Abstract
The endocardial cushions play a critical role in septation of the four-chambered mammalian heart and in the formation of the valve leaflets that control blood flow through the heart. Within the outflow tract (OFT), both cardiac neural crest and endocardial-derived mesenchymal cells contribute to the endocardial cushions. Bone morphogenetic protein 4 (BMP4) is required for endocardial cushion development and for normal septation of the OFT. In the present study, we show that anterior heart field (AHF)-derived myocardium is an essential source of BMP4 required for normal endocardial cushion expansion and remodeling. Loss of BMP4 from the AHF in mice results in an insufficient number of cells in the developing OFT endocardial cushions, defective cushion remodeling, ventricular septal defects, persistent truncus arteriosus, and abnormal semilunar valve formation.
Keywords: BMP4, anterior heart field, mouse, Cre, Mef2c, semilunar valve, endocardial cushion, outflow tract, ventricular septal defect
Introduction
The mammalian heart initially forms from a crescent-shaped region of anterior lateral mesoderm that is folded into a linear tube (Brand, 2003; Srivastava, 2006). This linear tube then undergoes rightward looping and expands dramatically through the addition of cells from the second heart field to the outflow and inflow poles (Buckingham et al., 2005; Abu-Issa and Kirby, 2007). A more restricted, anterior subset of progenitors from the splanchnic and pharyngeal mesoderm, referred to as the anterior heart field (AHF), are added only to the arterial pole and give rise to the myocardial and endothelial layers of the OFT, right ventricle (RV), and interventricular septum (Cai et al., 2003; Buckingham et al., 2005; Verzi et al., 2005; Abu-Issa and Kirby, 2007; Black, 2007). In addition, AHF-derived endothelial cells make extensive contributions to the endocardial cushion mesenchyme within the OFT (Cai et al., 2003; Verzi et al., 2005; Waldo et al., 2005; Song et al., 2007).
Endocardial cushion mesenchyme arises through a process known as endocardial to mesenchymal transformation (EMT) in which newly formed mesenchymal cells contribute to the endocardial cushions that form the membranous portion of the ventricular septum, the semilunar valves leaflets, and the mesenchyme that is responsible for septation of the common OFT (Nakajima et al., 2000; Delot, 2003). In addition, there is a substantial contribution of cardiac neural crest cells, which enter the OFT from the pharyngeal arches and play an essential role in dividing the common OFT and forming the semilunar valve leaflets (Waldo et al., 1998; Jiang et al., 2000; Brown and Baldwin, 2006; Hutson and Kirby, 2007). Despite the central importance of the endocardial cushions in heart development and congenital heart defects, much remains to be learned about the signals that regulate their development and subsequent remodeling.
Bone morphogenetic protein (BMP) signaling is essential early in development for cardiac myocyte specification and later is involved in endocardial cushion development and subsequent valvulogenesis (Delot, 2003; Schneider et al., 2003). BMPs bind to heterotetrameric receptor complexes, which exert their effects via receptor-mediated activation of Smad transcription factors (Derynck, 1994; Massague et al., 1994). Activated Smads translocate to the nucleus in response to BMP signaling and alter expression of downstream transcriptional targets (Delot, 2003; Chen et al., 2004). Based on their patterns of expression in the developing heart, Bmp2 and Bmp4 have been implicated in endocardial cushion development (Lyons et al., 1990; Yamagishi et al., 1999; Abdelwahid et al., 2001; Delot et al., 2003; Liu et al., 2004; Ma et al., 2005). Within the developing heart, Bmp4 is expressed in the myocardium of the OFT beginning at E8.5 and continuing throughout embryonic development (Jiao et al., 2003; Liu et al., 2004). As the OFT develops and remodels, Bmp4 expression is also notable within the endoderm and mesoderm of the pharyngeal arches (Liu et al., 2004). BMP4 is also present in the inflow pole of the heart beginning at E8.5 and is expressed in regions critical for OFT and atrioventricular septation (Jiao et al., 2003). The majority of Bmp4-null mice die early in development prior to gastrulation, making it difficult to define the role of this signaling molecule in the heart using conventional gene inactivation approaches (Winnier et al., 1995).
Recent work in which Bmp4 was conditionally inactivated in the heart within the Nkx2-5 expression domain indicated that BMP4 was required in either the myocardium or pharyngeal endoderm for OFT septation and formation of the membranous portion of the interventricular septum (Liu et al., 2004). However, the role of BMP4 in semilunar valve development has not been investigated previously, and the precise tissue source of BMP4 required for OFT cushion formation and maturation has not been identified.
In the studies presented here, we investigated the requirement of BMP4 in the AHF by inactivating Bmp4 in the Mef2c-AHF-Cre expression domain. Mef2c-AHF-Cre is expressed exclusively in the AHF and its derivatives within the myocardium and endocardium of the right ventricle and OFT (Verzi et al., 2005). We show that inactivation of Bmp4 in the AHF results in lethality at birth due to defects in endocardial cushion development. Bmp4 AHF conditional knockout mice have profound defects in OFT septation and in formation of the membranous portion of the interventricular septum. In addition, we show that BMP4 is required for normal expansion and remodeling of the endocardial cushions within the OFT and that BMP4 is required for normal semilunar valve formation and remodeling. Thus, these studies establish that AHF-derived myocardium is an essential source of BMP4 required for OFT septation, cushion remodeling, and semilunar valve maturation.
Results
Bmp4 is required in the AHF and its derivatives for postnatal survival
To examine the requirement of Bmp4 in the AHF and its derivatives, we employed a conditional inactivation approach to delete Bmp4 using the AHF-specific Cre transgenic line, Mef2c-AHF-Cre (Verzi et al., 2005). Mice homozygous for a floxed Bmp4 allele (Bmp4floxneo) (Liu et al., 2004) were crossed to mice heterozygous for a Bmp4 null allele and hemizygous for the Mef2c-AHF-Cre transgene (Bmp4+/-; Mef2c-AHF-CreTg/0), as depicted in Fig. 1A. These crosses resulted in disruption of Bmp4 expression in regions where Mef2c-AHF-Cre transgene activity and Bmp4 expression overlapped (Fig. 1B-E). Although we have previously described the Cre-derived fate map in Mef2c-AHF-Cre transgenic mice (Verzi et al., 2005; Ai et al., 2007), we examined the activity of the Mef2c-AHF-Cre transgene within the OFT and endocardial cushions in further detail to determine precisely where the Cre fate-map overlapped the expression of Bmp4 (Fig. 1B-E). As described previously, cells marked by the activity of the Mef2c-AHF-Cre transgene contributed extensively to the OFT, RV, and interventricular septum at E10.5 (Fig. 1B, C). At this stage, Bmp4 expression was clearly evident in control embryos in the developing outflow tract (OFT) as well as in the developing pharyngeal endoderm and forelimb bud (Fig. 1D). Within cardiogenic regions at this stage, Bmp4 expression was clearly restricted to the myocardial layer of the developing OFT and was absent from the endothelial layer and the endocardial cushions (Fig. 1E). These analyses demonstrate that Mef2c-AHF-Cre transgene activity overlaps with Bmp4 expression exclusively within the myocardium of the developing OFT.
