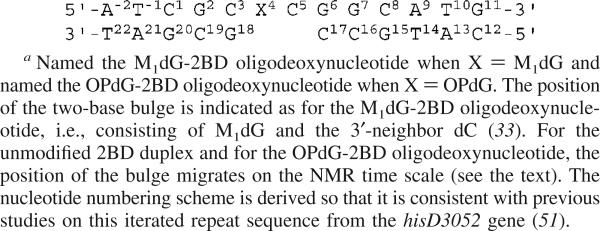Abstract
The OPdG adduct N2-(3-oxo-1-propenyl)dG, formed in DNA exposed to malondialdehyde, was introduced into 5′-d(ATCGCXCGGCATG)-3′•5′-d(CATGCCGCGAT)-3′ at pH 7 (X = OPdG). The OPdG adduct is the base-catalyzed rearrangement product of the M1dG adduct, 3-(β-d-ribofuranosyl)pyrimido[1,2-a]purin-10(3H)-one. This duplex, named the OPdG-2BD oligodeoxynucleotide, was derived from a frameshift hotspot of the Salmonella typhimuium hisD3052 gene and contained a two-base deletion in the complementary strand. NMR spectroscopy revealed that the OPdG-2BD oligodeoxynucleotide underwent rapid bulge migration. This hindered its conversion to the M1dG-2BD duplex, in which the bulge was localized and consisted of the M1dG adduct and the 3′-neighbor dC [Schnetz-Boutaud, N. C., Saleh, S., Marnett, L. J., and Stone, M. P. (2001) Biochemistry 40, 15638−15649]. The spectroscopic data suggested that bulge migration transiently positioned OPdG opposite dC in the complementary strand, hindering formation of the M1dG-2BD duplex, or alternatively, reverting rapidly formed intermediates in the OPdG to M1dG reaction pathway when dC was placed opposite from OPdG. The approach of initially formed M1dG-2BD or OPdG-2BD duplexes to an equilibrium mixture of the M1dG-2BD and OPdG-2BD duplexes was monitored as a function of time, using NMR spectroscopy. Both samples attained equilibrium in ∼140 days at pH 7 and 25 °C.
Introduction
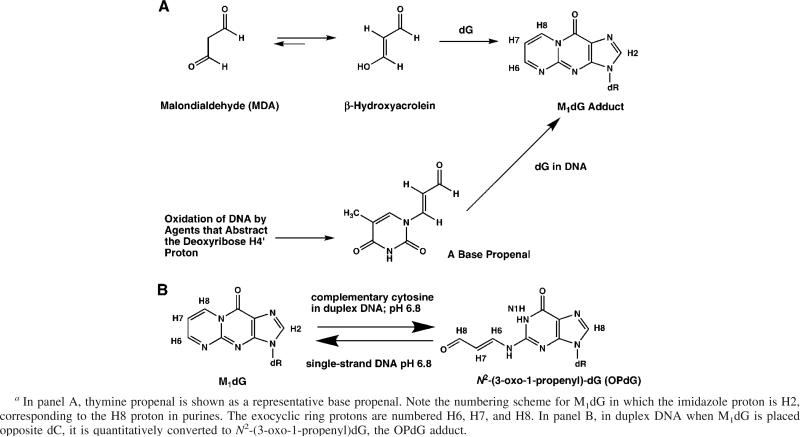
The exocyclic guanine adduct M1dG 1 [3-(2′-deoxy-β-d-erythro-pentofuranosyl)pyrimido[1,2-α]purin-10(3H)-one] arises in DNA from multiple sources. One source is exposure to malondialdehyde (MDA), a toxic and mutagenic metabolite produced by lipid peroxidation and prostaglandin biosynthesis (for a review, see refs 1 and 2). In aqueous solution, MDA exists as β-hydroxyacrolein. It reacts with DNA as a bis-electrophile to form M1dG (3–7). Alternatively, the M1dG adduct arises as a consequence of oxidative damage to DNA, resulting in the formation of base propenals that subsequently transfer their oxopropenyl group to dG (8) (Scheme 1). The base propenals are significantly more potent than MDA in forming M1dG (9).
Scheme 1.
(A) Formation of M1dG from MDA or from Base Propenals and (B) Depiction of M1dG Being Stable in Single-Stranded DNA a
The M1dG adduct has been identified in DNA from rodent (10) and human (11, 12) tissue samples, as have other exocyclic purine lesions (13–16), suggesting their presence in vivo. The levels of M1dG adducts in DNA have been quantitated by mass spectrometric (17, 18), postlabeling (19, 20), and immunochemical (21) techniques. This endogenously formed adduct is the most abundant exocyclic adduct present in human DNA (18–20, 22). It has also been detected at low levels in human urine (23). The low urinary levels of M1dG likely reflect metabolic conversion to the 6-oxo-M1dG derivative (24). M1dG is an efficient premutagenic lesion in Escherichia coli (25, 26), mammalian (26), and human (27) cells. Thus, the M1dG adduct is anticipated to mediate human carcinogenesis.
The M1dG adduct is stable in nucleotides and single-stranded DNA at neutral pH. Under basic conditions, it converts to the N2-(3-oxo-1-propenyl)dG (OPdG) derivative. In contrast, when M1dG is placed at neutral pH into duplex DNA opposite dC, a spontaneous conversion to the OPdG derivative is facilitated. Upon denaturation of the duplex, M1dG is regenerated (Scheme 2). Ring opening does not occur at neutral pH in duplex DNA if thymine is placed opposite from M1dG. These observations suggested that dC in duplex DNA catalyzes the transformation of M1dG to its ring-opened OPdG derivative (28). The ring opening of M1dG as a nucleoside or in oligodeoxynucleotides is a reversible second-order reaction with hydroxide ion (29). The reverse ring closure mechanism involves rapid formation of protonated OPdG and 8-hydroxy-6,7-propenodG intermediates that slowly converts to M1dG in a general acid-catalyzed reaction (30).
Scheme 2.
2BD Oligodeoxynucleotide Duplex Containing a Two-Nucleotide 5′-GpC-3′ Deletion in the Complementary Strand a
A refined structure was obtained for the OPdG adduct in 5′-d(ATCGCXCGGCATG)-3′•5′-d(CATGCCGCGCGAT)-3′ (X =OPdG) (31). This sequence contains the d(CpG)3 frameshift mutation hotspot located in the Salmonella typhimurium hisD3052 gene. The structure of the OPdG adduct embedded in this sequence was of interest because MDA, a small alkylating agent, induced frameshift mutations (32). In this structure, OPdG maintained stacking interactions with neighboring bases. It was not Watson–Crick hydrogen bonded. The cytosine complementary to OPdG was pushed toward the major groove but maintained partial stacking with its neighboring bases. The modified guanine remained in the anti conformation, while the OPdG 3-oxo-1-propenyl moiety was positioned in the minor groove of the duplex (31).
The frameshift mutations observed in vivo in E. coli and in COS-7 cells and associated with the d(CpG)3 iterated repeat included −2 bp deletions (26). Thus, it was of interest to examine oligodeoxynucleotides containinga2bp (CpG) deletion opposite either M1dG or OPdG. Additionally, replication bypass studies conducted in vitro suggested that DNA polymerase β induced two-base deletions when replicating past M1dG in this iterated sequence derived from the S. typhimuium hisD3052 gene. 2 Previous NMR structural studies examined the M1dG adduct in the 5′-d(A−2T−1C1G2C3X4C5G6G7C8A9T10G11)-3′•5′-d(C12A13T14G15C16C17G18C19G20A21T22)-3′ sequence containing a 2 bp deletion in the complementary strand (Scheme 2), named the M1dG-2BD oligodeoxynucleotide (33). In that freshly prepared sample, the two-base bulge in the M1dG-2BD oligodeoxynucleotide was reported to be localized and consisted of M1dG and the 3′-neighbor dC. The structure of the M1dG-2BD duplex suggested that the bulged sequence lacked a cytosine properly positioned to facilitate opening of M1dG and supported the conclusion that proper positioning of dC complementary to M1dG is necessary for promotion of ring opening of the exocyclic adduct in duplex DNA (33). The structure of the M1dG-2BD duplex was similar to that of the structural analogue 1,N2-propanodeoxyguanosine (PdG) in the corresponding PdG-2BD duplex (34). The fixed position of the bulged bases in both instances suggested (33) that these exocyclic adducts did not facilitate bulge migration, a phenomenon first reported by Woodson and Crothers (35, 36).
The NMR studies presented here examine the OPdG-2BD oligodeoxynucleotide, in which the OPdG adduct is placed opposite the two-nucleotide deletion (Scheme 1). In contrast to the M1dG-2BD oligodeoxynucleotide (33), the OPdG-2BD oligodeoxynucleotide undergoes rapid bulge migration (35, 36). This hinders its conversion to the M1dG-2BD duplex (33). The data suggest that bulge migration transiently positions OPdG opposite dC in the complementary strand or, alternatively, reverts rapidly formed intermediates in the OPdG to M1dG reaction pathway (30) when dC is placed opposite from OPdG. In contrast, the localized bulge in the M1dG-2BD duplex consisting of the M1dG adduct and the 3′-neighbor dC (33) hinders the conversion of M1dG to OPdG that is catalyzed by the positioning of cytosine opposite from M1dG (28, 29). The addition of either M1dG-2BD- or OPdG-2BD-containing samples to an equilibrium mixture was monitored as a function of time, using NMR spectroscopy. Both samples attained equilibrium, slightly favoring the M1dG-2BD duplex, in ∼140 days at pH 7 and 25 °C.
Materials and Methods
Oligodeoxynucleotide Synthesis
The unmodified oligodeoxynucleotide was synthesized by the Midland Certified Reagent Co. (Midland, TX) and purified by anion-exchange chromatography. M1dG was synthesized, purified, and incorporated into oligodeoxynucleotides using either an older (37, 38) or a more recently described and improved methodology (39). The extinction coefficients of the oligodeoxynucleotides were calculated on the basis of nearest-neighbor analysis and were 1.04 × 105 M−1 cm−1 for the M1dG-modified strand 5′-d(ATCGCXCGGCATG)-3′ and 1.01 × 105 M−1 cm−1 for the complementary strand 5′-d(CATGCCGCGAT)-3′ (40). To prepare the OPdG-2BD duplex, the 13-mer containing the M1dG adduct was combined with the 11-mer complementary strand at a 1:1 molar ratio in 0.1 M NaCl, 10 mM NaH2PO4, and 50 μM Na2EDTA at pH 11.8. After 3 h at room temperature, the buffer was rapidly adjusted to pH 7.0. The M1dG-2BD duplex was prepared as previously described (33). For spectroscopic examination of the nonexchangeable protons, the OPdG-2BD and M1dG-2BD samples were then lyophilized and exchanged three times with D2O and then suspended in 99.996% D2O. Samples used for spectroscopic examination of exchangeable protons were suspended in a 9:1 H2O/D2O mixture. The concentrations of both the OPdG-2BD and M1dG-2BD duplex samples were 0.52 mM. The concentration of the unmodified duplex sample was 1.1 mM.
