Abstract
Purpose
To investigate the feasibility and utility of arterial spin labeling (ASL) perfusion MRI in characterizing alterations of cerebral blood flow (CBF) in pediatric patients with arterial ischemic stroke (AIS).
Materials and Methods
Ten children with AIS were studied within 4–125 hours following symptom onset, using a pulsed ASL (PASL) protocol attached to clinically indicated MR examinations. The inter-hemisphere perfusion deficit (IHPD) was measured in predetermined vascular territories and infarct regions of restricted diffusion, which were compared with the degree of arterial stenosis and volumes of ischemic infarcts.
Results
Interpretable CBF maps were obtained in all 10 patients, showing simple lesion in 9 patients (5 hypoperfusion, 2 hyperperfusion, and 2 normal perfusion) and complex lesions in one patient. Both acute and follow-up infarct volumes were significantly larger in cases with hypoperfusion than in either hyper-or normal perfusion cases. The IHPD was found to correlate with the degree of stenosis, diffusion lesion and follow-up T2 infarct volumes. Mismatch between perfusion and diffusion lesions was observed. Brain regions presenting delayed arterial transit effects were tentatively associated with positive outcome.
Conclusion
This study demonstrates the clinical utility of ASL in the neuroimaging diagnosis of pediatric AIS.
Keywords: Ischemic stroke, Magnetic resonance imaging, Perfusion, Pediatrics
INTRODUCTION
Arterial ischemic stroke (AIS) affects 2 to 8 per 100,000 children per year in Europe and North America, and ranks among the top 10 causes of death in this age group (1–3). Nonatherosclerotic arteriopathies are increasingly identified as the most frequent cause of pediatric AIS (4, 5). Cerebral blood flow (CBF) mirrors cerebral metabolic demand and neuronal function and is therefore a vital parameter in the evaluation of pediatric brain injury and recovery. Studies in adult stroke have shown that multimodal MRI including diffusion and perfusion MRI can be used to quantify the initial extent of stroke and the volume of viable tissue at risk of infarct completion. The finding of perfusion-diffusion mismatch has been proposed as a criterion for using acute thrombolytic or perfusional interventions, and may be useful in predicting patient outcome (6, 7). While diffusion MRI is relatively mature and has been readily applied in both adults and children, perfusion MRI has been studied in a limited way in pediatric stroke.
Arterial spin labeling (ASL) perfusion MRI is an emerging technology for noninvasive measurement of CBF that utilizes arterial blood water as an endogenous tracer (8). ASL is a safe, economical and convenient MRI technology ideally suited for pediatric perfusion imaging because there is no need for administration of contrast agents or radioactive tracers. Unique aspects of pediatric physiology contribute to an excellent image quality of ASL perfusion MRI in children (8, 9). Additionally, ASL perfusion MRI offers the potential advantage over dynamic susceptibility contrast perfusion MRI for providing repeatable quantitative CBF measurements. To date this technique has not been applied to the evaluation of acute AIS in childhood. The purpose of this study was to investigate the utility of pulsed ASL (PASL) perfusion MRI in characterizing acute and subacute alterations of regional CBF in children with AIS, and to describe how hemodynamic alterations relate to cerebral anatomic and vascular abnormalities demonstrated by conventional MRI and MR angiography (MRA).
MATERIALS AND METHODS
Subjects
We performed a prospective consecutive cohort study of 87 infants and children with acute AIS enrolled from May 2002-Oct 2006. The present study of ASL perfusion MRI included children from that cohort study aged 1 year to 18 years who underwent ASL perfusion MRI as part of the acute brain MRI at the time of hospital admission. Children with infratentorial infarcts, or multiple infarcts of varying ages were excluded. AIS was defined as an acute focal neurologic deficit with evidence of acute cerebral infarction in an arterial distribution on brain imaging, regardless of duration of clinical symptoms (10). The diagnosis of AIS was determined by a pediatric neurologist based on results of standard clinical neurological assessment and neuroimaging according to an institutional stroke protocol. Written informed parental consent and patient assent were obtained according to a protocol approved by the institutional review committee. Among 37 children who met inclusion criteria, 10 patients underwent PASL perfusion imaging at the time of admission. The median age of study subjects was 9.2 years, range 1.1 to 16 years; 7 were males, 3 females. Table 1 provides the demographic and clinical information of the 10 patients. In addition, 9 children (6 males, 3 females, median age = 7.5years, range 1.1 to 17.6 years) without acute brain injury were recruited as comparison subjects. The comparison subjects were recruited from a list of children scheduled for clinical-indicated non-contrast MRI for the diagnosis of developmental delay and chronic headache disorders, and whose scans were found to be anatomically normal (see Table 2).
Table 1.
