Abstract
Endocannabinoids, such as anandamide and 2-arachidonoylglycerol, are synthesized from membrane phospholipids in the heart and other cardiovascular tissues. They activate cannabinoid CB1 and CB2 receptors, TRPV1, peroxisome proliferator-activated receptors and perhaps a novel vascular G-protein-coupled receptor. Inactivation is by cellular uptake and fatty acid amide hydrolase (FAAH). Endocannabinoids relax coronary and other arteries and decrease cardiac work, but seem not to be involved in tonic regulation of cardiovascular function. They act as a stress response system which is activated, for example, in myocardial infarction and circulatory shock. Endocannabinoids are largely protective; they decrease tissue damage and arrhythmia in myocardial infarction, may reduce progression of atherosclerosis (CB2 receptor stimulation inhibits lesion progression), and FAAH knockout mice (which have enhanced endocannabinoid levels) show decreased cardiac dysfunction with age compared to wild-types. However, endocannabinoids may mediate doxorubicin-induced cardiac dysfunction. Their signaling pathways are not fully elucidated but they can lead to changed expression of a variety of genes, including those involved in inflammatory responses. There is potential for therapeutic targeting of endocannabinoids and their receptors, but their apparent involvement in both protective and deleterious actions on the heart mean that careful risk assessment is needed before any treatment can be introduced.
Keywords: Endocannabinoid system, anandamide, cardioprotection, cannabinoid receptors, rimonabant, atherosclerosis
Introduction
Endocannabinoids are substances occurring naturally within the body which mimic the activity of Δ9-tetrahydrocannabinol, which was shown in the 1960s to be the primary psychoactive ingredient of cannabis.1 Most early interest in cannabinoids centered on their roles in the nervous system, and the two most actively pursued clinical applications, multiple sclerosis2 and obesity3, quite possibly arise from actions on nerves. Studies of Δ9-tetrahydrocannabinol in the cardiovascular system, as in the brain, were at first held back by the absence of tools to identify the receptors, and signaling mechanisms, mediating the observed responses.4 Increased availability of receptor agonists, antagonists, and inhibitors of endocannabinoid inactivation, has enabled wider study of the cannabinoid system.5 In particular, the introduction of rimonabant, a cannabinoid receptor antagonist, as an anti-obesity agent has focused attention on the potential for reducing cardiovascular risk.3 At the time of its discovery, first reported in 1994, rimonabant (then called SR141716A) was seen as an antagonist at central cannabinoid receptors6 but it is now clear that its target receptor, the CB1 receptor, occurs outside the brain. Indeed, though its actions as an anti-obesity agent can be ascribed to the principle of antagonism of the central orexigenic actions of endocannabinoids, it is now clear that its beneficial effects may also involve, to a greater or lesser extent, modification of endocrine activity through actions on peripheral CB1 receptors in the liver and adipose tissue.7 The years between the discovery of rimonabant and its introduction into the clinic have been exciting ones in the story of the cannabinoids and one outcome has been a great expansion of our knowledge of their actions on the heart and circulation.
Endocannabinoids and their receptors
Less than 20 years ago, Matsuda et al.8 cloned a receptor for Δ9-tetrahydrocannabinol from a rat cerebral cortex cDNA library. This started the search for the neurotransmitter system with which the cannabinoids interact and, very soon, arachidonoylethanolamide was identified in pig brain as a high affinity ligand for this receptor9. This agonist (Figure 1) is more commonly called anandamide (from the Sanskrit “a-nanda” for happiness, joy, or enjoyment) and this first receptor is referred to as the CB1 receptor, since a second receptor, designated CB2 and largely found in peripheral tissues, was later cloned from the HL60 human leukemia cell line.10 Both receptors couple through Gi/o proteins (for discussion of receptor signaling pathways, see reviews by Howlett et al.11 and Hiley & Ford5). CB1 receptors have been suggested to evoke the effects of cannabinoids in the myocardium of human,12 rat13 and mouse,14 and stimulation evokes a negative inotropic effect. However, some evidence from isolated rat hearts is inconsistent with this simple picture of cardiac effects being solely due to CB1 receptors (see under ‘Cardioprotection’ below).15
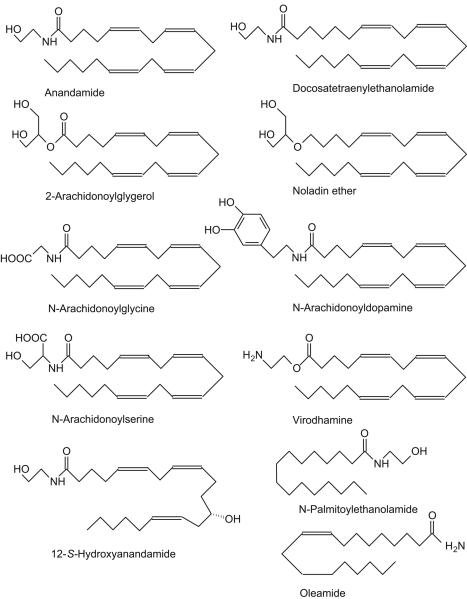
Figure 1.
Structures of the major endocannabinoids identified to date; most are based on the 20-carbon fatty acid, arachidonic acid though palmitoylethanolamide (the activity of which as an endocannabinoid is controversial) and the CB1 cannabinoid and TRPV1 agonist, oleamide, are exceptions with shorter chain lengths. The most frequently studied is anandamide (an agonist at CB1 and CB2 receptors as well as TRPV1 channels), which with 2-arachidonoylglycerol (an agonist at both CB1 and CB2 receptors with generally higher efficacy than anandamide11) is found to occur in many tissues of the cardiovascular system, including the heart and blood cells (especially platelets and monocytes). 12-S-hydroxyanandamide, which activates CB1 receptors, is synthesized by 12-lipoxygenase from anandamide taken up by platelets.21 Since 12-S-hydroxyanandamide is not a substrate for fatty acid amide hydrolase, then conversion of anandamide to this oxygenated derivative might have the effect of prolonging signaling due to anandamide synthesis.
Anandamide was reported to have a lower affinity for the CB2 than the CB1 receptor.10 A second naturally-occurring arachidonic acid derivative, 2-arachidonoylglycerol (2-AG), binds to cells transfected with either CB1 or CB2 receptor cDNA but generally shows somewhat a higher activity at CB2 receptors.16 Anandamide and 2-AG were the first identified members of an expanding family of putative endocannabinoids. These now include docosatetraenylethanolamide (CB1 receptor agonist),17 N-arachidonoyl dopamine (CB1 receptor-selective ligand)18, and virodhamine (O-arachidonoylethanolamine; CB1 receptor partial agonist/CB2 receptor agonist; see fig. 1 for structures).19 Noladin ether (2-arachidonoylglycerylether), which is structurally related to 2-AG, is a potent ligand at the CB1 receptor but only weakly associates with CB2 receptors20 and the anandamide derivative produced by 12-lipoxygenase, 12-S-hydroxyanandamide, is also a CB1 receptor agonist.21 N-palmitoylethanolamide has been suggested to be a selective CB2 receptor agonist22 but this is a controversial assertion since it has not been confirmed by other studies. For example, Griffin et al. reported that it was only weakly active at rat cloned CB2 receptors expressed in HEK293 cells while its stimulation of GTPgS binding to rat cerebellar membranes was sensitive to rimonabant and so is not likely to be due to activation of CB2 receptors.23 Certainly N-palmitoylethanolamide can act at peroxisome proliferator-activated receptors (PPAR), namely PPAR-a,24 and another G protein-coupled receptor, GPR55.25 Both of these types of receptor are discussed later in respect of the actions of anandamide.
