Abstract
Background
Cisplatin is one of the most effective chemotherapeutic agents in the treatment of several solid tumors including osteosarcoma (OS). Despite aggressive treatment, 25% of patients with OS continue to die from their disease. Since cisplatin based regimens have been uniformly used in OS therapy, treatment failure is likely due, at least in part, to cisplatin resistance.
Procedure
The objective of this study was to determine the relationship between MKP-1 expression and cisplatin sensitivity of osteosarcoma cell lines and to explore the mechanism underlying this relationship. Three OS cell lines were examined for their MKP-1 expression and cisplatin sensitivity. JNK phosphorylation and apoptosis induction was also measured. Western and Northern blot, flow cytometry, siRNA and MTT assays were used.
Results
U2OS cells, which express high level of MKP-1, are less sensitive to cisplatin-induced cell death. Inhibition of MKP-1 by siRNA silencing sensitizes U2OS cells to cisplatin-induced cell death. Furthermore, delayed apoptosis induction following cisplatin treatment was observed in U2OS, in parallel to decreased JNK activation, increased MKP-1 expression and relatively increased cisplatin resistance. Interestingly, triptolide, an MKP-1 inhibitor, blocks MKP-1 expression and enhances cisplatin-induced cell death.
Conclusion
High MKP-1 expression is associated with decreased sensitivity or increased resistance to cisplatin-induced cell death in OS cell lines, and MKP-1 could potentially be used as a marker of cisplatin resistance and a therapeutic target for molecular therapies.
Keywords: Drug resistance, Cisplatin, Chemotherapy, Osteosarcoma, MKP-1
Introduction
Cisplatin is one of the most effective chemotherapeutic agents and is widely used in the treatment of a variety of pediatric and adult solid tumors including osteosarcoma (OS), medulloblastoma and neuroblastoma. Cisplatin is a DNA-damaging agent that forms cisplatin-DNA adducts and kills cells via several mechanisms, including induction of apoptosis[1]. Resistance to cisplatin is multifactorial and leads to poor response to chemotherapy and treatment failure. Failure to induce apoptosis is believed to be one of the major mechanisms underlying cisplatin resistance[2,3].
Osteosarcoma is the most common malignant bone tumor in children. The introduction of chemotherapy in 1970's significantly increased the long-term survival rate of OS from 15% to 70%[4]. However, 30-40% of patients with OS relapse with current therapies. After relapse, only approximately one-quarter of patients survive beyond 5 years[5]. In the past several years, only minor improvements have been achieved by intensifying chemotherapy, frequently offset by significantly increasing toxicity. Efforts at identifying new risk factors for the stratification of OS treatment are ongoing [5-7]. Histological response after neoadjuvant chemotherapy assessed at the time of definitive surgery is the only reliable marker that can predict outcome[2,3,8]. Since cisplatin is one of the most effective chemotherapeutic agents that has been uniformly used in front line chemotherapy of OS, the poorer response to OS chemotherapy is likely due, at least in part, to cisplatin resistance.
It has been shown that cisplatin can activate the mitogen-activated protein kinase (MAPK) pathways, leading to apoptotic cell death[9]. There are three major pathways of MAPKs: stress-activated protein kinase (SPK)/c-Jun NH2-terminal kinase (JNK), p38 MAPK, and extra-cellular signal-regulated kinase (ERK)[10]. The importance of MAPK activation in cellular response to cisplatin has been gradually appreciated in recent years[11,12]. MAPKs control cell proliferation, differentiation and cell death. Their role in response to cisplatin is complex and the response varies with the type of cells as well as their proliferation and differentiation status[11-14]. It is believed that MAPK activities are regulated through reversible phosphorylation of both threonine and tyrosine residues by upstream kinases, as well as by the members of MAPK phosphatase (MKP) family, which can dephosphorylate both phospho-threonine and phospho-tyrosine residues and inactivate MAPKs[2,15]. Recent studies suggest that MKP-1, the founding member of MKP family, plays an important role in the response of cancer cells to chemotherapy, especially to cisplatin[11,14,16]. Therefore, further investigaton is warranted to study the role of MKP-1 in pediatric cancers including osteosarcoma.
