgl regulate neuroblast cell size and mitotic spindle asymmetry. Nat Cell Biol. 2003;5:166–170. doi: 10.1038/ncb922. [DOI] [PubMed] [Google Scholar]
- Atwood SX, Chabu C, Penkert RR, Doe CQ, Prehoda KE. Cdc42 acts downstream of Bazooka to regulate neuroblast polarity through Par-6 aPKC. J Cell Sci. 2007;120:3200–3206. doi: 10.1242/jcs.014902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello B, Reichert H, Hirth F. The brain tumor gene negatively regulates neural progenitor cell proliferation in the larval central brain of Drosophila. Development. 2006;133:2639–2648. doi: 10.1242/dev.02429. [DOI] [PubMed] [Google Scholar]
- Bello BC, Izergina N, Caussinus E, Reichert H. Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Develop. 2008;3:5. doi: 10.1186/1749-8104-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton R, St Johnston D. Drosophila PAR-1 and 14-3-3 inhibit Bazooka/PAR-3 to establish complementary cortical domains in polarized cells. Cell. 2003;115:691–704. doi: 10.1016/s0092-8674(03)00938-3. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. Asymmetric segregation of the tumor suppressor brat regulates self-renewal in Drosophila neural stem cells. Cell. 2006;124:1241–1253. doi: 10.1016/j.cell.2006.01.038. [DOI] [PubMed] [Google Scholar]
- Boone JQ, Doe CQ. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev Neurobiol. 2008;68:1185–1195. doi: 10.1002/dneu.20648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman SK, Rolland V, Betschinger J, Kinsey KA, Emery G, Knoblich JA. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev Cell. 2008;14:535–546. doi: 10.1016/j.devcel.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chabu C, Doe CQ. Dap160/intersectin binds and activates aPKC to regulate cell polarity and cell cycle progression. Development. 2008;135:2739–2746. doi: 10.1242/dev.024059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KW, Nam SC, Mukhopadhyay B. Par-1 and PP2A: Yin-Yang of Bazooka localization. Fly (Austin) 2007;1:235–237. doi: 10.4161/fly.4954. [DOI] [PubMed] [Google Scholar]
- Doe CQ. Neural stem cells: balancing self-renewal with differentiation. Development. 2008;135:1575–1587. doi: 10.1242/dev.014977. [DOI] [PubMed] [Google Scholar]
- Egger B, Boone JQ, Stevens NR, Brand AH, Doe CQ. Regulation of spindle orientation and neural stem cell fate in the Drosophila optic lobe. Neural Develop. 2007;2:1. doi: 10.1186/1749-8104-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannus M, Feiguin F, Heisenberg CP, Eaton S. Planar cell polarization requires Widerborst, a B’ regulatory subunit of protein phosphatase 2A. Development. 2002;129:3493–3503. doi: 10.1242/dev.129.14.3493. [DOI] [PubMed] [Google Scholar]
- Ikeshima-Kataoka H, Skeath JB, Nabeshima Y, Doe CQ, Matsuzaki F. Miranda directs Prospero to a daughter cell during Drosophila asymmetric divisions. Nature. 1997;390:625–629. doi: 10.1038/37641. [DOI] [PubMed] [Google Scholar]
- Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J. 2001;353:417–439. doi: 10.1042/0264-6021:3530417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail) Trends Biochem Sci. 2008;33:113–121. doi: 10.1016/j.tibs.2007.12.004. [DOI] [PubMed] [Google Scholar]
- Knoblich JA, Jan LY, Jan YN. Asymmetric segregation of Numb and Prospero during cell division. Nature. 1995;377:624–627. doi: 10.1038/377624a0. [DOI] [PubMed] [Google Scholar]
- Lee CY, Andersen RO, Cabernard C, Manning L, Tran KD, Lanskey MJ, Bashirullah A, Doe CQ. Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 2006a;20:3464–3474. doi: 10.1101/gad.1489406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006b;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Lee CY, Wilkinson BD, Siegrist SE, Wharton RP, Doe CQ. Brat is a Miranda cargo protein that promotes neuronal differentiation and inhibits neuroblast self-renewal. Dev Cell. 2006c;10:441–449. doi: 10.1016/j.devcel.2006.01.017. [DOI] [PubMed] [Google Scholar]
- Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126:4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- Nam SC, Mukhopadhyay B, Choi KW. Antagonistic functions of Par-1 kinase and protein phosphatase 2A are required for localization of Bazooka and photoreceptor morphogenesis in Drosophila. Dev Biol. 2007;306:624–635. doi: 10.1016/j.ydbio.2007.03.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunbhakdi-Craig V, Machleidt T, Ogris E, Bellotto D, White CL, 3rd, Sontag E. Protein phosphatase 2A associates with and regulates atypical PKC and the epithelial tight junction complex. J Cell Biol. 2002;158:967–978. doi: 10.1083/jcb.200206114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petronczki M, Knoblich JA. DmPAR-6 directs epithelial polarity and asymmetric cell division of neuroblasts in Drosophila. Nat Cell Biol. 2001;3:43–49. doi: 10.1038/35050550. [DOI] [PubMed] [Google Scholar]
- Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76:477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol. 2003;163:1089–1098. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schonthal AH. Role of serine/threonine protein phosphatase 2A in cancer. Cancer Lett. 2001;170:1–13. doi: 10.1016/s0304-3835(01)00561-4. [DOI] [PubMed] [Google Scholar]
- Siller KH, Serr M, Steward R, Hays TS, Doe CQ. Live imaging of Drosophila brain neuroblasts reveals a role for Lis1/dynactin in spindle assembly and mitotic checkpoint control. Mol Biol Cell. 2005;16:5127–5140. doi: 10.1091/mbc.E05-04-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith HA, Armstrong CG, Guo Y, Kaiser K, Cohen PT. Deficiency of protein phosphatase 2A uncouples the nuclear and centrosome cycles and prevents attachment of microtubules to the kinetochore in Drosophila microtubule star (mts) embryos. J Cell Sci. 1996;109(Pt 13):3001–3012. doi: 10.1242/jcs.109.13.3001. [DOI] [PubMed] [Google Scholar]
- Sousa-Nunes R, Chia W, Somers WG. Protein phosphatase 4 mediates localization of the Miranda complex during Drosophila neuroblast asymmetric divisions. Genes Dev. 2009;23:359–372. doi: 10.1101/gad.1723609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T, Shiomi K, Togashi S, Takeichi M. Mutation of twins encoding a regulator of protein phosphatase 2A leads to pattern duplication in Drosophila imaginal discs. Genes Dev. 1993;7:429–440. doi: 10.1101/gad.7.3.429. [DOI] [PubMed] [Google Scholar]
- Wang H, Ouyang Y, Somers WG, Chia W, Lu B. Polo inhibits progenitor self-renewal and regulates Numb asymmetry by phosphorylating Pon. Nature. 2007;449:96–100. doi: 10.1038/nature06056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Somers GW, Bashirullah A, Heberlein U, Yu F, Chia W. Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 2006;20:3453–3463. doi: 10.1101/gad.1487506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Grimm A, Knust E. Drosophila atypical protein kinase C associates with Bazooka and controls polarity of epithelia and neuroblasts. J Cell Biol. 2000;150:1361–1374. doi: 10.1083/jcb.150.6.1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wodarz A, Ramrath A, Kuchinke U, Knust E. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature. 1999;402:544–547. doi: 10.1038/990128. [DOI] [PubMed] [Google Scholar]
- Yasugi T, Umetsu D, Murakami S, Sato M, Tabata T. Drosophila optic lobe neuroblasts triggered by a wave of proneural gene expression that is negatively regulated by JAK/STAT. Development. 2008;135:1471–1480. doi: 10.1242/dev.019117. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Third instar larval twins mutant optic lobe showing ectopic Dpn-positive neuroblasts (red); cell membranes shown in green (Scrib).
(B–F) Third instar larval neuroblasts at mitosis stained for aPKC (top row) or Miranda (Mir) bottom row; the same neuroblast is shown in each column. Genotypes are indicated at top.
(G,H) Wild type clone (G) or twins mutant clone (H) stained for the neuroblast marker Asense. Wild type clone has a single Asense-positive neuroblast (white asterisk); twins mutant clone has multiple neuroblasts (red asterisks).