Abstract
Objectives
We hypothesized that diabetes mellitus and associated changes in bladder function triggered bladder wall tissue remodeling and concomitant alterations in the mechanical properties. We thus investigated the time-course changes in function and mechanical properties of diabetic and diuretic rat bladders using both in vivo and in vitro techniques.
Methods
Cystometry was performed at 2, 4 and 8 weeks on female SD rats that received either single injection of streptozotocin (65mg/kg, i.p.) or 5% sucrose in drinking water for the duration of experiments. At each time point, the biaxial mechanical properties of 10 mm × 10 mm tissue specimens obtained from the posterior part of bladder wall were quantified. Changes in overall tissue compliance and mechanical anisotropy as a function of time were examined.
Results
Both diabetic and diuretic conditions led to increases in bladder weight, bladder capacity and in vivo compliance compared to controls at all time points tested. Under biaxial loading all bladder wall tissues exhibited a non-linear stress-strain relationship and mechanical anisotropy, with greater tissue compliance in the circumferential direction compared to the longitudinal direction. While the compliance of bladder wall increased progressively and synchronously in both diabetic and diuretic bladders up to 4 weeks, only diabetic bladders continued to increase the compliance up to 8 weeks (diabetic: 0.64 ± 0.04 vs. diuretic: 0.48 ± 0.05, p=0.03).
Conclusions
Diuresis mainly contributes to the “early” changes of mechanical properties of the bladder, with diabetes inducing further “late” changes of mechanical properties of the rat bladders after 4 weeks.
Keywords: Diabetes, Bladder, Biomechanics
The prevalence of diabetes in the United States continues to increase. Over 50% of patients with diabetes exhibiting bladder dysfunction characterized by large bladder capacity, diminished bladder sensation, poor contractility and elevated post-void residual urine.1 These conditions, such as incomplete bladder emptying can lead to chronic urine retention, recurrent urinary tract infections and deterioration of the upper urinary tract.2 It has been suggested that the diabetic bladder dysfunction may be induced by diabetic neuropathy and/or diuresis associated with diabetes.3 Yet, to date, the impact of diabetes and diuresis at the tissue and organ levels on specific functional and mechanical changes in the bladder remains uncharacterized. Thus, to achieve a better understanding of the pathophysiology of diabetic cystopathy, it is first necessary to elucidate the biomechanical changes that underlie the progression of the diabetes cystopathy.
Since the bladder wall in vivo is stretched two-dimensionally during filling, planar biaxial mechanical testing is necessary for realistic characterization of bladder tissue mechanical properties.4 We previously conducted the first studies on the mechanical properties of neurogenic bladders using this technique and demonstrated that following spinal cord injury, bladder walls could undergo rapid structural and compositional remodeling, which resulted in profound changes in bladder wall biomechanical behavior.5,6 Since bladder wall remodeling and voiding dysfunction are also observed in diabetes,7,8 we hypothesize that the changes of mechanical properties of the bladder wall are an important indicator of the bladder dysfunction induced by diabetes. To gain insights into the relationship between bladder tissue mechanics and diabetic cystopathy, we investigated the time-course changes in cystometric parameters and passive biaxial mechanical properties of diabetic and diuretic rat bladder wall. This study was designed to isolate the diuretic effects from the additional neuropathic and other metabolic effects induced by diabetes on bladder tissue mechanics.
MATERIALS AND METHODS
Induction of diabetes and diuresis
Diabetes was induced in adult female Sprague-Dawley rats (weight 230 to 280gm; Hilltop Laboratory, Pittsburgh, PA) by a single intraperitoneal injection (65mg/kg) of Streptozotocin (STZ) (Sigma, St. Louis, MO) dissolved in an ice-cold 0.1 M citrate buffer. Blood glucose level of these animals was checked one week after STZ injection and rechecked before sacrifice to confirm diabetes (blood glucose >300 mg/dl). Chronic diuresis was induced by feeding 5% sucrose in water. Normal rats were used for controls. All animals were cared and handled in accordance with institutional guidelines and a protocol approved by Institutional Animal Care and Use Committee of University of Pittsburgh (Pittsburgh, PA).
