Abstract
Since melatonin production has been documented in extrapineal and extraneuronal tissues, we investigated the expression of molecular elements of the melatoninergic system in human RPE cells (ARPE-19). The expression of key enzymes for melatonin synthesis: tryptophan hydroxylases (TPH1 and TPH2); arylalkylamine N-acetyltransferase (AANAT) and hydroxyindole-O-methyltransferase (HIOMT)was detected in ARPE-19 cells using RT-PCR.TPH1 and AANAT proteins were detected in ARPE by Western blotting, while sequential metabolism of tryptophan, serotonin and N-acetylserotonin to melatonin was shown by RPHPLC. We also demonstrated, by means of RT-PCR, that ARPE expressed mRNA encoding the melatonin receptors: MT2 (but not MT1), two isoforms of nuclear receptor (RORα1 and RORα4/RZR1), and quinone oxidoreductase (NQO2). By analogy with other peripheral tissues, for example the skin, the expression of these metabolic elements in RPE cells suggests that the RPE represents an additional source of melatonin in the eye, to regulate local homeostasis and prevent from oxidative damage in intra-, auto- and/or paracrine fashions.
Keywords: Melatonin, Retinal pigment epithelium, HIOMT, AANAT, Tryptophan hydroxylase, Melatonin receptor
1. Introduction
Melatonin (N-acetyl-5-methoxytryptamine) is detected not only in vertebrates and invertebrates, but also in plants, bacteria, unicellular eukaryotes and algae (reviewed in Tan et al., 2003; Leon et al., 2004). In mammals, melatonin is produced in the pineal gland (Reiter, 1991) and in extracranial sites including the gastrointestinal tract, the retina, the immune system, the ovaries (Reiter, 1991, 2003; Yu and Reiter, 1993; Tan et al., 2003) and the skin (Slominski et al., 1996, 2003a,b,c, 2008; Slominski et al., 2005a). In the eye, melatonin plays a significant role in the physiology and rhythmic activities of retina and non-neuronal ocular tissue, such as corneal epithelium and the retinal pigment epithelium (RPE) (Wiechmann and Summers, 2008). These diurnal rhythms have been demonstrated at all levels of ocular organization, ranging from fundamental molecular events to whole organ/system level processes. In most vertebrate species studied to date, including humans, the photoreceptor cells produce melatonin. In addition, melatonin synthesis has also been shown in ciliary epithelial cells (Martin et al., 1992). It is synthesized and released predominantly at night (Pang et al., 1980; Yu et al., 1981). The nocturnal rise in melatonin levels appears to provide a paracrine circadian signal to various retinal cells to modulate their function. Melatonin is involved in rhythmic functions of the retina, such as photoreceptor outer segment disc shedding (Besharse and Dunis, 1983; White and Fisher, 1989; Strauss, 2005), photomechanical movements (Pierce and Besharse, 1985), modulation of neurotransmitter release (Dubocovich, 1983; Boatright et al., 1994), circadian changes in intra-ocular pressure (Pintor et al., 2001) and sensitivity to light (Wiechmann et al., 1988).
Melatonin acts as a hormone, neurotransmitter, biological modifier, immunomodulator and antioxidant (Yu and Reiter, 1993); in addition it also functions as an oncostatic molecule (Gupta et al., 1988; Lissoni et al., 1989, 1996, 2002; Cos and Sanchez-Barcelo, 2000). Furthermore, the fundamental role of melatonin in the protection of the cell from external and internal stresses, including high-energy ultraviolet wavelengths of solar radiation, and in maintenance of cellular homeostasis has been extensively studied over the last decade (Yu and Reiter, 1993; Reiter, 1996; Karbownik et al., 2001; Tan et al., 2003; Leon et al., 2004; Rodriguez et al., 2004). A protective effect of melatonin against UV radiation has already been documented in human keratinocytes, dermal fibroblasts, leukocytes and in the rat lens (Bardak et al., 2000; Nickel and Wohlrab, 2000; Fischer et al., 2001, 2002, 2004; Kim et al., 2001; Ryoo et al., 2001; Lee et al., 2003).
Melatonin is the product of a multistep metabolic pathway that starts with the hydroxylation of l-tryptophan by tryptophan hydroxylase (TPH, EC 1.14.16.4). Decarboxylation of hydroxytryptophan by aromatic amino acid decarboxylase (AAD, EC 4.1.1.28) generates serotonin, which can act as a neurotransmitter, besides its actions as a regulator of vascular tone, immunomodulator, growth factor; and a precursor for melatonin. In this metabolic pathway, acetylation of serotonin catalyzed by arylalkylamine N-acetyltransferase (AANAT, EC 2.3.1.87) generates N-acetylserotonin (NAS), which is further methylated by hydroxyindole-O-methyltransferase (HIOMT, EC 2.1.1.4) (Slominski et al., 2008).
Melatonin can act through membrane bound receptors: MTNR1A(MT1) andMTNR1B(MT2); nuclear orphan receptors from the RORα/RZRα family (Becker-Andre et al., 1994; Wiesenberg et al., 1998; Dubocovich et al., 2003). In addition, it binds to quinone reductase type 2 NQO2 (previously described as MT3) (Tan et al., 2007). Immunoreactivity characteristic for the MT2 receptor was observed in non-neuronal ocular tissues, such as the lens epithelium, and in cells located in the sclera and the apical microvillar cell membrane, but not in the basement membrane of the retinal pigment epithelium (RPE) cells (Wiechmannand Summers, 2008). This is particularly interesting because melatonin has a hypothesized role in photoreceptor outer segment disk shedding and phagocytosis (LaVail, 1976; Young and Bok, 1969; Ogino et al., 1983). It has been suggested that melatonin protects the photoreceptor outer segment of membranes from photooxidative stress (Marchiafava and Longoni, 1999; Siu et al., 1999) and counteracts ischemic injury to RPE cells (Ogino et al., 1983).
