Abstract
Purpose
To describe a new method of culturing mouse corneal epithelial cells (MCECs).
Methods
MCECs were isolated from C57/BL6 mouse corneas and cultured on type-I collagen-coated plastic dishes in low-calcium progenitor cell targeting medium (CnT-50). Expression of the mRNAs of N-terminal truncated isoform of p63 (DNp63), cytokeratin 12 (K12), and cytokeratin 14 (K14) were determined by reverse transcription-polymerase chain reaction (RT-PCR). To examine the differentiation capabilities, passage 3 (P3) MCECs at confluence were subcultured on amniotic membrane (AM) in a differentiation medium (CnT-30) until confluence. At confluence, 1 mM calcium was added and cultured for 4 more days. The expression of K12 in the stratified MCECs was analyzed by immunostaining.
Results
The MCECs cultured in CnT-50 proliferated until at least P10. The number of cells at confluency at P3 was 61.8 (SD ±9.4, n=5) times that at P0. MCECs cultured on AM in CnT-30 with addition of calcium were stratified up to two to three layers, and the stratified MCECs expressed K12. DNp63 mRNA was continuously expressed throughout the different passages, and K12 mRNA was detected in P0 cells and the stratified MCECs on AM.
Conclusions
Cultured MCECs maintain their proliferation and differentiation capabilities as well as their corneal epithelial cell characteristics. These results suggest that MCECs produced by this culturing method provide a useful experimental model which can enable further development of research of the corneal epithelium.
Introduction
Mice are used in many different types of studies for several reasons: they are well-suited for genetic manipulations such as genomic sequence analyses; different strains with different characteristics are readily available, and many transgenic (Tg) and knockout strains have been created and are commercially available. Furthermore, in vitro approaches with cultured mouse cells allow the investigation of tissue or cell specific properties.
To investigate the physiological and pathological conditions of corneal epithelial cells, many corneal epithelial cell lines and primary culture systems have been established for humans and rabbits [1-6]. However, there have been few reports regarding the creation of a corneal epithelial cell line and primary culture system for mice.
Hazlett et al. [7] developed a method for short-term cultures of primary mouse corneal epithelial cells (MCECs), although they failed to subculture the cells past passage three, and the cultures may have been contaminated by fibroblasts. Since then, some researchers reported on the results of in vitro examinations of primary MCECs, and they were able to study these cells without any effect from adjacent tissue cells and matrices [8,9]. Unfortunately, a large number of eyes were used to obtain sufficient number of cells for the primary cultures, and the culture conditions were not stable among the different experimental groups.
Recently, Kawakita et al. [10] and Ma et al. [11] demonstrated that long-term cultures of MCECs could be achieved by culturing MCECs in keratinocyte serum-free medium. Although their technique required several weeks to establish a stable cell line and the probability of the establishment was 55%, there was a possibility that their method could establish a MCEC line. However, there was still some concern on whether the cells in this cell line maintained corneal properties, e.g., expression of ketratin 12.
Because it is important to have sufficient number of MCECs to perform reproducible experiments and to reduce the number of experimental animals used, it is necessary to develop an easily repeatable method to culture and grow MCECs that maintain the properties of normal MCECs. Thus, the purpose of this study was to develop a simple and reproducible method for culturing MCECs that will allow the cells to retain their proliferation and differentiation capabilities. To accomplish this, we used a low-calcium, low-bovine pituitary extract (BPE), serum-free progenitor cell targeted medium to culture MCECs.