Material and Methods
Reagents
Cisplatin was the product of Sicor Pharmaceuticals (Irvine, CA). Triptolide was purchased from Calbiochem (Darmstadt, Germany). MKP-1 antibody was the product of Santa Crutz Biotechnology (Santa Cruz, CA). Antibodies against total and phosphorylated JNK (p-JNK) and PARP, were the products of Cell Signaling Technology (Beverly, MA). Anti-actin antibody (AC-74) was purchased from Sigma.
Cell lines and cell cultures
The human OS cell line U2OS, M189 and P16T were as previously described[17,18]. These cells were maintained in RPMI 1640 medium containing 10% of fetal bovine serum (FBS) (Hyclone, Logan, UT),and 1% penicillin-streptomycin (Invitrogen Life Technologies) in a humidified atmosphere of 5% CO2 at 37°C.
Polyacrylamide gel electrophoresis (PAGE) and Western blotting
Whole-cell lysates were prepared as described[14]. 30-100 μg of cell lysates were electorphoresed through 10-15% SDS-PAGE. Following electrophoresis, separated proteins on gels were electrotransfered to PVD membranes (Schleicher & Schuell, Inc., Keene, NH). Non-specific binding was blocked with blocking buffer containing 10% fat-free milk powder in Tris buffer saline (TBS) for 1 hour at room temperature, followed by incubation with primary antibodies in blocking buffer with 5% of fat-free milk powder overnight at 4°C and room temperature for 1 hour. The membrane was then washed 3 times with TBS, and incubated with secondary antibody for 1 hour at room temperature and washed 3 times. The bound antibody was detected using Chemilluminescence reagent (Amersham Pharmacia Biotech, Piscataway, NJ) according to the manufacturer's protocol.
Isolation of RNA and Northern blot analysis
Total cellular RNA was purified using the Trizol reagent (Invitrogen Life Technologies, Carlsbad, CA) according to the manufacturer's instructions. Total RNA was separated in a 1.5% formaldehyde agarose gel and blotted to Hybond-N+ membrane (Amersham). The blots were hybridized with 32P labeled human MKP-1 cDNA as described previously[14]. Radioactive signals were analyzed by autoradiography.
SiRNA assay
MKP-1 siRNA pooled oligonucleotides were purchased from Darmacon Research (Lafayette, CO). Briefly, U2OS cells were plated at 105 cells per well in 6-well plates and transfected with MKP-1 siRNA using Oligofectamine reagent (Invitrogen) according to the manufacturer's instruction and as described previously[14]. siGlow, which is Lamin A siRNA, was used as a non-target fluorescent control. The cells were harvested 48-72 hours after transfection for examination of MKP-1 expression by Western blot and determination of cisplatin sensitivity by MTT assays.
MTT Assays
MTT assays were performed as described previously[14]. Briefly, cells were treated with cisplatin at different concentrations for various times. In the experiments involving the triptolide, cells were pretreated with triptolide for 2 hours before the cisplatin treatment. After incubation with MTT solution, acid isopropanol was added to dissolve formazan crystals. Absorbance was measured using a Vmax microplate reader (Molecular Devices, Sunnyvale, CA) at 590 nm, and percentage of survival was calculated.