Thus, local synthesis of melatonin has obvious clinical implications as it may help to elucidate the pathogenesis of a number of disorders of the eye, including keratopathies, corneal healing, age-related macular degeneration (AMD), and UVB/light induced pathologies.
Here, using human adult RPE-19 cells as a model, we report the concomitant expression of fully functional enzymatic machinery for the local production of melatonin, together with specific melatonin receptors and melatonin binding proteins.
2. Materials and methods
2.1. Cell culture
The RPE cell line ARPE-19was cultivated in Dulbecco’s modified Eagles medium (DMEM, GIBCO, Invitrogen Corp., Carlsbad, CA) supplemented with 5% fetal bovine serum, insulin (50µg/mL), and an antibiotic-antimycotic solution (PSA, Sigma, St. Louis, MO) at 37 °C in an atmosphere of 95% air and 5% CO2. Culture passages (p) 24–37 were used in the experiments. Cells were plated at 250,000 cells/cm2 on 75cm2 flasks and allowed to become confluent. The cells were detached from the flask by trypsinization (0.05% trypsin/EDTA for 5 min),washed with PBS (pH 7.4) and used for Western blotting; or total RNA preparation or stored at −80 °C (see below).
2.2. cDNA preparation and RT-PCR assays
Total RNA isolation and cDNA preparation were performed as described previously (Zmijewski et al., 2007). Briefly, RNA was isolated using a total RNA extraction kit (Qiagen, Valencia, CA) supplemented with RNAse-free DNAse Set (Qiagen, Valencia, CA) and reverse transcribed with SuperScript First-Strand Synthesis System (Applied Biosystems, Foster City, CA). Quality and quantity of all samples were standardized by the amplification of the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and 18S rRNA subunit, as described previously (Pisarchik and Slominski, 2001; Slominski et al., 2005a,b,c). PCR reactions (30–35 cycles) were carried out using PCR Master Mix (Promega, Madison, WI) and 0.4mM of each primer. Primers used for PCR amplification were synthesized by Integrated DNA Technology Inc. (Coralville, IA) and were described previously (Slominski et al., 2002a, 2005a,b,c; Fischer et al., 2006), except of the primers set for TPH2 (Accession number: AY098914) detections. The primers MZ138 (forward) 5′-GGCTCTTTCAGGAAAAACGTG-3′ and MZ139 (reverse) 5′-GTCCTTAAATCCTGGGTGGTC-3′ covered the junctions of exons 2–3 and 4–5, respectively. RT-PCR corresponding with the fragment of 308 bp was sequenced to confirm proper detection of TPH2. Products of amplification were separated by agarose gel electrophoresis, visualized by ethidium bromide staining and analyzed with QuantityOne software (Bio-Rad Laboratories, Hercules, CA).
2.3. Western blotting
Whole cell lysates of ARPE-19 cells were prepared as described above, and protein concentrations measured by BCA reagent (Rockford, IL). Cellular homogenates were centrifuged at 16,000 ×g for 10 min at 4 °C and the supernatants used immediately for assays, or stored at −80 °C. Fifty micrograms of proteins were loaded on 12% SDS-PAGE, transferred to immobilion-P poly(vinylidene difluoride) membranes (Millipore Corp, Bedford, MA) for 1.5 h at 4 °C and blocked overnight at cold room in 5% non-fat powdered milk in TBST (50 mMTris, pH 7.5, 150 mMNaCl, 0.01% Tween-20). Immunodetection of the proteins was performed after a 3-h incubation with sheep anti-TPH1 antibodies (dilution 1:300, Chemicon, Temecula, CA) or rabbit anti-AANAT1–26 (1:5000, gift of Dr. Klein, NIH, MD). The membranes were washed once with 5% non-fat, drymilk in TBST, twice in TBST (10 min each time) and then incubated for 1 h with appropriate secondary antibodies coupled to horseradish peroxidase (anti-sheep 1:2000 or anti-rabbit 1:5000; both from Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed twice in TBST and once in TBS. Bands were visualized by Super Signal West Pico according to the manufacturer’s instructions (Pierce, Rockford, IL).
2.4. Melatonin synthesis in ARPE cells measured by reverse phase high performance liquid chromatography (RP-HPLC)
Human ARPE-19 cells (passage 25) were seeded in 6-well plates and incubated with melatonin precursors: tryptophan, serotonin or N-acetylserotonin for 6 or 24 h in DMEM medium supplemented with 5% charcoal-stripped serum and 1% of PSA. Melatonin and other indolic compounds were extracted (twice) from separated cells and media using methylene chloride and extracts dried under nitrogen. These samples were dissolved in methanol and subjected to RP-HPLC analysis using an HPLC system equipped with an Atlantis column (C18), a mobile phase of methanol and water, and a fluorometric detector (all equipment Waters Associates, Milford, MA). The characteristic fluorescence parameters for indolic compounds (285 nm excitation; 360 nm emission) were used for the detection. Metabolites were identified based upon their retention time relative to standard synthetic compounds subjected to the same analysis. These standards were tryptophan, 5-hydroxytryptophan, serotonin (5-hydroxytryptamine), N-acetylserotonin (5-hydroxy-N-acetyltryptamine), melatonin (5-methoxy-N-acetyltryptamine), N-acetyltryptamine, 6-OH melatonin, 5-methoxytryptophol, and 5-methoxyindole acetic acid (Sigma–Aldrich, St. Louis, MO).