Fig. 1. Bmp4 expression overlaps the activity of Mef2c-AHF-Cre in the myocardial layer of the outflow tract (OFT) and is abolished in the OFT in Bmp4 AHF conditional knockout mice.
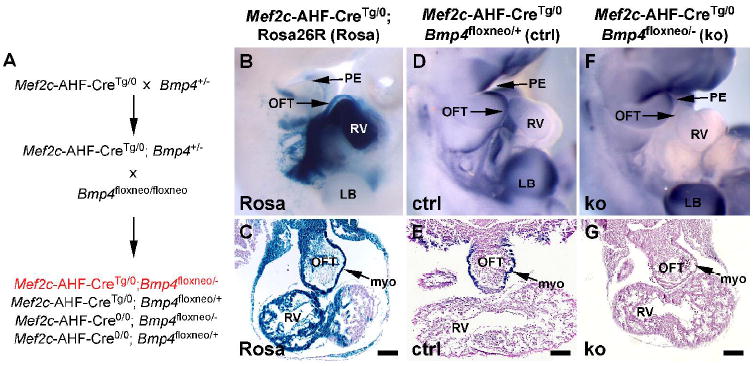
(A) Strategy for inactivation of Bmp4 in the Mef2c-AHF-Cre expression domain. (B, C) The activity of the Mef2c-AHF-Cre transgene is specific to the developing pharyngeal mesoderm, OFT, and RV at E10.5 as determined by crossing to ROSA26R Cre-dependent LacZ reporter mice (Rosa) stained with X-gal. (D, E) In situ hybridization for Bmp4 transcripts in control mice (ctrl) demonstrates Bmp4 expression in the pharyngeal endoderm (PE), OFT, and forelimb bud (LB) at E10.5. Within the heart, Bmp4 expression is limited to the myocardial layer (myo) (E) of the OFT and overlaps the activity of the Mef2c-AHF-Cre transgene only in this region. (F, G) In situ hybridization in Bmp4 AHF knockout embryos (ko) shows a complete loss of Bmp4 expression from the myocardial layer (myo) of the OFT at E10.5. Note that expression of Bmp4 in the PE and LB, which are outside the Mef2c-AHF-Cre expression domain, is retained. Genotypes are indicated above panels B and C, D and E, and F and G. The bars in the transverse sections in C, E, and G are equal to 100 μm.
Using the Mef2c-AHF-Cre transgene to inactivate Bmp4 resulted in a distinct loss of Bmp4 expression in the OFT at E10.5 (Fig. 1F, G). By contrast, Bmp4 expression was retained in the developing pharyngeal endoderm and forelimb bud since this is outside the Mef2c-AHF-Cre expression domain (Fig. 1F). These data indicate that Bmp4 expression was abolished in the myocardium of the OFT adjacent to the endocardial cushions in Bmp4floxneo/-; Mef2c-AHF-CreTg/0 embryos. Bmp4floxneo/-; Mef2c-AHF- CreTg/0 mice were born at the expected Mendelian frequency of 25% (Table 1). However, unlike their littermate controls, 100% of Bmp4 AHF knockout mice were severely cyanotic after delivery, and the majority (17/19) died within a few hours of birth (Table 2). These data demonstrate that BMP4 is required in AHF-derived tissues for postnatal viability. Taken together with the expression pattern of Bmp4, these data support the notion that AHF-derived myocardium is an important source of BMP4 signaling and is required for postnatal viability.
Table 1.
Bmp4 AHF knockout mice are born at normal Mendelian ratios. The asterisk denotes that 100% of Mef2c-AHF-CreTg/0; Bmp4floxneo/- neonates appeared cyanotic and greater than 85% died within a few hours of birth.
| Genotype | # of embryos (% of total) | # expected |
|---|---|---|
| Mef2c-AHF-Cre0/0; Bmp4floxneo/+ | 20 (27%) | 18.75 |
| Mef2c-AHF-CreTg/0; Bmp4floxneo/+ | 18 (24%) | 18.75 |
| Mef2c-AHF-Cre0/0; Bmp4floxneo/- | 18 (24%) | 18.75 |
| Mef2c-AHF-CreTg/0; Bmp4floxneo/- | 19 (25%)* | 18.75 |
| Total | 75 (100%) | 75 |
Table 2.
Summary of phenotypes observed in all Mef2c-AHF-CreTg/0; Bmp4floxneo/- neonatal mice. Type I persistent truncus arteriosus (PTA) refers to Collett and Edwards type I (Park, 2002). A plus (+) sign refers to the presence of the indicated defect. A minus (-) sign refers to the absence of a detectable defect. VSD, ventricular septal defect.