In-vitro Cytotoxicity Assay using Annexin-V/PI and Flow-Count Fluorospheres
P16T and U2OS cells were incubated in the presence of 5μg/ml of cisplatin for 4, 16, 20 and 24 hours in 35 mm Petri dishes. The experiment was designed to add the cisplatin at the different time points so that the cells could be harvested and assayed at the same time to minimize variation. At the end of treatment, the cisplatin treated and control cells (with no drug added), which served as a baseline control for spontaneous apoptosis, were trypsinized and resuspended in medium. Apoptosis detection was performed using the Apoptosis Annexin-V FITC Kit (Beckman Coulter; Brea, CA) per manufacturer's instruction and as previously described[19,20]. Briefly, 50 μL of resuspended cells were transferred to clean 12×75 culture tubes containing 50 μL of Annexin-V FITC/PI reagent and incubated in the dark for 15 minutes. At the end of incubation, an additional 0.4 ml 1X Binding Buffer containing 2.5% to 5% by volume Flow-Count Fluorospheres (Beckman Coulter; Brea, CA) was added to each tube, vortexed and then analyzed using a Coulter XL Flow Cytometer (Coulter; Miami, FL). Early apoptotic events were based on the annexin-V positive, PI negative population.
Statistical analysis
All data are expressed as the mean ± SEM. Statistical analyses were done using Student's t test. The data are presented as the mean ± SD, and p ≤ 0.05 was considered significant.
Results
High MKP-1 expressing OS cells are relatively more resistant to cisplatin
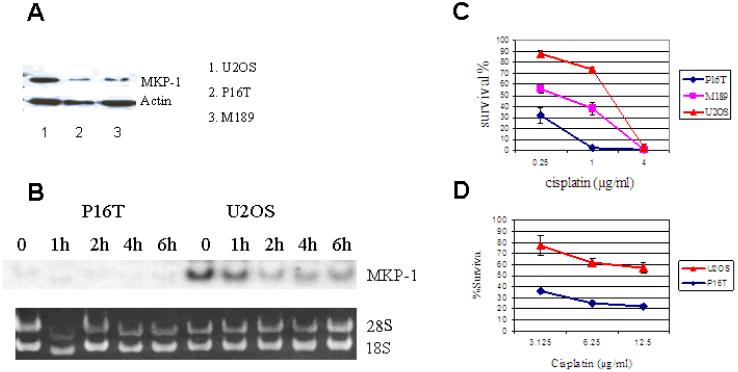
Three osteosarcoma cell lines, P16T, M189 and U2OS, were analyzed for their level of MKP-1 expression and cisplatin sensitivity (Figures 1A-D). U2OS cells, which expressed highest level of MKP-1, were most resistant to cisplatin-induced cell death. On the other hand, only minimal MKP-1 expression was detected in P16T cells which were most sensitive to cisplatin-induced cell death (Figures 1A-D). The above association of high MKP-1 expression and cisplatin resistance implies that MKP-1 could be an important determinant of cisplatin resistance. Of note, the concentrations of cisplatin used in Figure 1C were across the in vivo concentration spectrum when 100 mg/m2 of cisplatin was administered to patients as a 24-hour intravenous infusion [21,22]. Because higher concentrations of cisplatin are required for the siRNA assay and for induction of JNK activation as previously reported[14], we also used cisplantin at higher than clinically applicable concentrations for 24-hour MTT assays. Again, U2OS cells were more resistant to cisplatin than P16T cells (Figure 1D).
Figure 1. MKP-1 expression and cisplatin sensitivity in OS cell lines.
A: MKP-1 expression in 3 OS cell lines as determined by Western blot. The high level of MKP-1 expression was evident in U2OS cells, and only minimal MKP-1 expression was detected in P16T cells. Actin was a loading control. B: MKP-1 expression determined by Northern blot. U2OS and P16T cells were treated with 50μg/ml of cisplatin for 1, 2, 4 & 6 hours. MKP-1 expression was detected in U2OS, but not in P16T cells prior to and following cisplatin treatment. 18S and 28S were loading controls. C&D: Cisplatin-induced cell death in OS cell lines. The OS cells were incubated in the presence of cisplatin for 72 hours (C) at indicated concentrations across the pharmacologically achievable in vivo concentration spectrum. The cell survivals were determined by MTT assay. When OS cells were treated with 1μg/ml of cisplatin, almost all P16T cells were killed after 72 hours, but 74% of U2OS cells survived the treatment (C). U2OS cells were also more resistant than P16T cells when the cells were incubated in the higher concentrations of cisplatin for 24 hours (D).