3. Results and discussion
3.1. Expression of the functional melatoninergic system in ARPE-19 cells
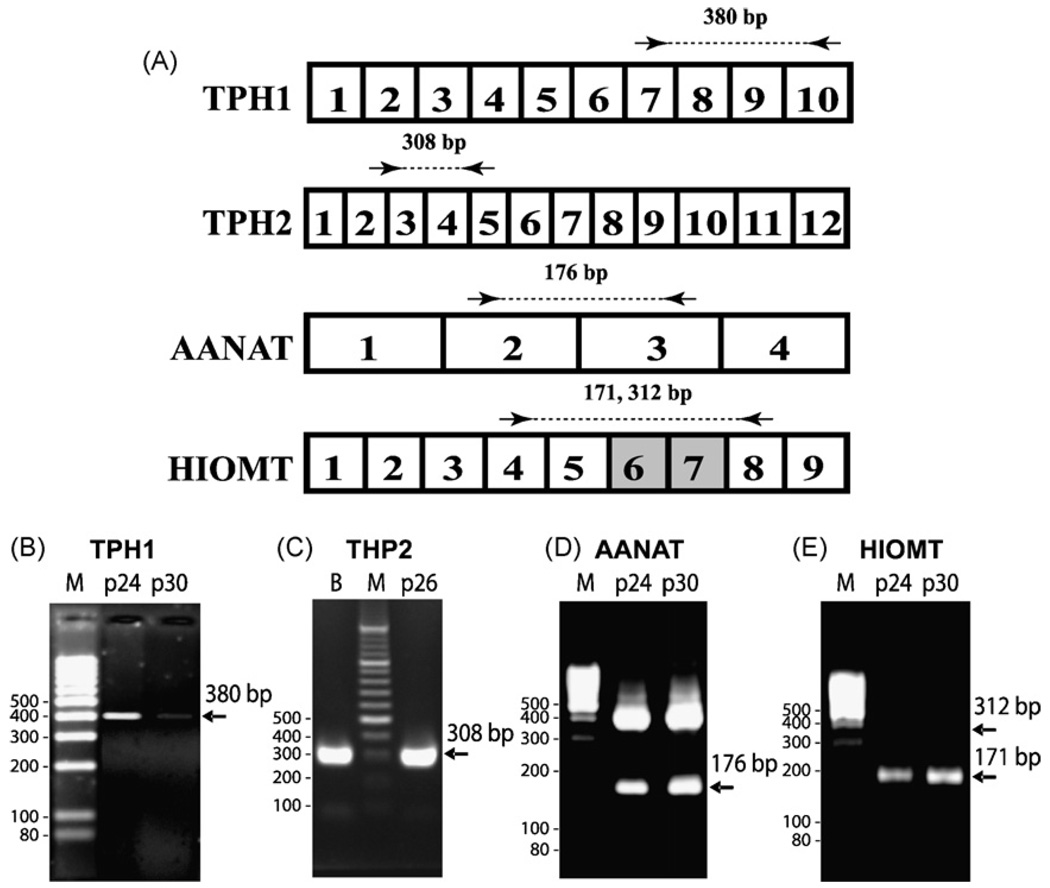
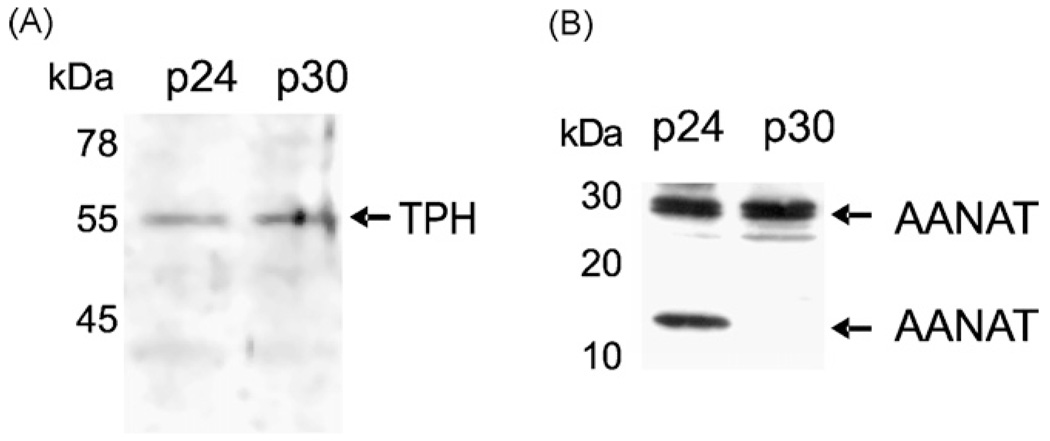
The presence of characteristic transcripts for tryptophan hydroxylases type 1 and 2 (TPH1 and TPH2), AANAT and HIOMT were detected in the RPE cell line (ARPE-19; derived from an adult human donor) by RT-PCR. The RT-PCR product of 380 bp corresponding to a predicted fragment spanning exons 7–10 of TPH1 (Slominski et al., 2002a,b)was detected in passages 24 and 30 of ARPE-19; its expression decreased in later passages (Fig. 1A and B). Detection of RT-PCR fragment of 308 bp spanning exons 3 and 4 of TPH2 (Fig. 1A and C) represents the first documentation that this gene is expressed in non-neuronal cells. As predicted for AANAT (exons 2–3 (Slominski et al., 2002a,b)) and HIOMT (exons 4–8 (Slominski et al., 2002a,b)), mRNA fragments of 176 and 171 bp, respectively, were detected in ARPE-19 cells (Fig. 1A,Dand E). Interestingly, the fragment of 171 bp is characteristic for a splicing variant of HIOMT, with the deletion of exons 6 and 7, as reported previously (Slominski et al., 2002a,b). Furthermore, using specific anti-TPH and anti-AANAT antibodies and Western blot analysis of cell extracts, we detected the expression of TPH1 and AANAT proteins with approximate molecular weights (MW) of 54 and 30 kDa, respectively (Fig. 2). An additional band of approximately 16 kDa detected in passage 24 might represent a splicing variant of AANAT (17.4 kDa) or posttranslational degradation of the full-length protein (Fig. 2B).
Fig. 1.