NMR Spectroscopy
1H NMR spectra were recorded at 500.13 and 800.13 MHz. Chemical shifts were referenced to the water resonance. Phase sensitive NOESY spectra used for resonance assignments were recorded at 25 ± 0.5 °C using TPPI phase cycling with a mixing time of 250 ms. For examination of exchangeable protons, phase sensitive NOESY experiments were carried out using a field gradient Watergate pulse sequence for water suppression (41). The spectra were recorded at 10 ± 0.5 °C with a mixing time of 150 ms. These experiments were generally recorded with 512 real data points in the d1 dimension and 2048 real data points in the d2 dimension. A relaxation delay of 1.9 s was used for these experiments. The data were processed using FELIX (Accelrys, Inc., San Diego, CA) on Octane workstations (Silicon Graphics, Inc., Mountain View, CA). The data in the d1 dimension were zero-filled to give a matrix of 2048 × 2048 real points. A skewed sine-bell square apodization function with a 90° phase shift and a skew factor of 1.0 was used in both dimensions.
Results
1H Resonance Assignments of Nonexchangeable DNA Protons for the Unmodified 2BD Duplex
The unmodified 2BD duplex contained eight cytosines. These each exhibited sharp COSY cross-peaks arising from H5–H6 scalar couplings, shown in Figure 1A. Although there was spectral overlap arising from the iterated C1G2C3G4C5G6 repeat sequence, at 800 MHz it was possible to resolve resonances with slightly different chemical shifts. Thus, the C3 H6 resonance was located at 7.35 ppm, slightly downfield of C17 H6, located at 7.36 ppm. The C3 H5 resonance was located at 5.46 ppm, slightly upfield from the C17 H5 resonance, located at 5.49 ppm. The C5 H6 resonance was located at 7.19 ppm, slightly upfield of the C19H6 resonance located at 7.22 ppm. The C8 H6 resonance was located at 7.28 ppm, slightly upfield of the C16 H6 resonance located at 7.31 ppm.
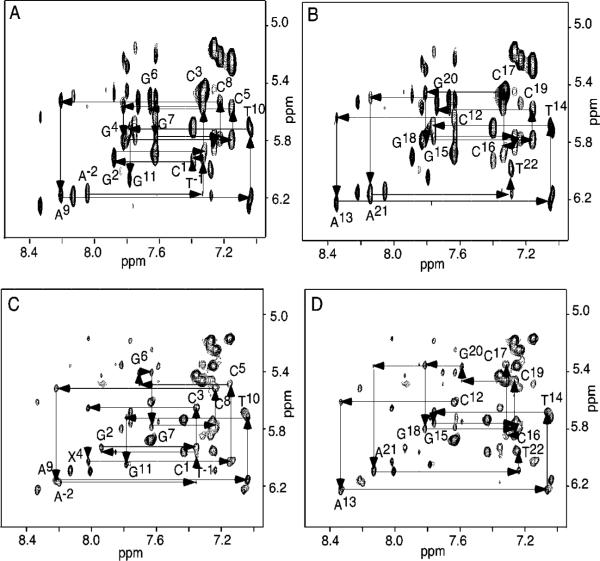
Figure 1.
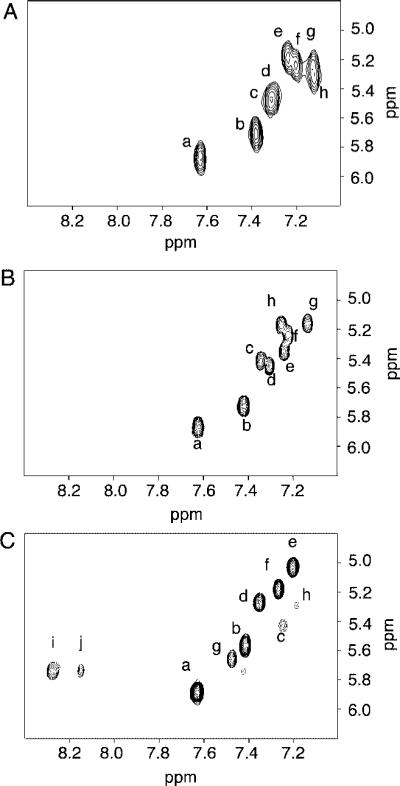
(A) COSY spectrum of the unmodified 2BD duplex. (B) COSY spectrum of the freshly prepared OPdG-2BD duplex. (C) COSY spectrum of the freshly prepared M1dG-2BD duplex. Cross-peaks: a, C12 H5 → C12 H6; b, C1 H5 → C1 H6; c, C3 H5 → C3 H6; d, C17 H5 → C17 H6; e, C16 H5 → C16 H6; f, C8 H5 → C8 H6; g, C5 H5 → C5 H6; h, C19 H5 → C19 H6; i, M1dG H7 → H8; j, M1dG H6 → H7. The experiments were performed at 800 MHz and 25 °C.
An expanded plot from the NOESY spectrum for the unmodified 2BD duplex, showing the sequential aromatic–anomeric proton connectivities, is also shown in Figure 2 A,B. The NOESY data were also characterized by sharp cross-peaks. The sequential NOE assignments were made using standard protocols (42, 43). The A−2 H1′ → T−1 H6 cross-peak was weak, reflecting fraying at the terminal A−2•T22 base pair. The T22 H1′ resonance was located at 6.01 ppm. The T−1 H1′ resonance was located at 5.97 ppm, the C1 H1′ resonance at 5.92 ppm, and the G2 H1′ resonance at 5.94 ppm. In the 13-mer strand, this resulted in overlap of the T−2 H6 → T−1 H1′ and G2 H1′ → C3 H6, T−1 H1′ → C1 H6 and C1 H6 → C1 H1′, and C1 H1′ → G2 H8 and G2 H8 → G2 H1′ NOESY cross-peaks. The C3 H6 → C3 H1′ cross-peak was partially superimposed upon the stronger C3 H5 → C3 H6 and C17 H5 → C17 H6 cross-peaks. The G4 H8 resonance was located at 7.87 ppm, slightly downfield of G18 H8, located at 7.84 ppm. This caused the G4 H8 → G4 H1′ and G18 H8 → G18 H1′ cross-peaks to be superimposed. The G4 H8 → C5 H6 cross-peak overlapped with the G18 H8 → C19 H6 cross-peak, and the C5 H6 → C5 H1′ cross-peak overlapped with the C19 H6 → C19 H1′ cross-peak. The C19 H1′ resonance was located at 5.58 ppm, slightly downfield of G20 H1′, located at 5.45 ppm. The chemical shifts of the nonexchangeable protons are listed in Table S1 of the Supporting Information.
Figure 2.
(A) Expanded plot showing sequential NOE connectivity between aromatic and anomeric protons for nucleotides A−2 → G11 of the unmodified 2BD duplex. (B) Expanded plot showing sequential NOE connectivity between aromatic and anomeric protons for nucleotides C12 → T22 of the unmodified 2BD duplex. (C) Expanded plot showing sequential NOE connectivity between aromatic and anomeric protons for nucleotides A−2 → G11 of the OPdG-2BD duplex. (D) Expanded plot showing sequential NOE connectivity between aromatic and anomeric protons for nucleotides C12 → T22 of the OPdG-2BD duplex. The 800 MHz 250 ms mixing time NOESY experiments were conducted at 25 °C.
1H Resonance Assignments of Exchangeable DNA Protons for the Unmodified 2BD Duplex
The far-downfield region of the NOESY spectrum showing the exchangeable resonances arising from the Watson–Crick base-paired imino protons is shown in Figure 3A. A complete sequential connectivity was traced in the noniterated portion of the oligodeoxynucleotide duplex, from G6 N1H → G7 N1H → G15 N1H → T14 N3H → T10 N3H using standard protocols (44). The G11 N1H resonance was not observed, presumably due to fraying of the G11•C12 terminal base pair, accompanied by rapid exchange with solvent. There were no unusual chemical shifts noted among this group of resonances. The imino resonance for the A−2•T22 terminal base pair at the other end of the oligodeoxynucletoide duplex was also not observed, presumably due to rapid exchange with solvent. In the reiterated portion of the 2BD duplex, the imino resonance arising from T−1 N3H was also not observed, suggesting its rapid exchange with solvent. In addition, a broad resonance, assigned as one or more of the imino proton resonances arising from nucleotides G20, G18, G2, and G4, was observed at ∼12.9 ppm. Consequently, the imino resonances from nucleotides T−1, and nucleotides G20, G18, G2, and G4, located in the iterated repeat portion of the duplex, could not be unequivocally identified. The exocyclic N4 amino protons of cytosines exhibit longer lifetimes with respect to solvent exchange than the N3 imino protons. Inspection of the amino region of the 1H NMR spectrum (Figure 3A) revealed the presence of resonances arising from hydrogen-bonded and non-hydrogen-bonded exocyclic amino protons of nucleotides C1, C19, C3, and C5. This indicated that on the NMR time scale, each of the nucleotides (C1, C19, C3, and C5) was involved in Watson–Crick hydrogen bonding with a deoxyguanosine in the complementary strand. Thus, the nucleotides in the complementary strand, G18, C19, and G20, must be transiently forming Watson–Crick hydrogen bonds with C1, G2, C3, G4, and C5 in the parent strand, i.e., bulge migration (35) (see Scheme 2). No cross-peaks were observed between the hydrogen-bonded and non-hydrogen-bonded N4 exocyclic amino protons of C1 and deoxyguanosine imino protons. Furthermore, the chemical shift difference between the hydrogen-bonded and non-hydrogen-bonded amino protons of C1 was smaller than that observed for the other deoxycytosine residues, with the exception of terminal deoxycytosine residue C12. This observation suggested that on the NMR time scale, nucleotides C1 and G2 were preferentially unpaired as compared to nucleotides G4 and C5. In effect, the unmodified duplex was undergoing sequential fraying from the 5′-terminus of the 13-mer strand, extending into the duplex and involving the A−2•T22, T−1•A21, C1•G20, and G2•C19 base pairs. The chemical shifts of the exchangeable protons are listed in Table S4 of the Supporting Information.
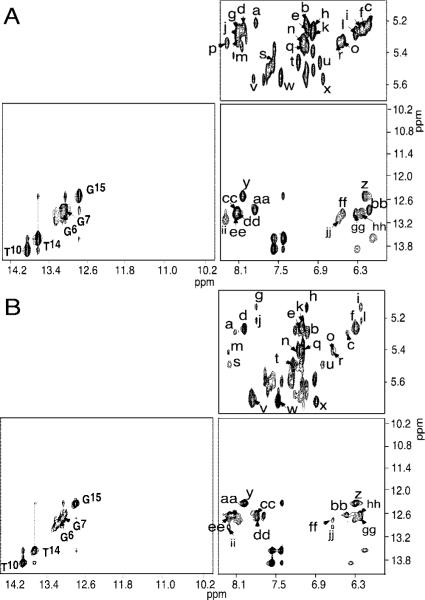
Figure 3.
