Abstract
Objectives
To further explore the oncogenic activity of Aurora A kinase while attempting to develop a useful mouse model for pancreatic cancer, Aurora A kinase was targeted to Pdx-1 positive cells.
Methods
Aurora A kinase overexpression was targeted to mouse pancreas tissue using the Pdx-1 promoter in a transgenic model. The pancreas tissue of 7–11 month old transgenic animals were evaluated for metastatic adenocarcinomas, preinvasive ductal neoplasia, or other histological anomalies.
Results
Examination of pancreatic tissue from Pdx-1-Aurora A transgenic mice revealed abnormalities, such as mild islet cell hyperplasia, lymphocytic infiltration, and general dysplasia between ductal/islet cell interfaces. However, the majority of the tissue from these transgenic mice was normal.
Conclusions
The overexpression of Aurora A can potentially initiate the development of mild abnormalities in pancreatic tissue; however, neither preinvasive ductal neoplasia nor fully metastatic adenocarcinomas were observed. Combining the Pdx-1-Aurora A transgenic model with other genetic alterations may provide additional insight.
Keywords: pancreatic adenocarcinoma, Aurora A kinase, transgenic mouse model
Introduction
Pancreas adenocarcinoma is diagnosed in 37,170 people a year in the United States. 1 Additionally, 33,370 patients are expected to die from pancreatic cancer annually, which is the worst prognosis of any tumor type. Poor survival is largely dependent upon the lack of highly effective therapies and diagnostic techniques, which has been historically due to the lack of an animal model that recapitulates human pancreatic cancer. However, significant progress in this area has been made in recent years that should help elucidate early detection systems and novel therapeutics.2, 3 Despite advancements in mouse models, the Aurora A kinase target has not been expressed in pancreas tissue to determine its potential in vivo oncogenic effects.
Aurora A is an oncogenic kinase whose gene amplification or otherwise overexpression has been associated with the initiation and progression of many tumor types, including pancreas4, 5, bladder6, glioma7, non-Hodgkin’s lymphoma8, esophageal9, 10, gastric11, breast12,13 and colon14 cancers. Aurora kinases are involved in key regulatory steps of mitosis, such as bipolar spindle formation (Aurora A), chromosome alignment during metaphase (Aurora B) and cytokinesis (Aurora B). It has been shown that the overexpression of Aurora A is sufficient to transform fibroblast cells in vitro and in vivo.14 Similarly, the upregulation of Aurora A in near-diploid human breast epithelial cells induces centrosomes amplification,15 which can lead to multipolar mitotic spindles and genomic instability (aneuploidy). Aurora A overexpression is an early event in carcinogenesis models16 and therefore, the up-regulation of Aurora A has been suggested to be a potential driving force in the development of genomic instability,17 a hallmark of most cancer cells.18 Although Aurora A is commonly amplified and overexpressed in pancreatic adenocarcinomas4,5 and it has been hypothesized to be an early event in tumorigenesis, it has not been definitively shown that Aurora A is overexpressed in premalignant lesions, such as PanINs (pancreatic intraepithelial neoplasia). However, Aurora A expression has been found to be elevated in high-grade PINs (prostatic intraepithelial neoplasia).19
Establishing novel mouse models of pancreatic cancer through previously untargeted genetic lesions, such as Aurora Kinase A will provide information regarding additional pathways that lead to PanIN and/or PDAC development. Nearly all mouse models of pancreatic cancer utilize a mutation in K-ras as an initiating event and loss of a tumor suppressor gene (p16, p53, DPC4/SMAD4, or TGFβRII) as a progressing event.23,20–23 How Aurora Kinase A plays a role in the process, if at all, has yet to be determined and is a major theme of this work.
Considering the need for developing additional mouse models of pancreatic cancer with other oncogenes besides mutant K-ras and the potential role for Aurora A kinase being a potential causative agent in the initiation and progression of pancreatic cancer, we targeted the overexpression of Aurora A kinase to pancreas tissue using the Pdx-1 promoter in a transgenic mouse system. Pdx-1 (pancreatic duodenal homeobox gene-1) is a transcription factor involved in the regulation of genes important for pancreas development and function, including insulin, glucose transporter type 2, glucokinase and other genes involved in glucose metabolism.24,25 It functions in pancreas formation during embryogenesis and plays a regulatory role in mature pancreatic islet cell physiology.
In summary, Pdx-1-Aurora A transgenic mice developed subtle ductal abnormalities and dysplasia in their pancreatic tissues, but did not develop tumors or preinvasive neoplasia. These findings complement results from transgenic studies where Aurora A was expressed in mammary epithelium26 (and our unpublished results).