Clinical characteristics and image acquisition parameters of the 10 study subjects
| Case No |
Age (yr) /Sex |
Major Stroke Risk Factors |
Clinical Presentation | Time to Scan (hr) |
Field strength |
Delay time (sec) |
Sedation for MRI (mg/kg) |
Time to FU (mo) |
|---|---|---|---|---|---|---|---|---|
| 1 | 16 | HIV vasculitis, | Right hemiparesis, | 5.3 | 1.5T | 1.0 | None | 14 |
| M | IV Ig infusion | homonymous hemianopsia, aphasia, | ||||||
| 2 | 14.8 | FIA, | Headache, transient | 120 | 1.5T | 1.5 | None | 3 |
| F | thrombophilia* | visual blurring, left hemipharesis | ||||||
| 3 | 10.3 | ASA/PFO | Left facial weakness, | 120 | 1.5T | 1.5 | None | 7 |
| F | Left hemichoreoathetosis | |||||||
| 4 | 6.9 | FIA† | Right hemiparesis, | 31 | 1.5T | 0.7 | None | 3 |
| M | expressive aphasia | |||||||
| 5 | 11.6 | FIA, | Aphasia, syncope, | 16 | 3T | 1.5 | None | 3 |
| M | thrombophilia‡ | headache, right hemiparesis | ||||||
| 6 | 2.8 | Moya-Moya | Right hemiparsis, | 4 | 3T | 1.5 | MDZ 0.05; | 6 |
| M | right focal seizure | PB 2.0 | ||||||
| 7 | 1.2 | SVT | Right hemiparesis, | 125 | 3T | 1.5 | MDZ 0.1; | 8 |
| M | pneumonia theombophilia§ | left homonymous hemianopsia | PB 6.0 | |||||
| 8 | 10 | FIA | Headache, aphasia, | 17.3 | 3T | 1.5 | Fent infusion | 8 |
| F | right hemiparesis | 1mg/kg/hr | ||||||
| 9 | 4.9 | Thrombophilia ∥ | Left homonymous | 44 | 3T | 1.5 | MDZ 0.04; | 3 |
| M | hemianopsia | PB 1.0 | ||||||
| 10 | 13.1 | Thrombophilia# | Dysarthria, mild right | 12 | 3T | 1.5 | None | 3 |
| M | hemiparesis, blurred vision |
Factor V leiden mutation
Focal intracranial arteriopathy post varicella
Positive lupus anticoagulant
Positive lupus anticoagulant
High lipoprotein A
Elevated lipoprotein A
FIA = Focal intracranial arteriopathy; ASA = Atrial Septal Aneurysm; PFO = Patent Foramen Ovale; SVT = Superaventricular tachycardia; RSV = Respiratory Syncytial Virus; MDZ = midazolam; PB = pentobarbital; Fent = fentanyl; FU = follow-up
Table 2.
Quantitative CBF values obtained in 9 comparison
| Case No. | Age (y) | Gender | gCBF (ml/ 100g/min) |
IHPD ACA (%) | IHPD MCA (%) |
IHPD PCA (%) |
Mean IHPD VT(%) |
|---|---|---|---|---|---|---|---|
| 1 | 1.1 | F | 157.1 | 22.3 | 8.7 | 24.5 | 18.5 |
| 2 | 1.2 | M | 117.1 | 7.1 | 1.5 | 14.7 | 7.8 |
| 3 | 3.8 | M | 83.9 | 15.5 | 6.7 | 8.5 | 10.2 |
| 4 | 3.8 | M | 103.4 | 4.6 | 2.8 | 15.0 | 7.5 |
| 5 | 7.5 | F | 124.8 | 9.3 | 0.6 | 6.3 | 5.4 |
| 6 | 8.7 | M | 87.9 | 1.4 | 1.7 | 12.0 | 5.0 |
| 7 | 9 | F | 67.5 | 1.0 | 6.3 | 1.2 | 2.9 |
| 8 | 15.2 | M | 89.3 | 6.0 | 3.5 | 20.0 | 9.8 |
| 9 | 17.6 | M | 81.3 | 5.2 | 5.1 | 6.1 | 5.4 |
| Mean | 7.5 | 101.4 | 8.1 | 4.1 | 12.0 | 8.1# | |
| SD | 5.8 | 27.6 | 6.9 | 2.8 | 7.4 | 5.7# |
gCBF = global CBF; IHPD = Inter-hemisphere perfusion deficit; ACA = anterior cerebral artery; MCA = middle cerebral artery; PCA = posterior cerebral artery; VT = vascular territory
We choose 20% as the criterion of significant IHPD in AIS patients based on the mean IHPD plus 2 SD (8.1+2 × 5.7 = 19.5%) in comparison children.
Image Acquisition
MR images were obtained for clinical indications according to an institutional clinical stroke protocol including diffusion-weighted imaging (DWI) with apparent diffusion coefficient (ADC) maps (TR/TE=5800/78ms, 128x128, 4.8mm slice, b=0 and 1000s/mm2), T1 weighted imaging (MPRAGE, TR/TI/TE=2050/1050/2ms, 256x256, 1mm slice), T2 weighted imaging (TSE, TR/TE= 6000/108ms, 256x256, 1.5mm slice), fluid-attenuation inversion recovery (FLAIR, TR/TI/TE=9000/ 2500/95ms, 256x256, 4.8mm slice) and MRA (TR/TE=36/3ms, 448x448, 1mm slice). MRI was obtained on Siemens whole-body 1.5T Vision or Sonata, or 3T Trio systems (Erlangen, Germany). An identical PASL sequence (9) was attached to clinically indicated MRI scans. Imaging parameters of the PASL scan were: FOV = 20 cm, 64x64 matrix, TR/TE = 3000/19 ms, slice thickness = 8 mm, 2 mm gap for 1.5 T; 5 mm and 1 mm gap for 3T. Eight (1.5T) or 16 (3.0T) slices were acquired using a gradient echo-planar imaging (EPI) sequence. A delay time (0.7–1.5 sec) was applied between the saturation and excitation pulses to reduce transit artifacts. A volume of M0 images (TR/TE=8000/19ms, TI=6s, same slice position as PASL scans) were acquired after the PASL scans using the gradient-echo EPI sequence. Total scan time for PASL with 80 acquisitions was 4 min 20 sec. Follow-up MR examinations obtained for clinical indications were available in all patients 3–14 months later (median = 4.5 months) and were used to calculate chronic infarct volumes. A summary of MR imaging features of each case is presented in Table 1.
For perfusion MRI data, the raw EPI images were separated into label and control pairs and pair-wise subtracted. Artifact due to patient movement during PASL scans was corrected using an algorithm based on principal component analysis. The subtracted difference images were then averaged across the image series to form the mean ASL difference perfusion image. Absolute CBF maps in units of ml/100g/min were generated based on a single-compartment PASL perfusion model using a blood T1 of 1.2 sec at 1.5T and 1.49 sec at 3.0T, as well as the acquired M0 image with a blood/brain water partition coefficient (λ) of 0.9 ml/g (11). Note these parameters were adopted from adult literature. Relative perfusion differences between the affected and unaffected hemispheres were used for the following analyses to avoid potential variations in absolute CBF quantification across age groups and two field strengths. Images of different MR modalities of the same patient were co-registered to the T1 weighted MRI and then normalized into a canonical space (Montreal Neurological Institute standard brain) using the SPM2 software package (Wellcome Department of Imaging Neuroscience, UCL).