It has been suggested that there is at least one other G-protein-coupled cannabinoid receptor in the cardiovascular system on the basis of the actions of abnormal cannabidiol. This compound is inactive at CB1 and CB2 receptors but lowers blood pressure, even in CB1- and CB1/CB2-receptor knockout mice.26 Furthermore, responses to anandamide in rat isolated mesenteric arteries have a pharmacology which is not consistent with actions at the cloned cannabinoid receptors.27 For example, even though the anandamide-induced relaxation of rat coronary arteries is sensitive to rimonabant (at the high concentration of 1 μM), it is not antagonized by another CB1 receptor antagonist, AM251.28 Therefore a new vascular ‘anandamide receptor’ has been proposed29 which could also mediate the actions of abnormal cannabidiol.26 Studies on this novel site have been facilitated by O-1918, a derivative of abnormal cannabidiol; it antagonizes the actions anandamide and abnormal cannabidiol but has no activity at the classical cannabinoid receptors.30 N-arachidonoylserine, a weak ligand at both CB1 and CB2 receptors has been suggested to be an endogenous ligand for this novel receptor. This, too, dilates the rat mesenteric artery and, like anandamide, stimulates phosphorylation of p44/42 mitogen-activated protein kinase (MAPK) and protein kinase B/Akt in cultured endothelial cells.31
The presumption of G-protein coupling for the new receptor, like that of the CB1 and CB2 receptors, arises from the actions it is thought to mediate being pertussis toxin-sensitive.27,30 Its identity is unclear, though the orphan receptor GPR55 is a possible candidate.32 Application of the synthetic cannabinoid, CP55,940, or the endocannabinoids, anandamide and virodhamine, to cells expressing GPR55 increases GTPgS incorporation.25 There is still considerable doubt that GPR55 is the vascular receptor for anandamide: a) it does not couple through Gi/o, as would be expected for a pertussis toxin-sensitive system, but through Ga13;25,33 b) the vasorelaxant response to abnormal cannabidiol in the mesenteric artery is still present in GPR55 null mice34; and c) other work suggests not only that the endogenous ligand is lysophosphatidylinositol, but that anandamide is inactive when using phosphorylation of extracellular signal-regulated kinase (ERK) as the assay.35
Anandamide can also activate TRPV1 vanilloid receptors on sensory nerve endings,36 a property it shares with Δ9-tetrahydrocannabinol and cannabinol.37 Activation of TRPV1 channels releases calcitonin gene-related peptide, and evokes vasodilatation, though the effect is clearly regional since, in contrast to the actions of anandamide on rat mesenteric resistance artery, rat hepatic artery and guinea-pig basilar artery,36 relaxation of rat coronary artery does not involve TRPV1.28 There are no reports of TRPV1 on myocardial cells but there is evidence that, on cardiac afferent nerves, it plays a role in signaling in ischemia (though the role of anandamide in this has not been investigated).38 Very high plasma levels of anandamide in mice are associated with TRPV1 and the Bezold-Jarisch reflex.39
The final targets for anandamide are the PPAR, though this has not yet been shown in the heart. Anandamide, noladin ether, virodhamine and the endocannabinoid-like oleoylethanolamine bind to PPAR-a40 (a mechanism that might be important in cytoprotection in cerebral infarction) and anandamide induces 3T3-L1 fibroblasts to differentiate into adipocytes by a process sensitive to the PPAR-g antagonist GW9662.41 Δ9-tetrahydrocannabinol has also been shown to initiate PPAR-g activity.42 Actions on any of these different types of receptors are often linked to signaling pathways which enable endocannabinoids to regulate gene expression. This gives them considerable potential to act as regulators of longer term processes such as inflammation and apoptosis.
Synthetic and degradative pathways
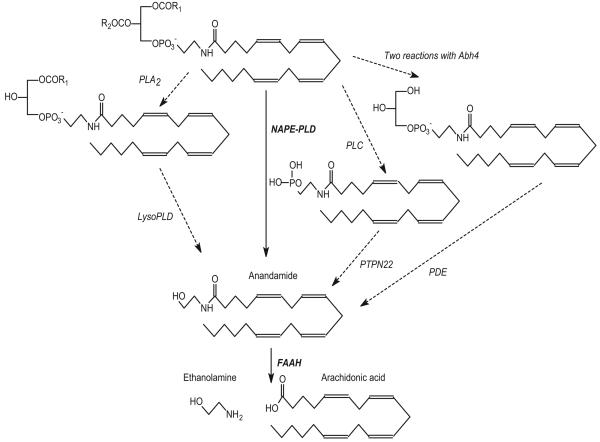
The synthetic pathways for anandamide and 2-AG are mainly thought to involve breakdown of pre-formed arachidonoylphospholipids in which glycerol is esterified with the fatty acid at the sn-1 position, rather than much more usual sn-2 position.43 2-AG is formed by the action of an sn-1-specific diacylglycerol lipase44. The primary route for anandamide synthesis is the formation of the N-acylethanolamine phospholipid (NAPE) precursor by a Ca2+-activated N-acyltransferase. The NAPE is then broken down by a specific phospholipase D (NAPE-PLD).45 NAPE-PLD has been isolated from rat heart,46 and both synthetic processes are found in myocardial microsomal fractions of many species. However, their activity ratio varies widely between species (from 0.002 to 15) with high NAPE-PLD activity in rats and guinea-pigs contrasting with very low N-acyltransferase activity47. Production of anandamide exhibits redundancy,48,49 but the other anandamide-generating pathways (shown in Figure 2) do not necessarily occur in the heart. In one of these synthetic routes (found in mouse brain and RAW264.7 macrophages), phospholipase C hydrolyzes NAPE to phosphoanandamide which is then acted upon by phosphatases, including the protein tyrosine phosphatase PTPN22, to give the endocannabinoid.50 Alternatively, in mouse brain and some peripheral tissues, NAPE is first subjected to sequential deacylations (possibly by the enzyme a/b-hydrolase 4 [Abh4]) to give a glycerophospho-N-acylethanolamine which is then acted upon by a metal-dependent phosphodiesterase to liberate anandamide.51 High levels of activity of NAPE-lipase activity are found in brain and testis, but somewhat less of this enzyme occurs in the heart (∼12% of that in the brain).
Figure 2.
Synthetic pathways for anandamide. The primary pathway involves the hydrolysis of an arachidonate-containing phospholipid, in which the arachidonic acid has already been converted to the ethanolamine derivative, by N-acylethanolamine-specific phospholipase D (NAPE-PLD). Anandamide is then inactivated by hydrolysis, mediated by fatty acid amide hydrolase (FAAH). Alternative pathways proposed include sequential action of a phospholipase A2 (PLA2) and lysophospholipase D; breakdown of the glycerophospholipid by phospholipase C (PLC) and then dephosphorylation by a protein tyrosine phosphatase (PTPN22); and de-esterification of the precursor by the action of (Abh4) with further processing by a phosphodiesterase (PDE).49 Anandamide might reach the FAAH site by means of a high affinity transporter.
Inactivation of anandamide is probably by cellular uptake by an anandamide membrane transporter52 (though this is not universally accepted)53 followed by hydrolysis into ethanolamide and arachidonic acid by fatty acid amide hydrolase (FAAH).54 2-AG is broken down by FAAH as well as a specific monoacyglycerol lipase.55 Messenger RNA for the lipase is expressed in the heart56 but there has been no direct visualization of FAAH or its expression in myocardial cells. There is indirect evidence for FAAH in the heart; levels of anandamide, but not 2-AG, are elevated in the hearts of FAAH−/− mice14. Interestingly, these mice also show decreased deterioration of cardiac function with age relative to their FAAH+/+ littermates (see later under ‘Cardiac dysfunction’)57 which might be due to enhancement of the effects of endogenous anandamide. The finding of measurable levels of anandamide in mouse heart contrasts with an earlier report that anandamide was undetectable in lipid extracts of normal rat heart, although 2-AG was present.58 Nevertheless, it seems that both synthetic and metabolic capacities for endocannabinoids are present in the heart, but the capacity for producing the NAPE precursor is very low in several species, including humans,47 and with the exception of mice the evidence for anandamide synthesis and activity is generally circumstantial.
Other cells of the cardiovascular system can synthesize endocannabinoids. Anandamide and its associated enzymes are found in endothelial cells from rat kidney,59 but it is not synthesized by bovine coronary artery endothelial cells.60 2-AG is produced by human umbilical vein endothelial cells stimulated by A23187, a calcium ionophore, or thrombin.61 Enhanced intracellular Ca2+ also causes release of anandamide and 2-AG from macrophage-derived cell lines.62 In platelets and neutrophils, anandamide is taken up and converted by lipoxygenase to 12-S-hydroxyanandamide.21 Thus cardiac function and coronary perfusion might be modulated by endocannabinoids derived not only from cardiac tissues but also from the circulating cells.