Methods
Transgene Construction and Generation of Transgenic Mice
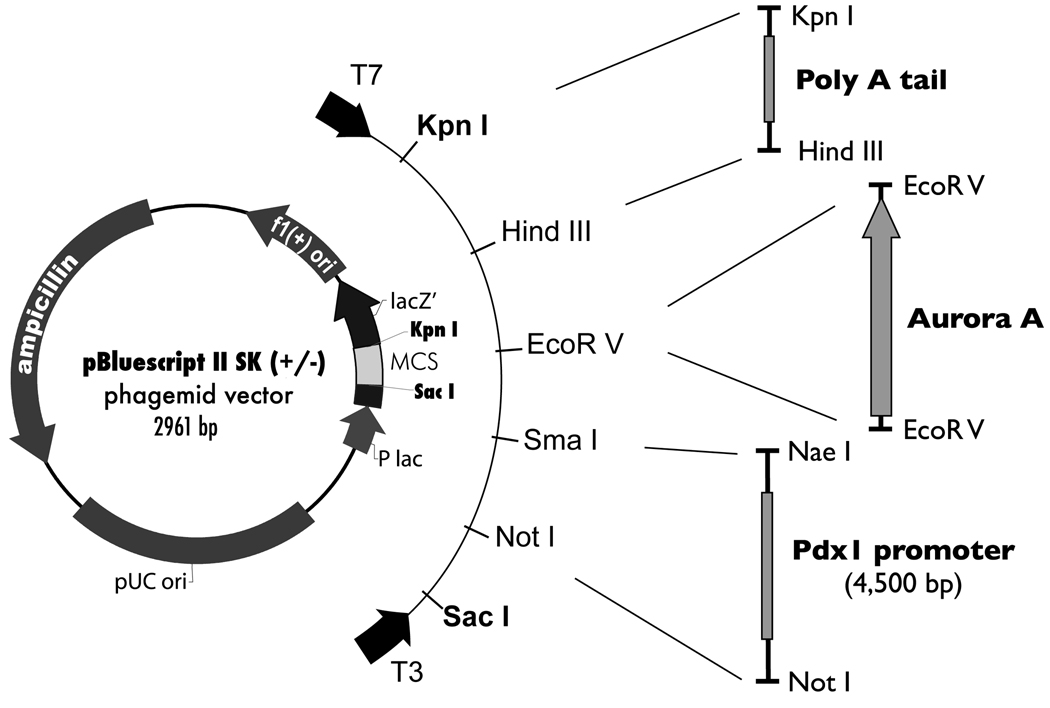
Aurora A was cloned from MIA PaCa-2 cDNA into a vector containing the Pdx-1 promoter27 (provided by Dr. Helen Edlund) using an EcoRV site and the following primer pair: 5'-AAAGATATCGAGGCGCCCTGTAGGATACT-3' and 5'- AAAGATATCTGGCTCAAGGATTTCTCCCC-3' (Fig. 1). The plasmid was linearized by XhoI digestion and microinjected into the pronucleus of fertilized single-cell BALB/c-C3H strain (The Jackson Laboratory, Bar Harbor, ME) eggs with assistance from the Genetically Modified Mice Shared Service at the Arizona Cancer Center (Tucson, AZ). DNAs from a 2-mm tail biopsy were used to identify transgenic mice. In a PCR reaction, 20 ng of genomic DNA was amplified with the following primer pairs: 5'-TAGCGAGGGGGAAGAGGAGAT-3' and 5'-ACTGACCACCCAAAATCTGC-3' to detect the Pdx-1-Aurora A transgene and 5'-CAAATGTTGCTTGTCTGGTG-3' and 5'-GTCAGTCGAGTGCACAGTTT-3' were used to detect endoTCR, an internal control. The PCR conditions were: 95°C for 5 min, 35 cycles of 94°C for 30 s, 53.4°C for 30 s and 72°C for 30 s and 72°C for 5 min to end the reaction. PCR products were analyzed on 1% agarose gels stained with ethidium bromide. Samples yielding a PCR product of the appropriate size were considered positive for Pdx-1-Aurora A. Mice were housed in the animal facility at the Arizona Health Science Center and cared for by the Genetically Modified Mice Shared Service at the Arizona Cancer Center.
Figure 1.
Strategy for Pdx-1-Aurora A transgene construction. Full-length Aurora A was inserted into a pBluescript II SK (+/−)-based vector downstream of the Pdx-1 promoter and upstream of a poly A tail. The Pdx-1/poly A vector was generously provided by Dr. Helen Edlund.