(A) Expanded plot showing sequential NOE connectivity of the base-paired imino protons for the unmodified 2BD duplex. (B) Expanded plot showing sequential NOE connectivity for the base-paired imino protons of the freshly prepared OPdG-2BD duplex. Cross-peaks: a, C16 N4H2 → C16 H5; b, C16 H6 → C16 H5; c, C16 N4H1 → C16 H5; d, C8 N4H2 → C8 H5; e, C8 H6 → C8 H5; f, C8 N4H1 → C8 H5; g, C5 N4H2 → C5 H5; h, C5 H6 → C5 H5; i, C5 N4H1 → C5 H5; j, C19 N4H2 → C19 H5; k, C19 H6 → C19 H5; l, C19 N4H1 → C19 H5; m, C17 N4H2 → C17 H5; n, C17 H6 → C17 H5; o, C17 N4H1 → C17 H5; p, C3 N4H2 → C3 H5; q, C3 H6 → C3 H5; r, C3 N4H1 → C3 H5; s, C1 N4H2 → C1 H5; t, C1 H6 → C1 H5; u, C1 N4H1 → C1 H5; v, C12 N4H2 → C12 H5; w, C12 H6 → C12 H5; x, C12 N4H1 → C12 H5; y, G15 N1H → C8 N4H2; z, G15 N1H → C8 N4H1; aa, G7 N1H → C16 N4H2; bb, G7 N1H → C16 N4H1; cc, G18 N1H → C5 N4H2; dd, G2 N1H and X4 N1H → C19 N4H2; ee, G6 N1H and X4 N1H → C17 N4H2; ff, G18 N1H → C5 N4H1; gg, G2 N1H and X4 N1H → C19 N4H1; hh, G6 N1H and X4 N1H → C17 N4H1; ii, G18 N1H and G20 N1H → C3 N4H2; jj, G18 N1H and G20 N1H → C3 N4H1. The 800 MHz, 250 ms mixing time NOESY experiments were performed at 10 °C.
1H Resonance Assignments of Nonexchangeable DNA Protons for the OPdG-2BD Duplex
As compared to those of the unmodified 2BD duplex, the resonances arising from the iterated C1G2C3G4C5G6 repeat sequence of the OPdG-2BD duplex exhibited better resolution. The eight cytosines each exhibited sharp COSY cross-peaks arising from H5–H6 scalar couplings, as shown in Figure 1 B.
The sequential NOE assignments shown in Figure 2 C,D were made using standard protocols (42, 43). No disruptions in sequential NOE connectivity were observed in either the modified stand or the complementary strand. In the modified 13-mer strand, the A−2 H1′ → T−1 H6, T−1 H6 → T−1 H1′, and T−1 H1′ → G2 H8 cross-peaks were weak, probably reflecting an increased level of fraying at terminal A−2•G22 and T−1•A21 base pairs. The C5 H1′ → G6 H8 cross-peak was also weak. In the complementary strand, the C16 H6 resonance was observed at 7.23 ppm while the C17 H6 resonance was observed at 7.30 ppm. The G15 H1′ resonance was observed at 5.73 ppm. This was slightly upfield of the C16 H1′ resonance. The G16 H1′ resonance was observed at 5.73 ppm. This caused both the G15 H1′ → C16 H6 and C16 H6 → C16 H1′ cross-peaks to be superimposed with the G18 H1′ → C19 H6 and C19 H6 → C19 H1′ cross-peaks. No unusual 1H shifts were noted in this region of the spectrum. The C19 H1′ → G20 H8 and G20 H1′ → A21 H8 cross-peaks were weak, probably due to fraying at the terminal base pairs. The chemical shifts of the nonexchangeable protons are listed in Table S2 of the Supporting Information.
1H Resonance Assignments of Exchangeable DNA Protons for the OPdG-2BD Duplex
The far-downfield region of the NOESY spectrum showing the exchangeable resonances arising from the Watson–Crick base-paired imino protons is shown in Figure 3 B. Significantly, it appeared substantially similar to the corresponding spectrum in Figure 3 A for the nonadducted 2BD duplex. For the non-reiterated portion of the duplex, sequential connectivity was traced from G6 N1H → G7 N1H → G15 N1H → T14 N3H → T10 N3H using standard protocols (44). There were no unusual chemical shifts noted among this group of resonances. The G11 N1H resonance was not observed, presumably due to fraying of the G11•C12 terminal base pair, accompanied by rapid exchange with solvent. At the other end of the duplex, the imino resonance for the A−2•T22 base pair was also not observed, suggesting rapid exchange with solvent. Additionally, the T−1 N3H resonance was not observed, presumably due to rapid exchange with solvent. A broad resonance, assigned as one or more of the imino proton resonances arising from nucleotides G20, G18, G2, and X4, was observed at ∼12.9 ppm. Consequently, the imino resonances from nucleotides T−1, and nucleotides G20, G18, G2, and X4, located in the iterated repeat portion of the duplex, could not be unequivocally identified. Inspection of the amino region of the 1H NMR spectrum (Figure 3B) revealed weaker resonances arising from hydrogen-bonded and non-hydrogen-bonded exo-cyclic amino protons of nucleotides C1, C19, C3, and C5, as compared to the unmodified 2BD duplex (Figure 3A). This was attributed, in part, to the adducted sample being less concentrated but, more significantly, to slower bulge migration in the OPdG-2BD duplex as compared to the unmodified 2BD duplex, resulting in exchange broadening. Nevertheless, as in the unmodified 2BD duplex, on the NMR time scale, each of the nucleotides (C1, C19, C3, and C5) exhibited evidence of being involved in Watson–Crick hydrogen bonding with a deoxyguanosine in the complementary strand. Thus, the nucleotides in the complementary strand, G18, C19, and G20, must be transiently forming Watson–Crick hydrogen bonds with C1, G2, C3, X4, and C5, in the parent strand (see Scheme 2). No cross-peaks were observed between the hydrogen-bonded and non-hydrogen-bonded N4 exocyclic amino protons of C1 and deoxyguanosine imino protons. However, the chemical shift difference between the hydrogen-bonded and non-hydrogen-bonded amino protons of C1 was now greater than that observed for C1 in the unmodified 2BD duplex (Figure 3A), approaching that observed for the other deoxycytosine residues, with the exception of terminal deoxycytosine residue C12. This suggested that on the NMR time scale, nucleotides C1 and G2 were paired more frequently than in the unmodified 2BD duplex; i.e., the presence of the OPdG lesion at X4 resulted in somewhat more favorable paring of base pairs C1•G20 and G2•C19 than was the case in the unmodified 2BD duplex, but the OPdG-2BD duplex maintained a more rapid rate of bulge migration than did the M1dG-2BD duplex, where the bulge was clearly localized at nucleotides X4 and C5. The chemical shifts of the exchangeable protons are listed in Table S4 of the Supporting Information.
Assignments of the OPdG Protons and NOEs between OPdG and DNA in the OPdG-2BD Duplex
The OPdG proton resonances were identified from a combination of NOESY and COSY experiments. Figure 4 shows the chemical shifts of OPdG protons and intermolecular NOEs between OPdG adduct protons and DNA. OPdG H8 was furthest downfield. At 8.96 ppm, it showed a strong NOE to OPdG H6 located at 7.95 ppm and a broad NOE to OPdG H7 located at 5.72 ppm. The OPdG H6 proton showed a broad NOE to OPdG H7. Six NOEs were observed between OPdG protons and DNA. All of these involved the adducted nucleotide X4 and 3′-neighbor nucleotide C5 in the modified strand. Both OPdG H8 and H6 exhibited NOEs to C5 H5′ and C5 H1′; OPdG H8 also exhibited a NOE to C5 H4′ and X4 H1′.
Figure 4.
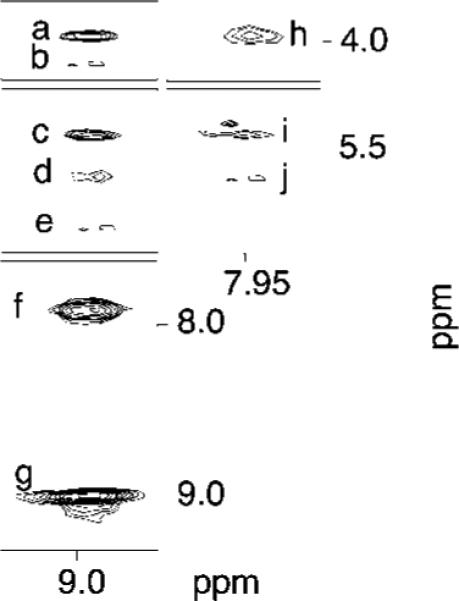
Tile plot showing NOE cross-peaks between OPdG protons and DNA protons in the OPdG-2BD duplex. Cross-peaks: a, OPdG H8 → C5 H5′; b, OPdG H8 → C5 H4′; c, OPdG H8 → C5 H1′; d, OPdG H8 → OpdG H6; e, OPdG H8 → X4 H1′; f, OPdG H8 → OPdG H7; g, OPdG H8 → OPdG H8; h, OPdG H7 → C5 H5′; i, OPdG H7 → C5 H1′; j, OPdG H7 → OPdG H6. The 800 MHz, 250 ms mixing time NOESY experiment was conducted at 25 °C.