Inhibition of the membrane transporter and FAAH provides the means for enhancing the activity of endocannabinoids produced by the tissues of the body. The transport system can be inhibited by several fatty acid analogues, including arvanil, olvanil, N-(4-hydroxyphenyl)arachidonoylamide (AM404), and N-(3-furanylmethyl)-5Z,8Z,11Z,14Z-eicosatetraenamide (UCM707).41,63 Many of these agents are also inhibit FAAH,53 but there are high affinity and relatively selective inhibitors available for the enzyme, as well as an endogenous inhibitor, 2-octyl-g-bromoacetoacetate, originally isolated from human cerebrospinal fluid.64 A detailed structure/activity study of fatty acid a-keto heterocycles produced some very potent compounds, including OL-92, which has a subnanomolar inhibition constant.65 URB532 and URB597, which have they have IC50 values around 1 μM, are carbamates and lack the characteristic long chain fatty acid moiety of both the endocannabinoids and the other FAAH inhibitors.66 In addition to hydrolyzing anandamide, FAAH acts upon other fatty acid amides including the primary amide, oleamide, which is active at CB1 receptors and TRPV1 and might have a role in cardiovascular signaling.67
Endocannabinoids in cardiovascular syndromes
Administration of anandamide, Δ9-tetrahydrocannabinol or synthetic cannabinoids causes hemodynamic changes which are complex, involving phases of both increased and decreased blood pressure as well as changes in heart rate. These effects are mainly mediated by CB1 receptors and include actions on the nervous system, both central and peripheral.68 Interestingly, in view of its actions on TRPV1 receptors, anandamide does not affect blood pressure in CB1 receptor-knockout mice, even though it evoked vanilloid receptor-dependent relaxation in isolated mesenteric beds from these animals.26 This suggests that regulatory effects of endocannabinoids in at least some beds may be purely local. Smoking cannabis in humans is associated with a small increase in the risk of heart attack, lasting 1-2 h which is probably due to increased sympathetic outflow.69 On the other hand, there is little evidence that the endocannabinoid system is normally activated in the healthy cardiovascular system. Administration of rimonabant, a CB1 receptor antagonist, does not affect heart rate or blood pressure in anesthetized rats70 and the Rimonabant in Obesity (RIO) clinical trials show only a tiny (<1mmHg), albeit significant, decrease in blood pressure in obese but normotensive patients over one year of treatment.71 This contrasts with the greater fall in blood pressure in hypertensive obese patients, and in those with type II diabetes.
In spontaneously hypertensive rats (SHR), CB1 receptor blockade increases blood pressure and left ventricular activity, suggesting that the endocannabinoid system modulates the effects of the condition13. CB1 receptor expression was increased in the heart in SHR, relative to Wistar-Kyoto normotensive animals, which translated into increased hypotensive effects in response to exogenous cannabinoids in the SHR. Further evidence for activation of the endocannabinoid system in hypertension came from the observation that inhibition of FAAH, with URB597, reduced blood pressure and cardiac function to the levels seen in the normotensive animals. These results present the intriguing possibility that the endocannabinoid system is one which is activated in the cardiovascular system only in disease states.
Shock
Just over 10 years ago, Kunos's laboratory reported that rimonabant increased blood pressure in rats subjected to hemorrhagic shock even though, as noted above, the cannabinoid receptor antagonist had no effect in controls.70 It was concluded not only that the endocannabinoid system was activated by hypovolemia, but also that endocannabinoids, generated by monocytes and platelets, were protective since rimonabant shortened survival time despite the pressor effect. Endocannabinoids are also generated by circulating cells in both endotoxic shock and the cardiogenic shock occurring after experimentally-induced myocardial infarction.72 The CB1 receptor does not seem to be involved in the deep depression of blood pressure following exposure to bacterial lipopolysaccharide since, although it can be attenuated by rimonabant, it cannot be reversed by AM251, another selective CB1 receptor antagonist.73 This separation of effects of the two antagonists is characteristic of the vasodilator responses to anandamide in mesenteric blood vessels from rats,74 although both CB1 blockers antagonize anandamide in rat coronary artery.15 As discussed above when considering the receptors for endocannabinoids, the identity of the receptor mediating the non-CB1, but rimonabant-sensitive, vascular responses has yet to be elucidated, but it is clear that endocannabinoid production can be induced when the cardiovascular system is functioning under deleterious conditions.
Cardioprotection: infarction and arrhythmia
Endotoxin administration not only produces cardiovascular shock, but can also protect the heart against ischemia/reperfusion injury.75 At least some of the protective effect is due to endocannabinoids since, although rimonabant had no effect, the highly selective CB2 receptor antagonist, SR144528, did block the response. Increased expression of the inducible and Ca2+-independent isoform of nitric oxide synthase (NOS Type II) is the response most usually associated with endotoxic shock,76,77 and therefore the question arises of whether or not there is an association between the endocannabinoid system and nitric oxide (NO) in this phenomenon. Lagneux and Lamontagne75 reported that the cardioprotection in their study was both sensitive to the NOS inhibitor, Nω-nitro-L-arginine, and could be mimicked by administering sodium nitroprusside. What is particularly interesting is that SR144528 reduced the protective effect of the NO donor suggesting that NO worked through the endocannabinoids rather than the cannabinoids through NO. This contrasts with the results of experiments using cultured cardiomyocytes from neonatal rats in which Δ9-tetrahydrocannabinol, acting through CB2 receptors (since SR144528 blocked the response but not rimonabant), induced expression of NOS Type II and elevated NO production.78 These results rule out a role for the postulated vascular cannabinoid receptor since rimonabant was used at concentrations (1 and 10 μM) sufficient to block actions at this site.79
CB2 receptors are also involved in heat stress preconditioning which can be attenuated by both blockade of these receptors and NOS inhibition.80 Activation of the CB2 receptor also reduces leukocyte activation in experimentally-induced myocardial infarction in mice.81 In this case the protection was induced by a synthetic cannabinoid, WIN55212-2 rather than an endocannabinoid and it was blocked by the CB2 antagonist AM630 while CB1 blockade, with AM251, was ineffective. WIN55212-2 treatment reduced levels of myeloperoxidase, interleukin-1b and the CXC chemokine ligand 8 in the damaged tissue.
More recently, Wagner et al. showed that endocannabinoids were involved in preconditioning by NO.82 They reported that delayed preconditioning, which occurs 24 h after pretreatment with NO derived from transdermal nitroglycerin, is mediated by CB1 receptors, since it was sensitive to AM251 but not AM630. It will be noted that the effect of AM251, and therefore that likely involvement of the CB1 receptor, is a major contrast with the results with endotoxic shock and heat stress where this antagonist was ineffective. Wagner and colleagues also showed that tissue levels of 2-AG in the heart, but not of anandamide, were elevated 2.6-fold by the NO preconditioning protocol, and that pretreatment with either 2-AG, or the metabolically stable noladin ether, reduced infarct size when either was given 30 min before no-flow ischemia.
The mechanisms involved are not yet worked out. Although Kola et al.83 used neither endocannabinoids nor cannabinoid receptor antagonists in their study, they proposed that cannabinoids (in their case Δ9-tetrahydrocannabinol) acting on cannabinoid receptors might act by stimulating AMP kinase activity, which causes a shift in metabolism to anaerobic glycolysis and fatty acid oxidation in reperfusion. In this context, it has to be noted that Δ9-tetrahydrocannabinol can act at non-cannabinoid receptors, since O'Sullivan and colleagues have shown that it acts as an agonist a PPAR-g receptors in blood vessels,42 and activation of these nuclear receptors can lead to AMPK activation.84 As noted previously, anandamide can activate PPAR-g, though this has not been shown in the heart. 41
Since Wagner and colleagues found anandamide levels were not elevated,82 a role for endogenous anandamide has yet to be proven. However, inclusion of it, or its metabolically stable analogue methanandamide, in the perfusion fluid reduced the area of infarction (but did not affect recovery of cardiac function) when administered from 5 min before induction of ischemia until the end of a 60-min reperfusion period.85 The effect was blocked by rimonabant and SR144528, suggesting that CB1, CB2 and/or the novel rimonabant-sensitive, non-CB1, receptor mediated the response. Both CB112,13,39 and CB2 receptors86 have been found in the myocardium but neither arachidonoylcyclopropylamide (a CB1-selective agonist) nor JWH133 (a CB2-selective agonist), alone or together, reduced the extent of infarction.