Microscopic Analysis and Histology
Mice were euthanized at ages ranging from 7 and 11 months of age. Mouse pancreatic tissues were harvested, visually examined for gross abnormalities and either fixed in formalin solution (10% neutral buffered AFIP formulation, Sigma Diagnostics, St. Louis, MO) or snap frozen in liquid nitrogen. Fixed tissues were embedded in paraffin, sectioned, mounted on slides and stained with hematoxylin and eosin (H&E) for microscopic examination.
RT-PCR Analysis
Frozen pancreas tissues were homogenized in lysis buffer and RNA was isolated using the NucleoSpin RNA II Kit (Clontech, Mountain View, CA). RNA concentration was determined using UV spectroscopy and 2 µg of RNA were used for cDNA preparation utilizing the First Strand cDNA Synthesis Kit (Fermentas, Hanover, MD). cDNA from each sample was amplified by PCR using Aurora A-specific primers (5'-TCGGCACCTGAAAATAATCC-3' and 5'- ACTGACCACCCAAAATCTGC-3'). The PCR conditions were: 95°C for 5 min, 30 cycles of 94°C for 30 s, 58°C for 30 s and 72°C for 30 s and 72°C for 5 min to end the reaction. PCR products were analyzed on 1% agarose gels stained with ethidium bromide. Primers for β-actin (Ambion, Austin, TX) were used to generate a PCR product used for loading controls and primers for Pdx-1-Aurora A (described above) were used to ensure there was no plasmid or genomic DNA contamination in the RNA samples.
Results
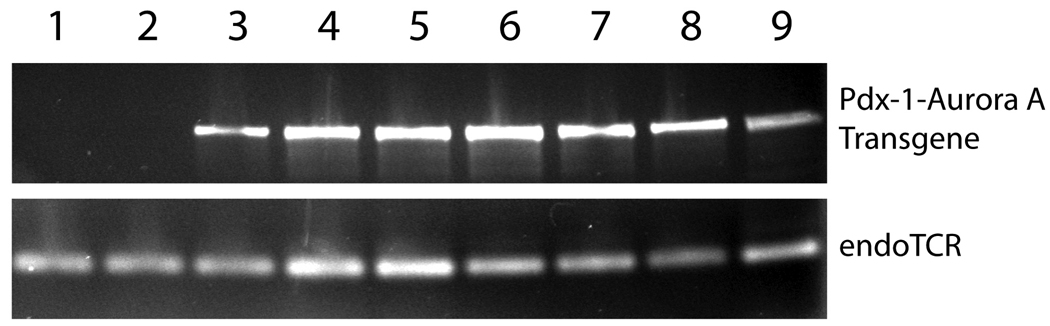
We identified three founder mice that carried the Pdx-1-Aurora A transgene. The founders were bred with negative littermates to generate four additional transgene positive mice making a total of seven positive mice (Fig. 2). Littermates negative for the transgene were retained as controls for the study. At birth and throughout development, all mice (transgene positive and negative) were normal in weight and appearance. All mice were euthanized at the same time when the founder mice were approximately 11 months old and their offspring at about 7 months of age. Gross examination of pancreas tissue by visual inspection at the time of harvesting showed no major abnormalities between positive and negative groups.
Figure 2.
PCR analysis on genomic DNA isolated from tail biopsy to identify transgenic mice. Mice 1–2 were not Pdx-1-Aurora A transgene positive and were used as controls for this study. Mice 3–9 were positive for the transgene.
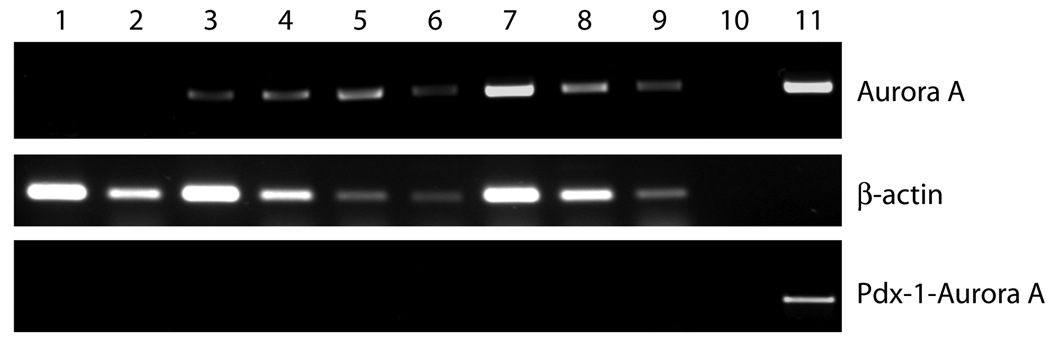
To confirm expression from the Pdx-1-Aurora A transgene, RT-PCR was performed on RNA isolated from frozen pancreas tissue. Primers for PCR were designed and used to detect transgenic human Aurora A expression and not the transcripts of the mouse homologue of Aurora A (ARK-1/IAK1). mRNAs for the Aurora A transgene were detected in all transgenic mice and not in negative mice (Fig. 3). Mouse β-actin was detected in all samples. Pdx-1-Aurora A-specific primers were used to detect plasmid or DNA contamination, which were not found in any of the samples. Taken together, these results confirm the expression of the Aurora A transgene.