| Neonate | Cyanosis | Death | PTA ; Type | VSD | Leaflet #; Valve defects |
|---|---|---|---|---|---|
| 1 | + | + | + ; Type I | + | 3; Thick leaflets |
| 2 | + | + | + ; Type I | + | 4; Thick leaflets |
| 3 | + | + | + ; Type I | + | 3; Thick leaflets |
| 4 | + | + | + ; Type I | + | 4; Thick leaflets |
| 5 | + | + | + ; Type I | + | 3; Thick leaflets |
| 6 | + | + | + ; Type I | + | 4; Thick leaflets |
| 7 | + | + | + ; Type I | + | 4; Thin leaflets |
| 8 | + | + | + ; Type I | + | 4; Thick leaflets |
| 9 | + | + | + ; Type I | + | 3; Thick leaflets |
| 10 | + | + | + ; Type I | + | 6; Thick leaflets |
| 11 | + | + | + ; Type I | + | 4; Thin, short leaflets |
| 12 | + | + | + ; Type I | + | 2; Thick leaflets |
| 13 | + | + | + ; Type I | + | 5; Thick, overlapping leaflets |
| 14 | + | + | + ; Type I | + | 4; Thick leaflets |
| 15 | + | + | + ; Type I | + | 5; Thick leaflets |
| 16 | + | + | + ; Type I | + | 4; Thick leaflets |
| 17 | + | + | + ; Type I | + | 3; Thick leaflets |
| 18 | + | - | - | - | 6; Thick leaflets |
| 19 | + | - | - | - | 6; Thick leaflets |
| Total | 19/19 | 17/19 | 17/19 | 17/19 | 19/19 abnormal |
Bmp4 AHF knockout mice exhibit profound outflow tract and ventricular septal defects
The complete prevalence of cyanosis in Bmp4 AHF knockout animals suggested the possibility of intracardiac mixing of oxygenated and deoxygenated blood. Therefore, we examined the hearts of these animals for defects that could account for cyanosis and compared conditional knockout mice to control littermates (Fig. 2). Neonatal hearts from control animals had a normally formed OFT with an intact aorta and pulmonary artery and normal septation of the ventricular chambers (Fig. 2A, C, E). By contrast, the vast majority (17/19) of Bmp4 AHF knockout animals displayed obvious persistent truncus arteriosus (PTA) with abnormal communication between the pulmonary artery and the aorta near the ventricular outflow in a region where the cushion mesenchyme underlies AHF-derived myocardium (Fig. 2D, Table 2). The aortic arch arteries appeared normal and had normal branching patterns in Bmp4 AHF knockout animals (data not shown).
Fig. 2. BMP4 is required in the AHF for septation of the ventricles and outflow tract.

(A, B) Transverse sections of neonatal hearts taken from control (ctrl) (A) and Bmp4 AHF knockout (ko) (B) neonatal mice showed a normal interventricular septum (IVS) in control mice and a ventricular septal defect (VSD) in knockout mice. (C, D) Transverse sections through the outflow tracts (OFT) of neonatal mice showed a normally septated pulmonary artery (PA) and aorta (Ao) in control mice (C), while Bmp4 AHF knockout mice exhibited persistent truncus arteriosus (PTA) (D). (E, F) High magnification images of the normally formed membranous IVS in control mice (E) compared to the absence of a membranous septum and a large VSD in Bmp4 AHF knockout mice (F). Note also the misshapen valve leaflets in Bmp4 AHF knockout mice (F). LV, left ventricle; RV, right ventricle. The bars are equal to 100 μm in all panels.
Bmp4 AHF knockout animals also had a large defect in the membranous ventricular septum, which resulted in a communication between the right and left ventricles (Fig. 2B, F). These ventricular septal defects were apparent in the same animals that had an obvious PTA (Table 2). Despite Mef2c-AHF-Cre activity in the myocardium of the right ventricle, the muscular portion of the ventricular septum, and the myocardial component of the OFT, there were no apparent phenotypic changes in these structures (Fig. 2). It is important to note that the Mef2c-AHF-Cre transgene is not expressed in the pharyngeal endoderm, indicating that AHF-derived myocardium is an essential source of BMP4 required for OFT septation and formation of the membranous portion of the interventricular septum.
Bmp4 is required in AHF-derived myocardium for semilunar valve development
The defects in both OFT septation and in the membranous portion of the interventricular septum suggested a primary defect in the development of the OFT endocardial cushions. Because the semilunar valve leaflets are also derived primarily from the OFT cushions (Nakajima et al., 2000), we hypothesized that the pulmonary and aortic valves might also be defective in Bmp4 AHF conditional knockout mice. Indeed, Bmp4 AHF knockout mice exhibited profound defects in the outflow valve leaflets, which were abnormal in both number and morphology (Fig. 3). Littermate control animals always displayed three properly formed valve leaflets in both the aorta and the pulmonary artery, and control valves always had leaflets with crisp edges and fine connections to the aortic or pulmonary artery walls (Fig. 3A, B). In contrast, although normally seated, the outflow valve leaflets of Bmp4 AHF knockout animals displayed an obviously abnormal, thickened morphology (Fig. 3C-F). Despite the abnormal shape of the valve leaflets in Bmp4 AHF knockout animals, the array of elastin and collagen fibers that comprise the extracellular matrix (ECM) portion of the valve leaflets was present, but the valve leaflets of Bmp4 AHF knockout mice appeared thicker than leaflets in control animals (Fig 3G, H). This thickened appearance did not appear to be due to increased cellularity but rather due to a lack of proper trilaminar organization. Valve leaflets from control neonates exhibited a trilaminar organization of ECM and mesenchymal cells typical of normal valve leaflets (Fig. 3G). By contrast, Bmp4 AHF knockout valve leaflets lacked the same level of organization, particularly in the spongiosa and ventricularis layers, which appeared disorganized and contained many loosely connected cells in both layers instead of a normal distribution of extracellular matrix in the spongiosa layer and a compact, cellularized ventricularis layer (Fig. 3H).
Fig. 3. BMP4 is required in the AHF for proper formation of the semilunar valve leaflets.
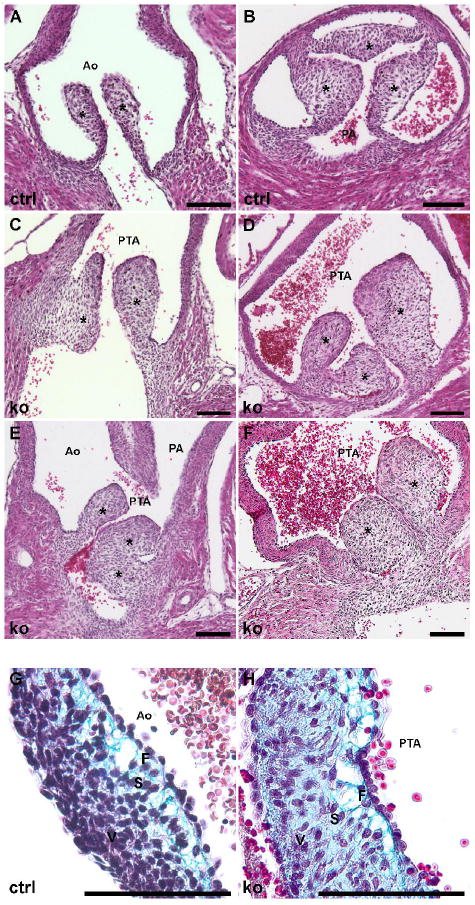
(A, B) Control neonatal mice (ctrl) have normal formation of the aortic valve (A) and pulmonary valve (B) leaflets with normal leaflet architecture and number. (C-F) Bmp4 AHF knockout (ko) mice have a single common outflow tract (OFT) valve with abnormal formation of the semilunar valve leaflets. The neonatal ko valve leaflets have irregular morphology and disrupted architecture. Note the single outflow valve in (E), which leads to the persistent truncus arteriosus (PTA) that then divides into the aorta (Ao) and pulmonary artery (PA). Modified Movat’s pentachrome staining of ctrl (G) and ko (H) neonatal valves shows the trilaminar extracellular matrix (ECM) and cellular organization of the neonatal leaflets. The cellular component of the control leaflet (G) has become condensed within the ventricularis (V) layer and the proteoglycan is focused within the spongiosa (S) layer. By contrast, the ko leaflet has cells and proteoglycan distributed throughout the leaflet. F, fibrosa layer. Asterisks mark the valve leaflets in all panels. The bars in all panels are equal to 100 μm.