Image Analysis
Post-acquisition analyses included visual inspection of PASL perfusion images and concurrently acquired DWI, T1/T2, FLAIR and MRA images. These data were assessed for perfusion abnormalities including both hypo- and hyperperfusion. CBF maps were further thresholded by the mean global CBF value plus two times the standard deviation (SD) of CBF values across pixels to identify delayed arterial transit effects (focal intravascular signals). These hyper-intensities were then confirmed by visual inspection.
Two approaches were employed for quantitative analysis. In the first approach, mean CBF in the region of infarct was determined by manually tracing the area of restricted diffusion from the DWI (b = 1000s/mm2) as a region of interest (ROI), and then superimposing the ROI on the CBF maps. A contralateral ROI was also generated by flipping the infarct ROI in the left/right direction to quantify the mean CBF within the mirror ROI in the unaffected hemisphere. In the second approach, CBF in the vascular territory involving the infarct was estimated using a published template (12) through automatic segmentation of normalized CBF maps into vascular territories in the left and right brain hemispheres. Mean CBF values were obtained in the standardized vascular territories on both affected and unaffected hemispheres. The inter-hemisphere perfusion difference (IHPD) was calculated as (unaffected - affected) / unaffected × 100% for both ROI and vascular territory based approaches. Therefore IHPD with positive values reflect hypoperfusion of the affected hemisphere and negative IHPD values demonstrate the converse. An IHPD of 20% was determined as the criterion for a significant perfusion difference between the two hemispheres. This was based on the mean IHPD values + 2 SD from 9 comparison children (Table 2). This criterion is comparable to that reported in adult stroke studies (13).
Acute infarct regions were identified on initial DWI images of maximum contrast (b = 1000 s/mm2) with confirmation of restricted diffusion on ADC maps. Follow-up infarct volumes were measured on the follow-up T2-weighted images. Two neuroradiologists, blind to all clinical information, independently traced the visible lesions on DWI maps and the follow-up T2-weighted images using the MRICRO software. The outer edge of the hyperintense lesional tissue was traced manually on each DWI and T2-weighted images. The whole brain volume was manually traced slice by slice on T2 images excluding the ventricles. Lesion and brain volumes were obtained by summing the number of outlined voxels and multiplying by the slice thickness (including inter-slice gap) of each slice. Inter-reader reliabilities of volumetric assessments were consistently above 0.99 (r = 0.991, P < 0.001 for DWI ROI volume, and r = 0.995, P < 0.001 for T2 volume), and mean values of the two readers were reported.
Analysis of perfusion and diffusion mismatch was carried out primarily for the hypoperfusion lesions. First, the volume of perfusion deficits was manually delineated on CBF maps. The manually delineated perfusion ROI was then thresholded to only include pixels with perfusion values below 60% (or above 140% in hyperperfusion cases) of the mean CBF value in the contralateral ROI. We chose 60% as the threshold range because it represented 2SD of CBF values across pixels in the control ROI. PWI/DWI mismatch was then defined as the volume difference between PWI and DWI greater than 20% of diffusion lesion volume (14).
The MRA findings were graded qualitatively according to severity of arterial stenosis into three groups: 1) normal – vessels of affected arterial territory had normal-appearing flow signal; 2) partial stenosis – vessels of affected arterial territory had luminal narrowing with some flow related enhancement of the vessels; 3) completed stenosis – vessels of affected arterial territory had complete occlusion with no flow related enhancement of the vessel distal to the stenosis. These groups were then analyzed to determine the relationship between the degree of stenosis and the IHPD or ischemic lesion volumes.
Statistical Analysis
Non-parametric Mann-Whitney Test (2-tailed) was used to evaluate the significance of observed differences in infarct volumes between hypoperfused lesions (IHPD ≥ 20%) vs. lesions with hyperperfusion (IHPD ≤ −20%) and normal perfusion (−20% < IHPD < 20%) (13, 15). Spearman rank correlation (2-tailed) was employed to assess the relationship between regional perfusion abnormalities, volumetric measures of ischemic infarcts and the degree of arterial stenosis defined by conventional MR sequences, using the SPSS 12.0 software package (SPSS Inc. Chicago, IL).
RESULTS
MRI Data
Quantitative CBF maps of diagnostic quality were obtained in all 10 patients. The initial scan was obtained at a median of 24.2 hours after symptom onset (range 4–125 hours). Nine patients had a single lesion and one patient had 2 lesions (complex, Patient #7) for a total of 11 AIS lesions. Among the 9 solitary lesions, 7 (78%) were located in the middle cerebral artery (MCA) and 2 (22%) in the posterior cerebral artery (PCA) territories. Perfusion abnormalities could be identified by visual inspection of the color scale CBF maps in 9 of the 10 cases (except for Patient #5). The lateralization of the perfusion abnormalities was compatible with anatomic abnormalities identified on DWI, T2, follow-up T2 and MRA images as well as the clinical localization of these 9 patients’ symptoms (Table 1).