Cystometry
Cystometry was performed on control, diuretic and diabetic rats at 2, 4 and 8 weeks post-treatment as described previously.9 Briefly, under urethane (0.1g./kg, s.c.) anesthesia, a polyethylene tube 50 with a cuff at the end was inserted into the bladder through the bladder dome after vertical midline laparotomy. Normal saline at room temperature (20 –22 °C) was infused at a rate of 0.08ml/min to elicit repetitive micturition cycles and software package (Windaq; Dataq Instruments, Akron, OH) was used for data collection. Saline voided from the urethral meatus was collected and measured to determine voided volume. After constant voided volumes were collected, the infusion was stopped temporarily; residual volume was measured by withdrawing intravesical fluid through the catheter, first by gravity and then by manually expressing the bladder.10 Bladder capacity (BC) was calculated as the sum of voided volume (VV) and volume of residual urine (collected by manual expression). Voiding efficiency (VE) was estimated: VE (%) = [(VV/BC) × 100]. The maximal voiding pressure was measured, and bladder compliance was calculated (BC divided by difference in the pressure threshold and baseline pressure).
Tissue preparation for biaxial mechanical testing
After cystometry, the bladders were harvested, placed immediately in modified Kreb’s solution (containing 113mM. NaCl, 4.7mM. KCl, 1.2mM MgSO4, 25mM NaHCO3, 1.2mM KH2PO4, 11.5mM glucose, and 1mM egtazic acid) and refrigerated at 4°C for 48 hours. The specimen preparation solution and protocol were developed to ensure that no spontaneous contractions would occur during testing but otherwise live, intact tissue was maintained.5 Before mechanical testing, each bladder was opened along the urachus, and the trigone and apex were removed. The posterior part of bladder wall was trimmed to a 1.0 × 1.0 cm square with the edges parallel to the orthogonal longitudinal (base-apex) and circumferential directions, which were in reference to the original anatomical orientation.
Passive Biaxial mechanical behavior
Biaxial testing device and procedures for the bladder wall have been previously described in detail.5 In brief, prepared test specimens were mounted on the biaxial device in a trampoline-like fashion using two suture line pairs per side, which allowed the edges to expand freely in the lateral direction. Loads were monitored along both orthogonal axes by two load cells during the testing. Stresses along the longitudinal (TL) and circumferential (TC) axes were determined in the Lagrange sense (measured force/unloaded cross-sectional area). The in-plane tissue deformation was determined by tracking the position of four markers affixed to the specimen surface, placed in the central region (about 2–3 mm square) of the specimen. From these deformation data the in-plane stretches (=current length/initial length) along longitudinal (λL) and circumferential (λC) axes were calculated.
The specimens were tested at room temperature in modified Kreb’s solution. An initial equibiaxial stress protocol was performed to mechanically precondition the tissue specimen. The preconditioning protocol consisted of 12 loading-unloading cycles from an initial 0.5g load (1–2kPa stress, depending on the thickness of the specimen) to the maximal stress (100 kPa) with a loading period of approximately 15 seconds. This stress level showed a repeatable mechanical response without evident tissue damage.5 All test protocols maintained a constant ratio of the axial stress (TC:TL) throughout cycling. To quantify the overall tissue compliance, the areal strain under 100 kPa equi-biaxial stress computed as λLλC −1, and expressed the fractional change in area between the unloaded and loaded states. The degree of mechanical anisotropy was measured by the ratio (λC−1)/(λL−1), which indicated the differences in mechanical response along the circumferential and longitudinal axes.
Statistical analysis
All data were expressed as mean ± standard error. Two-way analysis of variance with Bonferroni post-hoc test was used to examine the differences in cystometric parameters, bladder compliance and areal strain of control, diuretic and diabetic rats at 2, 4, and 8 weeks in each group. For all statistical tests, p≤0.05 was considered significant.
Results
General characteristics
Diabetic rats exhibited 12 ± 5% weight loss while diuretic rats exhibited 13 ± 7% weight gain from the initial body weight during the 8-week period (table 1). Blood glucose levels of diabetic rats were 4 to 5 times higher than those of control and diuretic rats. Bladder weights of diuretic and diabetic rats were approximately twice greater than those of control rats.
Table 1.