Figure 3.
RT-PCR analysis of Aurora A expression using RNA isolated from transgenic mouse pancreatic tissues. As indicated, the top band represents transgene Aurora A amplified message, but not endogenouse mouse Aurora A (ARK-1/IAK1). The middle band represents mouse β-actin amplified message. The bottom band represents direct amplification of the Pdx-1-Aurora A transgene, but not expressed message from the transgene. Lanes 1–2, nontransgenic mice; Lanes 3–9, Pdx-1-Aurora A positive mice; Lane 10, negative control; Lane 11, Pdx-1-Aurora A plasmid.
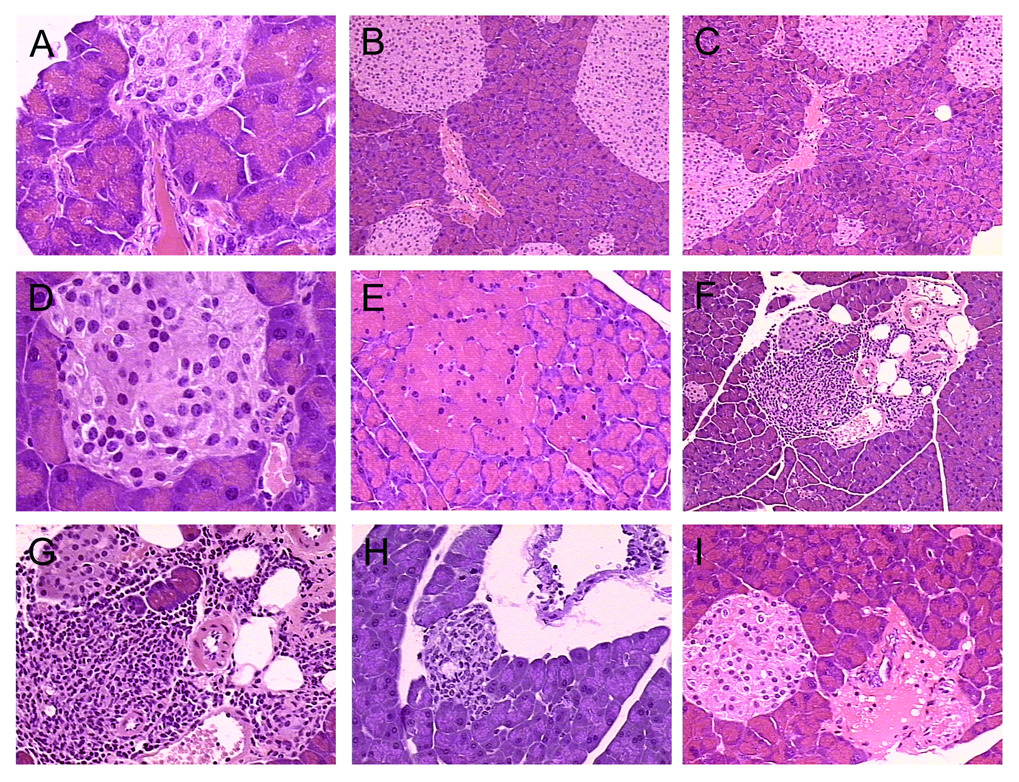
Microscopic evaluation revealed that the majority of the pancreas tissues from the Pdx-1-Aurora A transgenic mice were relatively normal, with most tissue being acinar cells arranged in acini-surrounding centroacinar cells which fenestrate into ducts (Fig. 4A). Most ducts were normal with a few having a thicker stromal layer around their epithelium. Islets were observed periodically imbedded around the parenchyma. A few samples appeared to have a slightly higher frequency and/or size of islets of Langerhans, perhaps a product of mild hyperplasia (Fig. 4B–D), though without any signs of abnormal islet cell architecture. A subtle change was observed in many samples in the area where small ducts, blood vessels and islets were proximal to each other (called an islet-ductal interface). There appeared to be some mild dysplasia with occasional loss of stromal tissue surrounding the neighboring islets. In the 7 transgenic mice evaluated, six (3–8) were generated from one line (A) and one (9) from another line (B) (Table 1). Of 6 mice from line A, 61% displayed some level of dysplasia in ducts near islets (these ducts are either immediately adjacent to or less than 30 microns from an islet). In one mouse from line B, there were no noticeable dysplasia in ducts in the ductal-islet interface. However, there was one prominent odd duct that was observed in this sample. Thus, there is some consistency between these lineages in that ductal dysplasia has been observed. Since only one mouse was analyzed from this line, it is difficult to conclude that ductal dysplasia was absent in ductal-islet interfaces. In all Pdx-1-Aurora A mice (both lines combined), 11 of 20 (55%) ductal-islet interfaces were found to have dysplastic ducts (Table 1). These evaluations were done using comparisons with non-transgenic mouse pancreas (n=3; 12 ductal-islet interfaces examined), where ducts near islets did not demonstrate any mild dysplasia. Despite the difference, it is possible that these lesions are within the boundary of abnormal structures that can develop in wild type mouse pancreas, especially considering how mild these changes appear. However, it is unlikely that they would develop at this higher frequency (55% of observed ductal-islet interfaces in multiple mice and lineages). Also, there are some accompanying tissue effects (i.e. inflammation, fibrosis, etc.) in a smaller subset of these lesions (see Table 1), which would indicate further pathology as a result of this dysplasia. In some cases, islets cells and epithelial cells (probably from ducts) overlapped with each other. In normal mouse pancreas, there were very distinct separations between islet and ductal cells, where a thin layer of cells provided a barrier for the islets.
