Abstract
Objective
The availability of self-report scales that accurately identify low adherers to antihypertensive medication in real time could improve outpatient management of this disease. We evaluated the association and concordance of the new 8-item self-report Morisky Medication Adherence Scale (MMAS) with pharmacy fill data in a sample of community dwelling seniors with hypertension.
Study Design
Cross-sectional study
Methods
Pharmacy records for antihypertensive medications were abstracted for managed care adult hypertensive patients, ≥65 years, who completed a survey that included the 8-item MMAS (n=87). Continuous single-interval medication availability (CSA), medication possession ratio (MPR), and continuous multiple-interval medication gaps (CMG) were calculated using pharmacy data. MMAS adherence was categorized as high, medium, and low (MMAS scores of 8, 6 to <8, and <6, respectively); pharmacy fill non-persistency was defined as <0.8 for CSA and MPR and >0.2 for CMG.
Results
Overall, 58%, 33%, and 9% of participants had high, medium, and low medication adherence by MMAS. After adjustment for demographics and compared to high adherers on MMAS, patients with low MMAS adherence were 6.89 (95% confidence interval (CI): 2.48 – 19.1) times more likely to have non-persistent pharmacy fill rates by CSA and 5.22 (95% CI:1.88 – 14.5) times more likely to have non-persistent pharmacy fill rates by MPR. Concordance between MMAS and CSA, MPR, and CMG was ≥75%.
Conclusions
The MMAS is significantly associated with anti-hypertensive pharmacy refill adherence. Although further validation of the MMAS is needed, it may be useful in identifying low medication adherers in clinical settings.
Keywords: medication adherence, hypertension, Morisky Medication Adherence Scale, pharmacy fill, managed care
Introduction
Despite the availability of effective medical treatment for hypertension, control of this chronic disease among adults is low [1]. Low adherence to prescribed antihypertensive medications is potentially a major barrier to adequate blood pressure control [2-4] and has been characterized by the National Council on Patient Information and Education as “America’s other drug problem.” [5]. Low medication adherence is associated with increased health care costs, and increased cardiovascular disease and hospitalization rates [6, 7]. Identifying non-adherent patients in outpatient settings is important in order to effectively increase hypertension control rates. Nevertheless, providers often do not ask about medication-taking behavior [8]. This may be, in part, because they do not have time, do not think of non-adherence as a likely cause for poor blood pressure control, are uncertain about quantifying non-adherent behavior [9], or are not in the habit of using this information in clinical practice. Approaches employed to assess medication adherence include patient self-report, pill counts, pharmacy records, drug levels, biological surrogates, and medication event monitoring system caps [10]. However, the most practical approach to apply in clinical practice is patient self-report. The advantages of assessing medication adherence by self report include simplicity, speed, and viability of use. Self report scales to assess antihypertensive medication adherence have been developed [11, 12, 13]. However, concordance of patients’ responses on previously developed self-report scales with objective measures of medication adherence has been variable [14,15]. The purpose of the current analysis was to evaluate the association and concordance of the new 8-item self-report Morisky Medication Adherence Scale (MMAS) [13] with prescription claims in a managed care population of older adults with hypertension.
Methods
Study Population
This analysis was part of a study designed to determine patient participation rates and factors associated with antihypertensive medication adherence and explore methods for analyzing medication adherence in older adults with chronic disease [16]. The study population was drawn from a large southern managed care organization which offered healthcare benefits to persons enrolled in the Medicare risk plans. Using a race-stratified sampling approach, two hundred study participants (100 white and 100 black patients) were randomly selected from an administrative database using the following inclusion criteria: member of the Medicare risk product, at least two documented encounters with a primary or secondary diagnosis of essential hypertension (International Classification of Disease-9th revision [ICD-9] code 401.XX) as recorded in the managed care organization’s administrative database, and continuous enrollment in the managed care organization for at least one year at the time of study participation. After excluding 23 patients (incapacitated n=8, invalid contact information n=13, institutionalized n=2), 177 patients were eligible for the survey. One hundred sixteen surveys were completed yielding an overall response rate of 65.5% with a slightly higher overall participation rate (68% versus 60%) among whites versus blacks [16]. Patients were excluded from the current analysis if they did not complete the MMAS (n=13), were missing data on age (n=1), if they did not have pharmacy fill data available (n=13) or had fewer than 3 antihypertensive medication pharmacy fills in the study time interval (n=2). After these exclusions, 87 patients were included in the current analysis. The age, gender, and race distributions were similar among those included and those excluded from the analysis (p>0.1 for each comparison). The study was approved by the Institutional Review Board of the Ochsner Clinic Foundation.