Methods
Tissue preparation and cell culture
C57/BL6 mice (CLEA Japan Inc, Tokyo, Japan), aged 4-8 weeks, were handled in accordance with the guidelines in the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Intact and viable MCEC sheets were prepared as described with some modifications [11]. In brief, the eyes were enucleated from the euthanized animals and incubated in DMEM/F12 (1:1 mixture; Invitrogen, Tokyo, Japan) containing 15 mg/ml dispase II (Roche Diagnostics, Basel, Switzerland), 100 mM sorbitol, and antibiotic-antimycotic (1X; Invitrogen) for 18 h at 4 °C. The loosened corneal epithelial sheets were peeled off with forceps and incubated in 100 µl of 0.25% trypsin (Invitrogen) for 10 min at 37 °C. To inhibit the activity of trypsin, 100 µl of 2 mg/ml soybean trypsin inhibitor (Roche Diagnostics) in PBS(-) was added to the medium, and the sheets were separated into single cells by pipetting. Then 2 ml of low-calcium, low- bovine pituitary extract (BPE), serum-free progenitor cell targeted medium (CnT-50; CELLnTEC, Bern, Switzerland) or low-calcium, serum- and BPE-free progenitor cell targeted medium (CnT-20; CELLnTEC) was added to the isolated cells. The cells were then transferred to type-I collagen coated 35 mm plastic dishes (Asahi Techno Glass, Funabashi, Japan). The cultures were incubated at 37 °C under 95% humidity and 5% CO2. The medium was changed every 2 to 3 days. Confluent cultures of MCECs were subcultured at a density 1×104 cells/cm2.
Preparation of cultured corneal epithelial cell sheets
To examine the differentiation potential of the MCECs, confluent cells at passage 3 (P3) were subcultured on amniotic membrane (AM) in low-calcium, serum- and BPE-free differentiation medium (CnT-30; CELLnTEC). When the cells had reached confluency, the medium (CnT-30) was changed to CnT-30 supplemented with calcium (1 mM) and the medium was changed daily for 4 days. The MCEC sheets on the AM were collected.
RNA extraction and reverse transcription-polymerase chain reaction (RT-PCR)
For RNA extraction, the epithelial surface of normal 4-week-old C57/BL6 mouse corneas were scraped with a scalpel, and the epidermal layer was excised with scissors. Total RNA was extracted from normal mouse corneal epithelia (MCE) and normal mice epidermal (MED) tissue, and also from MCEC sheets, and MCECs harvested from confluent primary culture (P0) and P3 with RNeasy Mini kit (Qiagen, Tokyo, Japan). cDNAs were made by reverse transcription of the total RNA with the SuperScript VILO cDNA Synthesis Kit (Invitrogen) according to the manufacturer’s protocol.
PCR was performed with AmpliTaq Gold PCR Master Mix (Applied Biosystems, Tokyo, Japan) in 20 µl containing 2 µl of cDNA, 0.25 µM concentrations of primer, and 10 µl of the 2X PCR Master Mix. The mixture was subjected to 30 cycles of 15 s at 95 °C for denaturation, 30 s at 60° C for primer annealing and extension. The PCR primers are listed in Table 1.
Table 1. PCR primers used in this study.
| Target | Primer | Primer sequence | Amplicon length | GenBank accession |
|---|---|---|---|---|
| K12 |
Forward |
ACTAGAGCCGACCTGGAAGC |
158 |
NM_010661 |
| Reverse |
ACCTTGGTGAGATCCACTCC |
|||
| K14 |
Forward |
ACCGCAAGGATGCTGAGGA* |
103 |
NM_016958 |
| Reverse |
GAAATCTCACTCTTGCCGCTCTG* |
|||
| DNp63 |
Forward |
CTGGAAAACAATGCCCAGACTCA |
126 |
AF075437
, AF075438, AF075439 |
| Reverse |
TGCGTGGTCTGTGTTGTAGG |
|||
| ß-actin | Forward |
CATCCGTAAAGACCTCTATGCCAAC* |
171 | NM_007393 |
| Reverse | ATGGAGCCACCGATCCACA* |
The asterisk indicates that the primer sequences for K14 and ß-actin were designed by Takara Bio Inc. (Otsu, Japan).
Immunohistochemical analyses
MCEC sheets were fixed in 4% paraformaldehyde/phosphate-buffered saline (PBS) at 4 °C. After fixation and dehydration in a graded series of ethanol, the samples were embedded in paraffin and cut into 5 µm sections. The sections were blocked in a solution containing 2% rabbit serum, 2% BSA, and 0.1% Triton-X 100 in PBS for 1 h, followed by incubation with 0.2 mg/ml of goat anti-cytokeratin 12 (Santa Cruz Biotechnology, Inc. Santa Cruz, CA) in CanGetSignal A (TOYOBO, Osaka, Japan) overnight at 4 °C. Immunoreactivity to primary antibodies was made visible by secondary antibody conjugated with FITC (Vector Laboratories, Burlingame, CA). After rinsing in PBS, the sections were mounted on glass slides with ProLong Gold Antifade Reagent with DAPI (Invitrogen).