Table 3 summarizes results of quantitative analysis of 11 lesions. Figure 1 shows the IHPD results measured in infarct ROIs and vascular territories. The IHPD values measured in infarct ROIs correlated with corresponding measures in vascular territories (r = 0.618, P = 0.043), suggesting considerable consistency in quantifying perfusion deficits with either manual or automated approaches. Due to the relatively small lesion sizes in both perfusion and diffusion images of patients #3 and #10, there was essentially no detectable perfusion difference between the affected and unaffected vascular territories. Given this potential discrepancy, we used IHPD measured in infarct ROIs for the final assessment of hypo-, hyper- and normal perfusion cases. This analysis resulted in 5 cases (56%) with hypoperfusion (IHPD ≥ 20%), 2 cases (22%) with hyperperfusion (IHPD ≤ −20%) and 2 cases (22%) with normal perfusion (−20% < IHPD < 20%) among the 9 patients with simple lesion.
Table 3.
Results of quantitative analysis of 11 lesions.
| Case | Les | VT | gCBF | Stenosis | Acute DWI | Ratio of DWI Inf | Perf Les | FU T2 | Ratio of FU T2 | IHPD (%) |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| No. | No. | affected | (ml/100g/ min) |
Grade | Inf Vol | Vol /Brain Vol (%) | Vol | Inf Vol | Inf Vol /Brain | VT | DWI |
| (mL) | (mL) | (mL) | Vol (%) | ROI | |||||||
| 1 | 1 | L MCA | 66.6 | 3 | 118.8 | 10.95 | 169.2 | 147 | 0.31 | 35.2 | 58.9 |
| 2 | 2 | R MCA | 53.4 | 2 | 6.3 | 0.47 | 20.3 | 4.2 | 0.17 | −110.3 | −65.4 |
| 3 | 3 | R MCA | 63.6 | 3 | 13.2 | 1.17 | 10.3 | 2.0 | 0.32 | −11.5 | 56.6 |
| 4 | 4 | L MCA | 36.1 | 3 | 32.8 | 2.73 | 66.5 | 3.9 | 0.14 | 26.5 | 17.5 |
| 5 | 5 | L MCA | 96.4 | 2 | 0.8 | 0.06 | N/A | 1.7 | 1.90 | 10.3 | 8.5 |
| 6 | 6 | L MCA | 93.6 | 3 | 69.5 | 6.41 | 98.9 | 20.6 | 0.48 | 32.1 | 64.7 |
| 7 | 7 | R PCA | 28.2 | 2 | 30.9 | 3.63 | 15.7 | 4.1 | 11.39 | -40.6 | -38.4 |
| 8 | L MCA | 3 | 141.9 | 16.66 | 93.5 | 97 | 0.55 | 32.7 | 93.8 | ||
| 8 | 9 | L MCA | 74.0 | 2 | 24.2 | 2.10 | 12.9 | 6.3 | 2.66 | 78.8 | 42.4 |
| 9 | 10 | R PCA | 26.9 | 2 | 89.3 | 8.00 | 62.4 | 29.7 | 0.02 | 53.3 | 49.0 |
| 10 | 11 | L PCA | 65.8 | 1 | 1.8 | 0.14 | 1.9 | 0.2 | 0.02 | 3.0 | −59.4 59.4 |
Les = lesion; VT = vascular territory; MCA = middle cerebral artery; PCA = posterior cerebral artery; DWI Inf Vol = acute infarct volume on admission diffusion-weighted images (DWI); FU = follow-up; FU T2 Inf Vol = chronic infarct volume on follow-up T2 images; IHPD = Inter-hemisphere perfusion deficit; ROI = the region of interest.
# mean ± SD of gCBF = 60.46 ± 24.63 ml/100g/min
Figure 1.
The inter-hemisphere perfusion deficit (IHPD) measured in ROIs of diffusion lesions and vascular territories (VT). Data are presented from the shortest to the longest imaging time following symptom onset in the 11 lesions. Each lesion is represented by a pair of bars, the red one for vascular territory, and green one for ROI. The positive and negative 20% lines are shown. Patient number is shown in the bracket.
Cases With Hypoperfusion
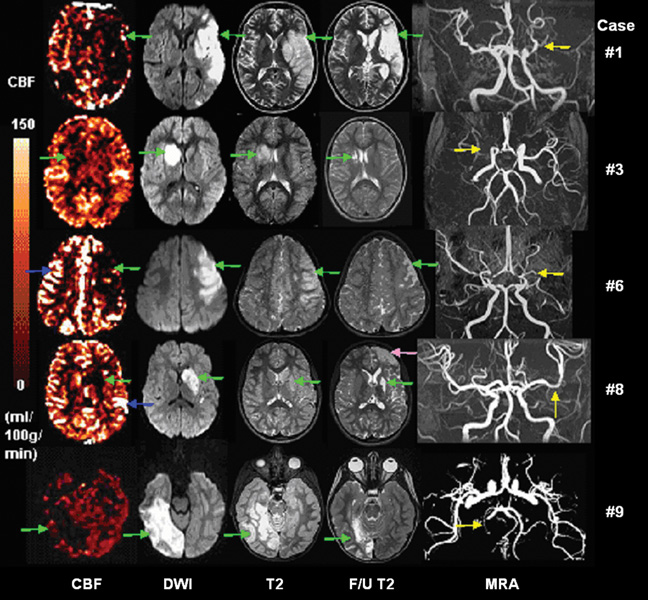
Figure 2 shows CBF, DWI, MRA, initial and follow-up T2-weighted images for the 5 hypoperfusion cases. The lesions in this group were associated with larger infarcts than those in the hyper- or normal perfusion groups. The median infarct volume on initial DWI images was 69.5 mL (range 13.2–118.8mL), corresponding to a median of 6.4 % of total brain volume (range 1.2–11.0%). The median infarct volume on follow-up T2 images was 20.6 mL (range 2.0–147mL), corresponding to 1.9% of total brain volume (range 0.2–13.5%). Three lesions (60%) developed a follow-up T2 infarct volume ≥ 20mL. One patient (Patient #8) experienced a second, clinically occult infarct in the ipsilateral anterior cerebral artery territory between the time of initial and follow-up imaging. We excluded this secondary lesion for statistical analysis.
Figure 2.