Figure 4.
Pdx-1-Aurora A transgenic mouse pancreatic lesions. (A) Most pancreas from these transgenic mice appeared normal with frequent mild dysplasia of ducts near islets, as evident in mouse 5. (B–D) Islet cell hyperplasia was evident in some of these transgenic mice including mouse 3 (B) and mouse 7 (C,D) . (E) Mild focal acinar hypertrophy was observed in mouse 6. (F) Mild fibrosis around an islet-ductal interface was detected in mouse 4. (G–H) Focal lymphocytic infiltration was observed around an islet-ductal interface in mouse 4 and a ductal lesion in mouse 9. (I) An odd fibrous lesion was evident near an islet-ductal interface in mouse 8.
Table 1.
Summary of ductal-islet interface abnormalities in Pdx-1-Aurora A transgenic mice
| Mouse # | Line | Ductal-Islet Interface | Comments | Other Tissue Effects | ||
|---|---|---|---|---|---|---|
| dysplasia | normal | total | ||||
| 3 | A | 2 | 2 | 2/4 | much lipoatrophy, many islets | |
| 4 | A | 1 | 2 | 1/3 | fairly normal parenchyma | focal lymphocytic infiltration & mild fibrosis |
| 5 | A | 3 | 0 | 3/3 | moderate ductal dysplasia* | |
| 6 | A | 0 | 1 | 0/1 | fairly normal parenchyma | mild acinar hypertrophy/dysplasia |
| 7 | A | 3 | 0 | 3/3 | mild ductal dysplasia | |
| 8 | A | 2 | 2 | 2/4 | mild ductal dysplasia | focal lymphocytic infiltration; fibrotic lesion |
| TOTAL | A | 11 | 7 | 11/18 | ||
| 9 | B | 0 | 2 | 0/2 | fairly normal parenchyma | odd ductal lesion; focal lymphocytic infiltration |
| TOTAL | B | 0 | 2 | 0/2 | ||
| TOTALS | A & B | 11 | 9 | 11/20 | ||
| Controls | N/A | 0 | 12 | 0/12 | normal parenchyma | |
most other ductal dysplasia was mild
The most prominent lesion observed in at least two separate samples of Pdx-1-Aurora A mice was the presence of lymphocytic infiltration in and around the islet ductal interface (Fig. 4F–H). It is not clear if this was a general immune response, though all other tissues in these samples were void of any lymphocytic infiltration. The presence of this infiltration surrounding the islet-ductal interface appears to confirm the more subtle difference where the architecture of these structures was mildly altered. Perhaps the immune response was a result of aberrant cell-cell interactions between islet and epithelial cells. It is important to note that the vast majority of parenchymal-mesenchymal cells in the pancreas of Pdx-1-Aurora A transgenic mice appeared relatively normal. The apparent influx of lymphocytes in a few islet ductal interfaces is probably indicative of altered cellular and molecular properties in these focal areas.