1H Resonance Assignments of the M1dG-2BD Duplex
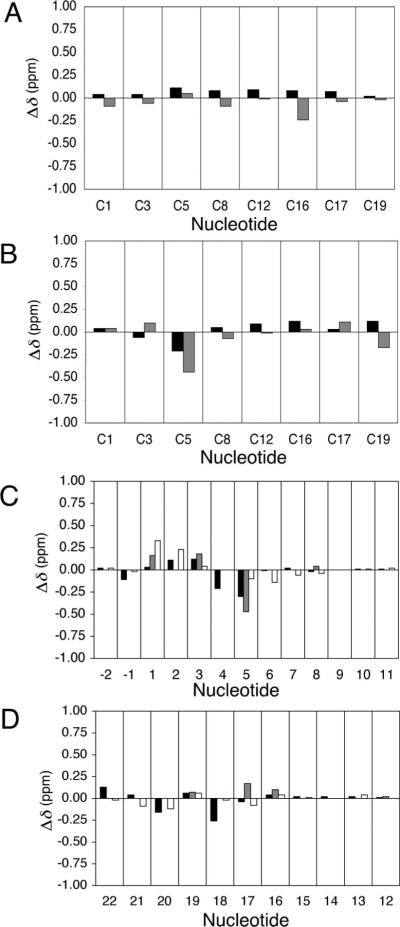
The detailed NMR assignments of the M1dG-2BD duplex were as previously reported (33). In contrast to the OPdG-2BD duplex and to the unmodified 2BD duplex, disruptions in sequential NOE connectivity were observed in both the modified and complementary strands. In the modified strand, the C3 H1′ → X4 H2 sequential NOE (X4 H2 is the imidazole proton of M1dG, corresponding to G4 H8 in the unmodified nucleotide) was missing. In the complementary strand, the C17 H1′ → G18 H8 NOE was not observed. Spectral line broadening was localized adjacent to the M1dG lesion. Line broadening was observed for the C3 and C5 H5 and H6 resonances. These were the 5′- and 3′-neighbors of the M1dG lesion, respectively. In the complementary strand, line broadening was observed for C19 H5 and H6 (Figure 1 C). The assignments for the base imino protons of the M1dG-2BD duplex showed an interruption in the sequential connectivity between imino protons at G18, the base pair 5′ to the M1dG lesion. No NOE connectivity was observed between G18 N1H and G6 N1H, which localized the M1dG adduct between base pairs C5•G18 and G6•C17. The exocyclic M1dG protons H6, H7, and H8 of M1G were identified as the characteristic aromatic spin system. M1dG H8 was observed at 8.4 ppm, M1dG H7 at 5.8 ppm, and M1dG H6 at 8.2 ppm. The following 3J coupling constants for the M1dG protons were measured in a DQF-COSY experiment: 3JH6,H7 ∼ 4 Hz and 3JH7,H8 ∼ 7 Hz. Figure 5 A shows chemical shift comparisons of cytosine protons H5 and H6, between the OPdG-2BD duplex and the unmodified 2BD duplex. No chemical shift perturbations were greater than 0.1 ppm except at position C16 H5 where a downfield shift of 0.24 ppm was observed. Figure 5 B shows chemical shift comparisons of cytosine proton H5–H6 cross-peaks, between the M1dG-2BD duplex and the unmodified 2BD duplex. No chemical shift perturbations were greater than 0.1 ppm except at position C5 in the modified strand and C19 in the complementary strand. A downfield shift of 0.44 ppm was observed for the C5 H5 resonance, a downfield shift of 0.21 ppm for the C5 H6 resonance, and a downfield shift of 0.17 ppm for the C19 H5 resonance.
Figure 5.
(A) Chemical shift differences of cytosine H5 and H6 protons of the OPdG-2BD duplex relative to the unmodified 2BD duplex. (B) Chemical shift differences of cytosine H5 and H6 protons of the M1dG-2BD duplex relative to the unmodified 2BD duplex. The data for the cytosine H5 protons are colored gray and the data for the cytosine H6 protons black. (C) Chemical shift differences of nucleotide base protons A−2 → G11 of the OPdG-2BD duplex relative to the M1dG-2BD duplex. (D) Chemical shift differences of nucleotide base protons C12 → T22 of the OPdG-2BD duplex relative to the M1dG-2BD duplex. The data for the nucleotide base aromatic H8/H6 protons are colored black, the data for the cytosine aromatic H5 protons gray, and the data for the sugar H1′ protons white. In all instances, Δδ = δmodified oligodeoxynucleotide – δunmodified oligodeoxynucleotide (parts per million).
Chemical Shift Comparisons between the OPdG-2BD and M1dG-2BD Duplexes
Panels C and D of Figure 5 show chemical shift differences for the pyrimidine H5 and H6 or purine H8 and deoxyribose H1′ protons, comparing the OPdG-2BD and M1dG-2BD duplexes. The 1H chemical shifts of the non-reiterated base pairs G6•C17, G7•C16, C8•G15, A9•T14, T10•A13, and G11•C12 were conserved in both duplexes. Significant chemical shift perturbations were observed for the reiterated base pairs associated with the two-nucleotide bulge, suggesting that the two-nucleotide bulge was differentially accommodated in the OPdG-2BD versus the M1dG-2BD duplex. The biggest chemical shift difference between the OPdG-2BD duplex and the M1dG-2BD duplex was 0.47 ppm, observed for the C5 H5 resonance. Other significant shift changes were observed in the modified strand, 0.33 ppm for the C1 H1′ resonance, 0.23 ppm for the G2 H1′ resonance, 0.18 ppm for the C3 H5 resonance, and 0.30 ppm for the C5 H6 resonance. In the complementary strand, the G18 H8 resonance exhibited a 0.26 ppm shift.
Equilibrium of the OPdG-2BD and M1dG-2BD Duplexes
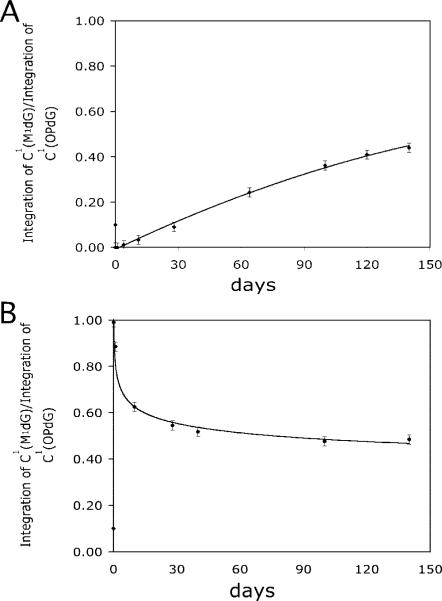
The initially formed OPdG-2BD and M1dG-2BD duplexes were not at equilibrium. The conversion of the OPdG-2BD duplex toward an equilibrium mixture of the M1dG-2BD and OPdG-2BD duplexes was monitored as a function of time, by monitoring the COSY spectrum. Figure 6 monitors the ratio of resonance intensities from the C1 H5–H6 COSY cross-peak of either OPdG or M1dG as a function of time, beginning with freshly prepared OPdG-2BD or M1dG-2BD duplexes. This cross-peak reflected the environment of the C1•G20 base pair, located at the 5′-terminus of the iterated repeat sequence with respect to the OPdG-modified nucleotide X4. Over a period of 140 days at 25 °C, the eight COSY cross-peaks arising from the OPdG-2BD duplex decreased in intensity and eight new cross-peaks corresponding to formation of the M1dG-2BD duplex appeared and grew in intensity. As noted above, for the OPdG-2BD duplex, the sequential connectivities in the reiterated region, T22 N3H → T−1 N3H → G20 N1H → G2 N1H → G18 N1H → G4 N1H → G6 N1H, were disturbed. As the OPdG duplex shifted toward the M1dG-2BD duplex, these sequential connectivities appeared in the spectrum. At equilibrium, the M1dG-2BD duplex was slightly favored. The equilibrium chemistry of a freshly prepared M1dG-2BD duplex was also monitored. At equilibrium, the COSY spectrum of the initially prepared M1dG-2BD duplex was identical to that of the OPdG-2BD duplex at equilibrium.
Figure 6.
Intensity ratios of the two C1 H5 → C1 H6 cross-peaks in the COSY spectrum, arising from M1dG and OPdG, as a function of time: (A) freshly prepared OpdG-2BD duplex and (B) freshly prepared M1dG-2BD duplex. The solid lines represent the best fits through the data in both plots. The errors in measuring the ratios are estimated to be ±2%.
Discussion
Interest in the structure of the OPdG and M1dG adducts embedded in the frameshift-prone hisD3052-iterated (CG)3 repeat sequence arose from the observation that MDA induced frameshift mutations (32). The M1dG and OPdG adducts are chemically distinct. One would predict that the biological processing of MDA- or base propenal-induced damage sites in DNA depends upon whether the damage exists as the exocyclic adduct M1dG or as its OPdG rearrangement product. Site-specific mutagenesis experiments involving M1dG (25) or its chemically stable analogue PdG (45), which cannot open to OPdG, indicated that PdG gave greater numbers of point mutations. Similarly, the acrolein-induced γ-hydroxyl-1,N2-PdG adduct, which also underwent ring opening (46), was found to not be miscoding in vivo (47, 48).
It has not been possible to examine the M1dG adduct with respect to structure in fully complementary DNA duplexes due to the fact that when placed opposite dC, it rapidly rearranges to the OPdG adduct (28). The saturated analogue 1,N2-propanodG (PdG adduct) (49) was used by our laboratory (30, 50–54) as well as by other laboratories (55–58) as a stable structural surrogate for exocyclic 1,N2-dG adducts such as the M1dG adduct and the acrolein γ-OH-PdG adduct. The PdG adduct reduced the thermal stability, transition enthalpy, and transition free energy of duplex DNA when positioned opposite dC or dA (59). When placed in the hisD3052 gene-iterated CG repeat sequence at pH 5.8, PdG rotated about the glycosyl bond into the syn conformation, and the 3′-neighbor base pair existed in a mixture of Watson–Crick and Hoogsteen conformations (50). In a noniterated duplex at pH 5.2, only PdG and not its 3′-neighbor base pair rotated about the glycosyl bond into the syn conformation (54). Presumably, the syn conformation of the glycosyl bond relieves steric strain associated with incorporation of the 1,N2-dG annelation product, which cannot participate in Watson–Crick hydrogen bonding, into the DNA duplex. In contrast, structural studies of the OPdG adduct in the fully complementary iterated repeat contained in the hisD3052 oligodeoxynucleotide showed that it remained in the anti orientation about the glycosyl bond and oriented in the minor groove of the DNA duplex such that it did not interfere with Watson–Crick base pairing (31).
The iterated CG repeat contained in the hisD3052 oligodeoxynucleotide is thought to be prone to frameshifts by slippage of either the template or the primer strand during DNA replication (60). The hisD3052 mutation arose from the histidinol dehydrogenase gene of S. typhimurium by deletion of a cytosine induced by ICR-191 (61, 62). It is reversed by additions and deletions that restore the reading frame but do not necessarily reverse the forward mutation (63). The most common reversion is a CG deletion in the reiterated sequence (64–67). The unmodified 2BD duplex and the M1dG-2BD (33) and OPdG-2BD duplexes model the intermediate structures leading to two-base deletions.
Bulge Migration in the Unmodified 2BD Duplex
Woodson and Crothers (35) first characterized the phenomenon of bulge migration within iterated DNA sequences. Several lines of evidence suggest that formation of a two-nucleotide bulge within the unmodified 2BD duplex was also accompanied by bulge migration. Significantly, a complete set of sequential NOE connectivities between nonexchangeable base aromatic and deoxyribose anomeric protons was observed (Figure 2 A,B). This observation was consistent with rapid migration of the two-nucleotide bulge in the hisD3052 sequence such that on the NMR time scale, a set of sequential NOEs consistent with a right-handed duplex is observed for nucleotides C1, G2, C3, G4, C5, and G6. The presence of a localized two-nucleotide bulge on the NMR time scale would have predicted a break in these NOE connectivities, which was observed for the M1dG-2BD duplex (33). In that instance, for the modified strand, the C3 H1′ → M1dG H2 sequential NOE (the imidazole proton of M1dG, corresponding to G H8 in the unmodified nucleotide) was missing. In the complementary strand, the C17 H1′ → G18 H8 NOE was missing (33).
The observation that the eight cytosine H6–H5 COSY cross-peaks in Figure 1 A were well resolved corroborated the conclusion that the bulge migrated rapidly compared to the NMR time scale. In contrast, for the M1dG-2BD duplex (Figure 1C), the localized bulge exhibited spectral line broadening adjacent to the M1dG lesion, for the C3 and C5 cytosine H5 and cytosine H6 resonances, the 5′- and 3′-neighbors of the M1dG lesion, respectively. In the complementary strand, line broadening was observed for C19 H5 and H6 (33).