CBF, DWI, initial and follow-up T2 images, and MRA for 5 hypoperfusion cases. Infarct lesions are marked by green arrows, arterial transit effects are marked by blue arrows, arterial stenosis is marked by yellow arrows. For Patient #8, secondary left frontal lobe infarct was marked by a rose arrow.
Using a semi-automatic segmentation approach, PWI/DWI mismatch was observed in the 5 hypoperfusion lesions: perfusion lesion volume was greater than diffusion lesion volume in 2 patients (#1 and #6), whereas perfusion lesion volume was smaller than diffusion lesion volume in the rest 3 cases (#3, #8 and #9) (see Fig. 3 and Table 3). The two PWI>DWI lesions had an earlier mean scan time (4.7 ± 0.9 hours after symptom onset) compared to the PWI<DWI lesions (60.4 ± 53.3 hours after symptom onset, P = 0.083, 2-tailed).
Figure 3.
CBF, DWI, and initial T2 images of a representative patient (#6) with PWI>DWI lesion (A), and another representative patient (#8) with PWI<DWI lesion. Regions of PWI lesion, DWI lesion and conjunction of PWI and DWI lesions are indicated by green, red and blue colors respectively, which are overlaid upon T2 images.
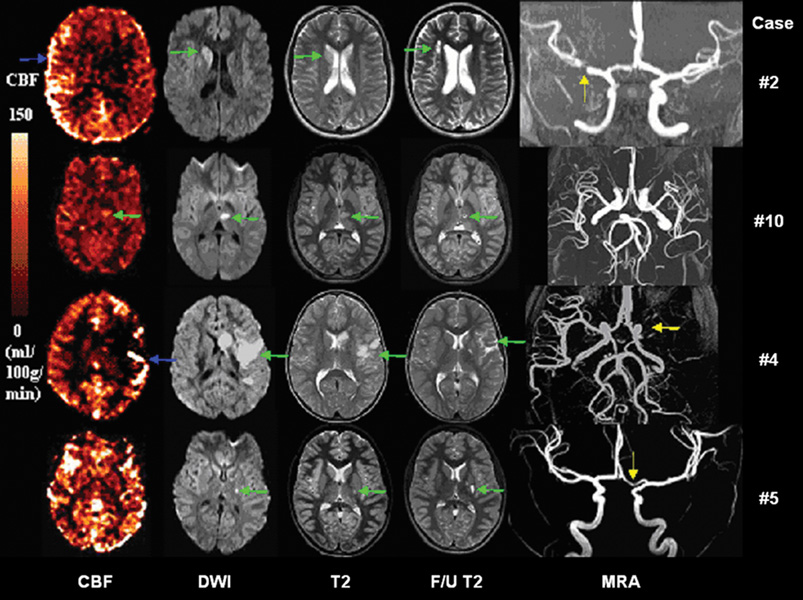
Cases With Hyper- & Normal Perfusion
Figure 4 shows CBF, DWI, MRA, initial and follow-up T2-weighted images for the 2 hyper- and 2 normal perfusion cases. There was no difference in time from symptom onset to presentation between the hyper- and hypoperfusion cases (52.6 ± 56 hours hyperperfusion, 60.8 ± 57 hours hypoperfusion, P = 0.81). Compared to the hypoperfusion cases, the infarcts on initial DWI in this group were smaller (P = 0.032), with a median of 4.1 mL (range 0.8–32.8 mL), corresponding to 0.31% of total brain volume (range 0.06–2.73%). Infarct volumes on follow-up T2 images were also smaller as compared to the hypoperfusion cases (P = 0.05), with a median volume of 2.8 mL (range 0.2 – 4.2mL), corresponding to 0.23% of total brain volume (range 0.02–0.32%). In particular, Patient #10’s symptoms began to resolve with intravenous (IV) fluids and head of bed positioning to optimize cerebral perfusion, and had completely resolved by the time of MRI scanning. Follow-up imaging on Patient #2 demonstrated slight expansion of the original lesion to include periventricular white matter regions within the right MCA territory.
Figure 4.
CBF, DWI, initial and follow-up T2 images, and MRA for 2 hyperperfusion (Patient #2 and #10) and 2 normal perfusion (Patient #4 and #5) cases. In Patient #2, CBF map shows delayed arterial transit in the left MCA territory (blue arrow). In Patient #10, CBF map shows hyperperfusion in the left thalamic region, and a corresponding lesion is present on both DWI and initial T2-weighted images. In Patient #4, CBF map shows hypoperfusion with delayed transit (blue arrow) in the left MCA territory. In Patient #5, CBF map shows normal perfusion, despite a small ischemic lesion in DWI, T2 and follow-up T2 images. Stenosis is present in M1 segment of Patient #2, #4 and #5 on MRA (yellow arrows).
Within the 4 hyper and normal perfusion lesions, Patient #4 also demonstrated PWI/DWI mismatch with perfusion volume greater than diffusion volume. Nevertheless, this case was acquired with a short post-labeling delay time of 700ms (by which time the label has not fully flown into affected cortical regions) and the follow-up T2 infarct was relatively small. The clinical development of this case was consistent with the IHPD value (<20%) and the presentation of delayed arterial transit effects (see discussion below).
Case With A Complex Lesion
One patient presented with multiple ischemic lesions involving the left MCA and right PCA territories (Fig. 5). The left MCA lesion showed hypoperfusion which was consistent with restricted diffusion and hyperintensity on T2 images. MRA showed narrowing in the left ICA and complete absence of the left MCA. In contrast, the right PCA lesion was manifested by relative hyperperfusion. DWI indicated restricted diffusion in corresponding areas, yet there was no visible abnormality on T2 images. MRA showed right PCA stenosis. Consequently, the follow-up T2 infarct volume was large (97mL) on the left side, and small (4.1mL) on the right side.
Figure 5.