Data collection
Patient surveys were conducted from December 2002 to March 2003 using a standardized data collection instrument. The survey data (including socio-demographic data and medication adherence) were entered into a Microsoft Access database and transferred to SAS 9.1 (SAS Inc. Cary, NC) for analysis; quality check revealed less than 1% data entry error. All patient identification information was collected and maintained according to HIPAA regulations and health plan privacy rules.
Self-reported Medication Adherence Scale
Self-reported medication adherence was measured with the new eight-item MMAS [13], which was developed from a previously validated four-item scale and supplemented with additional items to better capture barriers surrounding adherence behavior. Each of the 8 items measures a specific medication-taking behavior and not a determinant of adherence behavior. The 8-item MMAS is provided in the Appendix. The new scale has been determined to have higher reliability compared to the 4-item scale (α= 0.83 vs 0. 61) [11, 13] MMAS scores can range from zero to eight and have been trichotomized previously into three levels of adherence to facilitate use in clinical practice: high adherence - MMAS score of 8, medium adherence - MMAS scores of 6 to <8, and low adherence - MMAS scores of <6 [13]. Prior research revealed the new scale is significantly (p<0.05) associated with blood pressure control in patients with hypertension with 67.2% of low adherers having uncontrolled blood pressure versus 55.2% and 43.3% of medium and high adherers having uncontrolled blood pressure, respectively [13].
Pharmacy Adherence Measures
The managed care organization’s data warehouse system was the source of the pharmacy fill data for the current study. As an Oracle relational database, the data warehouse was populated with historic claims data, patient roster data, diagnosis and procedural codes and code descriptions. Data were extracted by informatics analysts using the Oracle Discoverer tool, and transported into SAS 9.1. The pharmacy data were abstracted on 87 patients and included 42 different antihypertensive medications with 1578 fills captured in the study period.
Pharmacy fill data were extracted for the 2002 calendar year and included a listing of all antihypertensive prescriptions filled, the date filled, generic and brand names of the drugs, and number of pills dispensed. Three measures of adherence were calculated: continuous single-interval medication availability (CSA), medication possession ratio (MPR), and continuous multiple-interval medication gaps (CMG) [17,18]. CSA was calculated by dividing the days’ supply obtained at a pharmacy fill by the number of days before the next pharmacy fill for that same medication. MPR was calculated as the sum of the days’ supply obtained between the first pharmacy fill and the last fill (supply obtained in the last fill was excluded) divided by the total number of days in this time period. CMG was calculated by dividing the total number of days without medications (i.e. treatment gaps) between the first and last pharmacy fill by the number of days in this time period. A graphical example of how CSA, MPR, CMG were calculated is provided in the Appendix.
For every participant, CSA was calculated for each pharmacy fill interval and MPR and CMG scores were calculated by class of antihypertensive medication being taken CSA and MPR values greater than one were truncated at the maximum value of one [19]. Given self-reported adherence reflects adherence to participants’ antihypertensive medication regimen, one CSA was assigned to each participant based on the mean of all CSAs calculated from all of their antihypertensive pharmacy fill intervals. One MPR and one CMG were assigned to each participant. For participants filling more than one class of antihypertensive medication, MPR and CMG were calculated for each class and then averaged across all classes to assign a single MPR and CMG to each participant. Given that a cut point of 0.8 has been previously used to define adequate medication adherence using pharmacy data [19-22] pharmacy fill nonpersistency was defined as <0.8 for CSA and MPR and >0.2 for CMG. Although other studies have reported continuous single interval gaps (CSG), this statistic is the inverse of CSA and, therefore, is not presented.
Statistical Analysis
This study constitutes a test of concordance and concurrent criterion-related validity, using pharmacy fill medication adherence as the criterion of interest and its association with self-reported medication-taking. Although our comparisons involved the same patients taking antihypertensive medications, the adherence measures were collected independent of each other.
Patient demographic characteristics, education, marital status, smoking status and number of antihypertensive medications filled were calculated by MMAS category (high, medium and low). The statistical significance of trends across categories was determined using least squares and maximum likelihood for continuous and categorical variables, respectively.