This unusual pharmacology was very close to that we had previously found for the coronary vasodilator and negative inotropic effects of anandamide, methanandamide and other cannabinoids.15 Therefore, although the actions of endogenous endocannabinoids can often be inferred to be at the known cannabinoid receptors by blockade with selective antagonists, it seems that actions of exogenous anandamide in the heart and vasculature can rarely be simply attributed to actions at one or both of these ‘classical’ cannabinoid receptors. Furthermore, the endocannabinoids can evoke protective effects by several signaling pathways, and Howlett and colleagues have recently discussed these in relation to the regulation of the production of reactive oxygen and nitrogen species during damage to the central nervous system.87
Further evidence for a cardioprotective role for CB2 receptors, in particular, comes from work on “remote preconditioning”. This is the phenomenon whereby ischemia in one vascular bed confers protection in another. In one model, a period of 15 min occlusion of the mesenteric artery, followed by 15 min reperfusion, is imposed on anesthetized rats before 30 min occlusion of the left coronary artery and 2 h reperfusion. This preconditioning reduced arrhythmia, as well as infarct size relative to hearts from sham-operated rats, and the protective effect was blocked by CB2 receptor blockade (with AM630) but not by CB1 receptor antagonism (with AM251).88 In experiments with isolated hearts, it will naturally be assumed that the endocannabinoids conferring the protection originate in the cells of the heart and its tissues but, in the case of remote preconditioning, the active agents could arise from circulating cells (e.g. platelets and monocytes) activated to collect or produce endocannabinoids when passing through the post-ischemic bed. The source of cardioprotective endocannabinoids is therefore an open question. It should be noted that, in this study, CB2 receptor blockade reduced the arrhythmias induced by the cardiac ischemia/reperfusion protocol. This accords with the data of Krylatov et al.89 that a non-selective cannabinoid receptor agonist, HU-210, reduces ischemia-induced arrhythmia by activation of the CB2 receptor. Also, some of the beneficial effects might be on non-myocardial cells. CB2 receptor activation (with synthetic cannabinoid agonists) of endothelial cells from human coronary artery decreases the pro-inflammatory activation of these cells by tumor necrosis factor-a (TNF-ạ) and this is manifested by reduced production of ICAM-1, VCAM-1 and monocyte chemoattractant protein.90
It is not only in the heart that endocannabinoids might have a role in regulation of post-ischemic injury. Readers are referred to the recent review by Pacher & Haskó for a wider discussion of their possible roles in modulating damage, including after stroke and hepatic ischemia.91
Cardiac dysfunction
From the above, it seems that endocannabinoids act as “good guys” throughout cardiovascular pathology. However, activation of CB1 receptors can be proarrhythmic as shown by Motobe et al. in their study of the increased risk of arrhythmia and sudden cardiac death associated with the use of bone cement in hip replacement surgery.92 A small clinical trial showed that plasma levels of anandamide and 2-AG were elevated in patients on whom the cement was used compared to those where the procedure took place without its use; this was interpreted as showing the increased risk arose as a result of the presence of the endocannabinoids. It should be noted that the study did not exclude the possibility that the endocannabinoids are a protective response to a threat induced by the presence of the cement.
However, the authors' assumption that the endocannabinoids were detrimental to cardiac function has received support from results with experimental models, in which cannabinoid antagonists can be used. For example, the antitumor agent doxorubicin impairs heart performance in mice and the dysfunction can be alleviated by CB1 receptor blockade with rimonabant or AM251. Doxorubicin treatment is associated with elevated myocardial anandamide levels, but not with changes in CB1 or CB2 receptor expression.86 When incubated with a myocardial cell line (H9c2), doxorubicin induced apoptosis which also was reduced by CB1 receptor blockade; but the effect was not sensitive to a CB2 blocker or CB1 and CB2 receptor agonists. Similarly, work on cardiac function suggests that endocannabinoids mediate the cardiac dysfunction associated with cirrhosis. Administration of AM251, to block CB1 receptors, improved cardiac function in carbon tetrachloride-induced cirrhosis in rats and it was also shown that anandamide levels were increased in the hearts of rats with cirrhosis compared to the controls.93
In contrast, aging-associated cardiac dysfunction is reduced in FAAH-null mice, which could be interpreted as showing a need for increased endocannabinoid activity in the heart.57 The homozygous knockout mice exhibited decreased levels of markers for oxidative stress and inflammation since the increased expression of gp91phox, TNF-a, and Type II NOS associated with age in wild-type mice was largely absent in the knockouts. The apparent benefit of increased endocannabinoid activity was ascribed to the inhibitory effects of cannabinoids on inflammatory conditions, which include, among others, atherosclerosis (see below), colitis and arthritis.94
Cannabinoids and cardiac remodeling
Endocannabinoids do appear to be beneficial following myocardial infarction, a severe consequence of which is remodeling of the heart. This results in increased risk of heart failure and arrhythmia and the usual approach to minimizing this is to treat patients with angiotensin converting enzyme inhibitors.95 Wagner et al.96 sought to determine whether the elevated levels of endocannabinoids following experimentally-induced infarction led to deleterious or beneficial changes in heart function. Administration of AM251 for 12 weeks after the insult exacerbated the decline in left ventricular capability whereas giving the non-selective cannabinoid agonist HU-210 enhanced left ventricular performance. However, the beneficial effects of HU-210 were greatest in cases of restricted degrees of infarction, which may reduce the potential utility of this approach.97
It has been considered for some time that cannabinoid receptors have the potential to regulate apoptosis since they can signal through both pro- and anti-apoptotic pathways.5 Their lipophilicity also means that they have the capability to penetrate cells and act intracellularly, even without the assistance of a membrane transporter. Recently, it has been shown that a number of cannabinoid agents, including HU-210, Δ9-tetrahydrocannabinol, and anandamide, decrease rat heart mitochondrial O2 consumption98 and that mitochondria are involved in marijuana-induced cell death.99 Furthermore, vanilloid receptor agonists, which overlap with endocannabinoids at least in the form of anandamide and oleamide, have pro-apoptotic actions on mitochondria.100 Coupled with the observations on apoptosis in FAAH-null mice,57 it is clear that endogenously produced cannabinoids have strong potential for the regulation of apoptosis, and hence remodeling, in the heart.
Coronary blood vessels, atherosclerosis and endothelium
In discussing the receptors mediating the responses of endocannabinoids, it has already been stated that anandamide and other cannabinoids relax the blood vessels of the heart.15 These responses in the rat are sensitive to CB1 receptor blockade but not to CB2 receptor or TRPV1 antagonism. Their physiological relevance remains unclear since there is no substantial evidence for spontaneous activity of the endocannabinoid system in the heart or its vessels under non-pathological conditions. However, the CB2 receptor is widely recognized as being associated with cells that mediate immune responses and inflammation10,22,94 and so it is not surprising that cannabinoids have been shown to reduce lesion progression in the apolipoprotein E knockout mouse model of atherosclerosis.101 In these experiments the mice were given a low oral dose of Δ9-tetrahydrocannabinol and the response was inhibited by the CB2 receptor antagonist SR144528. The target appears to be CB2 receptors on macrophages and T lymphocytes within the lesion and the treatment also reduced leukocyte adhesion in the mesenteric vasculature. Interestingly, increasing the dose beyond an optimal dose of 1 mg kg−1 per day resulted in a decreased effectiveness, which suggests either that Δ9-tetrahydrocannabinol is acting as a partial agonist or that there are opposing effects that can be activated by the cannabinoid. No data were provided for the effects of the CB2 antagonist alone, so it is not possible to state explicitly that the endocannabinoid system is, or is not, activated within the lesion. However, this seems unlikely since the antagonist did not increase lesion progression beyond the control level although it completely reversed the effects of Δ9-tetrahydrocannabinol. A potential effect for endocannabinoids on the progression of vascular lesions is indirectly indicated by the observation that estrogen increases anandamide release from endothelial cells and this inhibits platelet activation; this might be one of the mechanisms by which hormone replacement therapy and natural estrogens confer protection on the cardiovascular system.102
One mechanism by which endocannabinoids could affect the progression of atherosclerosis, or restenosis after treatment of coronary artery obstructions, is by modulation of the actions of inflammatory factors. It has already been pointed out (see under ‘Cardioprotection’) that stimulation of CB2 receptors reduces the inflammatory activation of endothelial cells by TNF-ã.90 This cytokine also induces proliferation and migration of cultured human coronary artery vascular smooth muscle cells together with activation of signaling molecules such as Ras, MAPK, ERK1/ERK2 and Akt.103 Interestingly, these actions are not only associated with greatly increased expression of CB2 receptors but also CB2 receptor agonists, such as JWH133 and HU-308, bring about a concentration-dependent reduction in the effects of TNF-a. As noted already, there is as yet no substantive evidence for local modulation of the progression of atherosclerotic lesions by endocannabinoids but the difficulties in constructing suitable studies are such that it is not currently possible to discount such an hypothesis.