CBF, DWI, initial and follow-up T2 images, and MRA for the complex infarct case. The hypoperfusion lesions locate in the left and right MCA territories, perfusion deficit is consistent with diffusion restricted lesion and T2 hyperintensity (green arrows). The hyperperfusion lesions locate in the right PCA and left ACA territories (blue arrows), where there is restricted diffusion and mildly abnormal T2 signal. MRA shows left ICA is small and left MCA is absent and right PCA stenosis (yellow arrows).
Integrated Analysis Of Simple And Complex Lesions
We subsequently included the above two complex lesions into the respective hypo- and hyperperfusion groups. Analysis of all the 11 lesions showed that hypoperfused lesions demonstrated significantly larger infarct volumes on both initial examinations (P = 0.026, N = 6 vs. 5) and follow-up scans (P = 0.028, N = 6 vs. 5), compared to the hyper-& normal perfusion group. The IHPD measured from acute infarct ROIs was found to correlate well with acute infarct volume on DWI (r = 0.745, P = 0.008) and follow-up T2 infarct volumes (r = 0.718, P = 0.013), when expressed as % of total brain volume. When the acute infarct volume on DWI and IHPD were concurrently included as covariates in regression analyses, the initial DWI lesion volume remained the dominant predictor for the follow-up T2 infarct volume (r = 0.809, P = 0.003).
Arterial Transit Effects
Thresholding in conjunction with visual inspection was used to confirm delayed arterial transit which presented as distinct linear hyper-intensities in CBF maps. We observed delayed arterial transit effects in 4 patients (patients #2, 4, 6, and 8). For Patient #2 and #4, the specific cortical areas where delayed arterial transit initially appeared did not evolve into an infarct (follow-up T2 infarct is 4.2mL and 3.9mL). For Patient #8, the ischemic lesion in left basal ganglia and left caudate resulted in a small follow-up T2 infarct (6.3mL). Note the left frontal lesion was due to a secondary infarct. Patient #6 was diagnosed with Moya-moya disease and perfusion images showed multiple arterial transit effects on both hemispheres. However, the lack of transit artifact on the lesional side was associated with relatively large follow-up T2 infarct volume (20.6mL), while the presence of transit artifact contralateral to the lesion seemingly spared the arterial territory.
In the 6 patients with a total of 7 lesions without apparent delayed arterial transit effects, 3 lesions (42.9%) (Patient #5, #7 - lesion7, and #10) presented relative normal or hyperperfusion, with follow-up T2 images showing relatively small infarcts (1.7mL, 4.1mL, and 0.2mL). Three lesions (42.9%) presented hypoperfusion with relatively large follow-up T2 infarcts (147mL, 97mL and 29.7mL). Only one patient (Patient #3) presented hypoperfusion with a relatively small follow-up T2 infarct (2.0mL).
Relationship Between Arterial Stenosis And Perfusion Deficits
According to the degree of arterial stenosis based on MRA findings, there was 1 affected artery in group 1 (normal MRA), 4 in group 2 (arterial stenosis), and 6 in group 3 (arterial occlusion). We found that a greater degree of arterial stenosis was associated with larger acute infarct volumes on DWI (r = 0.623, P = 0.041), and with greater IHPD measured in acute infarct ROIs (r = 0.774, P = 0.005). However, the association between the degree of stenosis and follow-up T2 volumes only showed a trend (r = 0.452, P = 0.163).
DISCUSSION
The present work represents our initial experience in applying PASL perfusion MRI in conjunction with conventional MRI modalities in a series of pediatric AIS patients. The imaging protocol (4.5 min) was well tolerated and has been readily combined with a routine clinical MRI examination at two magnetic field strengths across a time span of 4 years. In combination with MRA and T1/T2 images, PASL allows an integrated hemodynamic and structural assessment during a single MR session, without repeated doses of intravenous contrast (8). Further, the technical reliability of PASL demonstrated in the present study (across 4 years time span and two field strengths) may be potentially desirable for multi-center based clinical trials of pediatric stroke.
In this small cohort of patients there was an excellent agreement between ASL perfusion maps and clinical symptoms, acute infarct location, MRA abnormalities, and follow-up T2 findings. The perfusion deficit as expressed by IHPD correlated with the degree of arterial stenosis, acute DWI and follow-up T2 infarct volumes. These findings strongly support the clinical validity of PASL perfusion MRI which should add important information about cerebral circulatory function to the anatomical picture provided by conventional MRI and MRA. In the present study, hypoperfused lesions demonstrated significantly enlarged infarct volumes during both initial and follow-up examinations, compared to the hyper- & normal perfusion group.
Previous studies in animals and adults suggest that spontaneous post-ischemic hyperperfusion has been observed in about one-third of the cases 5–18 h after stroke (16). The generally used definition for post-ischemic hyperperfusion has been a significant increase in CBF relative to the homologous area of the contralateral hemisphere (18). Partial or complete reperfusion may occur through an ischemic zone despite persistent cerebral artery occlusion as a result of restoration of perfusion pressure through leptomeningeal collateral vessels. Early reperfusion (<24 h) may have significant prognostic benefits and has been associated with improved outcome and smaller infarct size. Late reperfusion of a vascular territory with damaged blood-brain barrier appears hazardous by promoting hemorrhagic transformation or fatal edema formation (16–18). Patients with normal perfusion, focal hyperperfusion or spontaneous recovery of neurological deficits may not benefit from thrombolytic therapy (16–18). Prior knowledge of perfusion recovery or hyperperfusion could help guide the clinician’s decision on the judicious use of thrombolysis and thus could avoid exposing the patient to potential risks.