Expression of TPH1, TPH2, AANAT, and HIOMT genes in human retinal pigment epithelium (RPE) cells. (A) Exonal organization of genes. Coding exons are identified by numbers. Arrows represent PCR primers. Dashed line encompasses PCR fragments. (B) Tryptophan hydroxylase I (TPH1), (C) tryptophan hydroxylase II (TPH2), (D) arylalkylamine N-acetyltransferase (AANAT), (E) hydroxyindole-O-methyltransferase (HIOMT). RT-PCR lane M—DNA ladder; p24, p26, p31 indicate RPE passages, B—human brain cDNA. RT-PCR was performed as previously described (Slominski et al., 2002a,b) except for TPH2 (see Section 2 for details).
Fig. 2.
Detection of TPH (Panel A), AANAT (Panel B) immunoreactive proteins in human retinal pigment epithelium (RPE) cells. 50 µg of whole cell lysates of RPE passages 24 (p24) and 30 (p30), were resolved using SDS-PAGE. Immunodetection was performed using: sheep anti-TPH (1:300); rabbit anti-AANAT1–26 (1:5000) antibodies followed by an appropriate secondary antibody coupled to horseradish peroxidase (1:1000 or 1:5000, respectively). The presence of immunoprecipitates was visualized with Super Signal West Pico Chemiluminescent Substrate (Pierce). The chemiluminescent signal was acquired on a Fluor-S MultiImager and analyzed with Quantity One software (Bio-Rad).
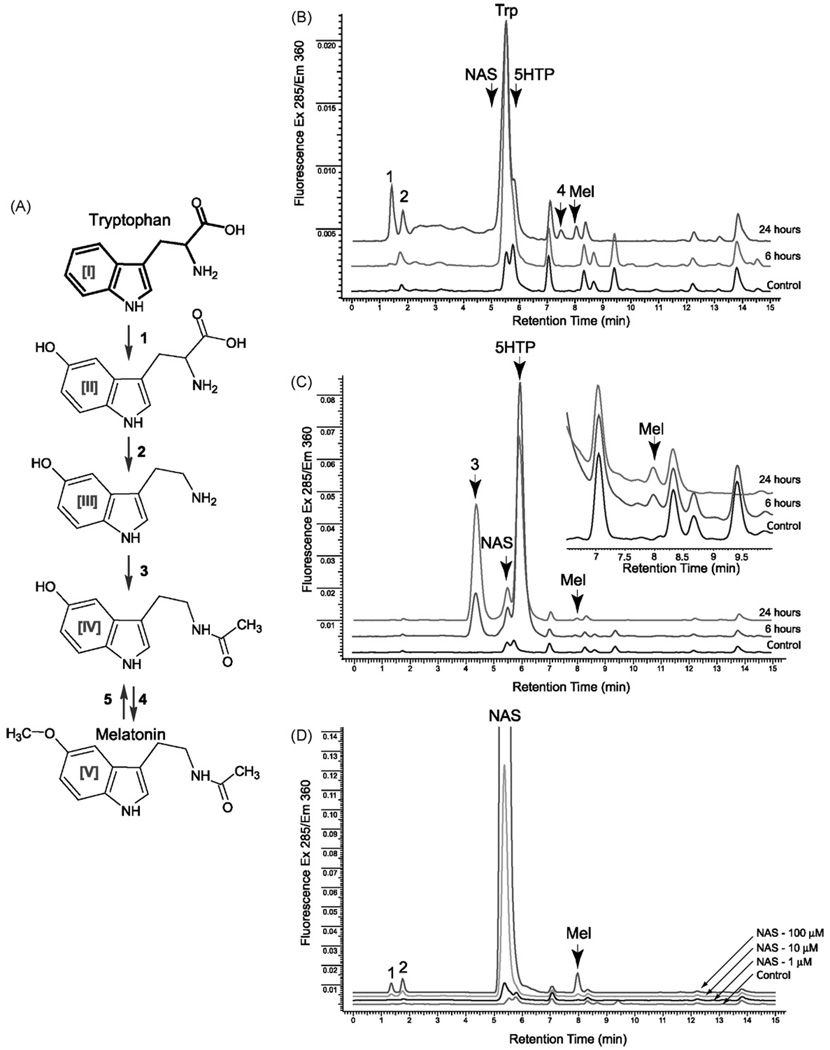
Having established the expression of molecular elements of the melatoninergic system in the ARPE-19 cells, we used a reverse phase HPLC (RP-HPLC) equipped with a fluorometric detector (285 nm excitation; 360 nm emission) to study the transformation of tryptophan to melatonin in cell culture (Fig. 3). The presence of indolic rings facilitated the monitoring of all steps in the production of melatonin from tryptophan (Fig. 3A). The substrates and products were identified by matching retention time with synthetic standards. The production of melatonin was undetectable in untreated ARPE cells (Fig. 3, control chromatograms), however, it became clearly evident when cultures were exposed to substrates of the pathway (Fig. 3). Specifically, when the ARPE-19 cells were incubated with l-tryptophan (100 µM)the characteristic peak for melatonin with retention time (RT) of 8 min was observed in samples incubated for 24 h (Fig. 5B). The detection of intermediates of melatonin synthesis was difficult, because the region between 5 and 7min contains at least 4 different indolic compounds including N-acetylserotonin (NAS, RT = 5.4 min), tryptophan (Trp, RT = 5.6 min), 5-hydroxytryptamine (5-HT, RT = 5.8 min), and 6-hydroxymelatonin (6.18 min). Nevertheless, a compound with RT = 5.8 min (assigned as serotonin) was clearly detected on the shoulder of the Trp peak after 24 h of culture in the presence of Trp. Subsequently, serotonin was used as a second precursor for melatonin and products of its metabolism were monitored in ARPE-19 cells after 6 and 24 h(Fig. 3C). Synthesis of melatonin from serotonin was found to be time-dependent, with highest production occurring after 24 h incubation. The production of a direct melatonin precursor N-acetylserotonin (NAS) was indicated by the detection of a peak with RT of 5.4 min corresponding to the NAS standard. An additional (main) product of serotonin metabolism was detected with a RT of 4.4 min (Peak 3); its relative amount increased with time. Finally, the production of melatonin from N-acetylserotonin was investigated (Fig. 3D). The production of melatonin was dose-and time-dependent, with maximum production observed using 100 µM of N-acetylserotonin after 24 h of incubation. Two additional uncharacterized products of metabolism with RT: 1.45 and 1.8 min were also detected in ARPE cells treated with tryptophan and N-acetylserotonin. Peak 4 (RT 7.4min) was characteristic for tryptophan metabolism, but this retention time did not correspond with any of the synthetic standards.