SiRNA silencing of MKP-1 increases cisplatin sensitivity in OS
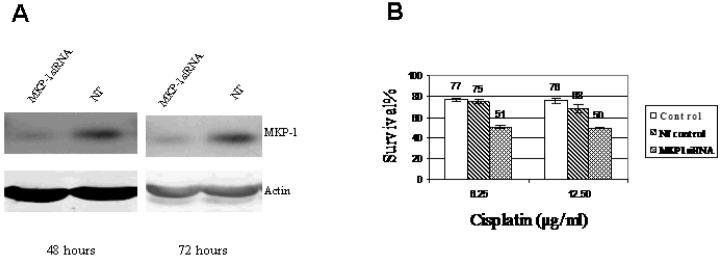
Since high MKP-1 expressing OS cells are relatively more resistant to cisplatin, we hypothesize that decreasing MKP-1 expression will render the cells less resistant. To test this hypothesis, the effect of MKP-1 knock down by siRNA on cisplatin sensitivity was examined. U2OS cells, which are relatively more resistant to cisplatin and express higher levels of MKP-1, were transfected with MKP-1 siRNA (Figure 2). The results showed that transfection with MKP-1 siRNA significantly inhibited MKP-1 expression, indicating the silencing effect of MKP-1 siRNA (Figure 2A). As hypothesized, MKP-1 knock down significantly increased the cisplatin-induced cytotoxicity (Fig. 2B). As knock down of MKP-1 renders OS cells less resistant to cisplatin, it is likely that MKP-1 plays an important role in cisplatin resistance. Of note, to catch the peak effect of transient MKP-1 knock down by siRNA, the 24-hour MTT assay with cisplatin at higher than clinically applicable concentrations was used in this experiment to demonstrate a significant difference between the control and siRNA transfected cells.
Figure 2. The effect of MKP-1 knock down on cisplatin sensitivity in U2OS cells.
U2OS cells were transfected with MKP-1 siRNA. 48 and 72 hours after transfection, MKP-1 expression was determined by Western blot and cisplatin cytotoxicity measured by MTT assay. MKP-1 siRNA and NT represent MKP-1 siRNA and non-target transfected cells respectively; Control represents U2OS cells without transfection. A: Significantly decreased MKP-1 expression was observed at both 48 and 72 hours after transfection of MKP-1 siRNA. Actin was a loading control. B: Significantly increased cisplatin induced cell death was observed in MKP-1 siRNA transfected cells. At 48 hours after siRNA transfection, the cells were treated with 6.25 and 12.5 μg/ml of cisplatin for 24 hours.
Cisplatin-induced cell death is associated with apoptosis
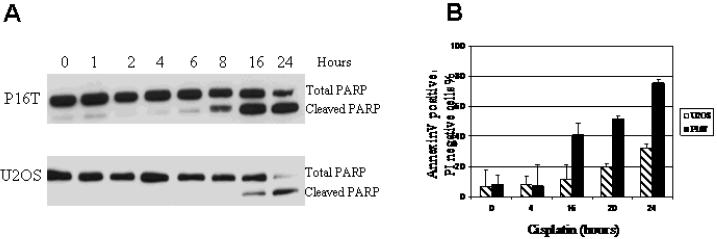
To further delineate the mechanisms of cisplatin-induced cytotoxicity and cisplatin resistance in OS, we examined PARP cleavage by Western blot and annexin V staining by flow cytometry as previously described[19,20]. As shown in Figures 3A & B, the increased percentage of annexin V positive/PI negative cell population and increased PARP cleavage were observed following cisplatin treatment. In addition, both PARP cleavage and percentage of annexin V positive/PI negative cell population were increased more robustly and earlier in the time course in P16T cells compared to U2OS cells. These results suggest that cisplatin-induced cell death is associated with apoptosis induction, and the delay of apoptosis induction in U2OS cells is associated with relative cisplatin resistance. Furthermore, increased apoptosis in P16T cells is associated with increased cisplatin sensitivity.