Conclusion: endocannabinoids as targets in cardiac therapy
The clinical use of rimonabant for the treatment of obesity3 marks the first manipulation of the endocannabinoid system for therapeutic purposes, but it has not been a run-away success as concerns have already been expressed about its use with respect to side effects arising from actions on the brain.104 Indeed, this review of endocannabinoid effects on the heart shows that it is not possible yet to define them simply as protective or injurious to the heart and its blood vessels, though the balance does seem to favor a protective role. Therefore, the use of cannabinoid receptor ligands as therapeutic agents needs careful consideration, not only with regard to potential central side effects. Since the endocannabinoid system appears to be activated in response to threatening pathophysiological circumstances, then its actions can be enhanced not only by the deployment of agonists but also by inhibitors of FAAH or cellular uptake. This approach has the potential advantage that the increased agonist activity would be largely confined to the sites at which the endogenous processes have already been activated to meet the threat.
The consideration of the field of cannabinoid therapies has moved on even since the appearance in 2006 of the major review by Pacher, Bátkai and Kunos of the prospects of the endocannabinoid system as a successful therapeutic target.94 Doxorubicin cardiotoxicity, for example, is newly identified as a condition in which endocannabinoid activity might be implicated.86 Therefore use of a CB1 receptor antagonist, preferably without central effects, might be beneficial for those patients undergoing chemotherapy with this cytotoxic agent since its mechanisms of attack on the tumor and on the heart are different.105 Such use would be limited to cancer patients, and therefore small numbers of individuals, but manipulation of endocannabinoid activity in more prevalent pathologies such as myocardial infarction, circulatory shock, and perhaps atherosclerosis will need careful balancing of actions. The circumstances of the condition, relating to the endocannabinoids produced, their cellular source, and the profile of gene expression of the receptors and enzymes involved in their production, action and inactivation, will need to be more fully understood before the system can be satisfactorily targeted for therapeutic purposes.
Acknowledgments
Supported in part by a University Translation Award (076001/Z/04/Z) from the Wellcome Trust.
Footnotes
The author reports no conflict of interest.
REFERENCES
- 1.Gaoni Y, Mechoulam R. Isolation, structure and partial synthesis of an active constituent of haschish. J Am Chem Soc. 1964;86:1646–1648. [Google Scholar]
- 2.Wade DT, Robson P, House H, Makela P, Aram J. A preliminary controlled study to determine whether whole-plant cannabis extracts can improve intractable neurogenic symptoms. Clin Rehabil. 2003;17:21–29. doi: 10.1191/0269215503cr581oa. [DOI] [PubMed] [Google Scholar]
- 3.Van Gaal LF, Rissanen AM, Scheen AJ, Ziegler O, Rossner S. Effects of the cannabinoid-1 receptor blocker rimonabant on weight reduction and cardiovascular risk factors in overweight patients: 1-year experience from the RIO-Europe study. Lancet. 2005;365:1389–1397. doi: 10.1016/S0140-6736(05)66374-X. [DOI] [PubMed] [Google Scholar]
- 4.Graham JD, Li DM. Cardiovascular and respiratory effects of cannabis in cat and rat. Br J Pharmacol. 1973;49:1–10. [PMC free article] [PubMed] [Google Scholar]
- 5.Hiley CR, Ford WR. Cannabinoid pharmacology in the cardiovascular system: potential protective mechanisms through lipid signalling. Biol Rev Camb Philos Soc. 2004;79:187–205. doi: 10.1017/s1464793103006201. [DOI] [PubMed] [Google Scholar]
- 6.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D, Ferrara P, Soubrie P, Brelière JC, Le Fur G. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 7.Osei-Hyiaman D, DePetrillo M, Pacher P, Liu J, Radaeva S, Batkai S, Harvey-White J, Mackie K, Offertaler L, Wang L, Kunos G. Endocannabinoid activation at hepatic CB1 receptors stimulates fatty acid synthesis and contributes to diet-induced obesity. J Clin Invest. 2005;115:1298–1305. doi: 10.1172/JCI23057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 9.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 10.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 11.Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, Felder CC, Herkenham M, Mackie K, Martin BR, Mechoulam R, Pertwee RG. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev. 2002;54:161–202. doi: 10.1124/pr.54.2.161. [DOI] [PubMed] [Google Scholar]
- 12.Bonz A, Laser M, Kullmer S, Kniesch S, Babin-Ebell J, Popp V, Ertl G, Wagner JA. Cannabinoids acting on CB1 receptors decrease contractile performance in human atrial muscle. J Cardiovasc Pharmacol. 2003;41:657–664. doi: 10.1097/00005344-200304000-00020. [DOI] [PubMed] [Google Scholar]
- 13.Bátkai S, Pacher P, Osei-Hyiaman D, Radaeva S, Liu J, Harvey-White J, Offertáler L, Mackie K, Rudd MA, Bukoski RD, Kunos G. Endocannabinoids acting at cannabinoid-1 receptors regulate cardiovascular function in hypertension. Circulation. 2004;110:1996–2002. doi: 10.1161/01.CIR.0000143230.23252.D2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pacher P, Batkai S, Osei-Hyiaman D, Offertaler L, Liu J, Harvey-White J, Brassai A, Jarai Z, Cravatt BF, Kunos G. Hemodynamic profile, responsiveness to anandamide, and baroreflex sensitivity of mice lacking fatty acid amide hydrolase. Am J Physiol. 2005;289:H533–541. doi: 10.1152/ajpheart.00107.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ford WR, Honan SA, White R, Hiley CR. Evidence of a novel site mediating anandamide-induced negative inotropic and coronary vasodilatator responses in rat isolated hearts. Br J Pharmacol. 2002;135:1191–1198. doi: 10.1038/sj.bjp.0704565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mechoulam R, Ben-Shabat S, Hanus L, Ligumsky M, Kaminski NE, Schatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 17.Barg J, Fride E, Hanus L, Levy R, Matus-Leibovitch N, Heldman E, Bayewitch M, Mechoulam R, Vogel Z. Cannabinomimetic behavioral effects of and adenylate cyclase inhibition by two new endogenous anandamides. Eur J Pharmacol. 1995;287:145–152. doi: 10.1016/0014-2999(95)00487-4. [DOI] [PubMed] [Google Scholar]
- 18.Bisogno T, Melck D, Bobrov M, Gretskaya NM, Bezuglov VV, De Petrocellis L, Di Marzo V. N-acyl-dopamines: novel synthetic CB1 cannabinoid-receptor ligands and inhibitors of anandamide inactivation with cannabimimetic activity in vitro and in vivo. Biochem J. 2000;351:817–824. [PMC free article] [PubMed] [Google Scholar]
- 19.Porter AC, Sauer JM, Knierman MD, Becker GW, Berna MJ, Bao J, Nomikos GG, Carter P, Bymaster FP, Leese AB, Felder CC. Characterization of a novel endocannabinoid, virodhamine, with antagonist activity at the CB1 receptor. J Pharmacol Exp Ther. 2002;301:1020–1024. doi: 10.1124/jpet.301.3.1020. [DOI] [PubMed] [Google Scholar]