Conditional knockout mice also had a variable number of valve leaflets, ranging from 2 to 6 apparent leaflets (Table 2). The morphologic abnormalities of the outflow valves varied widely among the different knockout mice, and the variability in the number of leaflets appeared to reflect, at least in part, the severity and location of the PTA. However, the morphological defects of the leaflets were apparent even in neonates in which an obvious PTA or VSD was not observed (Table 2), suggesting a primary defect in semilunar valve development and remodeling.
BMP4 function is required in AHF-derived myocardium for OFT cushion expansion and remodeling
Because each of the major defects observed in Bmp4 AHF knockout mice occurred in structures derived from the endocardial cushions, we examined the development and morphogenesis of the OFT cushions in further detail. To compare the presence of cardiac jelly in wild type and Bmp4 AHF knockout embryos, sections from embryonic hearts collected at E10.5 were stained with alcian blue (Fig. 4A, B). Alcian blue stains acidic glycosaminoglycans, including hyaluronic acid, a major component of the cardiac jelly, which is necessary for signaling between the myocardial and endocardial layers in the developing heart (Camenisch et al., 2000; Camenisch et al., 2002; McDonald and Camenisch, 2002). Alcian blue staining was easily detected in both control and AHF knockout embryos at this stage, suggesting that BMP4 is not required in AHF-derived tissues for cardiac jelly formation (Fig. 4A, B).
Fig. 4. BMP4 is required in the AHF for outflow tract endocardial cushion expansion.

(A, B) Alcian blue staining was used to detect the presence of acidic glycosaminoglycans, such as hyaluronic acid within the endocardial cushions. In comparison to control (ctrl) embryos (A), Bmp4 AHF knockout (ko) embryos (B) had a similar amount of alcian blue staining in both the atrioventricular (AVC) and outflow tract (OFT) endocardial cushions at E10.5. (C) Control embryos showed normal expansion of the AVC and OFT endocardial cushions at E10.5. (D) Bmp4 AHF knockout embryos showed deficient expansion of the OFT endocardial cushions at E10.5. Note the normal expansion of the AVC endocardial cushions, which do not develop adjacent to the OFT myocardium. (E, F) The difference in OFT endocardial cushion expansion was also evident at E12.5 in Bmp4 AHF knockout embryos (F) compared to control embryos (E). LA, left atrium; PA, pulmonary artery; RV, right ventricle; VSD, ventricular septal defect. Asterisks in panels E and F denote the endocardial cushions. The bars in all panels are equal to 100 μm.
Endocardial to mesenchymal transformation (EMT) begins in the developing endocardial cushion in the AVC at E9 and in the OFT cushion at E10 (Person et al., 2005). To assess EMT in Bmp4 AHF knockout embryos, an ex vivo explant assay on collagen matrix was employed (Potts et al., 1991; Camenisch et al., 2002; Sugi et al., 2004). Hearts were dissected at E9.5, and the endocardial layer was exposed and placed face down onto a collagen matrix such that the myocardial layer remained in contact with endocardial layer (Camenisch et al., 2002). After 48 h in culture, endocardial cells underwent EMT and migrated away from the initial explant into the collagen matrix. No obvious differences were seen in the migration of mesenchymal cells into the matrix when comparing Bmp4 AHF knockout and control explants (data not shown). These results are further supported by the observation that the endocardial cushions had begun to form by E10.5 in both Bmp4 AHF knockout and control embryos (Fig. 4C, D). Taken together, these results support the notion that cardiac jelly formation and EMT occur normally in Bmp4 AHF knockout embryos.
Although initial EMT in the OFT endocardial cushions was normal, cushion expansion and remodeling was defective in Bmp4 AHF knockout embryos (Fig. 4C, D). Compared to control embryos, which showed significant expansion of the OFT endocardial cushions at E10.5, the majority of Bmp4 AHF knockout embryos showed diminished expansion of the OFT endocardial cushions at E10.5 (Fig. 4C, D). In contrast to the OFT cushions, the AVC cushions appeared normal in Bmp4 AHF knockout embryos (Fig. 4C, D). The diminished OFT endocardial cushion expansion in Bmp4 AHF knockout embryos continued to be apparent at E12.5 (Fig. 4E, F).
Bmp4 is required in AHF-derived myocardium for normal endocardial cushion cell number
We reasoned that the apparent deficiency in OFT endocardial cushion expansion and remodeling in Bmp4 AHF conditional knockout embryos might be caused by a reduced number of cells in the endocardial cushions. To determine if this was the case, we quantified the total number of DAPI-stained cells within the endocardial cushions of the proximal OFT at E10.5 and E12.5 (Fig. 5A, B). These quantitative analyses showed that there was a significant difference in the total number of cells within the proximal OFT endocardial cushions in Bmp4 AHF knockout embryos compared to control embryos at E10.5 (Fig. 5A) and a highly significant difference at E12.5 (Fig. 5B). The difference in the total number of cells in the OFT cushions could be the result of increased apoptosis or decreased cellular proliferation. To determine if Bmp4 AHF knockout embryos had changes in proliferation within the endocardial cushions, BrdU incorporation was used as an indicator of cellular proliferation at E10.5 and E12.5. We counted BrdU-labeled cells as a fraction of the total DAPI-labeled nuclei. These quantitative analyses showed that there was no significant difference in the rate of proliferation within the OFT endocardial cushions at either E10.5 or E12.5 (Fig. 5C, D). To determine if Bmp4 AHF knockout embryos had alterations in the rate of apoptosis within the proximal OFT endocardial cushions, TUNEL staining was used. This method showed that there was no difference in the rate of apoptosis in AHF conditional knockout embryos and littermate controls at either E10.5 or E12.5 (Fig. 5E, F). Overall, our data demonstrate that the AHF is an essential source of BMP4 in the developing OFT and is required for normal endocardial cushion expansion and remodeling.