General characteristics and cystometry in control, diuretic and diabetic rats.
| N | Rat Weight (g) |
Bladder Weight (g) |
Blood Glucose (mg/dl) |
Bladder Capacity (mL) |
Voiding Efficiency (%) |
Max. Voiding Pressure (cmH2O) |
Bladder Compliance (mL/cmH2O) |
|
|---|---|---|---|---|---|---|---|---|
| Control | 7 | 258 ± 9 | 0.13 ± 0.01 | 94 ± 5 | 0.54 ± 0.07 | 92.4 ± 1.3 | 37.1 ± 3.7 | 0.07 ± 0.01 |
|
| ||||||||
| 2-w diuretic | 7 | 288 ± 6* | 0.19 ± 0.01* | 82 ± 5 | 1.31 ± 0.10* | 85.3 ± 2.4 | 34.4 ± 1.8 | 0.22 ± 0.03* |
| 4-w diuretic | 7 | 288 ± 1* | 0.24 ± 0.01*‡ | 90 ± 3 | 1.76 ± 0.21* | 84.3 ± 6.3 | 34.6 ± 3.2 | 0.36 ± 0.03*‡ |
| 8-w diuretic | 6 | 299 ± 7* | 0.25 ± 0.02*‡ | 90 ± 2 | 2.26 ± 0.19*‡ | 82.6 ± 5.6 | 33.8 ± 1.8 | 0.40 ± 0.05*‡ |
|
| ||||||||
| 2-w diabetic | 7 | 227 ± 8† | 0.24 ± 0.01*† | 400 ± 27*† | 1.17 ± 0.09* | 53.4 ± 4.6*† | 39.3 ± 1.7 | 0.31 ± 0.04* |
| 4-w diabetic | 6 | 230 ± 12† | 0.28 ± 0.02* | 393 ± 32*† | 1.80 ± 0.11*|| | 41.0 ± 5.5*† | 41.3 ± 2.9 | 0.44 ± 0.09* |
| 8-w diabetic | 7 | 222 ± 5*† | 0.29 ± 0.02*|| | 431 ± 24*† | 2.44 ± 0.15*||# | 39.6 ± 7.1*† | 40.5± 2.6 | 0.59 ± 0.06*†|| |
The symbol indicates that the value is significantly different from controls;
from diuretic rats of the same weeks;
from 2-week diuretic rats;
from 2-week diabetic rats;
from 4-week diabetic rats.
Cystometry
Cystometry revealed that mean bladder capacity and compliance increased in all treated rats compared with controls in table 1. Though diabetic rats had significantly decreased voiding efficiency, mean maximal voiding pressure was similar in all groups. The bladder compliance of diabetic and diuretic rats increased progressively and synchronously over the 4-week treatment period. The compliance of the 8-week diabetic bladders was significantly greater than that of the 8-week diuretic bladders (0.59±0.06 versus 0.40±0.05, p=0.03).
Biaxial mechanical behavior
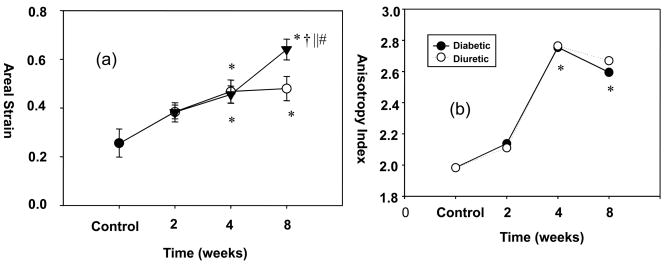
The cystometry results were most easily compared to the biaxial data by examining the areal strain (Fig. 1-a). Here, in diabetic and diuretic rats, areal strain increased progressively and synchronously over the 4-week treatment periods. Moreover, the areal strain was similar in 4-week and 8-week diuretic bladders (0.47±0.05 versus 0.48±0.05, p=0.87). However, the areal strain of 8-week diabetic bladders was significantly greater than that of 4-week diabetic bladders (0.64±0.04 versus 0.46±0.04, p=0.01) and 8-week diuretic bladders (0.64±0.04 versus 0.48±0.05, p=0.03). These results indicated continued remodeling of the bladder wall after 4 weeks in the diabetic state.
Figure 1.
(a) Changes in areal strain for both diuretic and diabetic bladders up to 8 weeks. Unlike the diuretic group, the diabetic group demonstrated continued increase in tissue compliance at 8 weeks compare to 4 weeks. (b) Both groups exhibited a progressive and synchronous increase in mechanical anisotropy up to 4 weeks, with no further changes observed afterwards. Legend: Black circle = control bladders; white circles = diuretic bladders; black triangles = diabetic bladders. * - Significant differences (p<0.05) from controls; † - from diuretic rats of the same week; || - from 2-week diabetic bladders; and # - from 4-week diabetic bladders.