The rapid bulge migration was also evident from the increased rate of solvent exchange of the dG N1H imino protons within the (CG)3 iterated repeat sequence. In the non-reiterated portion of the bulged oligodeoxynucleotide, the dG N1H and dT N3H imino resonances arising from Watson–Crick base pairs G6•C17, G7•C16, C8•G15, A9•T14, and T10•A13 were observed in the fardownfield region of the 1H NMR spectrum, indicating that these base pairs had long lifetimes with respect to exchange of the hydrogen-bonded protons with solvent. For these base pairs in the non-reiterated portion of the bulged oligodeoxynucleotide, the normal patterns of NOEs between the dG N1H and dC N4 exocyclic amino protons, characteristic of Watson–Crick base pairing, were observed in the 1H NMR spectrum. In contrast, the dG N1H imino resonances arising from nucleotides G20, G2, G18, and G4 in the reiterated portion of the bulged oligodeoxynucleotide exhibited significant spectral line broadening associated with an increased rate of solvent exchange. However, for nucleotides C1, C19, C3, and C5 in the reiterated portion of the bulged oligodeoxynucleotide, NOEs between the dC N4 exocyclic amino protons and the dG N1H protons of complementary deoxyguanosines, characteristic of individual Watson–Crick base pairs, could be assigned, indicating that transient Watson–Crick base pairing involving all four deoxycytosines in the reiterated portion of the duplex must be occurring. For the unmodified 2BD duplex, the T−1 N3H imino proton resonance arising from the T−1•A21 base pair adjacent to the iterated repeat was not observed. Also, no cross-peaks were observed between the exocyclic N4 amino protons of C1 and guanine imino protons. In contrast, for the M1dG-2BD duplex in which the two-nucleotide bulge was localized, an interruption in the sequential NOE connectivity between the dG N1H protons of adjacent base pairs was observed at G18, the base pair 5′ to the M1dG lesion, and no NOE was observed between G18 N1H and G6 N1H, which localized the M1dG adduct between base pairs C5•G18 and G6•C17 (33). For the M1dG-2BD duplex, the T−1 N3H imino proton resonance arising from the T−1•A21 base pair adjacent to the iterated repeat was observed. Also, cross-peaks from the N4 exocyclic amine protons of C1 to the G20 N1H imino proton were observed, indicating the presence of the C1•G20 base pair (33).
Minor Groove Orientation of the OPdG Adduct in the OPdG-2BD Duplex
The pattern of NOEs observed between the OPdG moiety and the DNA in the OPdG-2BD duplex was consistent with a minor groove orientation of the OPdG adduct. The NOEs shown in Figure 4 involved X4 H1′, and C5 H1′, H4′, and H5′. These NOEs served to locate the OPdG moiety within the minor groove. Thus, the orientation of OPdG in the OPdG-2BD duplex was similar to that in the fully complementary hisD3052 oligodeoxynucleotide (31). However, the pattern of NOEs within the minor groove was different. In the fully complementary duplex, OPdG exhibited NOEs to minor groove protons in the complementary strand, e.g., C19 H1′, G20 H1′, G20 H4′, and G20 H5′ and H5″ (31).
Bulge Migration in the OPdG-2BD Duplex
The spectra of the OPdG-2BD duplex were similar to those from the unmodified 2BD duplex and differed from the spectra obtained for the M1dG-2BD duplex (33). These observations suggested that formation of a two-nucleotide bulge within the OPdG-2BD duplex was also accompanied by bulge migration. The observation of a complete set of sequential NOE connectivities between nonexchangeable base aromatic and deoxyribose anomeric protons (Figure 2 C, D) was consistent with rapid migration of the two-nucleotide bulge such that on the NMR time scale, a set of sequential NOEs consistent with a right-handed duplex was observed for nucleotides C1, G2, C3, X4, C5, and G6. Eight well-resolved cytosine H6–H5 COSY cross-peaks were observed (Figure 1 B), also corroborating the conclusion that the bulge migrated rapidly on the NMR time scale. In the non-reiterated portion of the OPdG-2BD duplex, the dG N1H and dT N3H imino resonances from Watson–Crick base pairs G6•C17, G7•C16, C8•G15, A9•T14, and T10•A13 were observed, indicating that these base pairs had long lifetimes with respect to exchange of the hydrogen-bonded protons with solvent. Furthermore, for these base pairs, the normal patterns of NOEs between the dG N1H and dC N4 exocyclic amino protons, characteristic of Watson–Crick base pairing, were observed. In contrast, the dG N1H imino resonances arising from nucleotides G20, G2, G18, and X4 in the reiterated portion of the OPdG-2BD duplex exhibited spectral line broadening. For these nucleotides, the sequential NOEs between the dG N1H protons of adjacent base pairs and the NOEs between the dG N1H protons and the dC N4 exocyclic amino protons, characteristic of individual Watson–Crick base pairs, could not be unequivocally assigned. In addition, the T−1 N3H imino proton resonance arising from the T−1•A21 base pair adjacent to the iterated repeat was not observed, indicating that it also underwent solvent exchange at an increased rate (Figure 3B). The differential pattern of minor groove NOEs that was observed between the OPdG-2BD duplex and the fully complementary hisD3052 duplex containing the OPdG adduct (31) was also consistent with bulge migration in the OPdG-2BD duplex. Thus, the rapid migration of the bulge precluded observation of NOEs to minor groove protons in the complementary strand but maintained the observation of NOEs to the 3′-neighbor nucleotide C5 in the modified strand of the duplex.
The rapid rate of bulge migration is consistent with the observation that the OPdG adduct facilitates Watson–Crick base pairing, which was confirmed by Riggins et al. (30). Because OPdG does not interfere with Watson–Crick hydrogen bonding and is located in the minor groove, the location of the 2BD bulge is not localized. In contrast, M1dG does interfere with Watson–Crick hydrogen bonding, and its accommodation in duplex DNA presumably requires its reorientation into the syn conformation about the glycosyl bond, similar to the case with the PdG adduct. Accordingly, the localization of the two-nucleotide bulge in the M1dG-2BD duplex, involving the modified nucleotide X4 and its 3′-neighbor C5 (33), is favored.
Chemical Shift Differences among the Unmodified 2BD Duplex, the OPdG-2BD Duplex, and the M1dG-2BD Duplex
The notion that the OPdG and M1dG adducts existed in different environments within the iterated repeat sequence containing the two-nucleotide bulge was corroborated by examination of chemical shift effects (Figure 5). Only modest differences were observed when the chemical shifts for the cytosine H5 and H6 resonances of the unmodified 2BD duplex were compared to those of the OPdG-2BD duplex, consistent with the idea that these two duplexes behaved similarly. In contrast, a comparison of the unmodified 2BD duplex with the M1dG-2BD duplex showed a significant difference at nucleotide C5, the 3′-neighbor to the M1dG-adducted nucleotide. Likewise, large chemical shift differences were observed when the OPdG duplex was compared with the M1dG duplex. Significantly, these chemical shift differences were localized to the iterated repeat sections of the two bulged duplexes. Only minimal chemical shift differences were observed for the non-reiterated regions of the M1dG-2BD duplex versus the OPdG-2BD duplex, consistent with the conclusion that the two bulged duplexes differed in the manners in which the respective adducts were accommodated within the iterated repeat sequence.
Differential Rates of Bulge Migration in the Bulged M1dG-2BD and OPdG-2BD Duplexes Modulate the Rate at Which Equilibrium between M1dG and OPdG Is Achieved
The differential rates of bulge migration in the M1dG-2BD and OPdG-2BD duplexes affect the rate at which equilibrium between M1dG and OPdG is achieved, as compared to that of fully complementary duplexes containing the M1dG or OPdG adducts. For the fully complementary hisD3052 duplex, the M1dG adduct placed opposite dC rearranged to OPdG rapidly; its conversion was completed in minutes. For the M1dG-2BD duplex, the two-base bulge was localized and consisted of M1dG and the 3′-neighbor (33). As such, the M1dG-2BD bulge lacks a hydrated cytosine positioned to facilitate opening of M1dG (33); thus, at neutral pH, ring opening of M1dG to OPdG occurs slowly in the M1dG-2BD duplex. This study indicates that at 25 °C, equilibrium between M1dG and OPdG, favoring M1dG (33), was attained over a period of 140 days (Figure 6). This suggests that the localized bulge in the M1dG-2BD duplex undergoes a slow migration. This was not recognized in the earlier studies (33), which were conducted using a freshly prepared M1dG-2BD duplex. A slow migration of the bulge in the M1dG-2BD duplex would transiently relocate M1dG to a position opposite from nucleotide C19 in the complementary strand, which would facilitate ring opening of the OPdG adduct, via the mechanism proposed by Riggins et al. (29). Once the OPdG adduct forms at neutral pH, its more rapid bulge migration rate then slows the rate of its reversion back to the localized bulge in the M1dG-2BD duplex. We conclude that the step in the formation of M1dG, believed to be the dehydration of the initially formed 8-hydroxy-6,7-propenodG intermediate (30), is slow compared to bulge migration. Hence, it requires many days for the equilibrium involving OPdG and M1dG in the OPdG-2BD and M1dG-2BD duplexes to be attained.
Comparison to Other Oligodeoxynucleotides Containing Bulged Nucleotides
Previous studies examined oligodeoxynucleotides containing unpaired bases or bulges of various sequence contexts and length (68–75). Unpaired purines generally adopt intrahelical conformations in solution (74, 76, 77). For unpaired pyrimidines, both intrahelical (7879)and extrahelical conformations (6980)have been observed. The role of flanking DNA sequences in determining the conformation of bulged pyrimidines was demonstrated by studies demonstrating that bulged pyrimidines embedded in A•T tracts adopt an extrahelical conformation (70, 81).
Structure–Activity Relationships
The M1dG-induced frame-shift mutations observed in vivo in E. coli and in COS-7 cells, and associated with the d(CpG)3 iterated repeat, include −2 bp deletions (26). These are consistent with the observation that frameshift mutations in the iterated CG repeat sequence of the hisD3052 gene are typically −2 base deletions. These suggest mechanisms whereby the modified guanine and adjacent cytosine undergo transient dislocation during replication bypass (60, 82). This work demonstrates rapid bulge migration within the iterated repeat sequence of the hisD3052 gene containing the OPdG adduct. The occurrence of transient dislocation during replication could occur either prior to insertion of a nucleotide opposite M1dG or, alternatively, after nucleotide insertion and prior to extension (83). It could also involve dissociation or re-association with the replication complex.