The distributions of CSA, MPR, and CMG were plotted and the median, 25th and 75th percentiles, minimum and maximum values were determined, overall and by MMAS category. Due to skewed distributions for CSA, MPR, and CMG, quantile regression was used to determine the statistical significance of trends in median values for these measures across MMAS category. The prevalence of non-persistency determined by CSA, MPR and CMG scores were calculated, overall and by MMAS category. Log binomial regression models that included adjustment for age, gender, and race were used to determine the prevalence ratio of non-persistency (CSA, MPR, and CMG, separately) associated with MMAS category. Percent concordance between MMAS and pharmacy fill adherence was used to describe the agreement between the approaches for assessing adherence. Low adherers are likely at greatest risk for uncontrolled blood pressure and subsequent adverse outcomes and could benefit most from tailored interventions to overcome barriers to adherence; thus, we assessed the concordance between low adherence on MMAS with non-persistency by CSA, MPR, and CMG.
All statistical analyses were performed using SAS version 9.1.3 (Cary, NC).
Results
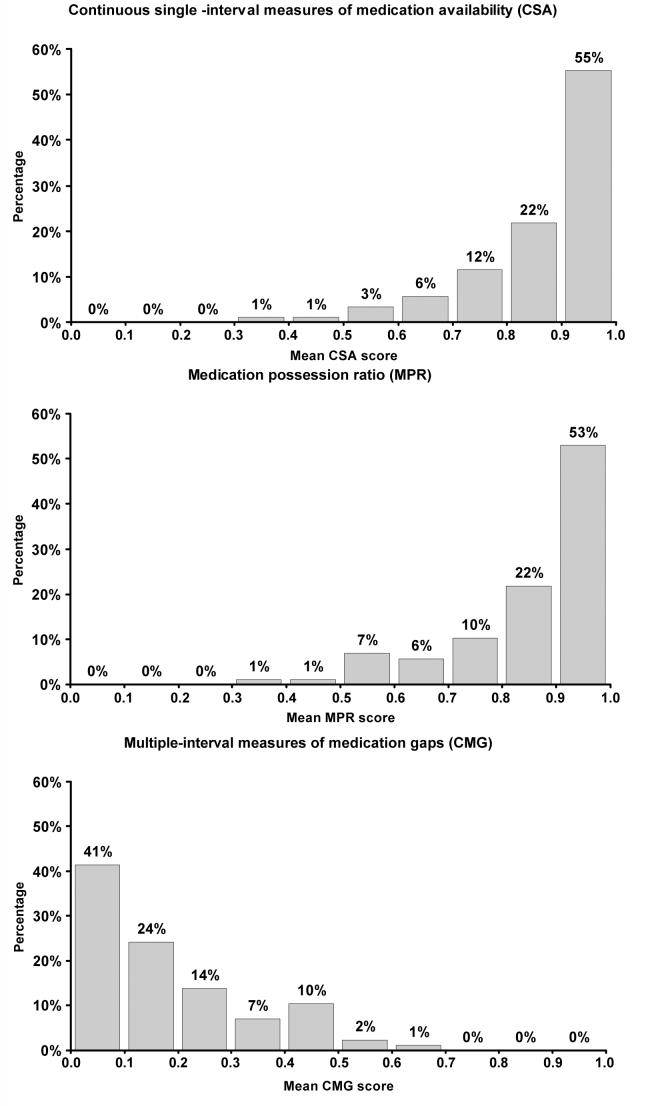
Of the 87 patients included in the study, the mean age was 76 years, 31% were men, 48% were black, 47% had graduated high school, 43% were married, 43% smoked cigarettes, and the mean number of antihypertensive medications being taken was 2.2 (range: 1 to 4 medications). The mean MMAS was 7.4 (standard deviation = 0.9). Demographic characteristics of study participants by MMAS category are presented in Table 1. A significantly higher percentage of black versus white patients were low adherers by MMAS. There were no other significant differences in patient demographics across MMAS categories. By self-report using MMAS, the distribution of participants’ adherence to prescribed medication in this study population was: 58% high, 33% medium and 9% low. The distributions of the pharmacy fill adherence measures are presented in Figure 1. Median CSA, MPR and CMG were 0.91, 0.91, and 0.12, respectively (Table 2). However, 23% and 25% of patients had non-persistent CSA and MPR, respectively, and 35% had non-persistent CMG.
Table 1.
Baseline characteristics by Morisky Medication Adherence Scale score category
| Morisky Medication Adherence Scale category (score range) | ||||
|---|---|---|---|---|
| Low (<6) N=8 |
Medium (6 to <8) N=29 |
High (8) N=50 |
P-value | |
| Mean (SE) age, years | 72.8 (1.8) | 76.8 (1.6) | 76.3 (0.9) | 0.41 |
| Male, % | 0 | 28 | 38 | 0.03 |
| Black, % | 88 | 48 | 42 | 0.04 |
| High school education, % | 63 | 48 | 44 | 0.61 |
| Currently married, % | 25 | 35 | 50 | 0.16 |
| Current smoker, % | 50 | 35 | 47 | 0.51 |
| Mean (SE) antihypertensive medications filled | 2.0 (0.2) | 2.1 (0.2) | 2.2 (0.2) | 0.55 |
SE - Standard Error
Figure 1.