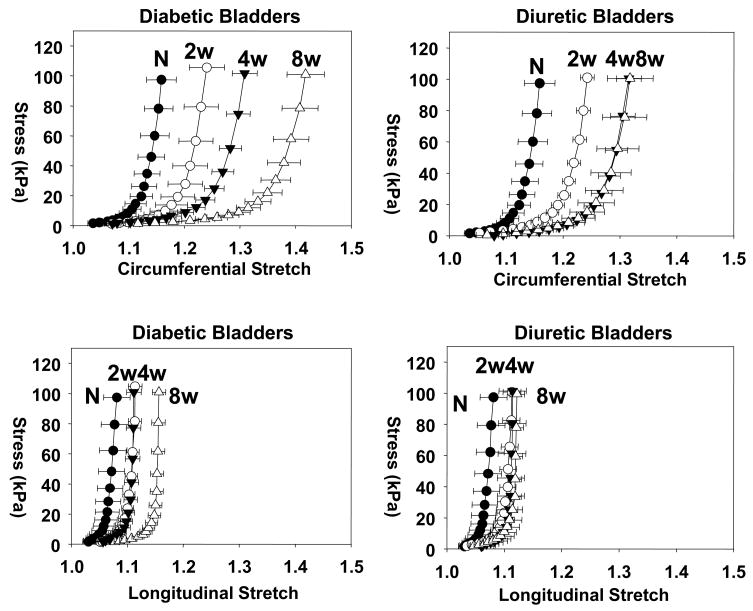
More detailed observations of the progressive changes in tissue mechanical behavior were apparent in the stress-stretch curves (Fig. 2). Similar to many soft biological tissues, the mechanical behaviors in both the circumferential and longitudinal directions were nonlinear with increasing stiffness at higher stretches. This behavior was maintained over all time points studied. The extensibility along the circumferential axis at both time points was greater than that along the longitudinal axis, indicating the presence of substantial mechanical anisotropy along the bladder walls. Interestingly, the degree of mechanical anisotropy increased progressively and synchronously in both groups up to 4 weeks, but did not exhibit any further changes afterwards (Fig. 1-b). No differences in the degree of mechanical anisotropy were observed between test groups at any time point.
Figure 2.
Mean stress-stretch responses for the equi-biaxial protocol for all test periods, demonstrating nearly identical response for both groups up to four weeks. Unlike the diuretic group, the diabetic group demonstrated continued increase in tissue compliance at 8 weeks compared to 4 weeks.
DISCUSSION
The present study provided evidence that compared to controls, diabetic and diuretic rats exhibited striking increases in bladder weight, bladder capacity and in vivo compliance at 2, 4, and 8 weeks as seen in previous studies.3,8,11,12 Though there were no age-match controls, we presumed that these increases were not due to aging of the animals based on a previous report by Tammela et al that demonstrated only a small and non-significant age-related increase in micturition volumes in Sprague-Dawley rats during the 60-day study period13. In addition, only the diabetic rats had decreased voiding efficiency. Since the maximal voiding pressure achieved by the diabetic groups was similar to that of the controls, decreased voiding efficiency may be due to elevated resistance or inability to relax the urethra caused by diabetic urethropathy,9 but not due to decreased bladder contractility. Further investigation, however, is necessary to determine the smooth muscle contractility and/or active state biomechanical properties of the normal and diabetic bladders.
Previous studies have showed that diabetic and sucrose-fed rats similarly increase water consumption and consequently increase urine output that lead to overloading of the bladder wall.3,8 In addition, patients with polyuric syndromes (e.g. nephrogenic diabetes insipidus and psychogenic polydipsia) develop enlarged and highly compliant bladders.14 Thus, these reports and our study suggest that diuresis per se may induce profound changes in the physical properties of the bladder wall.3,8 It should be noted, however, enlarged bladder and chronic urine retention are not necessarily the only causes of increased compliance, as evidenced by bladder outlet obstruction animals having large, but poorly compliant bladders.15
It has been reported previously that both STZ diabetic and sucrose-fed diuretic rat bladders are more compliant compared to normal, but there exists some discrepancy between reports.3,12 While one study showed that the pressure-volume data of diuretic rats were nearly identical to those from diabetic rats,3 a study by another group reported that STZ rats had more compliant bladders than diuretic rats.12 Our mechanical study demonstrated that both tissue and organ compliance of diabetic and diuretic rat bladders were similar up to 4 weeks, but that diabetic bladders were significantly more compliant at 8 weeks. Our previous study has also showed the time-dependent alternations are different in gene expression, nerve growth factor, collagen and elastin synthesis between diuretic and diabetic rat bladders.16,17 Based on the timing of these events, it can be speculated that the progression of neuropathy and tissue remodeling may be related to the diabetes-specific changes in the mechanical properties of bladder wall. Taken together, these data suggest that diuresis mainly contributes to the “early” changes of the mechanical properties of bladders during the 4-week treatment period, with further or “late” changes in 8-week diabetic bladders induced by other diabetic effects.