Fig. 3.
Metabolism of tryptophan, serotonin and N-acetylserotonin (NAS) in retinal pigment epithelium (RPE) cells. (A) The classical melatonin synthesis pathway starts with hydroxylation of tryptophan [I] by tryptophan-5-hydroxylase (1; TPH1 and TPH2) followed by decarboxylation of 5-hydroxytryptophan [II] by 5-HTP-decarboxylase or aromatic amino acids decarboxylase (AAD) (2) the product—serotonin [III] is acetylated by serotonin-N-acetyltransferase (3; AANAT, NAT-1). The final synthetic step is carried out by 4-hydroxyindole-O-methyl transferase (4; HIOMT) which converts N-acetylserotonin [IV] into melatonin [V] and the last step of the melatonin synthesis pleiotropic could be reverted by CYP450 (5; CYP2C19). The synthesis of melatonin in the human ARPE cells, passage 25, was studied by incubating cells with melatonin precursors Tryptophan (Trp) (B), Serotonin (5HTP) (C) or N-acetylserotonin (NAS) (D) for 0, 6 and 24 h. (B). Melatonin and other indolic compounds were extracted with methylene chloride and subjected to reverse phase HPLC analysis and fluorometric (285nm excitation; 360nm emission) detection, as described in Section 2. Metabolites were identified based on their retention times relative to the synthetic standards. The unknown products are shown as Peaks 1–4.
These results demonstrate for the first time that human RPE cells not only express the molecular apparatus governing transformation of tryptophan to melatonin, but that they can also produce melatonin using tryptophan, serotonin or N-acetylserotonin as substrates. Thus, the melatonin synthesis pathway is conserved in pigment cells that are either of neural crest origin (normal and malignant melanocytes), or derive from neuroectoderm of the optic cup, e.g., RPE. Furthermore, the above data indicates a redundancy for intra-ocular melatonin synthesis, with production by RPE in addition to the well-documented synthesis by the retina (Iuvone et al., 2005; Lundmark et al., 2006).
3.2. Expression of the receptors for melatonin in ARPE-19
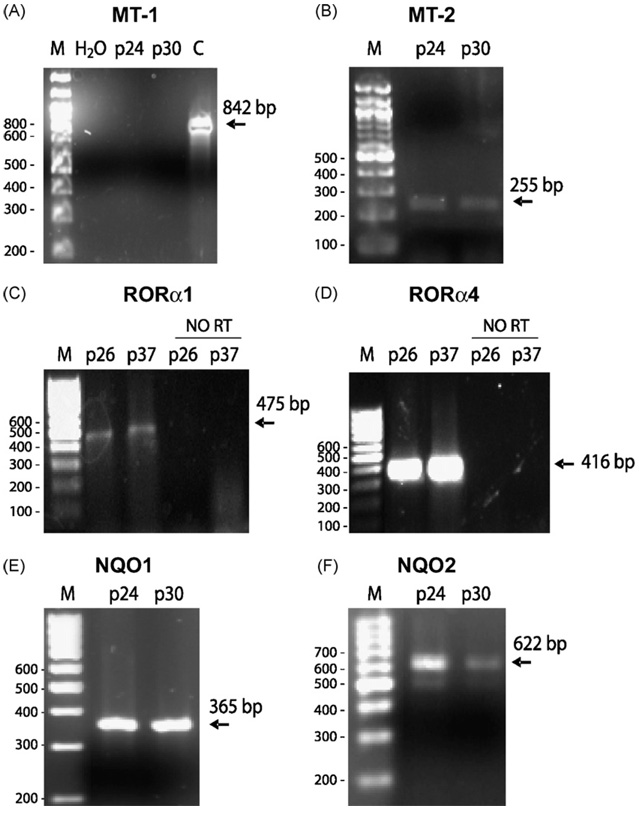
RT-PCR analysis revealed that ARPE-19 expressed melatonin receptor type 2 (MT2, Fig. 4B), but not type 1 (MT1) gene (Fig. 4A). This finding is in agreement with detection of MT2 immunoreactivity in RPE (Wiechmann et al., 2004, 2008). Furthermore, expression of mRNAs corresponding to two isoforms of nuclear receptor (RORα1 and RORα4/RZR1) was detected in passages p26 and p37 (Fig. 4C and D). The RT-PCR fragments of 416 and 475 bp corresponded to RORα1 and RORα4/RZR1, respectively (Pozo et al., 2004; Fischer et al., 2006). The expression of other splicing variants RORα2 and RORα3 was not detected (not shown). The melatonin binding quinone reductase type 2 (NQO2), previously described as melatonin receptor type 3, was also detected in human ARPE-19 cells (Fig. 4F), with relatively similar level of expression to quinone reductase type 1 (Fig. 4E). RT-PCR analysis showed that expression of NQO2 decreased with age of culture (passage 24 versus 30) but that the characteristic band of 622 bp (Fischer et al., 2006) was still detectable in the latter passage (Fig. 4F), whereas expression of NQO1 remained unchanged (Fig. 4E). These data suggest multiple mechanisms for the well-documented sensitivity of RPE to melatonin (Wiechmann et al., 2004, 2008; Strauss, 2005), e.g., interactions of membrane bound MT2, with at least two isoforms of nuclear receptor RORα1 and RORα4/RZR1 and non-receptor mediated modulation of NQO2 activity with subsequent attenuation of oxidative stress.
Fig. 4.