Results
Characteristics of cultured MCECs
Mouse corneal epithelial cells (MCECs), which were cultured on type-I collagen coated plastic dishes in low calcium, low BPE, serum-free medium (CnT-50), continued to proliferate until at least P10. The MCECs had a cobble stone-like appearance which did not change throughout the 10 passages (Figure 1A-C).
Figure 1.

Phase contrast photomicrographs of mouse corneal epithelial cells (MCECs). MCECs at confluence in CnT-50 medium at different passages; A, passage 0 (P0); B, P3, and C, P10. MCECs at confluency at P3 cultured in CnT20 (D). Scale bar; 100 μm.
The MCECs in low-calcium, serum- and BPE-free medium (CnT-20) were also able to proliferation until at least P3 (Figure 1D), but the rate of proliferation of MCECs in CnT-20 was considerably slower than in CnT-50 (data not shown). The proliferation curve of MCECs in CnT-50 is shown in Figure 2, and each value was the mean of five experiments. The number of cells was counted at confluence of each passage, and was normalized to the value at P0. The means±standard deviations of cells at P1, P2, and P3 were 6.7±5.2 (n=5) times, 16.7±3.6 (n=5) times, and 61.8±9.4 (n=5) times that at P0. The interval from primary cell seeding to confluence of P3 ranged from 24 to 52 days.
Figure 2.

Growth of MCECs cultured in CnT-50. Each point is the mean of five individual experiments. The number of cells at confluency at each passage was counted and normalized to the number at P0. Error bar represents the standard deviations.
RT-PCR
DNp63, K12, and K14 mRNA expression was detected in normal mouse cornea, and DNp63 and K14 but not K12 were detected in normal mouse epidermis (Figure 3). The expression of DNp63 and K14 mRNA was detected in cells from P0 and P3 of MCECs and MCEC sheets, while the expression of K12 mRNA was detected in MCECs at P0 and MCEC sheets but not at P3 (Figure 3).
Figure 3.
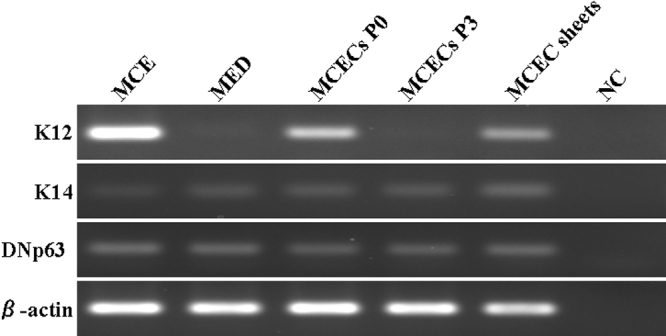
K12, K14, and DNp63 mRNA expression. K12, K14, and DNp63 mRNA expression in normal mouse corneal epithelia (MCE), normal mouse epidermis (MED), MCECs at confluence at P0 and P3, and MCEC sheets. PCR products were analyzed by 1.5% agarose gel electrophoresis. PCRs with no DNA template were used as negative control (NC).
Differentiation potential of cultured MCEs
MCECs were subcultured on AM in serum- and BPE-free differentiation medium (CnT-30) supplemented with 1 mM of calcium. The cells proliferated and were stratified to two to three layers by four days (Figure 4A). Immunoreactivity to K12, a corneal epithelium specific differentiation marker, was detected in the stratified MCECs as seen in normal mouse corneal epithelia (Figure 4B,D). The MCEC sheets did not stain with nonspecific goat IgG (Figure 4C).
Figure 4.