In addition to the changes in compliance, the results of the present study provided detailed information on the mechanical anisotropy of bladder tissues (a greater compliance in the circumferential direction than in the longitudinal direction), which could not be assessed by cystometry or conventional uniaxial tensile tests. Anisotropic mechanical behavior is characteristic of soft biological tissues that have an aligned network of smooth muscle fibers and the extracellular matrix.18 Using a mathematical model, Damaser et al have showed that oblate spheroid bladders can achieve greater compliance than either spherical or prolate spheroidal bladders.19 In contrast, low compliance bladders, often observed in patients with high grade deformity of bladder shape, were described as pine tree shape resembling prolate spheroid.20 It is, therefore, possible that the changes in the mechanical behavior may be a manifestation of morphological adaptation of the bladder from prolate to oblate spheroid in order to achieve greater compliance necessary for the increased urine storage volume.
The changes of mechanical properties induced by diabetes are not limited to simple increases in extensibility, but include more complex alterations in the biaxial mechanical behaviors. These phenomena may be due to the reorganization of constituents such as collagen, elastin and smooth muscle cell fibers in amount, orientation or perhaps in the way that they interact with each other. Using similar animal models, Liu and Daneshgari have showed that rapid, marked remodeling of the bladder wall and reorganization of the relative structural relations among the major tissue components in the morphology study.8 Moreover, Gray et al have demonstrated molecular-level changes in the extracellular matrix proteins of the bladder in diabetic animals.16 Together with the results of the present study, it can be concluded that the changes in the mechanical behavior of the bladder wall due to the complex tissue remodeling events are an important indicator of the diabetic bladder dysfunction.
CONCLUSIONS
The present study provided the first known data on the passive biaxial mechanical properties of diuretic and diabetic bladders for up to eight weeks. Our time-course study suggests that diuresis mainly contributes to the “early” changes of the bladder wall, with diabetes inducing further “late” changes of mechanical properties of the bladders. Thus controlling serum glucose levels with adequate diet and fluid intake may be an important step to prevent progressive bladder dysfunction caused by diuresis in the early stage of diabetes.
Acknowledgments
We gratefully acknowledge the support of NIH Grants HD39769 and DK55045, and the Paralyzed Veterans of America Spinal Cord Research Foundation (#2289-01 to JN). KKT was supported by a NIH T32 training grant (DK7774).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frimodt-Moller C. Diabetic cystopathy: epidemiology and related disorders. Ann Intern Med. 1980;92:318–321. doi: 10.7326/0003-4819-92-2-318. [DOI] [PubMed] [Google Scholar]
- 2.Sutaria PM, Staskin DR. Hydronephrosis and renal deterioration in the elderly due to abnormalities of the lower urinary tract and ureterovesical junction. Int Urol Nephrol. 2000;32:119–126. doi: 10.1023/a:1007115013407. [DOI] [PubMed] [Google Scholar]
- 3.Kudlacz EM, Chun AL, Skau KA, et al. Diabetes and diuretic-induced alterations in function of rat urinary bladder. Diabetes. 1988;37:949–955. doi: 10.2337/diab.37.7.949. [DOI] [PubMed] [Google Scholar]
- 4.Billiar KL, Sacks MS. Biaxial mechanical properties of the natural and glutaraldehyde treated aortic valve cusp--Part I: Experimental results. J Biomech Eng. 2000;122:23–30. doi: 10.1115/1.429624. [DOI] [PubMed] [Google Scholar]