In any case, relationships between DNA structure and dynamics, and the formation and accommodation of transient dislocation complexes during DNA replication, are anticipated to be polymerase-specific (84). Both M1dG and OPdG block replication by the Klenow fragment of DNA polymerase I, with M1dG being approximately 6-fold more blocking than OPdG (85). DNA polymerase β induces two-base deletions when replicating past M1dG in this iterated d(CpG)3 sequence derived from the S. typhimuium hisD3052 gene. 2 However, the interactions of M1dG and OPdG with various Y-family polymerases (86, 87) remain to be determined and are likely to be crucial for understanding the mechanisms by which these lesions induce both frameshift and base pair substitution mutations in human cells. Significantly, structures of the Sulfolobus solfataricus P2 DNA polymerase IV (Dpo4) have been obtained for binary and ternary complexes with primer templates site-specifically modified with 1,N2-ethenodeoxyguanosine (1,N2-εdG) (88, 89), an adduct that is structurally similar to M1dG. The Dpo4 structures with the 1,N2-εdG adduct suggest that it uses several mechanisms, including a variation of dNTP-stabilized misalignment, to generate frameshifts when it encounters the exocyclic DNA adduct (88).
Supplementary Material
Acknowledgment
Ms. Pamela Tamura and Dr. Ivan Kozekov synthesized the modified oligonucleotide. Mr. Markus Voehler assisted with the collection of NMR data. This work was supported by NIH Grants CA-55678 (M.P.S.) and CA-87819 (L.J.M.). Funding for the NMR spectrometers was supplied by Vanderbilt University, the Vanderbilt Center in Molecular Toxicology, Grant ES-00267, and by NIH Grant RR-05805. The Vanderbilt-Ingram Cancer Center is supported by NIH Grant CA-68485. Michael P. Stone grant RO1 CA-55678.
Footnotes
Abbreviations: MDA, malondialdehyde; M1dG, 3-(β-d-ribofuranosyl)pyrimido[1,2-a]purin-10(3H)-one; OPdG, N2-(3-oxo-1-propenyl)deoxyguanosine; PdG, 1,N2-propano-dG; HO-PdG, 8-hydroxy-6,7-propenodeoxyguanosine; EDTA, ethylenediaminetetraacetic acid; HPLC, high-performance liquid chromatography; NOE, nuclear Overhauser enhancement; NOESY, two-dimensional NOE spectroscopy; COSY, correlation spectroscopy; DQF-COSY, double-quantum-filtered correlation spectroscopy; TPPI, time-proportional phase increment; 1D, one-dimensional; 2D, two-dimensional. A right superscript refers to the numerical position in the sequence starting from the 5′-terminus of chain A and proceeding to the 3′-terminus of chain A and then from the 5′-terminus of chain B to the 3′-terminus of chain B. C2, C5, C6, C8, C1′, C2′, C2″, etc., represent specific carbon nuclei. H2, H5, H6, H8, H1′, H2′, H2″, etc., represent protons attached to these carbons.
Riggins, J. N., and Marnett, L. J. (2001) Malondialdehyde-deoxyguanosine Adducts M1dG and N2 OPdG Block Replication by Human DNA Polymerase β and Induce Frameshift Mutations in Vitro, 222nd American Chemical Society National Meeting, Chicago, IL, Division of Chemical Toxicology, Collected Abstracts.
Supporting Information Available: Chemical shifts of nonexchangeable protons for the OPdG- and M1dG-2BD duplexes (Tables S1 and S2, respectively) and chemical shifts of exchangeable protons for the OPdG- and M1dG-2BD duplexes (Tables S3 and S4, respectively). This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Marnett LJ. Lipid peroxidation-DNA damage by malondialdehyde. Mutat. Res. 1999;424:83–95. doi: 10.1016/s0027-5107(99)00010-x. [DOI] [PubMed] [Google Scholar]
- 2.Marnett LJ. Chemistry and biology of DNA damage by malondialdehyde. IARC Sci. Publ. 1999;150:17–27. [PubMed] [Google Scholar]
- 3.Basu AK, Essigmann JM. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem. Res. Toxicol. 1988;1:1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- 4.Marnett LJ, Basu AK, O'Hara SM, Weller PE, Rahman AFMM, Oliver JP. Reaction of malondialdehyde with guanine nucleosides: Formation of adducts containing oxadiazabicyclononene residues in the base-pairing region. J. Am. Chem. Soc. 1986;108:1348–1350. [Google Scholar]
- 5.Seto H, Okuda T, Takesue T, Ikemura T. Reaction of malonaldehyde with nucleic acid. I. Formation of fluorescent pyrimido [1,2-a]purin-10(3H)-one nucleosides. Bull. Chem. Soc. Jpn. 1983;56:1799–1802. [Google Scholar]
- 6.Seto H, Seto T, Takesue T, Ikemura T. Reaction of malonaldehyde with nucleic acid. III. Studies of the fluorescent substances released by enzymatic digestion of nucleic acids modified with malonaldehyde. Chem. Pharm. Bull. 1986;34:5079–5085. [PubMed] [Google Scholar]
- 7.Reddy GR, Marnett LJ. The mechanism of reaction of β-aryloxyacroleins with nucleosides. Chem. Res. Toxicol. 1996;9:12–15. doi: 10.1021/tx950105t. [DOI] [PubMed] [Google Scholar]
- 8.Dedon PC, Plastaras JP, Rouzer CA, Marnett LJ. Indirect mutagenesis by oxidative DNA damage: Formation of the pyrimidopurinone adduct of deoxyguanosine by base propenal. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11113–11116. doi: 10.1073/pnas.95.19.11113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Plastaras JP, Riggins JN, Otteneder M, Marnett LJ. Reactivity and mutagenicity of endogenous DNA oxopropenylating agents: Base propenals, malondialdehyde, and Nε-oxopropenyllysine. Chem. Res. Toxicol. 2000;13:1235–1242. doi: 10.1021/tx0001631. [DOI] [PubMed] [Google Scholar]
- 10.Wang MY, Liehr JG. Lipid hydroperoxide-induced endogenous DNA adducts in hamsters: Possible mechanism of lipid hydroperoxide-mediated carcinogenesis. Arch. Biochem. Biophys. 1995;316:38–46. doi: 10.1006/abbi.1995.1007. [DOI] [PubMed] [Google Scholar]
- 11.Chaudhary AK, Nokubo M, Reddy GR, Yeola SN, Morrow JD, Blair IA, Marnett LJ. Detection of endogenous malondialdehyde-deoxyguanosine adducts in human liver. Science. 1994;265:1580–1582. doi: 10.1126/science.8079172. [DOI] [PubMed] [Google Scholar]
- 12.Wang M, Dhingra K, Hittleman WN, Liehr JG, de Andrade M, Li D. Lipid peroxidation-induced putative malondialdehyde-DNA adducts in human breast tissues. Cancer Epidemiol. 1996;5:705–710. [PubMed] [Google Scholar]
- 13.Nath RG, Chung FL. Detection of 1,N2-propanodeoxyguanosine adducts in rodent and human liver DNA by 32P-postlabeling. Proc. Am. Assoc. Cancer Res. 1993;34:137. [Google Scholar]
- 14.Nath RG, Chung F-L. Detection of exocyclic 1,N2-propanodeoxyguanosine adducts as common DNA lesions in rodents and humans. Proc. Natl. Acad. Sci. U.S.A. 1994;91:7491–7495. doi: 10.1073/pnas.91.16.7491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Nair J, Barbin A, Guichard Y, Bartsch H. 1,N6-Ethenodeoxyadenosine and 3,N4-ethenodeoxycytidine in liver DNA from humans and untreated rodents detected by immunoaffinity/32P-postlabeling. Carcinogenesis. 1995;16:613–617. doi: 10.1093/carcin/16.3.613. [DOI] [PubMed] [Google Scholar]
- 16.Zhang S, Villalta PW, Wang M, Hecht SS. Analysis of crotonaldehyde- and acetaldehyde-derived 1,N2-propanodeoxyguanosine adducts in DNA from human tissues using liquid chromatography electrospray ionization tandem mass spectrometry. Chem. Res. Toxicol. 2006;19:1386–1392. doi: 10.1021/tx060154d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaudhary AK, Nokubo M, Marnett LJ, Blair IA. Analysis of the malondialdehyde-2′-deoxyguanosine adduct in rat liver DNA by gas chromatography/electron capture negative chemical ionization mass spectrometry. Biol. Mass Spectrom. 1994;23:457–464. doi: 10.1002/bms.1200230802. [DOI] [PubMed] [Google Scholar]
- 18.Rouzer CA, Chaudhary AK, Nokubo M, Ferguson DM, Reddy GR, Blair IA, Marnett LJ. Analysis of the malondialdehyde-2′-deoxyguanosine adduct pyrimidopurinone in human leukocyte DNA by gas chromatography/electron capture-negetiave chemical ionization/mass spectrometry. Chem. Res. Toxicol. 1997;10:181–188. doi: 10.1021/tx9601216. [DOI] [PubMed] [Google Scholar]
- 19.Vaca CE, Fang JL, Mutanen M, Valsta L. 32P-postlabelling determination of DNA adducts of malonaldehyde in humans: Total white blood cells and breast tissue. Carcinogenesis. 1995;16:1847–1851. doi: 10.1093/carcin/16.8.1847. [DOI] [PubMed] [Google Scholar]
- 20.Fang JL, Vaca CE, Valsta LM, Mutanen M. Determination of DNA adducts of malonaldehyde in humans: Effects of dietary fatty acid composition. Carcinogenesis. 1996;17:1035–1040. doi: 10.1093/carcin/17.5.1035. [DOI] [PubMed] [Google Scholar]
- 21.Sevilla CL, Mahle NH, Eliezer N, Uzieblo A, O'Hara SM, Nokubo M, Miller R, Rouzer CA, Marnett LJ. Development of monoclonal antibodies to the malondialdehyde-deoxyguanosine adduct, pyrimidopurinone. Chem. Res. Toxicol. 1997;10:172–180. doi: 10.1021/tx960120d. [DOI] [PubMed] [Google Scholar]
- 22.Chaudhary AK, Reddy RG, Blair IA, Marnett LJ. Characterization of an N6-oxopropenyl-2′-deoxyadenosine adduct in malondialdehyde-modified DNA using liquid chromatography/electrospray ionization tandem mass spectrometry. Carcinogenesis. 1996;17:1167–1170. doi: 10.1093/carcin/17.5.1167. [DOI] [PubMed] [Google Scholar]