- 20.Hanus L, Abu-Lafi S, Fride E, Breuer A, Vogel Z, Shalev DE, Kustanovich I, Mechoulam R. 2-arachidonyl glyceryl ether, an endogenous agonist of the cannabinoid CB1 receptor. Proc Natl Acad Sci U S A. 2001;98:3662–3665. doi: 10.1073/pnas.061029898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Edgemond WS, Hillard CJ, Falck JR, Kearn CS, Campbell WB. Human platelets and polymorphonuclear leukocytes synthesize oxygenated derivatives of arachidonylethanolamide (anandamide): their affinities for cannabinoid receptors and pathways of inactivation. Mol Pharmacol. 1998;54:180–188. doi: 10.1124/mol.54.1.180. [DOI] [PubMed] [Google Scholar]
- 22.Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. Proc Natl Acad Sci U S A. 1995;92:3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin G, Tao Q, Abood ME. Cloning and pharmacological characterization of the rat CB2 cannabinoid receptor. J Pharmacol Exp Ther. 2000;292:886–894. [PubMed] [Google Scholar]
- 24.Lo Verme J, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. The nuclear receptor peroxisome proliferator-activated receptor-a mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. 2005;67:15–19. doi: 10.1124/mol.104.006353. [DOI] [PubMed] [Google Scholar]
- 25.Ryberg E, Larsson N, Sjögren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 or CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White R, Ho WS, Bottrill FE, Ford WR, Hiley CR. Mechanisms of anandamide-induced vasorelaxation in rat isolated coronary arteries. Br J Pharmacol. 2001;134:921–929. doi: 10.1038/sj.bjp.0704333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagner JA, Varga K, Járai Z, Kunos G. Mesenteric vasodilation mediated by endothelial anandamide receptors. Hypertension. 1999;33:429–434. doi: 10.1161/01.hyp.33.1.429. [DOI] [PubMed] [Google Scholar]
- 30.Offertáler L, Mo FM, Bátkai S, Liu J, Begg M, Razdan RK, Martin BR, Bukoski RD, Kunos G. Selective ligands and cellular effectors of a G protein-coupled endothelial cannabinoid receptor. Mol Pharmacol. 2003;63:699–705. doi: 10.1124/mol.63.3.699. [DOI] [PubMed] [Google Scholar]
- 31.Milman G, Maor Y, Abu-Lafi S, Horowitz M, Gallily R, Bátkai S, Mo FM, Offertáler L, Pacher P, Kunos G, Mechoulam R. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc Natl Acad Sci U S A. 2006;103:2428–2433. doi: 10.1073/pnas.0510676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baker D, Pryce G, Croxford JL, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- 33.Baker D, Pryce G, Davies WL, Hiley CR. In silico patent searching reveals a new cannabinoid receptor. Trends Pharmacol Sci. 2006;27:1–4. doi: 10.1016/j.tips.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 34.Johns DG, Behm DJ, Walker DJ, Ao Z, Shapland EM, Daniels DA, Riddick M, Dowell S, Staton PC, Green P, Shabon U, Bao W, Aiyar N, Yue TL, Brown AJ, Morrison AD, Douglas SA. The novel endocannabinoid receptor GPR55 is activated by atypical cannabinoids but does not mediate their vasodilator effects. Br J Pharmacol. 2007;152:825–831. doi: 10.1038/sj.bjp.0707419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oka S, Nakajima K, Yamashita A, Kishimoto S, Sugiura T. Identification of GPR55 as a lysophosphatidylinositol receptor. Biochem Biophys Res Commun. 2007;362:928–934. doi: 10.1016/j.bbrc.2007.08.078. [DOI] [PubMed] [Google Scholar]
- 36.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. 1999;400:452–457. doi: 10.1038/22761. [DOI] [PubMed] [Google Scholar]
- 37.Zygmunt PM, Andersson DA, Hogestatt ED. Δ9-tetrahydrocannabinol and cannabinol activate capsaicin-sensitive sensory nerves via a CB1 and CB2 cannabinoid receptor-independent mechanism. J Neurosci. 2002;22:4720–4727. doi: 10.1523/JNEUROSCI.22-11-04720.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pan HL, Chen SR. Sensing tissue ischemia: another new function for capsaicin receptors? Circulation. 2004;110:1826–1831. doi: 10.1161/01.CIR.0000142618.20278.7A. [DOI] [PubMed] [Google Scholar]
- 39.Pacher P, Bátkai S, Kunos G. Haemodynamic profile and responsiveness to anandamide of TRPV1 receptor knock-out mice. J Physiol. 2004;558:647–657. doi: 10.1113/jphysiol.2004.064824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun Y, Alexander SP, Garle MJ, Gibson CL, Hewitt K, Murphy SP, Kendall DA, Bennett AJ. Cannabinoid activation of PPARa; a novel neuroprotective mechanism. Br J Pharmacol. 2007;152:734–743. doi: 10.1038/sj.bjp.0707478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rockwell CE, Kaminski NE. A cyclooxygenase metabolite of anandamide causes inhibition of interleukin-2 secretion in murine splenocytes. J Pharmacol Exp Ther. 2004;311:683–690. doi: 10.1124/jpet.104.065524. [DOI] [PubMed] [Google Scholar]
- 42.O'Sullivan SE, Tarling EJ, Bennett AJ, Kendall DA, Randall MD. Novel time-dependent vascular actions of Δ9-tetrahydrocannabinol mediated by peroxisome proliferator-activated receptor gamma. Biochem Biophys Res Commun. 2005;337:824–831. doi: 10.1016/j.bbrc.2005.09.121. [DOI] [PubMed] [Google Scholar]
- 43.Sugiura T, Kobayashi Y, Oka S, Waku K. Biosynthesis and degradation of anandamide and 2-arachidonoylglycerol and their possible physiological significance. Prostaglandins Leukot Essent Fatty Acids. 2002;66:173–192. doi: 10.1054/plef.2001.0356. [DOI] [PubMed] [Google Scholar]
- 44.Bisogno T, Howell F, Williams G, Minassi A, Cascio MG, Ligresti A, Matias I, Schiano-Moriello A, Paul P, Williams EJ, Gangadharan U, Hobbs C, Di Marzo V, Doherty P. Cloning of the first sn1-DAG lipases points to the spatial and temporal regulation of endocannabinoid signaling in the brain. J Cell Biol. 2003;163:463–468. doi: 10.1083/jcb.200305129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 46.Okamoto Y, Morishita J, Tsuboi K, Tonai T, Ueda N. Molecular characterization of a phospholipase D generating anandamide and its congeners. J Biol Chem. 2004;279:5298–5305. doi: 10.1074/jbc.M306642200. [DOI] [PubMed] [Google Scholar]
- 47.Moesgaard B, Petersen G, Mortensen SA, Hansen HS. Substantial species differences in relation to formation and degradation of N-acyl-ethanolamine phospholipids in heart tissue: an enzyme activity study. Comp Biochem Physiol B Biochem Mol Biol. 2002;131:475–482. doi: 10.1016/s1096-4959(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 48.Leung D, Saghatelian A, Simon GM, Cravatt BF. Inactivation of N-acyl phosphatidylethanolamine phospholipase D reveals multiple mechanisms for the biosynthesis of endocannabinoids. Biochemistry. 2006;45:4720–4726. doi: 10.1021/bi060163l. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Marzo V, Bisogno T, De Petrocellis L. Endocannabinoids and related compounds: walking back and forth between plant natural products and animal physiology. Chem Biol. 2007;14:741–756. doi: 10.1016/j.chembiol.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 50.Liu J, Wang L, Harvey-White J, Osei-Hyiaman D, Razdan R, Gong Q, Chan AC, Zhou Z, Huang BX, Kim HY, Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simon GM, Cravatt BF. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for a/b-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- 52.Beltramo M, Stella N, Calignano A, Lin SY, Makriyannis A, Piomelli D. Functional role of high-affinity anandamide transport, as revealed by selective inhibition. Science. 1997;277:1094–1097. doi: 10.1126/science.277.5329.1094. [DOI] [PubMed] [Google Scholar]