Frequency distributions of pharmacy fill adherence scores.
Table 2.
Median, 25th and 75th percentiles, minimum and maximum pharmacy fill adherence scores
| Adherence method | Median | 25th% | 75th% | Minimum | Maximum |
|---|---|---|---|---|---|
| CSA | 0.91 | 0.82 | 0.96 | 0.35 | 1.00 |
| MPR | 0.91 | 0.79 | 0.99 | 0.35 | 1.00 |
| CMG | 0.12 | 0.05 | 0.26 | 0.00 | 0.65 |
CSA - Continuous, single-interval measures of medication availability, MPR - Medication possession ratio, CMG - Continuous, multiple-interval measures of medication gaps
Association of MMAS with Non-persistency determined by Pharmacy Fill
MMAS was significantly associated with non-persistency determined by pharmacy fill adherence (Table 3). After adjustment for age, race, and gender, patients with medium and low adherence by MMAS were 2.58 (95% CI: 1.08 – 6.17) and 6.89 (95% CI: 2.48 – 19.1) times, respectively, more likely to have non-persistent pharmacy fill adherence by CSA, than patients with high adherence by MMAS (p-trend<0.001). Also, patients with medium and low adherence by MMAS were 2.31 (95% CI: 0.94 – 5.66) and 5.22 (95% CI: 1.88 – 14.5) times, respectively, more likely to have non-persistent pharmacy fill adherence by MPR than patients with high adherence by MMAS (p-trend=0.001; Table 3). The prevalence of non-persistent CMG for patients with low adherence on MMAS was 100%.
Table 3.
Median, prevalence and adjusted prevalence ratio of non-persistent pharmacy fill adherence score by Morisky aMedication Adherence Scale category.
| Pharmacy fill adherence method |
Morisky Medication Adherence Scale Category (score range) |
|||
|---|---|---|---|---|
| Low (<6) | Medium (6 to <8) | High (8) | p-trend | |
| CSA | ||||
| Median (25th – 75th percentile) | 0.66 (0.56 - 0.72) | 0.88 (0.76- 0.93) | 0.94 (0.89 - 0.97) | <0.001 |
| Non-persistent CSA (<0.8) | ||||
| Prevalence, % | 88 | 31 | 8 | <0.001 |
| Prevalence ratio* (95% CI) | 6.89 (2.48 - 19.1) | 2.58 (1.08 - 6.17) | 1.00 (ref) | <0.001 |
| MPR | ||||
| Median (25th – 75th percentile) | 0.65 (0.58 - 0.79) | 0.89 (0.72 - 0.97) | 0.98 (0.86 - 1.00) | 0.001 |
| Non-persistent MPR (<0.8) | ||||
| Prevalence, % | 75 | 31 | 14 | 0.002 |
| Prevalence ratio* (95% CI) | 5.22 (1.88 - 14.5) | 2.31 (0.94 - 5.66) | 1.00 (ref) | 0.001 |
| CMG | ||||
| Median (25th – 75th percentile) | 0.43 (0.29 - 0.52) | 0.13 (0.09 - 0.31) | 0.09 (0.04 - 0.20) | 0.002 |
| Non-persistent CMG (>0.2) | ||||
| Prevalence, % | 100 | 35 | 24 | <0.001 |
| Prevalence ratio* (95% CI) | ---¥ | 1.14 (0.74 - 1.77) | 1.00 (ref) | 0.0755 |
Adjusted for age, race and gender
Prevalence ratio could not be calculated as the prevalence of non-persistent CMG is 100%
CSA - Continuous, single -interval measures of medication availability, MPR - Medication possession ratio, CMG - Continuous, multiple-interval measures of medication gaps
Ref- Reference category; CI- Confidence interval
Concordance of Low Adherence on MMAS with Non-persistency Determined by Pharmacy Fill
Seven patients with low adherence by MMAS had non-persistence by CSA and 66 patients with medium or high adherence with MMAS had persistence by CSA yielding 84% concordance (Table 4). The concordance of low adherence by MMAS with non-persistency by MPR and CMG was 79%, and 75%, respectively.
Table 4.