- 5.Gloeckner DC, Sacks MS, Fraser MO, et al. Passive biaxial mechanical properties of the rat bladder wall after spinal cord injury. J Urol. 2002;167:2247–2252. [PubMed] [Google Scholar]
- 6.Nagatomi J, Gloeckner DC, Chancellor MB, et al. Changes in the biaxial viscoelastic response of the urinary bladder following spinal cord injury. Ann Biomed Eng. 2004;32:1409–1419. doi: 10.1114/b:abme.0000042228.89106.48. [DOI] [PubMed] [Google Scholar]
- 7.Pitre DA, Ma T, Wallace LJ, et al. Time-dependent urinary bladder remodeling in the streptozotocin-induced diabetic rat model. Acta Diabetol. 2002;39:23–27. doi: 10.1007/s005920200008. [DOI] [PubMed] [Google Scholar]
- 8.Guiming Liu, Daneshgari Temporal diabetes and diuresis induced remodeling of the urinary bladder in the rat. Am J Physiol Regul Integr Comp Physiol. 2006;291:837–843. doi: 10.1152/ajpregu.00917.2005. [DOI] [PubMed] [Google Scholar]
- 9.Torimoto K, Fraser MO, Hirao Y, et al. Urethral dysfunction in diabetic rats. J Urol. 2004;171:1959–1964. doi: 10.1097/01.ju.0000121283.92963.05. [DOI] [PubMed] [Google Scholar]
- 10.Sasaki K, Chancellor MB, Goins WF, Phelan MW, Glorioso JC, de Groat WC, Yoshimura Gene therapy using replication-defective herpes simplex virus vectors expressing nerve growth factor in a rat model of diabetic cystopathy. Diabetes. 2004;53:2723–2730. doi: 10.2337/diabetes.53.10.2723. [DOI] [PubMed] [Google Scholar]
- 11.Andersson PO, Malmgren A, Uvelius B. Cystometrical and in vitro evaluation of urinary bladder function in rats with streptozotocin-induced diabetes. J Urol. 1988;139:1359–1362. doi: 10.1016/s0022-5347(17)42919-3. [DOI] [PubMed] [Google Scholar]
- 12.Eika B, Levin RM, Longhurst PA. Comparison of urinary bladder function in rats with hereditary diabetes insipidus, streptozotocin-induced diabetes mellitus, and nondiabetic osmotic diuresis. J Urol. 1994;151:496–502. doi: 10.1016/s0022-5347(17)35001-2. [DOI] [PubMed] [Google Scholar]
- 13.Tammela TL, Leggett RE, Levin RM, Longhurst PA. Temporal changes in micturition and bladder contractility after sucrose diuresis and streptozotocin-induced diabetes. J Urol. 1995;153:2014. [PubMed] [Google Scholar]
- 14.Singh H, Linas SL. Compulsive water drinking in the setting of anticholinergic drug use: an unrecognized cause of chronic renal failure. Am J Kidney Dis. 1995;26:586–589. doi: 10.1016/0272-6386(95)90593-6. [DOI] [PubMed] [Google Scholar]
- 15.Damaser MS, Arner A, Uvelius B. Partial outlet obstruction induces chronic distension and increased stiffness of rat urinary bladder. Neurourol Urodyn. 1996;15:650–665. doi: 10.1002/(SICI)1520-6777(1996)15:6<650::AID-NAU6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 16.Gray MA, Wang CC, Sacks MS, et al. Time-dependent alterations of select genes in streptozotocin-induced diabetic rat bladder. Urology. 2008;71:1214–1219. doi: 10.1016/j.urology.2007.11.054. [DOI] [PubMed] [Google Scholar]
- 17.Sasaki K, Chancellor MB, Phelan MW, et al. Diabetic cystopathy correlates with a long-term decrease in nerve growth factor levels in the bladder and lumbosacral dorsal root Ganglia. J Urol. 2002;168:1259–1264. doi: 10.1016/S0022-5347(05)64636-8. [DOI] [PubMed] [Google Scholar]
- 18.Fung YC. Biomechanics: Mechanical Properties of Living Tissues. 2. New York: Springer Verlag; 1993. pp. 466–497. [Google Scholar]
- 19.Damaser MS, Lehman SL. The effect of urinary bladder shape on its mechanics during filling. J Biomech. 1995;28:725–732. doi: 10.1016/0021-9290(94)00169-5. [DOI] [PubMed] [Google Scholar]
- 20.Ogawa T. Bladder deformities in patients with neurogenic bladder dysfunction. Urol Int. 1991;47:59–62. doi: 10.1159/000282252. [DOI] [PubMed] [Google Scholar]