RT-PCR detection of melatonin receptors and binding proteins in ARPE-19. mRNA of APRE cells passages p24 and p30 (Panels A, B, E and F) or p26 and p37 (Panels C and D) were used to study the expression of MT-1 (A) or MT-2 (B) membrane bound receptors; alternative splicing variants of melatonin nuclear receptor: RORα1 (C); RORα4 (RZRα) (D); quinone reductases NQO1 (Panel E, not binding) and NQO2 (Panel F, previously described as melatonin receptor MT-3). M—DNA ladder; p24, p26, p30, p37 indicating RPE passages; C—control, human brain cDNA. In Panel A, water and melanomaWM164 were used as negative and positive controls, respectively. RT reactions performed using the same amount of RNA without reverse transcriptase, followed by standard PCR (Panels C and D) were used as additional negative controls.
Thus, the detection of genes coding receptor or enzyme targets for melatonin action suggests that the melatonin synthesis in human retinal pigment epithelial can be utilized locally in an intra-, auto- or paracrine fashions. This is consistent with similar observations made in normal skin melanocytes and melanoma cells (Slominski et al., 2002a,b, 2003a,b,c, 2004). It is well established that the retina produces melatonin for intra-ocular use (Ivanova and Iuvone, 2003; Iuvone et al., 2005). As reported above, the RPE cells represent a second source of melatonin, which may also be involved in the regulation of surrounding cells, including cones and rods. In addition, the antioxidant properties of melatonin and it’s metabolites can protect pigmented cells from oxidative stress associated with melanin synthesis (Slominski et al., 2004).
In conclusion, based on a ARPE-19 model, we hypothesize that that RPE represents an additional (redundant) source for melatonin in the eye to regulate local homeostasis in an intra-, auto- and/or paracrine fashion that is analogous to other peripheral tissues, such as skin, exposed to environmental stress.
Acknowledgments
The authors would like to thank Dr Rajesh K. Sharma for providing the ARPE-19 cell line and for his helpful suggestions. Supported in part by grant # AR052190 from NIH/NIAMS to AS.
References
- Bardak Y, Ozerturk Y, Ozguner F, Durmus M, Delibas N. Effect of melatonin against oxidative stress in ultraviolet-B exposed rat lens. Curr. Eye Res. 2000;20:225–230. [PubMed] [Google Scholar]
- Becker-Andre M, Wiesenberg I, Schaeren-Wiemers N, Andre E, Missbach M, Saurat JH, Carlberg C. Pineal gland hormone melatonin binds and activates an orphan of the nuclear receptor superfamily. J. Biol. Chem. 1994;269:28531–28534. [PubMed] [Google Scholar]
- Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: activation of rod shedding. Science. 1983;219:1341–1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- Boatright JH, Rubim NM, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis. Neurosci. 1994;11:1013–1018. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- Cos S, Sanchez-Barcelo EJ. Melatonin and mammary pathological growth. Front. Neuroendocrinol. 2000;21:133–170. doi: 10.1006/frne.1999.0194. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- Dubocovich ML, Rivera-Bermudez MA, Gerdin MJ, Masana MI. Molecular pharmacology, regulation and function of mammalian melatonin receptors. Front. Biosci. 2003;8:d1093–d1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Scholz G, Knoll B, Hipler UC, Elsner P. Melatonin reduces UV-induced reactive oxygen species in a dose-dependent manner in IL-3-stimulated leukocytes. J. Pineal Res. 2001;31:39–45. doi: 10.1034/j.1600-079x.2001.310106.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Scholz G, Knoll B, Hipler UC, Elsner P. Melatonin suppresses reactive oxygen species in UV-irradiated leukocytes more than vitamin C and trolox. Skin Pharmacol. Appl. Skin Physiol. 2002;15:367–373. doi: 10.1159/000064543. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Scholz G, Knoll B, Hipler UC, Elsner P. Melatonin suppresses reactive oxygen species induced by UV irradiation in leukocytes. J. Pineal Res. 2004;37:107–112. doi: 10.1111/j.1600-079X.2004.00142.x. [DOI] [PubMed] [Google Scholar]
- Fischer TW, Zmijewski MA, Zbytek B, Sweatman TW, Slominski RM, Wortsman J, Slominski A. Oncostatic effects of the indole melatonin and expression of its cytosolic and nuclear receptors in cultured human melanoma cell lines. Int. J. Oncol. 2006;29:665–672. doi: 10.3892/ijo.29.3.665. [DOI] [PubMed] [Google Scholar]
- Gupta D, Atanasio A, Reiter RJ. The Pineal Gland and Cancer. Oxford: Brain Research Promotion; 1988. [Google Scholar]
- Iuvone PM, Tosini G, Pozdeyev N, Haque R, Klein DC, Chaurasia SS. Circadian clocks, clock networks, arylalkylamine N-acetyltransferase, and melatonin in the retina. Prog. Retin. Eye Res. 2005;24:433–456. doi: 10.1016/j.preteyeres.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Ivanova TN, Iuvone PM. Melatonin synthesis in retina: circadian regulation of arylalkylamine N-acetyltransferase activity in cultured photoreceptor cells of embryonic chicken retina. Brain Res. 2003;973:56–63. doi: 10.1016/s0006-8993(03)02540-x. [DOI] [PubMed] [Google Scholar]
- Karbownik M, Lewinski A, Reiter RJ. Anticarcinogenic actions of melatonin which involve antioxidative processes: comparison with other antioxidants. Int. J. Biochem. Cell Biol. 2001;33:735–753. doi: 10.1016/s1357-2725(01)00059-0. [DOI] [PubMed] [Google Scholar]