Cross tabulation of anti-hypertensive medication adherence by MMAS and pharmacy fill measures (CSA and MPR).
| Adherence by CSA | Adherence by MMAS |
||
|---|---|---|---|
| Low (<6) | Medium (6 to <8) | High (8) | |
| N (%) | N (%) | N (%) | |
| Persistent (≥0.8) | 1 (12%) | 20 (69%) | 46 (92%) |
| Non-persistent (<0.8) | 7 (88%) | 9 (31%) | 4 (8%) |
| Total | 8 (100%) | 29 (100%) | 50 (100%) |
| Adherence by MPR | Adherence by MMAS |
||
|---|---|---|---|
| Low (<6) | Medium (6 to <8) | High (8) | |
| N (%) | N (%) | N (%) | |
| Persistent (≥0.8) | 2 (25%) | 20 (69%) | 43 (86%) |
| Non-persistent (<0.8) | 6 (75%) | 9 (31%) | 7 (14%) |
| Total | 8 (100%) | 29 (100%) | 50 (100%) |
| Adherence by CMG | Adherence by MMAS |
||
|---|---|---|---|
| Low (<6) | Medium (6 to <8) | High (8) | |
| N (%) | N (%) | N (%) | |
| Persistent (≥0.8) | 0 (0%) | 19 (65%) | 38 (76%) |
| Non-persistent (<0.8) | 8 (100%) | 10 (35%) | 12 (24%) |
| Total | 8 (100%) | 29 (100%) | 50 (100%) |
MMAS = Morisky Medication Adherence Scale; CSA = Continuous, Single-interval measures of medication Availability; MPR = Medication Possession Ratio; CMG= Continuous, multiple-interval measures of medication gaps
Discussion
Hypertension, a public health challenge in the United States and worldwide, is a modifiable risk factor for cardiovascular events [23,24]. Clinicians require information on antihypertensive medication adherence to draw proper conclusions about the effectiveness of treatment [25]. The goal is to have access to a quick, reasonably accurate self-report adherence measure for use in outpatient settings to facilitate clinical decision-making. We evaluated the accuracy of a new self-report measure by assessing its association and concordance with pharmacy fill rates. To our knowledge, the concordance between the MMAS and pharmacy fill for anti-hypertensive medications has not been previously evaluated. In this study, the MMAS maintained a strong, graded, statistically significant association with pharmacy fills. Using CSA, MPR and CMG for comparison, MMAS correctly classified ≥ 75% of patients as being adherent or not. Furthermore, patients classified as low adherers by MMAS were significantly more likely to be non-persistent by each measure of pharmacy fill. Thus, MMAS may be a practical and valid approach for identifying low adherers to chronic medications in outpatient settings.
Low adherence poses unique challenges for clinicians trying to determine if prescribed treatment is effective. If clinicians are able to accurately identify patients with low adherence, then appropriate and timely interventions can be implemented. Several modifiable factors have been reported to negatively impact adherence to prescribed therapies. These include forgetfulness [11], depression [26], lack of knowledge regarding hypertension and its treatment [27], complexity of medication regimen [28], health care system perceptions by the patient [29], sexual dysfunction [30], side effects of medication [31] and poor quality of life [32]. The MMAS provides information on behaviors associated with low adherence that may be unintentional (e.g. forgetfulness) or intentional (e.g. not taking medications when one feels worse). Identification of these behaviors can facilitate tailoring of interventions to specific patient issues [33]. For example, if a patient is identified as a low adherer by the MMAS and the responses indicate forgetfulness as a major barrier, then the clinician may suggest the patient use weekly pill boxes and engage a family member or friend to assist with medication reminders. If a patient is identified as a low adherer and responds that she stops taking medications when she feels better or worse, then the clinician can address knowledge barriers, medication side effects and educate the patient about the chronic nature of hypertension and the importance of taking medication as prescribed. On the other hand, if patients are found to be high adherers and their blood pressure remains uncontrolled, then the clinician should consider increasing medication dose or adding a medication to their regimen [2, 34]. It may be that use of a simple tool (e.g., the 8-item MMAS) in the outpatient setting may allow clinicians to eliminate low adherence as a contributing factor to poor blood pressure control.