Perfusion-diffusion mismatch is a central concept in the neuroimaging diagnosis of ischemic stroke in adults, and may represent a situation of ischemic but viable tissue that could be salvaged by timely reperfusion. Penumbra is a region evolving over time toward infarction with CBF values below those needed to sustain electrical activity but above those required to maintain cellular ionic gradients (19, 20). In this study, 2/5 hypoperfusion cases showed perfusion volume larger than diffusion volume. Both patients were among the earliest to present for clinical evaluation with a mean time of 4.7 hours from symptom onset to scan. The remaining 3 cases with hypoperfusion presented at a mean scan time of 60.4 hours. This finding is consistent with adult studies reporting that the perfusion-diffusion mismatch pattern occurs in approximately 70% of patients in the first 24 hours (21, 22). The greater overlap in MRI values between the operational penumbra and ischemic core at later time points (> 6 hours after stroke onset) could be due to partial reperfusion of the core region or the continued deterioration of the operational ischemic penumbra, or both (19). In general, one can assume that infarct volume at 24 hours after symptom onset has matured significantly, so that infarct volume on DWI at 24 hours or later should be stabilized and would remain the dominant predictor of the follow-up T2 infarct volume (as observed in our study). Nevertheless, ASL provided complementary information about the pathophysiology responsible for lesion size. Our data suggest that hyperperfusion in the acute and subacute phase may limit infarct volume, and therapies targeted to support or increase cerebral perfusion may be beneficial in pediatric strokes. The capability to detect perfusion-diffusion mismatch and to quantify penumbra volumes using ASL, as demonstrated in our study, may be particularly useful for pediatric patients with acute presentations (in time for thrombolysis therapies).
The present study also demonstrates that PASL perfusion MRI can provide clinically relevant information that is otherwise not available using conventional MRI sequences. We observed delayed arterial transit effects (focal intravascular signal) in 4 cases (Patient #2, 4, 6, and 8), which resulted from labeled arterial blood that hasn’t transited into brain tissue at the time of image acquisition (6, 15). The specific cortical areas where delayed arterial transit initially was seen appeared to have a protective effect. For patients #2, 4 and 8, the follow-up T2 infarct volumes were relatively small. In Patient #6 who had a history of Moya-moya disease, the lack of widespread arterial transit effects on the lesional side was associated with a relatively large follow-up T2 infarct volume. The collateral circulation is an important factor determining the viability of the ischemic region whenever the blood supply is inadequate from primary vessels (23). While transit effects were previously considered a hindrance to PASL perfusion MRI, we hypothesize that the presence of delayed arterial transit may potentially be a useful element for assessing collateral flow to hypoperfused areas and may predict a positive clinical outcome. This hypothesis remains to be tested in future studies in children, although previous ASL studies in adult strokes support this view (6, 15). Another caveat of delayed arterial transit is potential underestimation of perfusion in infarct regions. Performing ASL at high magnetic fields (longer blood T1) allows the use of prolonged postlabeling delay times and may provide a solution for this issue.
The unique information offered by PASL regarding the hemodynamic status of microcirculation and/or collateral blood supply complements the evaluation of vascular integrity by MRA, and the ischemic lesions by DWI. For instance, in Patient #4 and Patient #7-lesion #7, there were relatively large acute infarcts on DWI along with left M1 occlusion and right P1 significant stenosis, while PASL images indicated collateral blood supply with normal and hyperperfusion in these two lesions respectively. Consistent with perfusion findings, the follow up T2 images of the two lesions showed relatively small final infarction volume. This observation, in conjunction with a growing literature on the role of focal arteriopathy in the etiology of pediatric AIS, suggests a greater role for integrating vascular imaging, perfusion MRI and DWI for assessing the neuroanatomy and hemodynamics of cerebral blood supply and ischemic lesions in pediatric AIS (20).
Our study has several limitations. First, the number of patients included is small. Second, the use of follow-up T2 lesion size as a morphologic endpoint is limited and of uncertain clinical significance. The measurement of an area of T2 hyperintensity or encephalopmalacia fails to represent the tissue injury manifest as atrophy or transsynaptic degeneration, which is the typical chronic sequela of injury in the brain from any cause (24). Underestimation of chronic infarct volume may also occur due to the growth of a child’s brain over time. In Patient #2, periventricular white matter encephalomalacia was recognized as expansion of the original stroke lesion. Third, due to the relatively low frequency of stroke in children, ASL data was acquired with different MR systems at 1.5 and 3T across a time span of 4 years. The imaging parameters (e.g., post-labeling delay time) were not identical for all patients over the course of this study. However, because comparisons were made to the contralateral hemisphere, each patient served as their own control and the main statistical results reported were not affected when we excluded Patient #4 with a short delay time of 0.7sec.
In conclusion, PASL perfusion MRI is not only feasible but also provides unique physiologic information on the hemodynamic status that complements conventional MRI examinations. Recognition of stroke in children is increasing and there is improved availability of specialized acute stroke care in children. The inclusion of ASL perfusion MRI in acute imaging protocols could provide a means to quantify perfusion deficit and lesion volume, as well as to better understand the factors in children which determine reperfusion and collateralization, and how these factors affect outcome. Given the small number of highly selected patients studied, the presented findings must be taken as preliminary and further studies involving larger series of patients and serial imaging are necessary to validate our preliminary findings, and to further demonstrate the clinical utility of this imaging technology. Public awareness of pediatric stroke likewise must be improved so that children with ischemic brain injury come to medical attention earlier, in time for more aggressive therapies such as thrombolysis.