- Kim BC, Shon BS, Ryoo YW, Kim SP, Lee KS. Melatonin reduces X-ray irradiation-induced oxidative damages in cultured human skin fibroblasts. J. Dermatol. Sci. 2001;26:194–200. doi: 10.1016/s0923-1811(01)00088-3. [DOI] [PubMed] [Google Scholar]
- LaVail MM. Rod outer segment disk shedding in rat retina: relationship to cyclic lighting. Science. 1976;194:1071–1074. doi: 10.1126/science.982063. [DOI] [PubMed] [Google Scholar]
- Lee CK, Moon DH, Shin CS, Kim H, Yoon YD, Kang HS, Lee BJ, Kang SG. Circadian expression of Mel1a and PL-II genes in placenta: effects of melatonin on the PL-II gene expression in the rat placenta. Mol. Cell. Endocrinol. 2003;200:57–66. doi: 10.1016/s0303-7207(02)00414-8. [DOI] [PubMed] [Google Scholar]
- Leon J, Acuna-Castroviejo D, Sainz RM, Mayo JC, Tan DX, Reiter RJ. Melatonin and mitochondrial function. Life Sci. 2004;75:765–790. doi: 10.1016/j.lfs.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Barni S, Crispino S, Tancini G, Fraschini F. Endocrine and immune effects of melatonin therapy in metastatic cancer patients. Eur. J. Cancer Clin. Oncol. 1989;25:789–795. doi: 10.1016/0277-5379(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Brivio O, Brivio F, Barni S, Tancini G, Crippa D, Meregalli S. Adjuvant therapy with the pineal hormone melatonin in patients with lymph node relapse due to malignant melanoma. J. Pineal Res. 1996;21:239–242. doi: 10.1111/j.1600-079x.1996.tb00292.x. [DOI] [PubMed] [Google Scholar]
- Lissoni P, Vaghi M, Ardizzoia A, Malugani F, Fumagalli E, Bordin V, Fumagalli L, Bordoni A, Mengo S, Gardani GS, Tancini G. A phase II study of chemoneuroimmunotherapy with platinum, subcutaneous low-dose interleukin-2 and the pineal neurohormone melatonin (P. I. M.) as a second-line therapy in metastatic melanoma patients progressing on dacarbazine plus interferon-alpha. In Vivo. 2002;16:93–96. [PubMed] [Google Scholar]
- Lundmark PO, Pandi-Perumal SR, Srinivasan V, Cardinali DP. Role of melatonin in the eye and ocular dysfunctions. Vis. Neurosci. 2006;23:853–862. doi: 10.1017/S0952523806230189. [DOI] [PubMed] [Google Scholar]
- Marchiafava PL, Longoni B. Melatonin as an antioxidant in retinal photoreceptors. J. Pineal Res. 1999;26:184–189. doi: 10.1111/j.1600-079x.1999.tb00582.x. [DOI] [PubMed] [Google Scholar]
- Martin XD, Malina HZ, Brennan MC, Hendrickson PH, Lichter PR. The ciliary body—the third organ found to synthesize indoleamines in humans. Eur. J. Ophthalmol. 1992;2:67–72. doi: 10.1177/112067219200200203. [DOI] [PubMed] [Google Scholar]
- Nickel A, Wohlrab W. Melatonin protects human keratinocytes from UVB irradiation by light absorption. Arch. Dermatol. Res. 2000;292:366–368. doi: 10.1007/s004030000141. [DOI] [PubMed] [Google Scholar]
- Ogino N, Matsumura M, Shirakawa H, Tsukahara I. Phagocytic activity of cultured retinal pigment epithelial cells from chick embryo: inhibition by melatonin and cyclic AMP, and its reversal by taurine and cyclic GMP. Ophthalmic Res. 1983;15:72–89. doi: 10.1159/000265239. [DOI] [PubMed] [Google Scholar]
- Pang SF, Yu HS, Suen HC, Brown GM. Melatonin in the retina of rats: a diurnal rhythm. J. Endocrinol. 1980;87:89–93. doi: 10.1677/joe.0.0870089. [DOI] [PubMed] [Google Scholar]
- Pierce ME, Besharse JC. Circadian regulation of retinomotor movements. I. Interaction of melatonin and dopamine in the control of cone length. J. Gen. Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintor J, Martin L, Pelaez T, Hoyle CH, Peral A. Involvement of melatonin MT(3) receptors in the regulation of intraocular pressure in rabbits. Eur. J. Pharmacol. 2001;416:251–254. doi: 10.1016/s0014-2999(01)00864-0. [DOI] [PubMed] [Google Scholar]
- Pisarchik A, Slominski AT. Alternative splicing of CRH-R1 receptors inhuman and mouse skin: identification of new variants and their differential expression. FASEB J. 2001;15:2754–2756. doi: 10.1096/fj.01-0487fje. [DOI] [PubMed] [Google Scholar]
- Pozo D, Garcia-Maurino S, Guerrero JM, Calvo JR. mRNA expression of nuclear receptor RZR/RORalpha, melatonin membrane receptor MT, and hydroxindole-O-methyltransferase in different populations of human immune cells. J. Pineal Res. 2004;37:48–54. doi: 10.1111/j.1600-079X.2004.00135.x. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Pineal melatonin: cell biology of its synthesis and of its physiological interactions. Endocr. Rev. 1991;12:151–180. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Functional diversity of the pineal hormone melatonin: its role as an antioxidant. Exp. Clin. Endocrinol. Diabetes. 1996;104:10–16. doi: 10.1055/s-0029-1211415. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Melatonin: clinical relevance. Best Pract. Res. Clin. Endocrinol. Metab. 2003;17:273–285. doi: 10.1016/s1521-690x(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Rodriguez C, Mayo JC, Sainz RM, Antolin I, Herrera F, Martin V, Reiter RJ. Regulation of antioxidant enzymes: a significant role for melatonin. J. Pineal Res. 2004;36:1–9. doi: 10.1046/j.1600-079x.2003.00092.x. [DOI] [PubMed] [Google Scholar]
- Ryoo YW, Suh SI, Mun KC, Kim BC, Lee KS. The effects of the melatonin on ultraviolet-B irradiated cultured dermal fibroblasts. J. Dermatol. Sci. 2001;27:162–169. doi: 10.1016/s0923-1811(01)00133-5. [DOI] [PubMed] [Google Scholar]