Fig. 5. Bmp4 AHF knockout embryos have fewer proximal outflow tract (OFT) endocardial cushion cells than littermate controls at E10.5 and E12.5.

(A, B) Quantification of DAPI-stained nuclei in the proximal OFT endocardial cushions of control (ctrl) and Bmp4 AHF knockout (ko) embryos showed a significant reduction in the number of cells within the OFT endocardial cushions of knockout embryos at both E10.5 (A) and E12.5 (B) (p < 0.001 at E10.5 and p < 0.0001 at E12.5, n=10 at each stage). (C, D) Quantification of BrdU-labeled cells as a percentage of total DAPI-stained nuclei showed no difference in the rate of proliferation of proximal OFT endocardial cushion mesenchymal cells at E10.5 (C) and E12.5 (D) (p = 0.7172 at E10.5 and p= 0.6967 at E12.5, n=10 at each stage). (E, F) TUNEL staining of the endocardial cushions in the proximal OFT showed no difference in the number of apoptotic cells between control and Bmp4 AHF knockout embryos at E10.5 (E) and E12.5 (F). LA, left atrium; PA, pulmonary artery; RV, right ventricle. Asterisks mark the endocardial cushions in panels E and F. The bars in panels E and F are equal to 100 μm.
Discussion
Division of the mammalian heart and outflow tract into the pulmonary and systemic circulation is required to prevent mixing of oxygenated and deoxygenated blood and to ensure appropriate delivery of oxygen to the tissues. The endocardial cushions play an essential role in this division by forming the septal divisions of the heart and outflow tract, yet much of the genetic regulation that directs endocardial cushion formation remains undefined. In the present study, we show that inactivation of Bmp4 within AHF-derived myocardium results in neonatal lethality due to severe defects in OFT and ventricular septation, and we demonstrate that BMP4 is required for appropriate expansion and remodeling of the endocardial cushions into the mature leaflets of the semilunar valves.
The semilunar valves serve a critical role in regulation of blood flow from the ventricles to the systemic and pulmonary circulations, and abnormalities in the development of these valves are common (Hoffman and Kaplan, 2002). The semilunar valves initially form from the OFT endocardial cushions and receive an important contribution from cardiac neural crest cells (Nakajima et al., 2000; Delot, 2003; Brown and Baldwin, 2006; Hutson and Kirby, 2007). As the valve leaflets mature, they take on a characteristic trilaminar structure that consists of extracellular matrix (ECM), including collagen, elastin, and proteoglycans, which is organized in coordination with endocardial-and neural crest-derived mesenchymal cells (Hinton et al., 2006). In Bmp4 AHF conditional knockout mice, the semilunar valve leaflets failed to take on the trilaminar organization observed in control animals (Fig. 3). Thus, it is likely that the dysmorphic, thickened appearance of the leaflets in conditional knockout mice was due to abnormal remodeling rather than an increase in cellularity.
Early in development, BMPs play a role in specification of the cardiomyocyte lineage, and disruption of BMP signaling leads to reduction or absence of heart formation (Frasch, 1995; Schultheiss et al., 1997; Shi et al., 2000). As the heart begins its complex morphogenesis, BMPs act as morphogens to direct the process of selective rightward looping of the heart (Chen et al., 1997; Ramsdell and Yost, 1999). Then, as the endocardial cushions begin to form within the developing heart, BMP signaling is involved in the initiation of endocardial to mesenchymal transformation, as well as in the proliferation of the mesenchymal cells within the endocardial cushions (Nakajima et al., 2000; Allen et al., 2001; Delot et al., 2003; Liu et al., 2004; Ma et al., 2005). In addition, BMPs have been implicated in valve leaflet formation, but have not been previously implicated in semilunar valve leaflet remodeling (Delot, 2003; Armstrong and Bischoff, 2004).
Inactivation of BMP receptor function in endothelial cells suggests that the endocardium is a critical recipient tissue of BMP signaling (Gaussin et al., 2005; Ma et al., 2005; Song et al., 2007). These studies showed that when BMP signaling to the endocardium was interrupted, EMT did not occur and as a result the AVC cushions that ultimately separate the atria from the ventricles, did not form properly. These studies also demonstrated that endocardial Alk3 is required for normal proliferation of the mesenchymal cells in the cushions. Furthermore, removal of BMP receptor components, Alk2 or Alk3, in the Wnt1 expression domain results in defective OFT septation (Kaartinen et al., 2004; Stottmann et al., 2004). Taken together, these receptor knockout studies establish that BMP signaling to both the endocardium and neural crest is required for AVC and OFT endocardial cushion development. Furthermore, these previous studies suggest that BMP signaling may be required to recruit cardiac neural crest cells into the developing OFT and that those cells then participate in the proper division of the OFT into the aorta and pulmonary artery. Despite this, these receptor knockout studies did not identify the BMP ligands or the source tissues that are responsible for directing these processes.
Other studies have addressed the role of BMP4 in heart development using conditional approaches in which Cre expression was directed by regulatory elements from the Nkx2-5 or cardiac troponin T (cTnT) genes (Jiao et al., 2003; Liu et al., 2004). Interestingly, OFT defects were not observed when Bmp4 was inactivated in the cTNT expression domain, and Nkx2-5Cre results in Bmp4 deletion in a broader expression domain, including the pharyngeal endoderm (Jiao et al., 2003; Liu et al., 2004). Our studies refine the source of BMP4 required for OFT cushion expansion and remodeling to the Mef2c-AHF-Cre expression domain, which overlaps with Bmp4 expression only in AHF-derived myocardium in the OFT. Furthermore, while both the Mef2c-AHF-Cre and the Nkx2-5Cre conditional inactivation of Bmp4 resulted in incomplete septation of the OFT and VSD, only Nkx2-5Cre-mediated inactivation resulted in abnormal branching of the aortic arch arteries (Liu et al., 2004). Together, these data suggest that BMP4 is required in AHF-derived myocardium for septation of the common OFT and ventricular chamber and that the pharyngeal endoderm is the source of BMP4 required for normal aortic arch artery remodeling.