Discussion
Our findings demonstrate that Aurora A kinase overexpression can potentially initiate the development of mild abnormalities in the pancreas of adult mice; however, neither preinvasive ductal neoplasia nor fully metastatic adenocarcinomas were observed. The majority of the pancreas tissues were normal and the observed anomalies were present only at low frequencies. Interestingly, most of the unusual lesions could be a consequence of hyperplasia, which is a likely result of Aurora A kinase overexpression.
As with most studies, these results raised more questions than answers. Although we feel confident that Aurora A overexpression is not sufficient to drive pancreas tumorigenesis in this model, this conclusion may only be valid for the promoter and the genetic background of the mice used in this study. Although expression from the Pdx-1 promoter is pancreas-specific (there is some low-level expression in other tissues including gut), it is not specific to a single cell type within the pancreas. During organogenesis, Pdx-1 is widely expressed in all cells differentiating toward the exocrine and endocrine components of the pancreas. In adult pancreas, Pdx-1 expression is predominately restricted to the insulin-producing islet β-cells and a subset of somatostatin-producing islet δ-cells but is almost undetectable in other pancreatic cell types.28,29 The observed islet cell hyperplasia is consistent with this expression pattern (We have tried to detect Aurora kinase A expression in the mouse islets using immunostaining but were not successful due to technical reasons). It would have been interesting to know if the Pdx-1-Aurora A transgenic mice had altered glucose metabolism. Unfortunately, we did not collect information on the glucose tolerance or glucose plasma levels in these animals. Except for the few examples described above, acinar and ductal epithelial cells in the transgenic mice did not show significant abnormalities, and it is in these cells that human pancreatic neoplasms most likely originate.30 Therefore, it would be interesting to target Aurora A overexpression using another strategy such as the well-characterized acinar cell-specific elastase (Ela) enhancer/promoter.3
Although switching to a different promoter may well alter the results, it still may not be enough to induce aggressive pancreatic adenocarcinomas in an Aurora A transgenic mouse model. This speculation is based on two similar attempts to transgenically target the overexpression of Aurora A to a specific tissue. First, Cre-loxP-controlled overexpression of Aurora A to mouse mammary tissue did not yield mammary tumors,26 although mitotic abnormalities and hyperplasia were reported in the study. Secondly, MMTV-controlled overexpression of Aurora A did not reproducibly induce the development of mouse mammary tumors (our observation). One MMTV-Aurora A transgenic mouse developed a mammary tumor at seven months of age. However, we were unable to repeat that result in other mice including some approaching the age of 12 months, suggesting it was a spontaneous tumor arising from any number of factors.
Recent studies have revealed a direct relationship between Aurora A and p53. One report concluded that p53 interacts with Aurora A and suppresses the oncogenic activity of Aurora A, such as centrosome amplification and cellular transformation.31 These data may partially explain why Aurora A overexpression was insufficient in inducing tumor formation. However, a separate study reported an Aurora A-induced degradation of p53 suggesting that Aurora A overexpression can stifle the tumor suppressing function of p53.32 Although these reports appear to be contradictory, an interesting relationship between Aurora A and p53 does likely exist and exciting findings may result from crossing an Aurora A transgenic mouse with a p53 knockout mouse.
An activating mutation of the k-ras oncogene is the most frequent genetic alteration associated with pancreatic cancer, having been identified in up to 90% of all pancreatic adenocarcinomcas.30, 33 Therefore, another interesting study would be to cross an Aurora A transgenic line with a k-rasG12D transgenic line. Furthermore, crossing the transgenic mice into p16 knockout, DPC4 knockout or any number of other genetic backgrounds may yield interesting results. It is noteworthy that although the overexpression of Aurora A transformed immortalized rodent cell lines,14, 15 Aurora A-overexpressing primary mouse embryonic fibroblasts did not form colonies in soft agar.34 A major difference between these two models is that immortalized cell lines possess preexisting genetic lesions in genes that control cell growth, which makes them more susceptible to transformation compared to primary cells generally lacking in such mutations. Even though Aurora A overexpression was insufficient at inducing cancer development in this model, it may play an important role in tumorigenesis when combined with additional genetic alterations.
Acknowledgements
We thank Dr. Bob Erickson and members of the Genetically Modified Shared Service group for their assistance in generating and caring for transgenic mice. Specifically, we appreciate Diana Strnatka for performing embryo microinjections and Loretta Barbercheck for assisting with tail biopsies.