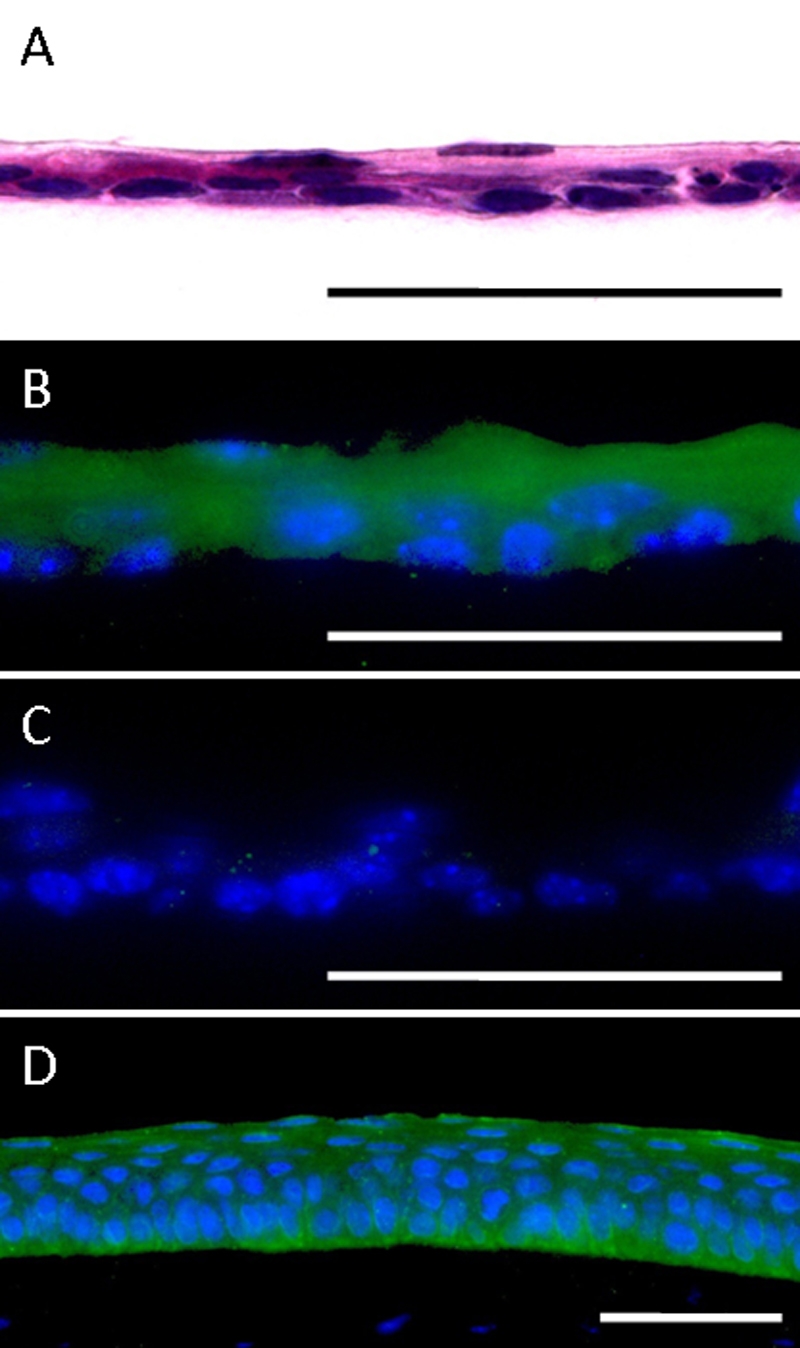
Immunohistochemical staining of MCEC sheets. Paraffin sections (5 µm) were stained with; A = H&E, B = immunostained with anti-cytokeratin 12, and C = normal goat IgG. Normal mouse cornea was immunostained with anti-cytokeratin 12 (D). Scale bar; 50 µm.
Discussion
Our results demonstrated that a simple culture method can maintain MCECs up to P10. The MCECs were cultured on type-I collagen-coated plastic dishes in low-calcium serum-free medium, which is commercially available. The MCECs maintained their proliferation and differentiation capabilities, and had corneal epithelial cell characteristics.
Low-calcium, serum-free, or no BPE human corneal epithelial progenitor cell targeted (PCT) media (CnT-50 or CnT-20) have been used for isolation and proliferation studies of early passage human CECs [12,13]. Although the number of studies using these media has been limited, the PCT media appears to be effective for culturing human CECs. Because we were not aware of any studies using the PCT medium for MCECs, we have examined its effectiveness for MCECs. We found that the MCECs cultured in CnT-50 continued to proliferate until at least P10, and the morphology of the cells was not altered throughout the ten passages (Figure 1). A relatively faster rate of proliferation was observed in a medium containing low concentrations of BPE (CnT-50) than in a complete defined medium (CnT-20), although the morphology of the cells was not different (Figure 1).