Although the concordance between self-report and pharmacy fill was good in this study, it was not perfect. Shortcomings of self-report include reliance on recall and social desirability bias with a tendency to overestimate adherence [10]. In addition, pharmacy fill rates may not capture some nuances of medication adherence behavior [9] and are not practical to capture in real time in outpatient clinical encounters It is generally assumed that patients who fill medications also take them, unless they have been instructed by their provider otherwise or have side effects that limit their medication intake [19]. It is possible that patients’ medication adherence varies by drug class. In the current study, 21 patients had one or more drug classes with an MPR < 0.8 and an MPR ≥ 0.8 for other drug classes. Even after accounting for this using a generalized estimating equation, a strong and statistically significant association between self-reported medication adherence and MPR and CMG non-persistency pharmacy fill rates remained present (data not shown). This suggests that averaging the pharmacy measures did not mask any drug class-specific relationships.
Stroupe and colleagues [35] reported that more than 20% oversupply (i.e., MPR ≥ 1.2) is related to a similar risk of future hospitalization as experienced by patients with undersupply of medication (i.e., MPR < 0.8). In the present study, only five patients had an average MPR ≥ 1.2, none of whom had low adherence on the MMAS. After excluding these five patients, the associations between non-persistence and self-report medication adherence were very similar to the original analysis. When we reclassified these patients as non-persistent, the results were also similar (data not shown).
Study Limitations and Strengths
This study was limited to older community dwelling adults with managed care insurance and may not be representative of patients from other socioeconomic backgrounds. However, the restriction of our sample to older adults in the managed care organization minimized some of the confounding effects of health insurance, access to medical care, and employment status in the elderly. Because hypertension is prevalent in the elderly nationwide, results of this study may be useful in the evaluation and management of a substantial segment of the population. In addition, clinical prescribing considerations (e.g., pill splitting, taking medications on alternate days, stopping a medication because of adverse drug reactions or side effects, hospitalization) or changes in patients schedules (stockpiling medications to accommodate a prolonged vacation) may not have been accurately reflected in pharmacy fill data. Further research is warranted to identify approaches for correctly classifying patients based on pharmacy fill with respect to medication adherence that takes into account prescribing nuances. Also, due to a relatively modest sample size, we did not differentiate level of adherence by drug class. Similar to previously reported studies [9], patients in this study were relatively high adherers, and further research is needed to explore the associations between self-report and pharmacy fill at lower adherence levels. Although we did not measure blood pressure as part of the study protocol and, thus, cannot determine the relationship between self-reported medication adherence, pharmacy fill, and blood pressure control, a previous study has demonstrated a significant association between MMAS and blood pressure control [13]. Strengths of this study include the use of a standardized data collection instrument by trained staff, inclusion of a racially diverse sample, and access to pharmacy records as an objective gold standard for comparison.
Conclusion
There are several important clinical implications of our findings. Compared to pharmacy fill, the self-reported MMAS performed well in identifying patients with low adherence to antihypertensive medications. These patients are likely at greatest risk for uncontrolled blood pressure and subsequent adverse outcomes and could benefit most from tailored interventions to overcome barriers to adherence. Although pharmacy fill data represent an objective assessment of medication adherence, it is impractical for use in clinical settings and does not provide information on reasons for low adherence. The MMAS tool is simple and economical to use in routine outpatient settings and may provide clinicians with important information (i.e. barriers to adherence) to guide treatment decisions for patients with hypertension. Scores on MMAS maintained a strong, graded, association with antihypertensive pharmacy fill adherence in community-dwelling seniors receiving healthcare through a managed care organization. This association suggests that patients’ self-report of adherence behavior is consistent with the rate at which they fill their antihypertensive medications. The current study extends prior work demonstrating the internal reliability and predictive validity of MMAS with respect to blood pressure control. This 8-item tool is simple and feasible to incorporate in clinical practice and may be useful in identifying patients at risk for medication adherence issues including low adherence in outpatient settings.
Supplementary Material
Acknowledgments
Source of Support: The project described was supported in part by Grant Number R01 AG022536 from the National Institute on Aging and by Ochsner Clinic Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Footnotes
- Compared to pharmacy fill, the self-reported MMAS performed well in identifying patients with low adherence to antihypertensive medications.
- Low adherers are likely at greatest risk for uncontrolled blood pressure and subsequent adverse outcomes and could benefit most from tailored interventions to overcome barriers to adherence.
- Although pharmacy fill rates represent an objective assessment of medication adherence, they are currently impractical for real-time use in most clinical settings.
- The MMAS scale is simple and economical to use in routine outpatient settings and may provide clinicians and administrators with important information to guide treatment decisions for patients with hypertension.