Financial support: Predoctoral fellowship to S.L.W from the American Chemical Society Division of Medicinal Chemistry and Wyeth. The National Institutes of Health for support from grant CA 95031.
Contributor Information
Steven L. Warner, College of Pharmacy, University of Arizona, Tucson, AZ.
Ruben M. Muñoz, Translational Genomics Research Institute, Phoenix, AZ.
David J. Bearss, Arizona Cancer Center, University of Arizona, Tucson, AZ.
Paul Grippo, Department of Surgery, Feinberg School of Medicine, Northwestern University, Chicago, IL.
Haiyong Han, Translational Genomics Research Institute, Phoenix, AZ.
Daniel D. Von Hoff, Translational Genomics Research Institute, Phoenix, AZ; Arizona Cancer Center, University of Arizona, Tucson, AZ.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57(1):43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Hingorani SR, Petricoin EF, Maitra A, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4(6):437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 3.Grippo PJ, Nowlin PS, Demeure MJ, et al. Preinvasive pancreatic neoplasia of ductal phenotype induced by acinar cell targeting of mutant Kras in transgenic mice. Cancer Res. 2003;63(9):2016–2019. [PubMed] [Google Scholar]
- 4.Li D, Zhu J, Firozi PF, et al. Overexpression of oncogenic STK15/BTAK/Aurora A kinase in human pancreatic cancer. Clin Cancer Res. 2003;9(3):991–997. [PubMed] [Google Scholar]
- 5.Rojanala S, Han H, Munoz RM, et al. The mitotic serine threonine kinase, Aurora-2, is a potential target for drug development in human pancreatic cancer. Mol Cancer Ther. 2004;3(4):451–457. [PubMed] [Google Scholar]
- 6.Sen S, Zhou H, Zhang RD, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94(17):1320–1329. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 7.Reichardt W, Jung V, Brunner C, et al. The putative serine/threonine kinase gene STK15 on chromosome 20q13.2 is amplified in human gliomas. Oncol Rep. 2003;10(5):1275–1279. [PubMed] [Google Scholar]
- 8.Yakushijin Y, Hamada M, Yasukawa M. The expression of the aurora-A gene and its significance with tumorgenesis in non-Hodgkin’s lymphoma. Leuk Lymphoma. 2004;45(9):1741–1746. doi: 10.1080/10428190410001683615. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka E, Hashimoto Y, Ito T, et al. The clinical significance of Aurora-A/STK15/BTAK expression in human esophageal squamous cell carcinoma. Clin Cancer Res. 2005;11(5):1827–1834. doi: 10.1158/1078-0432.CCR-04-1627. [DOI] [PubMed] [Google Scholar]
- 10.Tong T, Zhong Y, Kong J, et al. Overexpression of Aurora-A contributes to malignant development of human esophageal squamous cell carcinoma. Clin Cancer Res. 2004;10(21):7304–7310. doi: 10.1158/1078-0432.CCR-04-0806. [DOI] [PubMed] [Google Scholar]
- 11.Sakakura C, Hagiwara A, Yasuoka R, et al. Tumour-amplified kinase BTAK is amplified and overexpressed in gastric cancers with possible involvement in aneuploid formation. Br J Cancer. 2001;84(6):824–831. doi: 10.1054/bjoc.2000.1684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sen S, Zhou H, White RA. A putative serine/threonine kinase encoding gene BTAK on chromosome 20q13 is amplified and overexpressed in human breast cancer cell lines. Oncogene. 1997;14(18):2195–2200. doi: 10.1038/sj.onc.1201065. [DOI] [PubMed] [Google Scholar]
- 13.Tanaka T, Kimura M, Matsunaga K, et al. Centrosomal kinase AIK1 is overexpressed in invasive ductal carcinoma of the breast. Cancer Res. 1999;59(9):2041–2044. [PubMed] [Google Scholar]
- 14.Bischoff JR, Anderson L, Zhu Y, et al. A homologue of Drosophila aurora kinase is oncogenic and amplified in human colorectal cancers. Embo J. 1998;17(11):3052–3065. doi: 10.1093/emboj/17.11.3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou H, Kuang J, Zhong L, et al. Tumour amplified kinase STK15/BTAK induces centrosome amplification, aneuploidy and transformation. Nat Genet. 1998;20(2):189–193. doi: 10.1038/2496. [DOI] [PubMed] [Google Scholar]