Figure 3. Cisplatin-induced apoptosis in OS cells.
P16T and U2OS cells were treated with 5μg/ml of cisplatin for the indicated time, and the cells with no drug added (0 hour) served as controls. The total and cleaved PARP was determined by Western blot analysis, annexin V/PI staining was assayed by flow cytometry. A: Increased cleavage of PARP is evident 8 hours following cisplatin treatment in P16T cells but not until 16 hours in U2OS cells. B: Significantly increased percentage of annexin V positive/PI negative cell population was obvious 16 hours following cisplatin treatment in P16T cells, but not until 20 hours in U2OS cells. In addition, the more robust increase in annexin V positive/PI negative cell population was observed in P16T compared to U2OS cells.
Cisplatin induces apoptosis by activating JNK pathways and expression of MKP-1 is associated with delay of both JNK phosphorylation and apoptosis induction
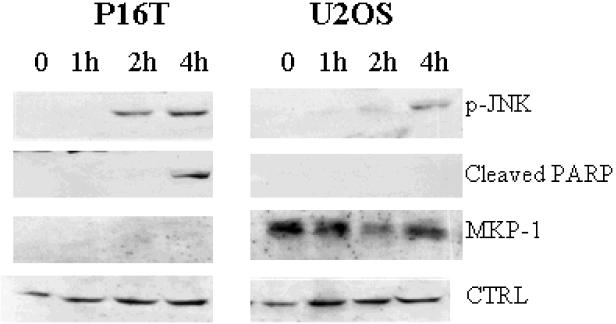
To determine the relationship between cisplatin-induced JNK activation and cell death in OS, we evaluated the phosphorylation status of JNK and cleavage of PARP in response to cisplatin treatment in U2OS and P16T cells. Delay of JNK phosphorylation as well as PARP cleavage was observed in U2OS cells when compared to P16T cells (Figure 4). This suggests that delayed JNK activation is associated with delayed apoptosis induction and relative resistance to cisplatin in U2OS cells.
Figure 4. JNK activation and PRAR cleavage following cisplatin treatment.
P16T and U2OS cells were treated with cisplatin at 50μg/ml for 1, 2 & 4 hours. JNK phosphorylation (p-JNK) was undetectable in both P16T and U2OS cells prior to cisplatin treatment, but became evident at 2 hours after cisplatin treatment in P16T cells followed by PARP cleavage at 4 hours. In U2OS cells, p-JNK was not observed until 4 hours after cisplatin treatment and no PARP cleavage detected. MKP-1 expression was detected throughout the course in U2OS but not in P16T cells. p-JNK, cleaved PARP and MKP-1 were determined by Western blot. CTRL was a loading control.
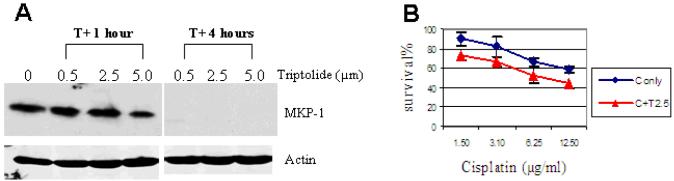
Inhibition of MKP-1 expression by triptolide enhances cisplatin-induced cell death in OS cells
Triptolide has been reported to inhibit MKP-1 expression in immortalized murine alveolar macrophages and macrophage cell line RAW 264.7[23,24]. However, its effect on the MAPK pathways in tumor cells has not been previously studied. We have demonstrated that MKP-1 silencing by siRNA enhances cisplatin-induced cell death and renders U2OS cells more sensitive to cisplatin (Figure 2). We expect that triptolide, by inhibiting MKP-1 expression, may enhance cisplatin-induced cell death in high MKP-1-expressing OS cells. To this end, MKP-1 expression, prior to and following triptolide treatment, in U2OS cells was examined. While MKP-1 expression was evident before triptolide treatment in U2OS cells, it became undetectable after four hours of treatment (Figure 5A). This indicates that triptolide is a potent MKP-1 inhibitor in OS cells. We then compared cisplatin-induced cell death in the presence and absence of triptolide. Indeed, significantly increased cytotoxicity was observed with the combination of cisplatin and triptolide compared to cisplatin alone (Figure 5B). These findings again suggest that MKP-1 inhibition may enhance cisplatin-induced cell death in OS.