References
- 1.Ong KL, Cheung BM, Man YB, Lau CP, Lam KS.Prevalence, awareness, treatment, and control of hypertension among United States adults 1999-2004 Hypertension 2007. January;49169–75. [DOI] [PubMed] [Google Scholar]
- 2.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ, National Heart, Lung, and Blood Institute Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National High Blood Pressure Education Program Coordinating Committee The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. The JNC 7 Report. JAMA. 2003;289:2560–2572. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 3.Krousel-Wood MA, Thomas S, Muntner P, Morisky DE. Medication adherence: a key factor in achieving blood pressure control and good clinical outcomes in hypertensive patients. Curr Opin Cardiol. 2004;19:357–362. doi: 10.1097/01.hco.0000126978.03828.9e. [DOI] [PubMed] [Google Scholar]
- 4.Burnier M, Santschi V, Favrat B, Brunner HR.Monitoring compliance in resistant hypertension: an important step in patient management J Hypertens Suppl 2003. May;212S37–S42. [DOI] [PubMed] [Google Scholar]
- 5.Bond WS, Hussar DA. Detection methods and strategies for improving medication compliance. Am J Hosp Pharm. 1991;48:1978–1988. [PubMed] [Google Scholar]
- 6.Sokol MC, McGuigan KA, Verbrugge RR, Epstein RS.Impact of medication adherence on hospitalization risk and healthcare cost Med Care 2005. June;436521–30. [DOI] [PubMed] [Google Scholar]
- 7.Schiff GD, Fung S, Speroff T, McNutt RA.Decompensated heart failure: symptoms, patterns of onset, and contributing factors Am J Med 2003. June 1;1148625–30. [DOI] [PubMed] [Google Scholar]
- 8.Bokhour BG, Belowitz DR, Long JA, Kressin NR. How do providers assess antihypertensive medication adherence in medical encounters ? J Gen Intern Med. 2006;21:577–583. doi: 10.1111/j.1525-1497.2006.00397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grymonpre R, Cheang M, Mmath, Fraser M, Metge C, Sitar DS. Validity of a prescription claims database to estimate medication adherence in older persons. Med Care. 2006;44:471–477. doi: 10.1097/01.mlr.0000207817.32496.cb. [DOI] [PubMed] [Google Scholar]
- 10.Hawkshead J, Krousel-Wood MA. Techniques for Measuring Medication Adherence in Hypertensive Patients in Outpatient Settings: Advantages and Limitations. Disease Management and Health Outcomes. 2007;15:109–118. [Google Scholar]
- 11.Morisky DE, Green W, Levine DM, et al. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Kim MT, Hill MN, Bone LR, Levine DM. Development and testing of the Hill-Bone Compliance to High Blood Pressure Therapy Scale. Prog Cardiovasc Nurs. 2000;15(3):90–6. doi: 10.1111/j.1751-7117.2000.tb00211.x. [DOI] [PubMed] [Google Scholar]
- 13.Morisky DE, Ang A, Krousel-Wood MA, Ward H. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Cook CL, Wade WE, Martin BC, Perri M. Concordance among three self-reported measures of medication adherence and pharmacy refill records. J Am Pharm Assoc. 2005;45:151–159. doi: 10.1331/1544345053623573. [DOI] [PubMed] [Google Scholar]
- 15.Garber MC, Nau DP, Erickson SR, Aikens JE, Lawrence JB. The concordance of self-report with other measures of medication adherence: a summary of the literature. Med Care. 2004;42:649–652. doi: 10.1097/01.mlr.0000129496.05898.02. [DOI] [PubMed] [Google Scholar]
- 16.Krousel-Wood MA, Muntner P, Jannu A, Hyre A, Breault J. Does Waiver of Written Informed Consent from the Institutional Review Board Affect Response Rate in a Low Risk Research Study? J Invest Med. 2006;54(4):174–179. doi: 10.2310/6650.2006.05031. [DOI] [PubMed] [Google Scholar]
- 17.Steiner JF, Prochazka AV. The assessment of refill adherence using pharmacy records: methods, validity, and applications. J. Clin. Epidemiol. 1997;50:105–116. doi: 10.1016/s0895-4356(96)00268-5. [DOI] [PubMed] [Google Scholar]
- 18.Lau DT, Nau DP. Oral antihyperglycemic medication nonadherence and subsequent hospitalizaion among individuals with type 2 diabetes. Diabetes Care. 2004;27:2149–2153. doi: 10.2337/diacare.27.9.2149. [DOI] [PubMed] [Google Scholar]