- 23.Hoberg AM, Otteneder M, Marnett LJ, Poulsen HE. Measurement of the malondialdehyde-2′-deoxyguanosine adduct in human urine by immuno-extraction and liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometry. J. Mass Spectrom. 2004;39:38–42. doi: 10.1002/jms.547. [DOI] [PubMed] [Google Scholar]
- 24.Otteneder MB, Knutson CG, Daniels JS, Hashim M, Crews BC, Remmel RP, Wang H, Rizzo C, Marnett LJ. In vivo oxidative metabolism of a major peroxidation-derived DNA adduct, M1dG. Proc. Natl. Acad. Sci. U.S.A. 2006;103:6665–6669. doi: 10.1073/pnas.0602017103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fink SP, Reddy GR, Marnett LJ. Mutagenicity in Escherichia coli of the major DNA adduct derived from the endogenous mutagen malondialdehyde. Proc. Natl. Acad. Sci. U.S.A. 1997;94:8652–8657. doi: 10.1073/pnas.94.16.8652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.VanderVeen LA, Hashim MF, Shyr Y, Marnett LJ. Induction of frameshift and base pair substitution mutations by the major DNA adduct of the endogenous carcinogen malondialdehyde. Proc. Natl. Acad. Sci. U.S.A. 2003;100:14247–14252. doi: 10.1073/pnas.2332176100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Niedernhofer LJ, Daniels JS, Rouzer CA, Greene RE, Marnett LJ. Malondialdehyde, a product of lipid peroxidation, is mutagenic in human cells. J. Biol. Chem. 2003;278:31426–31433. doi: 10.1074/jbc.M212549200. [DOI] [PubMed] [Google Scholar]
- 28.Mao H, Schnetz-Boutaud NC, Weisenseel JP, Marnett LJ, Stone MP. Duplex DNA catalyzes the chemical rearrangement of a malondialdehyde deoxyguanosine adduct. Proc. Natl. Acad. Sci. U.S.A. 1999;96:6615–6620. doi: 10.1073/pnas.96.12.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Riggins JN, Daniels JS, Rouzer CA, Marnett LJ. Kinetic and thermodynamic analysis of the hydrolytic ring-opening of the malondialdehyde-deoxyguanosine adduct, 3-(2′-deoxy-β-d-erythro-pentofuranosyl)-pyrimido[1,2-α]purin-10(3H)-one. J. Am. Chem. Soc. 2004;126:8237–8243. doi: 10.1021/ja040009r. [DOI] [PubMed] [Google Scholar]
- 30.Riggins JN, Pratt DA, Voehler M, Daniels JS, Marnett LJ. Kinetics and mechanism of the general-acid-catalyzed ring-closure of the malondialdehyde-DNA adduct, N2-(3-oxo-1-propenyl)deoxyguanosine (N2OPdG-), to 3-(2′-deoxy-β-d-erythro-pentofuranosyl)pyrimido[1,2-α]purin-10(3H)-one (M1dG). J. Am. Chem. Soc. 2004;126:10571–10581. doi: 10.1021/ja040010q. [DOI] [PubMed] [Google Scholar]
- 31.Mao H, Reddy GR, Marnett LJ, Stone MP. Solution structure of an oligodeoxynucleotide containing the malondialdehyde deoxyguanosine adduct N2-(3-oxo-1-propenyl)-dG (ring-opened M1G) positioned in a (CpG)3 frameshift hotspot of the Salmonella typhimuriumhisD3052 gene. Biochemistry. 1999;38:13491–13501. doi: 10.1021/bi9910124. [DOI] [PubMed] [Google Scholar]
- 32.O'Hara SM, Marnett LJ. DNA sequence analysis of spontaneous and β-methoxy-acrolein-induced mutations in Salmonella typhimuriumhisD3052. Mutat. Res. 1991;247:45–56. doi: 10.1016/0027-5107(91)90032-j. [DOI] [PubMed] [Google Scholar]
- 33.Schnetz-Boutaud NC, Saleh S, Marnett LJ, Stone MP. The exocyclic 1,N2-deoxyguanosine pyrimidopurinone M1G is a chemically stable DNA adduct when placed opposite a two-base deletion in the (CpG)3 frameshift hotspot of the Salmonella typhimuriumhisD3052 gene. Biochemistry. 2001;40:15638–15649. doi: 10.1021/bi011242u. [DOI] [PubMed] [Google Scholar]
- 34.Weisenseel JP, Moe JG, Reddy GR, Marnett LJ, Stone MP. Structure of a duplex oligodeoxynucleotide containing propanodeoxyguanosine opposite a two-base deletion in the (CpG)3 frameshift hotspot of Salmonella typhimurium hisD3052 determined by 1H NMR and restrained molecular dynamics. Biochemistry. 1995;34:50–64. doi: 10.1021/bi00001a007. [DOI] [PubMed] [Google Scholar]
- 35.Woodson SA, Crothers DM. Preferential location of bulged guanosine internal to a G:C tract by 1H NMR. Biochemistry. 1988;27:436–445. doi: 10.1021/bi00401a065. [DOI] [PubMed] [Google Scholar]
- 36.Woodson SA, Crothers DM. Proton nuclear magnetic resonance studies on bulge-containing DNA oligonucleotides from a mutational hot-spot sequence. Biochemistry. 1987;26:904–912. doi: 10.1021/bi00377a035. [DOI] [PubMed] [Google Scholar]
- 37.Reddy GR, Marnett LJ. Synthesis of an oligodeoxynucleotide containing the alkaline labile malondialdehyde-deoxyguanosine adduct pyrimido[1,2-a]purin-10(3H)-one. J. Am. Chem. Soc. 1995;117:5007–5008. [Google Scholar]
- 38.Schnetz-Boutaud NC, Mao H, Stone MP, Marnett LJ. Synthesis of oligonucleotides containing the alkali-labile pyrimidopurinone adduct, M1G. Chem. Res. Toxicol. 2000;13:90–95. doi: 10.1021/tx990141i. [DOI] [PubMed] [Google Scholar]
- 39.Wang H, Kozekov ID, Kozekova A, Tamura PJ, Marnett LJ, Harris TM, Rizzo CJ. Site-specific synthesis of oligonucleotides containing malondialdehyde adducts of deoxyguanosine and deoxyadenosine via a postsynthetic modification strategy. Chem. Res. Toxicol. 2006;19:1467–1474. doi: 10.1021/tx060137o. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cavaluzzi MJ, Borer PN. Revised UV extinction coefficients for nucleoside-5′-monophosphates and unpaired DNA and RNA. Nucleic Acids Res. 2004;32:e13. doi: 10.1093/nar/gnh015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piotto M, Saudek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solutions. J. Biomol. NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 42.Reid BR. Sequence-specific assignments and their use in NMR studies of DNA structure. Q. Rev. Biophys. 1987;20:2–28. doi: 10.1017/s0033583500004212. [DOI] [PubMed] [Google Scholar]
- 43.Patel DJ, Shapiro L, Hare D. DNA and RNA: NMR studies of conformations and dynamics in solution. Q. Rev. Biophys. 1987;20:35–112. doi: 10.1017/s0033583500004224. [DOI] [PubMed] [Google Scholar]
- 44.Boelens R, Scheek RM, Dijkstra K, Kaptein R. Sequential assignment of imino- and amino-proton resonances in 1H NMR spectra of oligonucleotides by two-dimensional NMR spectroscopy. Application to a lac operator fragment. J. Magn. Reson. 1985;62:378–386. [Google Scholar]
- 45.Fink SP, Reddy GR, Marnett LJ. Relative contribution of cytosine deamination and error-prone replication to the induction of propanodeoxyguanosine to deoxyadenosine mutations in Escherichia coli. Chem. Res. Toxicol. 1996;9:277–283. doi: 10.1021/tx950060w. [DOI] [PubMed] [Google Scholar]
- 46.de los Santos C, Zaliznyak T, Johnson F. NMR characterization of a DNA duplex containing the major acrolein-derived deoxyguanosine adduct γ-OH-1,-N2-propano-2′-deoxyguanosine. J. Biol. Chem. 2001;276:9077–9082. doi: 10.1074/jbc.M009028200. [DOI] [PubMed] [Google Scholar]
- 47.Yang IY, Hossain M, Miller H, Khullar S, Johnson F, Grollman A, Moriya M. Responses to the major acrolein-derived deoxyguanosine adduct in Escherichia coli. J. Biol. Chem. 2001;276:9071–9076. doi: 10.1074/jbc.M008918200. [DOI] [PubMed] [Google Scholar]
- 48.VanderVeen LA, Hashim MF, Nechev LV, Harris TM, Harris CM, Marnett LJ. Evaluation of the mutagenic potential of the principal DNA adduct of acrolein. J. Biol. Chem. 2001;276:9066–9070. doi: 10.1074/jbc.M008900200. [DOI] [PubMed] [Google Scholar]
- 49.Marinelli ER, Johnson F, Iden CR, Yu PL. Synthesis of 1,N2-(1,3-propano)-2′-deoxyguanosine and incorporation into oligodoexynucleotides: A model for exocyclic acrolein-DNA adducts. Chem. Res. Toxicol. 1990;3:49–58. doi: 10.1021/tx00013a009. [DOI] [PubMed] [Google Scholar]
- 50.Singh US, Moe JG, Reddy GR, Weisenseel JP, Marnett LJ, Stone MP. 1H NMR of an oligodeoxynucleotide containing a propanodeoxyguanosine adduct positioned in a (CG)3 frameshift hotspot of Salmonella typhimuriumhisd3052: Hoogsteen base-pairing at pH 5.8. Chem. Res. Toxicol. 1993;6:825–836. doi: 10.1021/tx00036a012. [DOI] [PubMed] [Google Scholar]
- 51.Moe JG, Reddy GR, Marnett LJ, Stone MP. 1H NMR characterization of a duplex oligodeoxynucleotide containing propanodeoxyguanosine opposite a two-base deletion in the (CpG)3 frameshift hotspot of Salmonella typhimuriumhisD3052. Chem. Res. Toxicol. 1994;7:319–328. doi: 10.1021/tx00039a008. [DOI] [PubMed] [Google Scholar]
- 52.Weisenseel JP. Ph.D. Thesis. Vanderbilt University; Nashville, TN: 2000. [Google Scholar]
- 53.Weisenseel JP, Reddy GR, Marnett LJ, Stone MP. Structure of the 1,N2-propanodeoxyguanosine adduct in a three-base DNA hairpin loop derived from a palindrome in the Salmonella typhimuriumhisD3052 gene. Chem. Res. Toxicol. 2002;15:140–152. doi: 10.1021/tx010107f. [DOI] [PubMed] [Google Scholar]
- 54.Weisenseel JP, Reddy GR, Marnett LJ, Stone MP. Structure of an oligodeoxynucleotide containing a 1,N2-propanodeoxyguanosine adduct positioned in a palindrome derived from the Salmonella typhimuriumhisD3052 gene: Hoogsteen pairing at pH 5.2. Chem. Res. Toxicol. 2002;15:127–139. doi: 10.1021/tx0101090. [DOI] [PubMed] [Google Scholar]
- 55.Kouchakdjian M, Marinelli E, Gao X, Johnson F, Grollman A, Patel D. NMR studies of exocyclic 1,N2-propanodeoxyguanosine adducts (X) opposite purines in DNA duplexes: Protonated X(syn):A(anti) pairing (acidic pH) and X(syn):G(anti) pairing (neutral pH) at the lesion site. Biochemistry. 1989;28:5647–5657. doi: 10.1021/bi00439a047. [DOI] [PubMed] [Google Scholar]