- 53.Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci U S A. 2003;100:4269–4274. doi: 10.1073/pnas.0730816100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cravatt BF, Giang DK, Mayfield SP, Boger DL, Lerner RA, Gilula NB. Molecular characterization of an enzyme that degrades neuromodulatory fatty-acid amides. Nature. 1996;384:83–87. doi: 10.1038/384083a0. [DOI] [PubMed] [Google Scholar]
- 55.Vandevoorde S, Lambert DM. Focus on the three key enzymes hydrolysing endocannabinoids as new drug targets. Curr Pharm Des. 2005;11:2647–2668. doi: 10.2174/1381612054546914. [DOI] [PubMed] [Google Scholar]
- 56.Karlsson M, Contreras JA, Hellman U, Tornqvist H, Holm C. cDNA cloning, tissue distribution, and identification of the catalytic triad of monoglyceride lipase. Evolutionary relationship to esterases, lysophospholipases, and haloperoxidases. J Biol Chem. 1997;272:27218–27223. doi: 10.1074/jbc.272.43.27218. [DOI] [PubMed] [Google Scholar]
- 57.Bátkai S, Rajesh M, Mukhopadhyay P, Haskó G, Liaudet L, Cravatt BF, Csiszar A, Ungvári Z, Pacher P. Decreased age-related cardiac dysfunction, myocardial nitrative stress, inflammatory gene expression, and apoptosis in mice lacking fatty acid amide hydrolase. Am J Physiol. 2007;293:H909–918. doi: 10.1152/ajpheart.00373.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmid PC, Schwartz KD, Smith CN, Krebsbach RJ, Berdyshev EV, Schmid HH. A sensitive endocannabinoid assay. The simultaneous analysis of N-acylethanolamines and 2-monoacylglycerols. Chem Phys Lipids. 2000;104:185–191. doi: 10.1016/s0009-3084(99)00124-3. [DOI] [PubMed] [Google Scholar]
- 59.Deutsch DG, Goligorsky MS, Schmid PC, Krebsbach RJ, Schmid HH, Das SK, Dey SK, Arreaza G, Thorup C, Stefano G, Moore LC. Production and physiological actions of anandamide in the vasculature of the rat kidney. J Clin Invest. 1997;100:1538–1546. doi: 10.1172/JCI119677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pratt PF, Hillard CJ, Edgemond WS, Campbell WB. N-arachidonylethanolamide relaxation of bovine coronary artery is not mediated by CB1 cannabinoid receptor. Am J Physiol. 1998;274:H375–381. doi: 10.1152/ajpheart.1998.274.1.H375. [DOI] [PubMed] [Google Scholar]
- 61.Sugiura T, Kodaka T, Nakane S, Kishimoto S, Kondo S, Waku K. Detection of an endogenous cannabimimetic molecule, 2-arachidonoylglycerol, and cannabinoid CB1 receptor mRNA in human vascular cells: is 2-arachidonoylglycerol a possible vasomodulator? Biochem Biophys Res Commun. 1998;243:838–843. doi: 10.1006/bbrc.1998.8187. [DOI] [PubMed] [Google Scholar]
- 62.Di Marzo V, Bisogno T, De Petrocellis L, Melck D, Orlando P, Wagner JA, Kunos G. Biosynthesis and inactivation of the endocannabinoid 2-arachidonoylglycerol in circulating and tumoral macrophages. Eur J Biochem. 1999;264:258–267. doi: 10.1046/j.1432-1327.1999.00631.x. [DOI] [PubMed] [Google Scholar]
- 63.De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett. 2000;483:52–56. doi: 10.1016/s0014-5793(00)02082-2. [DOI] [PubMed] [Google Scholar]
- 64.Patricelli MP, Patterson JE, Boger DL, Cravatt BF. An endogenous sleep-inducing compound is a novel competitive inhibitor of fatty acid amide hydrolase. Bioorg Med Chem Lett. 1998;8:613–618. doi: 10.1016/s0960-894x(98)00073-0. [DOI] [PubMed] [Google Scholar]
- 65.Boger DL, Sato H, Lerner AE, Hedrick MP, Fecik RA, Miyauchi H, Wilkie GD, Austin BJ, Patricelli MP, Cravatt BF. Exceptionally potent inhibitors of fatty acid amide hydrolase: the enzyme responsible for degradation of endogenous oleamide and anandamide. Proc Natl Acad Sci U S A. 2000;97:5044–5049. doi: 10.1073/pnas.97.10.5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kathuria S, Gaetani S, Fegley D, Valino F, Duranti A, Tontini A, Mor M, Tarzia G, La Rana G, Calignano A, Giustino A, Tattoli M, Palmery M, Cuomo V, Piomelli D. Modulation of anxiety through blockade of anandamide hydrolysis. Nat Med. 2003;9:76–81. doi: 10.1038/nm803. [DOI] [PubMed] [Google Scholar]
- 67.Hiley CR, Hoi PM. Oleamide: a fatty acid amide signaling molecule in the cardiovascular system? Cardiovasc Drug Rev. 2007;25:46–60. doi: 10.1111/j.1527-3466.2007.00004.x. [DOI] [PubMed] [Google Scholar]
- 68.Randall MD, Kendall DA, O'Sullivan S. The complexities of the cardiovascular actions of cannabinoids. Br J Pharmacol. 2004;142:20–26. doi: 10.1038/sj.bjp.0705725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mittleman MA, Lewis RA, Maclure M, Sherwood JB, Muller JE. Triggering myocardial infarction by marijuana. Circulation. 2001;103:2805–2809. doi: 10.1161/01.cir.103.23.2805. [DOI] [PubMed] [Google Scholar]
- 70.Wagner JA, Varga K, Ellis EF, Rzigalinski BA, Martin BR, Kunos G. Activation of peripheral CB1 cannabinoid receptors in haemorrhagic shock. Nature. 1997;390:518–521. doi: 10.1038/37371. [DOI] [PubMed] [Google Scholar]
- 71.Ruilope LM, Despres JP, Scheen A, Pi-Sunyer X, Mancia G, Zanchetti A, Van Gaal L. Effect of rimonabant on blood pressure in overweight/obese patients with/without co-morbidities: analysis of pooled RIO study results. J Hypertens. 2008;26:357–367. doi: 10.1097/HJH.0b013e3282f2d625. [DOI] [PubMed] [Google Scholar]
- 72.Wagner JA, Hu K, Bauersachs J, Karcher J, Wiesler M, Goparaju SK, Kunos G, Ertl G. Endogenous cannabinoids mediate hypotension after experimental myocardial infarction. J Am Coll Cardiol. 2001;38:2048–2054. doi: 10.1016/s0735-1097(01)01671-0. [DOI] [PubMed] [Google Scholar]
- 73.Bátkai S, Pacher P, Járai Z, Wagner JA, Kunos G. Cannabinoid antagonist SR-141716 inhibits endotoxic hypotension by a cardiac mechanism not involving CB1 or CB2 receptors. Am J Physiol. 2004;287:H595–600. doi: 10.1152/ajpheart.00184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ho WS, Hiley CR. Endothelium-independent relaxation to cannabinoids in rat-isolated mesenteric artery and role of Ca2+ influx. Br J Pharmacol. 2003;139:585–597. doi: 10.1038/sj.bjp.0705280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lagneux C, Lamontagne D. Involvement of cannabinoids in the cardioprotection induced by lipopolysaccharide. Br J Pharmacol. 2001;132:793–796. doi: 10.1038/sj.bjp.0703902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kilbourn RG, Jubran A, Gross SS, Griffith OW, Levi R, Adams J, Lodato RF. Reversal of endotoxin-mediated shock by NG-methyl-L-arginine, an inhibitor of nitric oxide synthesis. Biochem Biophys Res Commun. 1990;172:1132–1138. doi: 10.1016/0006-291x(90)91565-a. [DOI] [PubMed] [Google Scholar]
- 77.Schulz R, Nava E, Moncada S. Induction and potential biological relevance of a Ca2+-independent nitric oxide synthase in the myocardium. Br J Pharmacol. 1992;105:575–580. doi: 10.1111/j.1476-5381.1992.tb09021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Shmist YA, Goncharov I, Eichler M, Shneyvays V, Isaac A, Vogel Z, Shainberg A. Delta-9-tetrahydrocannabinol protects cardiac cells from hypoxia via CB2 receptor activation and nitric oxide production. Mol Cell Biochem. 2006;283:75–83. doi: 10.1007/s11010-006-2346-y. [DOI] [PubMed] [Google Scholar]
- 79.White R, Hiley CR. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Joyeux M, Arnaud C, Godin-Ribuot D, Demenge P, Lamontagne D, Ribuot C. Endocannabinoids are implicated in the infarct size-reducing effect conferred by heat stress preconditioning in isolated rat hearts. Cardiovasc Res. 2002;55:619–625. doi: 10.1016/s0008-6363(02)00268-7. [DOI] [PubMed] [Google Scholar]