Both the endocardial layer and the myocardial layer of the RV, interventricular septum, and OFT are AHF-derived (Kelly et al., 2001; Verzi et al., 2005). Interestingly, removal of BMP4 in the Mef2c-AHF-Cre expression domain does not appear to affect the developing myocardium of these AHF-derived structures. This finding is consistent with other targeted knockout approaches of Bmp4, such as Nkx2.5Cre and cTnt-Cre, in which the myocardium was similarly unaffected (Jiao et al., 2003; Liu et al., 2004). These findings suggest that once cardiac progenitors have adopted a cardiac fate in response to early BMP signaling, the myocardium can develop independently of BMP4. However, the endocardium of the OFT, which is also derived from the AHF, appears to require BMP4 signaling from AHF-derived myocardium to allow for normal expansion of the endocardial cushions within the OFT. Ultimately, AHF-derived myocardium likely signals to both endocardial-derived cells and cardiac neural crest cells to direct septation of the OFT and formation of the semilunar valve leaflets (Verzi et al., 2005; Park et al., 2006). Dual fate-mapping approaches may prove useful for exploring further how AHF-derived endocardium and cardiac neural crest cells behave in response to loss of signaling from the myocardium.
The AHF is responsible for the formation of the OFT and interventricular septum (Kelly et al., 2001; Mjaatvedt et al., 2001; Waldo et al., 2001; Cai et al., 2003; Verzi et al., 2005), and abnormalities in these structures result in severe cardiac malformations that are often lethal. Indeed, in humans, congenital heart defects are the most significant cause of morbidity and mortality in the first year of life, and defects in outflow tract (OFT) development are among the most severe congenital heart anomalies, while defects in ventricular septation and valve development are among the most common heart defects (Hoffman and Kaplan, 2002). Thus, it will be interesting to identify downstream targets of BMP4 signaling in the OFT cushions, as it is plausible that aberrant expression of these genes may result in abnormal formation of the OFT or semilunar valves leaflets and may contribute to congenital heart defects in humans. These studies will help to understand further the embryologic explanation for the observation that patient populations with congenital heart defects often appear to have normal development of certain regions of the heart and abnormal development within other regions.
Experimental Procedures
Transgenic and knockout mice
Mice carrying a floxed allele of Bmp4 (Bmp4floxneo) have been described previously (Liu et al., 2004). These mice were used to generate a null allele of Bmp4 by crossing to ß-actin-Cre transgenic mice, which resulted in conversion of the floxed allele to a null allele in the germline (Lewandoski et al., 1997). Mef2c-AHF-CreTg/0 mice, which direct Cre expression exclusively to the AHF and its derivatives in the RV, OFT, and interventricular septum, have also been described previously (Verzi et al., 2005). ROSA26R Cre-dependent lacZ reporter mice have been described previously (Soriano, 1999). All experiments using animals were approved by the UCSF Institutional Animal Care and Use Committee and complied with federal and institutional guidelines.
Histology, immunohistochemistry, and in situ hybridization
For basic histological analyses, neonatal mice were dissected and fixed in 4% paraformaldehyde overnight at 4°C and were then embedded in paraffin, sectioned at a thickness of 8 μm, and stained with hematoxylin and eosin (H&E). For genetic fate mapping studies, embryos were collected at E10.5 and stained with 5-bromo-4-chloro-3-indolyl ß-D-galactopyranoside (X-Gal) to detect ß-galactosidase activity as previously described (Dodou et al., 2004). Stained embryos were then fixed in 4% paraformaldehyde overnight, embedded in paraffin, sectioned at a thickness of 5 μm, and counterstained with neutral fast red for visualization by light microscopy and photography.
Alcian blue staining was used to visualize acidic glycosaminoglycans in the cardiac jelly. Paraffin embedded 5-μm sections of E9.5 and E10.5 embryos were cleared with xylene, rehydrated, and then stained in a 1% alcian blue, 3% acetic acid solution (pH=2.5) for 30 min. The slides were rinsed with water, counterstained with neutral fast red, dehydrated through a series of ethanol dilutions, cleared with xylene, and mounted for viewing by light microscopy and photography.
Modified Movat’s pentachrome staining was performed to visualize extracellular matrix (ECM) and valvular mesenchymal cell organization in neonatal valve leaflets (Jones, 2002). Briefly, paraffin embedded 8-μm sections of neonatal hearts were cleared with xylene, rehydrated, and stained with 1% alcian blue in 1% acetic acid for 20 min. Slides were then washed and stained with Verheoff’s hematoxylin solution for 20 min, rinsed in water and differentiated in 2% aqueous ferric chloride for 30 s. Slides were then treated with 5% sodium thiosulfate for 1 min, washed in tap water for 5 min, and stained with crocein scarlet-acid fuchsin (8:2) for 90 s. Slides were then rinsed in water, differentiated with 5% aqueous phosphotungstic acid for 5 min, rinsed in 0.5% acetic acid for 1 min and then rinsed 3 times in 100% ethanol. The slides were then stained in 6% alcoholic saffron solution for 30 min, rinsed in 100% ethanol, cleared in xylene, and mounted for viewing by light microscopy and photography as described (Jones, 2002).
Cellular proliferation was assayed by using BrdU incorporation. Pregnant mice were injected intraperitoneally with 2 mg of 5-bromo-2-deoxyuridine (BrdU, Sigma B9285) dissolved in saline, and embryos were collected 2 h later. Embryos were subsequently fixed in 4% paraformaldehyde overnight, embedded in paraffin, and sectioned at a thickness of 5 μm. BrdU incorporation was detected with a primary rat anti-BrdU antibody (Serotec MCA2060) at a 1:200 dilution in 3% normal goat serum followed by treatment with secondary Alexa Fluor 594 anti-rat antibody (Molecular Probes) at a 1:300 dilution. Apoptosis was assessed by terminal UTP labeling using the Chemicon TUNEL kit, following the manufacturer’s protocol.
Whole mount in situ hybridization was performed according to standard methods by using digoxigenin-labeled antisense probes as described previously (Rojas et al., 2005). The Bmp4 exon 4 probe plasmid has been described previously (Liu et al., 2004).