- 56.Kouchakdjian M, Eisenberg M, Live D, Marinelli E, Grollman AP, Patel DJ. NMR studies of an exocyclic 1N2-propanodeoxyguanosine adduct (X) located opposite deoxyadenosine (A) in DNA duplexes at basic pH: Simultaneous partial intercalation of X and A between stacked bases. Biochemistry. 1990;29:4456–4465. doi: 10.1021/bi00470a028. [DOI] [PubMed] [Google Scholar]
- 57.Huang P, Eisenberg M. The three-dimensional structure in solution (pH 5.8) of a DNA 9-mer duplex containing 1,N2-propanodeoxyguanosine opposite deoxyadenosine. Restrained molecular dynamics and NOE-based refinement calculations. Biochemistry. 1992;31:6518–6532. doi: 10.1021/bi00143a023. [DOI] [PubMed] [Google Scholar]
- 58.Huang P, Patel DJ, Eisenberg M. Solution structure of the exocyclic 1,N2-propanodeoxyguanosine adduct opposite deoxyadenosine in a DNA nonamer duplex at pH 8.9. Model of pH-dependent conformational transition. Biochemistry. 1993;32:3852–3866. doi: 10.1021/bi00066a004. [DOI] [PubMed] [Google Scholar]
- 59.Plum GE, Grollman AP, Johnson F, Breslauer KJ. Influence of an exocyclic guanine adduct on the thermal stability, conformation, and melting thermodynamics of a DNA duplex. Biochemistry. 1992;31:12096–12102. doi: 10.1021/bi00163a019. [DOI] [PubMed] [Google Scholar]
- 60.Streisinger G, Okada Y, Enrich J, Newton J, Tsugita A, Terzaghi E, Inouye M. Frameshift mutations and the genetic code. Cold Spring Harbor Symp. Quant. Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- 61.Hartman PE, Ames BN, Roth JR, Barnes WM, Levin DE. Target sequences for mutagenesis in Salmonella histidine-requiring mutants. Environ. Mutagen. 1986;8:631–641. doi: 10.1002/em.2860080414. [DOI] [PubMed] [Google Scholar]
- 62.Oeschger NS, Hartman PE. ICR-induced frameshift mutations in histidine operon of Salmonella. J. Bacteriol. 1970;101:490–504. doi: 10.1128/jb.101.2.490-504.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McCann J, Spingarn NE, Koburi J, Ames BN. Detection of carcinogens as mutagens: Bacterial tester strains with R-factor plasmids. Proc. Natl. Acad. Sci. U.S.A. 1975;72:979–983. doi: 10.1073/pnas.72.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.DeMarini DM, Abu-Shakra A, Gupta R, Hendee LJ, Levine JG. Molecular analysis of mutations induced by the intercalating agent ellipticine at the hisD3052 allele of Salmonella typhimurium TA98. Environ. Mol. Mutagen. 1992;20:12–18. doi: 10.1002/em.2850200104. [DOI] [PubMed] [Google Scholar]
- 65.Bell DA, Levine JG, DeMarini DM. DNA sequence analysis of revertants of the hisD3052 allele of Salmonella typhimurim TA98 using the polymerase chain reaction and direct sequencing: Application to 1-nitropyrene-induced revertants. Mutat. Res. 1991;252:35–44. doi: 10.1016/0165-1161(91)90249-8. [DOI] [PubMed] [Google Scholar]
- 66.Fuscoe JC, Wu R, Shen NH, Healy SK, Felton JS. Change analysis of revertants of the hisD3052 allele in Salmonella typhimurium. Mutat. Res. 1988;201:241–251. doi: 10.1016/0027-5107(88)90131-5. [DOI] [PubMed] [Google Scholar]
- 67.Isono K, Yourno J. Chemical carcinogens as frameshift mutagens: Salmonella DNA sequence sensitive to mutagenesis by polycyclic carcinogens. Proc. Natl. Acad. Sci. U.S.A. 1974;71:1612–1617. doi: 10.1073/pnas.71.5.1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Patel DJ, Kozlowski SA, Marky LA, Rice JA, Broka C, Itakura K, Breslauer KJ. Extra adenosine stacks into the self-complementary d(CGCAGAATTCGCG) duplex in solution. Biochemistry. 1982;21:445–451. doi: 10.1021/bi00532a004. [DOI] [PubMed] [Google Scholar]
- 69.Morden KM, Chu YG, Martin FH., Jr Unpaired cytosine in the deoxyoligonucleotide duplex dCA3CA3G:dCT6G is outside of the helix. Biochemistry. 1983;22:5557–5563. [Google Scholar]
- 70.Morden KM, Gunn BM, Maskos K. NMR studies of a deoxyribodecanucleotide containing an extrahelical thymidine surrounded by an oligo(dA):oligo(dT) tract. Biochemistry. 1990;29:8835–8845. doi: 10.1021/bi00489a047. [DOI] [PubMed] [Google Scholar]
- 71.Rosen MA, Live D, Patel DJ. Comparative NMR study of An-bulge loops in DNA duplexes: Intrahelical stacking of A, A-A, and A-A-A bulge loops. Biochemistry. 1992;31:4004–4014. doi: 10.1021/bi00131a016. [DOI] [PubMed] [Google Scholar]
- 72.Rosen MA, Shapiro L, Patel DJ. Solution structure of a trinucleotide A-T-A bulge loop within a DNA duplex. Biochemistry. 1992;31:4015–4026. doi: 10.1021/bi00131a017. [DOI] [PubMed] [Google Scholar]
- 73.Joshua-Tor L, Frolow F, Appella E, Hope H, Rabinovich D, Sussman JL. Three-dimensional structures of bulge-containing DNA fragments. J. Mol. Biol. 1992;225:397–431. doi: 10.1016/0022-2836(92)90929-e. [DOI] [PubMed] [Google Scholar]
- 74.Morden KM, Maskos K. NMR studies of an extrahelical cytosine in an A•T rich region of a deoxyribodecanucleotide. Biopolymers. 1993;33:27–36. doi: 10.1002/bip.360330104. [DOI] [PubMed] [Google Scholar]
- 75.Aboul-ela F, Murchie AI, Homans SW, Lilley DM. Nuclear magnetic resonance study of deoxyoligonucleotide duplex containing a three base bulge. J. Mol. Biol. 1993;229:173–188. doi: 10.1006/jmbi.1993.1016. [DOI] [PubMed] [Google Scholar]
- 76.Patel DJ, Kozlowski SA, Ikuta S, Itakura K, Bhatt R, Hare DR. NMR studies of DNA conformation and dynamics in solution. Cold Spring Harbor Symp. Quant. Biol. 1982;97:197–206. doi: 10.1101/sqb.1983.047.01.025. [DOI] [PubMed] [Google Scholar]
- 77.Nikonowicz EP, Meadows RP, Gorenstein DG. NMR structural refinement of an extrahelical adenosine tridecamer d(CGCAGAATTCGCG)2 via a hybrid relaxation matrix procedure. Biochemistry. 1990;29:4193–4204. doi: 10.1021/bi00469a024. [DOI] [PubMed] [Google Scholar]
- 78.van den Hoogen YT, van Beuzekom AA, de Vroom E, Van Der Marel GA, van Boom JH, Altona C. Bulge-out structures in the single-stranded trimer AUA and in the duplex (CUGGUGCGG):(CCGCCCAG). A model-building and NMR study. Nucleic Acids Res. 1988;16:5013–5030. doi: 10.1093/nar/16.11.5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van den Hoogen YT, van Beuzekom AA, van den Elst H, van der Marel GA, van Boom JH, Altona C. Extra thymidine stacks into the d(CTGGTGCGG):d(CCGCCCAG) duplex. An NMR and model-building study. Nucleic Acids Res. 1988;16:2971–2986. doi: 10.1093/nar/16.7.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kalnik MW, Norman DG, Li BF, Swann PF, Patel DJ. Conformational transitions in thymidine bulge-containing deoxytridecanucleotide duplexes: Role of flanking sequence and temperature in modulating the equilibrium between looped out and stacked thymidine bulge states. J. Biol. Chem. 1990;265:636–647. [PubMed] [Google Scholar]
- 81.Maskos K, Gunn BM, LeBlanc DA, Morden KM. NMR study of G•A and A•A pairing in (dGCGAATAAGCG)2. Biochemistry. 1993;32:3583–3595. doi: 10.1021/bi00065a009. [DOI] [PubMed] [Google Scholar]
- 82.Bebenek K, Kunkel TA. Streisinger revisited: DNA synthesis errors mediated by substrate misalignments. Cold Spring Harbor Symp. Quant. Biol. 2000;65:81–91. doi: 10.1101/sqb.2000.65.81. [DOI] [PubMed] [Google Scholar]
- 83.Benamira M, Singh U, Marnett LJ. Site-specific frameshift mutagenesis by a propanodeoxyguanosine adduct positioned in the (CpG)4 hot-spot of Salmonella typhimuriumhisD3052 carried on an M13 vector. J. Biol. Chem. 1992;267:22392–22400. [PubMed] [Google Scholar]
- 84.Tippin B, Kobayashi S, Bertram JG, Goodman MF. To slip or skip, visualizing frameshift mutation dynamics for error-prone DNA polymerases. J. Biol. Chem. 2004;279:45360–45368. doi: 10.1074/jbc.M408600200. [DOI] [PubMed] [Google Scholar]
- 85.Hashim MF, Riggins JN, Schnetz-Boutaud N, Voehler M, Stone MP, Marnett LJ. In vitro bypass of malondialdehyde-deoxyguanosine adducts: Differential base selection during extension by the Klenow fragment of DNA polymerase I is the critical determinant of replication outcome. Biochemistry. 2004;43:11828–11835. doi: 10.1021/bi049360f. [DOI] [PubMed] [Google Scholar]
- 86.Tippin B, Pham P, Goodman MF. Error-prone replication for better or worse. Trends Microbiol. 2004;12:288–295. doi: 10.1016/j.tim.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 87.Prakash S, Johnson RE, Prakash L. Eukaryotic translesion synthesis DNA polymerases: Specificity of structure and function. Annu. Rev. Biochem. 2005;74:317–353. doi: 10.1146/annurev.biochem.74.082803.133250. [DOI] [PubMed] [Google Scholar]
- 88.Zang H, Goodenough AK, Choi JY, Irimia A, Loukachevitch LV, Kozekov ID, Angel KC, Rizzo CJ, Egli M, Guengerich FP. DNA adduct bypass polymerization by Sulfolobus solfataricus DNA polymerase Dpo4. Analysis and crystal structures of multiple base-pair substitution and frameshift products with the adduct 1,N2-ethenoguanine. J. Biol. Chem. 2005;280:29750–29764. doi: 10.1074/jbc.M504756200. [DOI] [PubMed] [Google Scholar]
- 89.Irimia A, Zang H, Loukachevitch LV, Eoff RL, Guengerich FP, Egli M. Calcium is a cofactor of polymerization but inhibits pyrophosphorolysis by the Sulfolobus solfataricus DNA polymerase Dpo4. Biochemistry. 2006;45:5949–5956. doi: 10.1021/bi052511+. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.