- 81.Di Filippo C, Rossi F, Rossi S, D'Amico M. Cannabinoid CB2 receptor activation reduces mouse myocardial ischemia-reperfusion injury: involvement of cytokine/chemokines and PMN. J Leukoc Biol. 2004;75:453–459. doi: 10.1189/jlb.0703303. [DOI] [PubMed] [Google Scholar]
- 82.Wagner JA, Abesser M, Harvey-White J, Ertl G. 2-Arachidonylglycerol acting on CB1 cannabinoid receptors mediates delayed cardioprotection induced by nitric oxide in rat isolated hearts. J Cardiovasc Pharmacol. 2006;47:650–655. doi: 10.1097/01.fjc.0000211752.08949.eb. [DOI] [PubMed] [Google Scholar]
- 83.Kola B, Hubina E, Tucci SA, Kirkham TC, Garcia EA, Mitchell SE, Williams LM, Hawley SA, Hardie DG, Grossman AB, Korbonits M. Cannabinoids and ghrelin have both central and peripheral metabolic and cardiac effects via AMP-activated protein kinase. J Biol Chem. 2005;280:25196–25201. doi: 10.1074/jbc.C500175200. [DOI] [PubMed] [Google Scholar]
- 84.Ceolotto G, Gallo A, Papparella I, Franco L, Murphy E, Iori E, Pagnin E, Fadini GP, Albiero M, Semplicini A, Avogaro A. Rosiglitazone reduces glucose-induced oxidative stress mediated by NAD(P)H oxidase via AMPK-dependent mechanism. Arterioscler Thromb Vasc Biol. 2007;27:2627–2633. doi: 10.1161/ATVBAHA.107.155762. [DOI] [PubMed] [Google Scholar]
- 85.Underdown NJ, Hiley CR, Ford WR. Anandamide reduces infarct size in rat isolated hearts subjected to ischaemia-reperfusion by a novel cannabinoid mechanism. Br J Pharmacol. 2005;146:809–816. doi: 10.1038/sj.bjp.0706391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mukhopadhyay P, Bátkai S, Rajesh M, Czifra N, Harvey-White J, Haskó G, Zsengeller Z, Gerard NP, Liaudet L, Kunos G, Pacher P. Pharmacological inhibition of CB1 cannabinoid receptor protects against doxorubicin-induced cardiotoxicity. J Am Coll Cardiol. 2007;50:528–536. doi: 10.1016/j.jacc.2007.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Howlett AC, Mukhopadhyay S, Norford DC. Endocannabinoids and reactive nitrogen and oxygen species in neuropathologies. J Neuroimmune Pharmacol. 2006;1:305–316. doi: 10.1007/s11481-006-9022-6. [DOI] [PubMed] [Google Scholar]
- 88.Hajrasouliha AR, Tavakoli S, Ghasemi M, Jabehdar-Maralani P, Sadeghipour H, Ebrahimi F, Dehpour AR. Endogenous cannabinoids contribute to remote ischemic preconditioning via cannabinoid CB2 receptors in the rat heart. Eur J Pharmacol. 2008;579:246–252. doi: 10.1016/j.ejphar.2007.09.034. [DOI] [PubMed] [Google Scholar]
- 89.Krylatov AV, Ugdyzhekova DS, Bernatskaya NA, Maslov LN, Mekhoulam R, Pertwee RG, Stephano GB. Activation of type II cannabinoid receptors improves myocardial tolerance to arrhythmogenic effects of coronary occlusion and reperfusion. Bull Exp Biol Med. 2001;131:523–525. doi: 10.1023/a:1012381914518. [DOI] [PubMed] [Google Scholar]
- 90.Rajesh M, Mukhopadhyay P, Bátkai S, Haskó G, Liaudet L, Huffman JW, Csiszar A, Ungvari Z, Mackie K, Chatterjee S, Pacher P. CB2-receptor stimulation attenuates TNF-a-induced human endothelial cell activation, transendothelial migration of monocytes, and monocyte-endothelial adhesion. Am J Physiol. 2007;293:H2210–2218. doi: 10.1152/ajpheart.00688.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pacher P, Haskó G. Endocannabinoids and cannabinoid receptors in ischaemia-reperfusion injury and preconditioning. Br J Pharmacol. 2008;153:252–262. doi: 10.1038/sj.bjp.0707582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Motobe T, Hashiguchi T, Uchimura T, Yamakuchi M, Taniguchi N, Komiya S, Maruyama I. Endogenous cannabinoids are candidates for lipid mediators of bone cement implantation syndrome. Shock. 2004;21:8–12. doi: 10.1097/01.SHK.0000094766.36694.49. [DOI] [PubMed] [Google Scholar]
- 93.Bátkai S, Mukhopadhyay P, Harvey-White J, Kechrid R, Pacher P, Kunos G. Endocannabinoids acting at CB1 receptors mediate the cardiac contractile dysfunction in vivo in cirrhotic rats. Am J Physiol. 2007;293:H1689–1695. doi: 10.1152/ajpheart.00538.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pacher P, Bátkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev. 2006;58:389–462. doi: 10.1124/pr.58.3.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jugdutt BI. Prevention of ventricular remodelling post myocardial infarction: timing and duration of therapy. Can J Cardiol. 1993;9:103–114. [PubMed] [Google Scholar]
- 96.Wagner JA, Hu K, Karcher J, Bauersachs J, Schafer A, Laser M, Han H, Ertl G. CB1 cannabinoid receptor antagonism promotes remodeling and cannabinoid treatment prevents endothelial dysfunction and hypotension in rats with myocardial infarction. Br J Pharmacol. 2003;138:1251–1258. doi: 10.1038/sj.bjp.0705156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hiley CR, Ford WR. Endocannabinoids as mediators in the heart: a potential target for therapy of remodelling after myocardial infarction? Br J Pharmacol. 2003;138:1183–1184. doi: 10.1038/sj.bjp.0705155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Athanasiou A, Clarke AB, Turner AE, et al. Cannabinoid receptor agonists are mitochondrial inhibitors: a unified hypothesis of how cannabinoids modulate mitochondrial function and induce cell death. Biochem Biophys Res Commun. 2007;364:131–137. doi: 10.1016/j.bbrc.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 99.Sarafian TA, Habib N, Oldham M, et al. Inhaled marijuana smoke disrupts mitochondrial energetics in pulmonary epithelial cells in vivo. Am J Physiol. 2006;290:L1202–1209. doi: 10.1152/ajplung.00371.2005. [DOI] [PubMed] [Google Scholar]
- 100.Athanasiou A, Clarke AB, Turner AE, Kumaran NM, Vakilpour S, Smith PA, Bagiokou D, Bradshaw TD, Westwell AD, Fang L, Lobo DN, Constantinescu CS, Calabrese V, Loesch A, Alexander SP, Clothier RH, Kendall DA, Bates TE. Vanilloid receptor agonists and antagonists are mitochondrial inhibitors: how vanilloids cause non-vanilloid receptor mediated cell death. Biochem Biophys Res Commun. 2007;354:50–55. doi: 10.1016/j.bbrc.2006.12.179. [DOI] [PubMed] [Google Scholar]
- 101.Steffens S, Veillard NR, Arnaud C, Pelli G, Burger F, Staub C, Karsak M, Zimmer A, Frossard JL, Mach F. Low dose oral cannabinoid therapy reduces progression of atherosclerosis in mice. Nature. 2005;434:782–786. doi: 10.1038/nature03389. [DOI] [PubMed] [Google Scholar]
- 102.Maccarrone M, Bari M, Battista N, Finazzi-Agro A. Estrogen stimulates arachidonoylethanolamide release from human endothelial cells and platelet activation. Blood. 2002;100:4040–4048. doi: 10.1182/blood-2002-05-1444. [DOI] [PubMed] [Google Scholar]
- 103.Rajesh M, Mukhopadhyay P, Hasko G, Huffman JW, Mackie K, Pacher P. CB2 cannabinoid receptor agonists attenuate TNF-a-induced human vascular smooth muscle cell proliferation and migration. Br J Pharmacol. 2008;153:347–357. doi: 10.1038/sj.bjp.0707569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Christensen R, Kristensen PK, Bartels EM, Bliddal H, Astrup A. Efficacy and safety of the weight-loss drug rimonabant: a meta-analysis of randomised trials. Lancet. 2007;370:1706–1713. doi: 10.1016/S0140-6736(07)61721-8. [DOI] [PubMed] [Google Scholar]
- 105.Fajardo G, Bernstein D. Endocannabinoid inhibition: a new cardioprotective strategy against doxorubicin cardiotoxicity. J Am Coll Cardiol. 2007;50:537–539. doi: 10.1016/j.jacc.2007.04.052. [DOI] [PubMed] [Google Scholar]