Figure 5. MKP-1 expression and cisplatin cytotoxicity following triptolide treatment.
A: MKP-1 expression following triptolide treatment. U2OS cells were incubated in the presence of triptolide at 0.5, 2.5 and 5.0 μM for 1 and 4 hours. While MKP-1 expression was essentially unchanged after 1 hour of triptolide treatment, it became undetectable after 4 hours of treatment. Decreased MKP-1 level was also observed after 2 hours of treatment with 2.5 μM of triptolide (data not shown). Actin was a loading control. B: Cisplatin cytotoxicity following triptolide treatment. U2OS cells were first incubated in the presence of 2.5 μM of triptolide or medium. Two hours later, the triptolide-containing medium was replaced with fresh medium and cisplatin was added at indicated concentrations for a 24 hour MTT assay. Increased cisplatin cytotoxicity was observed when U2OS cells were exposed to the combination of triptolide and cisplatin compared to cisplantin alone. C only: Cisplatin alone, C+T 2.5: cisplatin and 2.5 μM of triptolide.
Discussion
Cisplatin is effective against a variety of solid tumors including OS, neuroblastoma, and medulloblastoma. One of the drawbacks of cisplatin treatment is development of resistance. The mechanism underlying this resistance is incompletely understood. In the current study, we have chosen OS as a model to study cisplatin resistance because of the important role cisplatin plays in the treatment of OS. We showed that MKP-1 highexpressing U2OS cells are relatively more resistant to cisplatin-induced cell death compared to MKP-1 low-expressing P16T cells. The association of high MKP-1 expression and increased cisplatin resistance implies that MKP-1 could be an important determinant of cisplatin resistance. However, since each cell line may have different genetic alterations that could also affect cisplatin sensitivity, the relationship of MKP-1 expression and cisplatin sensitivity in different cell lines may not fully reflect the role of MKP-1 expression in cisplatin resistance. To this end, MKP-1 siRNA silencing in U2OS cells was performed to minimize the effect of other genetic variations on cisplatin sensitivity. The results showed that MKP-1 knockdown sensitizes osteosarcoma cells to cispaltin-induced cell death. Furthermore, decreased MKP-1 expression and increased cisplatin-induced cell death was observed when the OS cells were pre-treated with triptolide, a recently identified MKP-1 inhibitor. These data suggest that high level of MKP-1 expression contributes to cisplatin resistance of OS.
MKP-1 dephosphorylates JNK and negatively regulates its activity[11,25]. Since JNK has been shown to be an important mediator of cisplatin-mediated killing, it has been postulated that inhibition of JNK activation is one of the mechanisms underlying the MKP-1 mediated cisplatin resistance[25]. The results of current study are in support of that hypothesis, as high MKP-1 expression in U2OS cells is associated with delayed JNK activation and apoptosis induction alone with decreased cytotoxicity induced by cisplatin. We have used clinically applicable concentrations of cisplatin in the current study to measure cisplatin-induced cell death and apoptosis induction in order to mimic in vivo condition as much as possible. However, no persistent JNK activation was observed when the cells were treated with 5μg/ml of cisplatin, therefore, a higher concentration was used for these experiments as previously described in the literature [14]. In order not to miss the peak effect of transient knock down of MKP-1 expression in the siRNA assay, higher concentrations of cisplatin were also used to demonstrate difference in cisplatin-induced killing between the control and transfected cells in a 24 hour MTT assay.