Acknowledgments
Funded by: Thrasher Research Fund, NIH grants HD049893, MH072576, K23NS052380, HD049883 and P41-RR02305
REFERENCES
- 1.Fullerton HJ, Chetkovich DM, Wu YW, Smith WS, Johnston SC. Deaths from stroke in US children, 1979 to 1998. Neurology. 2002;59:34–39. doi: 10.1212/wnl.59.1.34. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan V, Prengler M, McShane MA, Wade AM, Kirkham FJ. Investigation of risk factors in children with arterial ischemic stroke. Ann Neurol. 2003;53:167–173. doi: 10.1002/ana.10423. [DOI] [PubMed] [Google Scholar]
- 3.Fullerton HJ, Wu YW, Zhao S, Johnston SC. Risk of stroke in children: ethnic and gender disparities. Neurology. 2003;61:189–194. doi: 10.1212/01.wnl.0000078894.79866.95. [DOI] [PubMed] [Google Scholar]
- 4.Lynch JK, Hirtz DG, DeVeber G, Nelson KB. Report of the National Institute of Neurological Disorders and Stroke workshop on perinatal and childhood stroke. Pediatrics. 2002;109:116–123. doi: 10.1542/peds.109.1.116. [DOI] [PubMed] [Google Scholar]
- 5.Braun KP, Rafay MF, Uiterwaal CS, Pontigon AM, DeVeber G. Mode of onset predicts etiological diagnosis of arterial ischemic stroke in children. Stroke. 2007;38:298–302. doi: 10.1161/01.STR.0000254484.10680.c6. [DOI] [PubMed] [Google Scholar]
- 6.Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680–687. doi: 10.1161/01.str.31.3.680. [DOI] [PubMed] [Google Scholar]
- 7.Rivers CS, Wardlaw JM, Armitage PA, et al. Do acute diffusion- and perfusion-weighted MRI lesions identify final infarct volume in ischemic stroke? Stroke. 2006;37:98–104. doi: 10.1161/01.STR.0000195197.66606.bb. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Licht DJ. Pediatric perfusion MR imaging using arterial spin labeling. Neuroimaging Clin N Am. 2006;16:149–167. doi: 10.1016/j.nic.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Wang J, Licht DJ, Jahng GH, et al. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18:404–413. doi: 10.1002/jmri.10372. [DOI] [PubMed] [Google Scholar]
- 10.Ganesan V, Prengler M, Wade A, Kirkham FJ. Clinical and radiological recurrence after childhood arterial ischemic stroke. Circulation. 2006;114:2170–2177. doi: 10.1161/CIRCULATIONAHA.105.583690. [DOI] [PubMed] [Google Scholar]
- 11.Wang J, Alsop DC, Li L, et al. Comparison of quantitative perfusion imaging using arterial spin labeling at 1.5 and 4.0 Tesla. Magn Reson Med. 2002;48:242–254. doi: 10.1002/mrm.10211. [DOI] [PubMed] [Google Scholar]
- 12.Tatu L, Moulin T, Bogousslavsky J, Duvernoy H. Arterial territories of the human brain: cerebral hemispheres. Neurology. 1998;50:1699–1708. doi: 10.1212/wnl.50.6.1699. [DOI] [PubMed] [Google Scholar]
- 13.Hosoda K, Kawaguchi T, Ishii K, et al. Prediction of hyperperfusion after carotid endarterectomy by brain SPECT analysis with semiquantitative statistical mapping method. Stroke. 2003;34:1187–1193. doi: 10.1161/01.STR.0000068781.31429.BE. [DOI] [PubMed] [Google Scholar]
- 14.Butcher KS, Parsons M, MacGregor L, et al. Refining the perfusion-diffusion mismatch hypothesis. Stroke. 2005;36:1153–1159. doi: 10.1161/01.str.0000166181.86928.8b. [DOI] [PubMed] [Google Scholar]
- 15.Detre JA, Alsop DC, Vives LR, Maccotta L, Teener JW, Raps EC. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology. 1998;50:633–641. doi: 10.1212/wnl.50.3.633. [DOI] [PubMed] [Google Scholar]
- 16.Guadagno JV, Calautti C, Baron JC. Progress in imaging stroke: emerging clinical applications. Br Med Bull. 2003;65:145–157. doi: 10.1093/bmb/65.1.145. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen TB, Lum C, Eastwood JD, Stys PK, Hogan M, Goyal M. Hyperperfusion on perfusion computed tomography following revascularization for acute stroke. Acta Radiol. 2005;46:610–615. doi: 10.1080/02841850510021607. [DOI] [PubMed] [Google Scholar]
- 18.Marchal G, Young AR, Baron JC. Early postischemic hyperperfusion: pathophysiologic insights from Positron Emission Tomography. J Cereb Blood Flow Metab. 1999;19:467–482. doi: 10.1097/00004647-199905000-00001. [DOI] [PubMed] [Google Scholar]
- 19.Schlaug G, Benfield A, Baird AE, et al. The ischemic penumbra: operationally defined by diffusion and perfusion MRI. Neurology. 1999;53:1528–1537. doi: 10.1212/wnl.53.7.1528. [DOI] [PubMed] [Google Scholar]
- 20.Surikova I, Meisel S, Siebler M, Wittsack HJ, Seitz RJ. Significance of the perfusion-diffusion mismatch in chronic cerebral ischemia. J Magn Reson Imaging. 2006;24:771–778. doi: 10.1002/jmri.20686. [DOI] [PubMed] [Google Scholar]
- 21.Hernandez MJ, Vannucci RC, Salcedo A, Brennan RW. Cerebral blood flow and metabolism during hypoglycemia in newborn dogs. J Neurochem. 1980;35:622–628. doi: 10.1111/j.1471-4159.1980.tb03701.x. [DOI] [PubMed] [Google Scholar]
- 22.Baird AE, Warach S. Magnetic resonance imaging of acute stroke. J Cereb Blood Flow Metab. 1998;18:583–609. doi: 10.1097/00004647-199806000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Zhu M, Dai J, Li S. Cerebral angiography and MR perfusion images in patients with ischemic cerebral vascular disease. Chin Med J (Engl) 2002;115:1687–1691. [PubMed] [Google Scholar]
- 24.Doege CA, Kerskens CM, Romero BI, et al. MRI of small human stroke shows reversible diffusion changes in subcortical gray matter. Neuroreport. 2000;11:2021–2024. doi: 10.1097/00001756-200006260-00043. [DOI] [PubMed] [Google Scholar]