Recently, Kawakita, et al. [11] and Ma, et al. [10] reported on methods for culturing MCECs, however, it required several weeks to establish a stable cell line of MCECs, and the probability of the establishing the cell line was 55%. On the other hand, we attempted to culture MCECs five times by our method, and were successful each time. Our analyses showed that the MCECs had proliferated to 61.8 times (ranged from 54.5 to 76.9) that at P0 within the three passages (Figure 2).
In the cultured MCECs, the expression of DNp63 mRNA was detected throughout the passages examined (Figure 3). DNp63 is known to be a transcription factor that is essential for the differentiation of progenitor stratified squamous epithelial cells, e.g., corneal epithelial cells and epidermal cells [14,15]. During the differentiation of stratified squamous epithelial cells, DNp63 is expressed in the basal cells, but not in differentiated cells [14,15]. These results suggest that the differentiation capability was well maintained in MCECs cultured in CnT-50.
It has been recognized that multiple passaged cultured cells may alter their cellular properties. Indeed at P3, the MCECs cultured in CnT-50 did not express K12 mRNA. Therefore, we next examined whether the MCECs maintain the corneal epithelial characteristics as well as the differentiation potential. Confluent P3 MCECs cultured on AM in differentiation medium (CnT-30) supplemented with 1 mM calcium expressed K12 and were stratified up to two to three layers. Ma et al. [10] also demonstrated the differentiation potential and K12 expression in cultured MCECs, however they failed to detect K12 expression at the protein level. On the other hand, our immunohistochemical results clearly showed positive staining of K12 in the stratified MCECs grown on AM as seen in normal mouse cornea. It is well recognized that AMs provide a better microenvironment for corneal epithelial cells, and it has been reported that the use of AM promotes the corneal epithelial cell differentiation [16-18]. Thus, the expression of K12 might be better with the AM, however MCECs cultured in CnT-50 still maintain the corneal epithelial characteristics after P3.
Our analyses also showed that the MCECs proliferated an average of 61.8 times more (range: 54.5 - 76.9) at P3 than at P0 (Figure 2). When cells were collected from ten mouse eyes and cultured to confluence in CnT-50, the number of cells reached 3.0×107 by the third passage. This number is more than sufficient for most in vitro experiments.
In conclusion, a commercially available low-calcium, low-BPE, serum-free medium (CnT-50) made it possible to grow MCECs on type-I collagen coated plastic dishes. The relatively simple and reproducible results of culturing MCECs with CnT-50 should be valuable for laboratory experiments.
Acknowledgments
The authors would like to acknowledge a Grant-in-Aid for Scientific Research (C) 20592079 from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
References
- 1.Araki-Sasaki K, Ohashi Y, Sasabe T, Hayashi K, Watanabe H, Tano Y, Handa H. An SV40-immortalized human corneal epithelial cell line and its characterization. Invest Ophthalmol Vis Sci. 1995;36:614–21. [PubMed] [Google Scholar]
- 2.Adhikary G, Crish J, Lass J, Eckert RL. Regulation of involucrin expression in normal human corneal epithelial cells: a role for activator protein one. Invest Ophthalmol Vis Sci. 2004;45:1080–7. doi: 10.1167/iovs.03-1180. [DOI] [PubMed] [Google Scholar]
- 3.Dey S, Patel J, Anand BS, Jain-Vakkalagadda B, Kaliki P, Pal D, Ganapathy V, Mitra AK. Molecular evidence and functional expression of P-glycoprotein (MDR1) in human and rabbit cornea and corneal epithelial cell lines. Invest Ophthalmol Vis Sci. 2003;44:2909–18. doi: 10.1167/iovs.02-1142. [DOI] [PubMed] [Google Scholar]