EMT Collagen Matrix Assay
An ex vivo assay for EMT by migration into collagen matrix was carried out as adapted from Bernanke and Markwald (1982) as described previously (Potts et al., 1991; Camenisch et al., 2002). OFT regions of E9.5 mouse hearts were dissected and cut longitudinally to expose the endocardium, which was placed on drained collagen gels (1mg/ml, rat type I collagen, BD bioscience) that were saturated with Opti-MEM (Gibco/BRL) supplemented with 1% heat-inactivated fetal calf serum (HyClone), 100 U/ml penicillin, 100 μg/ml streptomycin (Cellgro), and 1% each of insulin, transferrin, and selenium (Gibco BRL). The tissues were cultured on the collagen gel at 37°C in 5% CO2 for 48 h, and migration of mesenchymal cells away from the site of the explanted tissue was measured and photographed.
Acknowledgments
We thank Todd Camenisch for providing probes and advice and Pooja Agarwal and Anabel Rojas for assistance with in situ hybridization and for helpful advice. DJM was supported by training grant T32 HD007162-26 from the NIH. JK was supported in part by a postdoctoral fellowship from the American Heart Association, Western States Affiliate. This work was supported by grant HL64658 from the NIH to BLB.
Grant Sponsors: NIH; Grant numbers HL64658; HD12324.
References
- Abdelwahid E, Rice D, Pelliniemi LJ, Jokinen E. Overlapping and differential localization of Bmp-2, Bmp-4, Msx-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 2001;305:67–78. doi: 10.1007/s004410100399. [DOI] [PubMed] [Google Scholar]
- Abu-Issa R, Kirby ML. Heart field: from mesoderm to heart tube. Annu Rev Cell Dev Biol. 2007;23:45–68. doi: 10.1146/annurev.cellbio.23.090506.123331. [DOI] [PubMed] [Google Scholar]
- Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, Klein WH, Martin JF. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104:9319–9324. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen SP, Bogardi JP, Barlow AJ, Mir SA, Qayyum SR, Verbeek FJ, Anderson RH, Francis-West PH, Brown NA, Richardson MK. Misexpression of noggin leads to septal defects in the outflow tract of the chick heart. Dev Biol. 2001;235:98–109. doi: 10.1006/dbio.2001.0291. [DOI] [PubMed] [Google Scholar]
- Armstrong EJ, Bischoff J. Heart valve development: endothelial cell signaling and differentiation. Circ Res. 2004;95:459–470. doi: 10.1161/01.RES.0000141146.95728.da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black BL. Transcriptional pathways in second heart field development. Semin Cell Dev Biol. 2007;18:67–76. doi: 10.1016/j.semcdb.2007.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand T. Heart development: molecular insights into cardiac specification and early morphogenesis. Dev Biol. 2003;258:1–19. doi: 10.1016/s0012-1606(03)00112-x. [DOI] [PubMed] [Google Scholar]
- Brown CB, Baldwin HS. Neural crest contribution to the cardiovascular system. Adv Exp Med Biol. 2006;589:134–154. doi: 10.1007/978-0-387-46954-6_8. [DOI] [PubMed] [Google Scholar]
- Buckingham M, Meilhac S, Zaffran S. Building the mammalian heart from two sources of myocardial cells. Nat Rev Genet. 2005;6:826–835. doi: 10.1038/nrg1710. [DOI] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenberger-de Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- Chen JN, van Eeden FJ, Warren KS, Chin A, Nusslein-Volhard C, Haffter P, Fishman MC. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. doi: 10.1242/dev.124.21.4373. [DOI] [PubMed] [Google Scholar]
- Delot EC. Control of endocardial cushion and cardiac valve maturation by BMP signaling pathways. Mol Genet Metab. 2003;80:27–35. doi: 10.1016/j.ymgme.2003.07.004. [DOI] [PubMed] [Google Scholar]
- Delot EC, Bahamonde ME, Zhao M, Lyons KM. BMP signaling is required for septation of the outflow tract of the mammalian heart. Development. 2003;130:209–220. doi: 10.1242/dev.00181. [DOI] [PubMed] [Google Scholar]
- Derynck R. TGF-beta-receptor-mediated signaling. Trends Biochem Sci. 1994;19:548–553. doi: 10.1016/0968-0004(94)90059-0. [DOI] [PubMed] [Google Scholar]
- Dodou E, Verzi MP, Anderson JP, Xu SM, Black BL. Mef2c is a direct transcriptional target of ISL1 and GATA factors in the anterior heart field during mouse embryonic development. Development. 2004;131:3931–3942. doi: 10.1242/dev.01256. [DOI] [PubMed] [Google Scholar]
- Frasch M. Induction of visceral and cardiac mesoderm by ectodermal Dpp in the early Drosophila embryo. Nature. 1995;374:464–467. doi: 10.1038/374464a0. [DOI] [PubMed] [Google Scholar]
- Gaussin V, Morley GE, Cox L, Zwijsen A, Vance KM, Emile L, Tian Y, Liu J, Hong C, Myers D, Conway SJ, Depre C, Mishina Y, Behringer RR, Hanks MC, Schneider MD, Huylebroeck D, Fishman GI, Burch JB, Vatner SF. Alk3/Bmpr1a receptor is required for development of the atrioventricular canal into valves and annulus fibrosus. Circ Res. 2005;97:219–226. doi: 10.1161/01.RES.0000177862.85474.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton RB, Jr, Lincoln J, Deutsch GH, Osinska H, Manning PB, Benson DW, Yutzey KE. Extracellular matrix remodeling and organization in developing and diseased aortic valves. Circ Res. 2006;98:1431–1438. doi: 10.1161/01.RES.0000224114.65109.4e. [DOI] [PubMed] [Google Scholar]
- Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- Hutson MR, Kirby ML. Model systems for the study of heart development and disease. Cardiac neural crest and conotruncal malformations. Semin Cell Dev Biol. 2007;18:101–110. doi: 10.1016/j.semcdb.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones ML. Connective tissues and stains. In: Bancroft JD, Gamble M, editors. Theory and practice of histological techniques. London: Churchill Livingstone; 2002. pp. 139–162. [Google Scholar]
- Kaartinen V, Dudas M, Nagy A, Sridurongrit S, Lu MM, Epstein JA. Cardiac outflow tract defects in mice lacking ALK2 in neural crest cells. Development. 2004;131:3481–3490. doi: 10.1242/dev.01214. [DOI] [PubMed] [Google Scholar]