- 19.Sikka R, Xia F, Aubert RE. Estimating medication adherence using administrative claims data. Am J Manag Care. 2005;11:449–457. [PubMed] [Google Scholar]
- 20.Rizzo JA, Simons WR. Variations in compliance among hypertensive patients by drug class : Implications for health care costs. Clin Ther. 1997;19:1446–1457. doi: 10.1016/s0149-2918(97)80018-5. [DOI] [PubMed] [Google Scholar]
- 21.Kopjar B, Sales AEB, Pineros SL, Sun H, Yu-Fang L, Hedeen AN. Adherence with statin therapy in secondary prevention of coronary heart disease in Veterans Administration male population. Am J Cardiol. 2003;92:1106–1108. doi: 10.1016/j.amjcard.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 22.Simpson E, Beck C, Richard H, Eisenberg MJ, Pilote L. Drug prescriptions after acute myocardial infarction : Dosage, compliance, and persistence. Am Heart J. 2003;145:438–44. doi: 10.1067/mhj.2003.143. [DOI] [PubMed] [Google Scholar]
- 23.Fields LE, Burt VL, Cutler JA, Hughes J, Roccella EJ, Sorlie P. The burden of adult hypertension in the United States 1999 to 2000. Hypertension. 2004;44:398–404. doi: 10.1161/01.HYP.0000142248.54761.56. [DOI] [PubMed] [Google Scholar]
- 24.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK, He J. Global burden of hypertension: analysis of worldwide data. Lancet. 2005;365:217–223. doi: 10.1016/S0140-6736(05)17741-1. [DOI] [PubMed] [Google Scholar]
- 25.Feinstein AR. Compliance bias and the interpretation of therapeutic trials. In: Haynes RB, Taylor DW, Sackett DL, editors. Compliance in Health Care. Johns Hopkins Press; Baltimore MD: 1979. p. 309. [Google Scholar]
- 26.Wang PS, Bohn RL, Knight E, Glynn RJ, Mogun H, Avorn J. Noncompliance with antihypertensive medications: the impact of depressive symptoms and psychosocial factors. J Gen Intern Med. 2002;17:504–511. doi: 10.1046/j.1525-1497.2002.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Egan BH, Lackland DT, Cutler NE. Awareness, knowledge and attitudes of older Americans about high blood pressure. Arch Intern Med. 2003;163:681–687. doi: 10.1001/archinte.163.6.681. [DOI] [PubMed] [Google Scholar]
- 28.Iskedjian M, Einarson TR, MacKeigan LD, Shear N, Addis A, Mittmann N, Ilersich AL. Relationship between daily dose frequency and adherence to antihypertensive pharmacotherapy: evidence from meta-analysis. Clin Ther. 2002;24:302–316. doi: 10.1016/s0149-2918(02)85026-3. [DOI] [PubMed] [Google Scholar]
- 29.World Health Organization World Health Organization: Hypertension in adherence to long-term therapies evidence for action. 2003:107–114. www.who.int/chronic_conditions/adherencereport/en/print.html
- 30.Wassertheil-Smoller S, Blaufox MD, Oberman A, et al. Effect of antihypertensives on sexual function and quality of life: the TAIM Study. Ann Intern Med. 1991;114:613–20. doi: 10.7326/0003-4819-114-8-613. [DOI] [PubMed] [Google Scholar]
- 31.Gregoire JP, Moisan J, Guibert R, et al. Tolerability of antihypertensive drugs in a community-based setting. Clin Ther. 2001;23:715–726. doi: 10.1016/s0149-2918(01)80021-7. [DOI] [PubMed] [Google Scholar]
- 32.Krousel-Wood MA, Thomas S, Jannu A, Muntner P, Morisky DE, Re RN. Low Adherence to Prescribed Antihypertension Medication and Poorer Quality of Life in Elderly Hypertensive Patients. Poster presented at American Heart Association; May 2004. [Google Scholar]
- 33.Harmon G, Lefante J, Krousel-Wood MA. Overcoming Barriers: The Role of Providers in Improving Patient Adherence to Antihypertensive Medications. Curr Opin Cardiol. 2006;21:310–315. doi: 10.1097/01.hco.0000231400.10104.e2. [DOI] [PubMed] [Google Scholar]
- 34.O’Connor PJ. Overcome clinical inertia to control systolic blood pressure. Arch Intern Med. 2003;163:2677–2678. doi: 10.1001/archinte.163.22.2677. [DOI] [PubMed] [Google Scholar]
- 35.Stroupe KT, Teal EY, Tu W, Weiner M, Murray MD. Association of refill adherence and health care use among adults with hypertension in an urban health care system. Pharmacotherapy. 2006;26:779–89. doi: 10.1592/phco.26.6.779. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.