- 4.Esco MA, Hazlett LD, Kurpakus-Wheater M. Pseudomonas aeruginosa binds to extracellular matrix deposited by human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2002;43:3654–9. [PubMed] [Google Scholar]
- 5.Morita S, Shirakata Y, Shiraishi A, Kadota Y, Hashimoto K, Higashiyama S, Ohashi Y. Human corneal epithelial cell proliferation by epiregulin and its cross-induction by other EGF family members. Mol Vis. 2007;13:2119–28. [PubMed] [Google Scholar]
- 6.O'Brien WJ, Krema C, Heimann T, Zhao H. Expression of NADPH oxidase in rabbit corneal epithelial and stromal cells in culture. Invest Ophthalmol Vis Sci. 2006;47:853–63. doi: 10.1167/iovs.05-1063. [DOI] [PubMed] [Google Scholar]
- 7.Hazlett L, Masinick S, Mezger B, Barrett R, Kurpakus M, Garrett M. Ultrastructural, immunohistological and biochemical characterization of cultured mouse corneal epithelial cells. Ophthalmic Res. 1996;28:50–6. doi: 10.1159/000267873. [DOI] [PubMed] [Google Scholar]
- 8.Ramaesh T, Ramaesh K, Leask R, Springbett A, Riley SC, Dhillon B, West JD. Increased apoptosis and abnormal wound-healing responses in the heterozygous Pax6+/- mouse cornea. Invest Ophthalmol Vis Sci. 2006;47:1911–7. doi: 10.1167/iovs.05-1028. [DOI] [PubMed] [Google Scholar]
- 9.Levin MH, Verkman AS. Aquaporin-3-dependent cell migration and proliferation during corneal re-epithelialization. Invest Ophthalmol Vis Sci. 2006;47:4365–72. doi: 10.1167/iovs.06-0335. [DOI] [PubMed] [Google Scholar]
- 10.Ma X, Shimmura S, Miyashita H, Yoshida S, Kubota M, Kawakita T, Tsubota K. Long-term culture and growth kinetics of murine corneal epithelial cells expanded from single corneas. Invest Ophthalmol Vis Sci. 2009;50:2716–21. doi: 10.1167/iovs.08-2139. [DOI] [PubMed] [Google Scholar]
- 11.Kawakita T, Espana EM, He H, Yeh LK, Liu CY, Tseng SC. Calcium-induced abnormal epidermal-like differentiation in cultures of mouse corneal-limbal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:3507–12. doi: 10.1167/iovs.04-0266. [DOI] [PubMed] [Google Scholar]
- 12.Qi H, Li DQ, Bian F, Chuang EY, Jones DB, Pflugfelder SC. Expression of glial cell-derived neurotrophic factor and its receptor in the stem-cell-containing human limbal epithelium. Br J Ophthalmol. 2008;92:1269–74. doi: 10.1136/bjo.2007.132431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qi H, Li DQ, Shine HD, Chen Z, Yoon KC, Jones DB, Pflugfelder SC. Nerve growth factor and its receptor TrkA serve as potential markers for human corneal epithelial progenitor cells. Exp Eye Res. 2008;86:34–40. doi: 10.1016/j.exer.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blanpain C, Fuchs E. p63: revving up epithelial stem-cell potential. Nat Cell Biol. 2007;9:731–3. doi: 10.1038/ncb0707-731. [DOI] [PubMed] [Google Scholar]
- 15.Di Iorio E, Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–8. doi: 10.1073/pnas.0503437102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grueterich M, Espana E, Tseng SC. Connexin 43 expression and proliferation of human limbal epithelium on intact and denuded amniotic membrane. Invest Ophthalmol Vis Sci. 2002;43:63–71. [PubMed] [Google Scholar]
- 17.Grueterich M, Espana EM, Tseng SC. Modulation of keratin and connexin expression in limbal epithelium expanded on denuded amniotic membrane with and without a 3T3 fibroblast feeder layer. Invest Ophthalmol Vis Sci. 2003;44:4230–6. doi: 10.1167/iovs.02-0943. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi N, Fullwood NJ, Bairaktaris G, Inatomi T, Kinoshita S, Quantock AJ. Cultivation of corneal epithelial cells on intact and denuded human amniotic membrane. Invest Ophthalmol Vis Sci. 2000;41:2506–13. [PubMed] [Google Scholar]