- Kelly RG, Brown NA, Buckingham ME. The arterial pole of the mouse heart forms from Fgf10-expressing cells in pharyngeal mesoderm. Dev Cell. 2001;1:435–440. doi: 10.1016/s1534-5807(01)00040-5. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Meyers EN, Martin GR. Analysis of Fgf8 gene function in vertebrate development. Cold Spring Harb Symp Quant Biol. 1997;62:159–168. [PubMed] [Google Scholar]
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci U S A. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- Ma L, Lu MF, Schwartz RJ, Martin JF. Bmp2 is essential for cardiac cushion epithelial-mesenchymal transition and myocardial patterning. Development. 2005;132:5601–5611. doi: 10.1242/dev.02156. [DOI] [PubMed] [Google Scholar]
- Massague J, Attisano L, Wrana JL. The TGF-beta family and its composite receptors. Trends Cell Biol. 1994;4:172–178. doi: 10.1016/0962-8924(94)90202-x. [DOI] [PubMed] [Google Scholar]
- McDonald JA, Camenisch TD. Hyaluronan: genetic insights into the complex biology of a simple polysaccharide. Glycoconj J. 2002;19:331–339. doi: 10.1023/A:1025369004783. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Nakaoka T, Moreno-Rodriguez R, Norris RA, Kern MJ, Eisenberg CA, Turner D, Markwald RR. The outflow tract of the heart is recruited from a novel heart-forming field. Dev Biol. 2001;238:97–109. doi: 10.1006/dbio.2001.0409. [DOI] [PubMed] [Google Scholar]
- Nakajima Y, Yamagishi T, Hokari S, Nakamura H. Mechanisms involved in valvuloseptal endocardial cushion formation in early cardiogenesis: roles of transforming growth factor (TGF)-beta and bone morphogenetic protein (BMP) Anat Rec. 2000;258:119–127. doi: 10.1002/(SICI)1097-0185(20000201)258:2<119::AID-AR1>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Park EJ, Ogden LA, Talbot A, Evans S, Cai CL, Black BL, Frank DU, Moon AM. Required, tissue-specific roles for Fgf8 in outflow tract formation and remodeling. Development. 2006;133:2419–2433. doi: 10.1242/dev.02367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK. Pediatric cardiology for practitioners. St Louis, MO: Mosby Elsevier; 2002. Cyanotic congenital heart defects; pp. 174–240. [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell biology of cardiac cushion development. Int Rev Cytol. 2005;243:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Potts JD, Dagle JM, Walder JA, Weeks DL, Runyan RB. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991;88:1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsdell AF, Yost HJ. Cardiac looping and the vertebrate left-right axis: antagonism of left-sided Vg1 activity by a right-sided ALK2-dependent BMP pathway. Development. 1999;126:5195–5205. doi: 10.1242/dev.126.23.5195. [DOI] [PubMed] [Google Scholar]
- Rojas A, De Val S, Heidt AB, Xu SM, Bristow J, Black BL. Gata4 expression in lateral mesoderm is downstream of BMP4 and is activated directly by Forkhead and GATA transcription factors through a distal enhancer element. Development. 2005;132:3405–3417. doi: 10.1242/dev.01913. [DOI] [PubMed] [Google Scholar]
- Schneider MD, Gaussin V, Lyons KM. Tempting fate: BMP signals for cardiac morphogenesis. Cytokine Growth Factor Rev. 2003;14:1–4. doi: 10.1016/s1359-6101(02)00053-9. [DOI] [PubMed] [Google Scholar]
- Schultheiss TM, Burch JB, Lassar AB. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- Shi Y, Katsev S, Cai C, Evans S. BMP signaling is required for heart formation in vertebrates. Dev Biol. 2000;224:226–237. doi: 10.1006/dbio.2000.9802. [DOI] [PubMed] [Google Scholar]
- Song L, Fassler R, Mishina Y, Jiao K, Baldwin HS. Essential functions of Alk3 during AV cushion morphogenesis in mouse embryonic hearts. Dev Biol. 2007;301:276–286. doi: 10.1016/j.ydbio.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srivastava D. Making or breaking the heart: from lineage determination to morphogenesis. Cell. 2006;126:1037–1048. doi: 10.1016/j.cell.2006.09.003. [DOI] [PubMed] [Google Scholar]
- Stottmann RW, Choi M, Mishina Y, Meyers EN, Klingensmith J. BMP receptor IA is required in mammalian neural crest cells for development of the cardiac outflow tract and ventricular myocardium. Development. 2004;131:2205–2218. doi: 10.1242/dev.01086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269:505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- Verzi MP, McCulley DJ, De Val S, Dodou E, Black BL. The right ventricle, outflow tract, and ventricular septum comprise a restricted expression domain within the secondary/anterior heart field. Dev Biol. 2005;287:134–145. doi: 10.1016/j.ydbio.2005.08.041. [DOI] [PubMed] [Google Scholar]
- Waldo K, Miyagawa-Tomita S, Kumiski D, Kirby ML. Cardiac neural crest cells provide new insight into septation of the cardiac outflow tract: aortic sac to ventricular septal closure. Dev Biol. 1998;196:129–144. doi: 10.1006/dbio.1998.8860. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Hutson MR, Ward CC, Zdanowicz M, Stadt HA, Kumiski D, Abu-Issa R, Kirby ML. Secondary heart field contributes myocardium and smooth muscle to the arterial pole of the developing heart. Dev Biol. 2005;281:78–90. doi: 10.1016/j.ydbio.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Waldo KL, Kumiski DH, Wallis KT, Stadt HA, Hutson MR, Platt DH, Kirby ML. Conotruncal myocardium arises from a secondary heart field. Development. 2001;128:3179–3188. doi: 10.1242/dev.128.16.3179. [DOI] [PubMed] [Google Scholar]
- Winnier G, Blessing M, Labosky PA, Hogan BL. Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev. 1995;9:2105–2116. doi: 10.1101/gad.9.17.2105. [DOI] [PubMed] [Google Scholar]
- Yamagishi T, Nakajima Y, Miyazono K, Nakamura H. Bone morphogenetic protein-2 acts synergistically with transforming growth factor-beta3 during endothelial-mesenchymal transformation in the developing chick heart. J Cell Physiol. 1999;180:35–45. doi: 10.1002/(SICI)1097-4652(199907)180:1<35::AID-JCP4>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]