In summary, we have shown that high MKP-1 expressing OS cells are more resistant to cisplatin and MKP-1 inhibition enhances cisplatin-induced cell death. Therefore, MKP-1 could potentially be used as a marker of cisplatin resistance, and the combination of MKP-1 inhibitors and cisplatin could be a novel strategy to overcome cisplatin resistance. The current study is focused on OS; however, the result may have implication beyond OS based on the broad application of cisplatin in the treatment of other pediatric and adult solid tumors, such as neuroblastoma.
Acknowledgements
We thank Michael U. Callaghan (Children's Hospital of Michigan) and Andrew M. Fribley (University of Michigan) for their critical reading of this manuscript. This work was supported by Kovan Fund, NIH grant R01 CA100073 (G.S.W.) and new investigator grant of Children's Research Center of Michigan (Z.J.W.). Z.J.W. was a recipient of Amgen Oncology Fellowship. Y.R. is supported by Georgie Ginopolis Endorsement Chair for Pediatric Cancer and Hematology.
References
- 1.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4(4):307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 2.Brozovic A, Osmak M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett. 2006 doi: 10.1016/j.canlet.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 3.Gorlick R, Anderson P, Andrulis I, et al. Biology of childhood osteogenic sarcoma and potential targets for therapeutic development: meeting summary. Clin Cancer Res. 2003;9(15):5442–5453. [PubMed] [Google Scholar]
- 4.Pizzo PA, Poplack DG. Principle and Practice of Pediatric Oncology. Lippincott Williams & Wilkins; Philadelphia, PA: 2006. [Google Scholar]
- 5.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 6.Man TK, Chintagumpala M, Visvanathan J, et al. Expression profiles of osteosarcoma that can predict response to chemotherapy. Cancer Res. 2005;65(18):8142–8150. doi: 10.1158/0008-5472.CAN-05-0985. [DOI] [PubMed] [Google Scholar]
- 7.Goorin AM, Harris MB, Bernstein M, et al. Phase II/III trial of etoposide and high-dose ifosfamide in newly diagnosed metastatic osteosarcoma: a pediatric oncology group trial. J Clin Oncol. 2002;20(2):426–433. doi: 10.1200/JCO.2002.20.2.426. [DOI] [PubMed] [Google Scholar]
- 8.Provisor AJ, Ettinger LJ, Nachman JB, et al. Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol. 1997;15(1):76–84. doi: 10.1200/JCO.1997.15.1.76. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Perez I, Murguia JR, Perona R. Cisplatin induces a persistent activation of JNK that is related to cell death. Oncogene. 1998;16(4):533–540. doi: 10.1038/sj.onc.1201578. [DOI] [PubMed] [Google Scholar]
- 10.Cano E, Mahadevan LC. Parallel signal processing among mammalian MAPKs. Trends Biochem Sci. 1995;20(3):117–122. doi: 10.1016/s0968-0004(00)88978-1. [DOI] [PubMed] [Google Scholar]
- 11.Wu GS. Role of mitogen-activated protein kinase phosphatases (MKPs) in cancer. Cancer Metastasis Rev. 2007 doi: 10.1007/s10555-007-9079-6. [DOI] [PubMed] [Google Scholar]
- 12.Losa JH, Parada Cobo C, Viniegra JG, et al. Role of the p38 MAPK pathway in cisplatin-based therapy. Oncogene. 2003;22(26):3998–4006. doi: 10.1038/sj.onc.1206608. [DOI] [PubMed] [Google Scholar]