- Siu AW, Reiter RJ, To CH. Pineal indoleamines and vitamin E reduce nitric oxide-induced lipid peroxidation in rat retinal homogenates. J. Pineal Res. 1999;27:122–128. doi: 10.1111/j.1600-079x.1999.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Baker J, Rosano TG, Guisti LW, Ermak G, Grande M, Gaudet SJ. Metabolism of serotonin to N-acetylserotonin, melatonin, and 5-methoxytryptamine in hamster skin culture. J. Biol. Chem. 1996;271:12281–12286. doi: 10.1074/jbc.271.21.12281. [DOI] [PubMed] [Google Scholar]
- Slominski A, Fischer TW, Zmijewski MA, Wortsman J, Semak I, Zbytek B, Slominski RM, Tobin DJ. On the role of melatonin in skin physiology and pathology. Endocrine. 2005a;27:137–148. doi: 10.1385/ENDO:27:2:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Johansson O, Jing C, Semak I, Slugocki G, Wortsman J. Tryptophan hydroxylase expression in human skin cells. Biochim. Biophys. Acta. 2003a;1639:80–86. doi: 10.1016/s0925-4439(03)00124-8. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J. Characterization of the serotoninergic system in the C57BL/6 mouse skin. Eur. J. Biochem. 2003b;270:3335–3344. doi: 10.1046/j.1432-1033.2003.03708.x. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Semak I, Sweatman T, Wortsman J, Szczesniewski A, Slugocki G, McNulty J, Kauser S, Tobin DJ, Jing C, Johansson O. Serotoninergic and melatoninergic systems are fully expressed in human skin. FASEB J. 2002a;16:896–898. doi: 10.1096/fj.01-0952fje. [DOI] [PubMed] [Google Scholar]
- Slominski A, Pisarchik A, Zbytek B, Tobin DJ, Kauser S, Wortsman J. Functional activity of serotoninergic and melatoninergic systems expressed in the skin. J. Cell. Physiol. 2003c;196:144–153. doi: 10.1002/jcp.10287. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J. Conversion of l-tryptophan to serotonin and melatonin in human melanoma cells. FEBS Lett. 2002b;511:102–106. doi: 10.1016/s0014-5793(01)03319-1. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Shibahara S, Wortsman J. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 2004;84:1155–1228. doi: 10.1152/physrev.00044.2003. [DOI] [PubMed] [Google Scholar]
- Slominski A, Tobin DJ, Zmijewski MA, Wortsman J, Paus R. Melatonin in the skin: synthesis, metabolism and functions. Trends Endocrinol. Metab. 2008;19:17–24. doi: 10.1016/j.tem.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Slominski A, Wortsman J, Tobin DJ. The cutaneous serotoninergic/melatoninergic system: securing a place under the sun. FASEB J. 2005b;19:176–194. doi: 10.1096/fj.04-2079rev. [DOI] [PubMed] [Google Scholar]
- Slominski A, Zbytek B, Szczesniewski A, Semak I, Kaminski J, Sweatman T, Wortsman J. CRH stimulation of corticosteroids production in melanocytes is mediated by ACTH. Am. J. Physiol. Endocrinol. Metab. 2005c;288:E701–E706. doi: 10.1152/ajpendo.00519.2004. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, Reiter RJ. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J. Pineal Res. 2003;34:75–78. doi: 10.1034/j.1600-079x.2003.02111.x. [DOI] [PubMed] [Google Scholar]
- Tan DX, Manchester LC, Terron MP, Flores LJ, Tamura H, Reiter RJ. Melatonin as a naturally occurring co-substrate of quinone reductase-2, the putative MT3 melatonin membrane receptor: hypothesis and significance. J. Pineal Res. 2007;43:317–320. doi: 10.1111/j.1600-079X.2007.00513.x. [DOI] [PubMed] [Google Scholar]
- White MP, Fisher LJ. Effects of exogenous melatonin on circadian disc shedding in the albino rat retina. Vision Res. 1989;29:167–179. doi: 10.1016/0042-6989(89)90122-3. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Summers JA. Circadian rhythms in the eye: the physiological significance of melatonin receptors in ocular tissues. Prog. Retin. Eye Res. 2008;27:137–160. doi: 10.1016/j.preteyeres.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Udin SB, Summers Rada JA. Localization of Mel1b melatonin receptor-like immunoreactivity in ocular tissues of Xenopus laevis. Exp. Eye Res. 2004;79:585–594. doi: 10.1016/j.exer.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Wiechmann AF, Yang XL, Wu SM, Hollyfield JG. Melatonin enhances horizontal cell sensitivity in salamander retina. Brain Res. 1988;453:377–380. doi: 10.1016/0006-8993(88)90182-5. [DOI] [PubMed] [Google Scholar]
- Wiesenberg I, Missbach M, Carlberg C. The potential role of the transcription factor RZR/ROR as a mediator of nuclear melatonin signaling. Restor. Neurol. Neurosci. 1998;12:143–150. [PubMed] [Google Scholar]
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J. Cell Biol. 1969;42:392–403. doi: 10.1083/jcb.42.2.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu HS, Pang SF, Tang PL. Increase in the level of retinal melatonin and persistence of its diurnal rhythm in rats after pinealectomy. J. Endocrinol. 1981;91:477–481. doi: 10.1677/joe.0.0910477. [DOI] [PubMed] [Google Scholar]
- Yu HS, Reiter RJ. Melatonin Biosynthesis, Physiological Effects, and Clinical Implications. Boca Raton: CRC Press; 1993. [Google Scholar]
- Zmijewski MA, Sharma RK, Slominski AT. Expression of molecular equivalent of hypothalamic–pituitary–adrenal axis in adult retinal pigment epithelium. J. Endocrinol. 2007;193:157–169. doi: 10.1677/joe.1.06927. [DOI] [PMC free article] [PubMed] [Google Scholar]