- 16.Goepfert TM, Adigun YE, Zhong L, et al. Centrosome amplification and overexpression of aurora A are early events in rat mammary carcinogenesis. Cancer Res. 2002;62(14):4115–4122. [PubMed] [Google Scholar]
- 17.Warner SL, Bearss DJ, Han H, et al. Targeting Aurora-2 kinase in cancer. Mol Cancer Ther. 2003;2(6):589–595. [PubMed] [Google Scholar]
- 18.Mitelman F, Johansson B, Mertens F. Catalog of Chromosome Aberrations in cancer. New York: Wiley-Liss; 1994. [Google Scholar]
- 19.Buschhorn HM, Klein RR, Chambers SM, et al. Aurora-A over-expression in high-grade PIN lesions and prostate cancer. Prostate. 2005;64(4):341–346. doi: 10.1002/pros.20247. [DOI] [PubMed] [Google Scholar]
- 20.Hingorani SR, Wang L, Multani AS, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7(5):469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre AJ, Bardeesy N, Sinha M, et al. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17(24):3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Izeradjene K, Combs C, Best M, et al. Kras(G12D) and Smad4/Dpc4 haploinsufficiency cooperate to induce mucinous cystic neoplasms and invasive adenocarcinoma of the pancreas. Cancer Cell. 2007;11(3):229–243. doi: 10.1016/j.ccr.2007.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Ijichi H, Chytil A, Gorska AE, et al. Aggressive pancreatic ductal adenocarcinoma in mice caused by pancreas-specific blockade of transforming growth factor-beta signaling in cooperation with active Kras expression. Genes Dev. 2006;20(22):3147–3160. doi: 10.1101/gad.1475506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Offield MF, Jetton TL, Labosky PA, et al. PDX-1 is required for pancreatic outgrowth and differentiation of the rostral duodenum. Development. 1996;122(3):983–995. doi: 10.1242/dev.122.3.983. [DOI] [PubMed] [Google Scholar]
- 25.Jonsson J, Carlsson L, Edlund T, et al. Insulin-promoter-factor 1 is required for pancreas development in mice. Nature. 1994;371(6498):606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 26.Zhang D, Hirota T, Marumoto T, et al. Cre-loxP-controlled periodic Aurora-A overexpression induces mitotic abnormalities and hyperplasia in mammary glands of mouse models. Oncogene. 2004;23(54):8720–8730. doi: 10.1038/sj.onc.1208153. [DOI] [PubMed] [Google Scholar]
- 27.Apelqvist A, Ahlgren U, Edlund H. Sonic hedgehog directs specialised mesoderm differentiation in the intestine and pancreas. Curr Biol. 1997;7(10):801–804. doi: 10.1016/s0960-9822(06)00340-x. [DOI] [PubMed] [Google Scholar]
- 28.Guz Y, Montminy MR, Stein R, et al. Expression of murine STF-1, a putative insulin gene transcription factor, in beta cells of pancreas, duodenal epithelium and pancreatic exocrine and endocrine progenitors during ontogeny. Development. 1995;121(1):11–18. doi: 10.1242/dev.121.1.11. [DOI] [PubMed] [Google Scholar]
- 29.Sander M, German MS. The beta cell transcription factors and development of the pancreas. J Mol Med. 1997;75(5):327–340. doi: 10.1007/s001090050118. [DOI] [PubMed] [Google Scholar]
- 30.Hilgers W, Kern SE. Molecular genetic basis of pancreatic adenocarcinoma. Genes Chromosomes Cancer. 1999;26(1):1–12. [PubMed] [Google Scholar]
- 31.Chen SS, Chang PC, Cheng YW, et al. Suppression of the STK15 oncogenic activity requires a transactivation-independent p53 function. Embo J. 2002;21(17):4491–4499. doi: 10.1093/emboj/cdf409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Q, Kaneko S, Yang L, et al. Aurora-A abrogation of p53 DNA binding and transactivation activity by phosphorylation of serine 215. J Biol Chem. 2004;279(50):52175–52182. doi: 10.1074/jbc.M406802200. [DOI] [PubMed] [Google Scholar]
- 33.Almoguera C, Shibata D, Forrester K, et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell. 1988;53(4):549–554. doi: 10.1016/0092-8674(88)90571-5. [DOI] [PubMed] [Google Scholar]
- 34.Anand S, Penrhyn-Lowe S, Venkitaraman AR. AURORA-A amplification overrides the mitotic spindle assembly checkpoint, inducing resistance to Taxol. Cancer Cell. 2003;3(1):51–62. doi: 10.1016/s1535-6108(02)00235-0. [DOI] [PubMed] [Google Scholar]