- 13.Seki K, Yoshikawa H, Shiiki K, et al. Cisplatin (CDDP) specifically induces apoptosis via sequential activation of caspase-8, -3 and -6 in osteosarcoma. Cancer chemotherapy and pharmacology. 2000;45(3):199–206. doi: 10.1007/s002800050030. [DOI] [PubMed] [Google Scholar]
- 14.Wang Z, Xu J, Zhou JY, et al. Mitogen-Activated Protein Kinase Phosphatase-1 Is Required for Cisplatin Resistance. Cancer Res. 2006;66(17):8870–8877. doi: 10.1158/0008-5472.CAN-06-1280. [DOI] [PubMed] [Google Scholar]
- 15.Yan M, Dai T, Deak JC, et al. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372(6508):798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]
- 16.Small GW, Shi YY, Higgins LS, et al. Mitogen-activated protein kinase phosphatase-1 is a mediator of breast cancer chemoresistance. Cancer Res. 2007;67(9):4459–4466. doi: 10.1158/0008-5472.CAN-06-2644. [DOI] [PubMed] [Google Scholar]
- 17.Isfort RJ, Cody DB, Lovell G, et al. Analysis of oncogenes, tumor suppressor genes, autocrine growth-factor production, and differentiation state of human osteosarcoma cell lines. Mol Carcinog. 1995;14(3):170–178. doi: 10.1002/mc.2940140306. [DOI] [PubMed] [Google Scholar]
- 18.Inaba H, Glibetic M, Buck S, et al. Interferon-gamma sensitizes osteosarcoma cells to Fas-induced apoptosis by up-regulating Fas receptors and caspase-8. Pediatric blood & cancer. 2004;43(7):729–736. doi: 10.1002/pbc.20151. [DOI] [PubMed] [Google Scholar]
- 19.Ozgen U, Savasan S, Buck S, et al. Comparison of DiOC(6)(3) uptake and annexin V labeling for quantification of apoptosis in leukemia cells and non-malignant T lymphocytes from children. Cytometry. 2000;42(1):74–78. doi: 10.1002/(sici)1097-0320(20000215)42:1<74::aid-cyto11>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 20.Savasan S, Buck S, Ozdemir O, et al. Evaluation of cytotoxicity by flow cytometric drug sensitivity assay in childhood T-cell acute lymphoblastic leukemia. Leukemia & lymphoma. 2005;46(6):833–840. doi: 10.1080/10428190500079951. [DOI] [PubMed] [Google Scholar]
- 21.Peng B, English MW, Boddy AV, et al. Cisplatin pharmacokinetics in children with cancer. Eur J Cancer. 1997;33(11):1823–1828. doi: 10.1016/s0959-8049(97)00341-9. [DOI] [PubMed] [Google Scholar]
- 22.Veal GJ, Dias C, Price L, et al. Influence of cellular factors and pharmacokinetics on the formation of platinum-DNA adducts in leukocytes of children receiving cisplatin therapy. Clin Cancer Res. 2001;7(8):2205–2212. [PubMed] [Google Scholar]
- 23.Zhao Q, Shepherd EG, Manson ME, et al. The role of mitogen-activated protein kinase phosphatase-1 in the response of alveolar macrophages to lipopolysaccharide: attenuation of proinflammatory cytokine biosynthesis via feedback control of p38. J Biol Chem. 2005;280(9):8101–8108. doi: 10.1074/jbc.M411760200. [DOI] [PubMed] [Google Scholar]
- 24.Chen TH, Kao YC, Chen BC, et al. Dipyridamole activation of mitogen-activated protein kinase phosphatase-1 mediates inhibition of lipopolysaccharide-induced cyclooxygenase-2 expression in RAW 264.7 cells. European journal of pharmacology. 2006;541(3):138–146. doi: 10.1016/j.ejphar.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Perez I, Martinez-Gomariz M, Williams D, et al. CL100/MKP-1 modulates JNK activation and apoptosis in response to cisplatin. Oncogene. 2000;19(45):5142–5152. doi: 10.1038/sj.onc.1203887. [DOI] [PubMed] [Google Scholar]