Abstract
Objective
Physical activity has been shown to enhance quality of life, however, few investigations of these effects exist in women undergoing the menopausal transition. The present study examined the long-term effects of physical activity on menopause-related quality of life (QOL) and tested the mediating effects of physical self-worth and positive affect in this relationship.
Design
Middle-aged women previously enrolled in a 4-month randomized controlled trial involving walking, yoga, and a control group completed a follow-up mail-in survey two years following the end of the trial. The survey included a battery of psychological and physical activity measures, including measures of menopausal symptoms and menopause-related quality of life. Longitudinal linear panel analysis was conducted within a covariance modeling framework to test whether physical self-worth and positive affect mediated the physical activity - quality of life relationship over time.
Results
At the end of the trial, physical activity and menopausal symptoms were related to physical self-worth and positive affect, and in turn, greater levels of physical self-worth and positive affect were associated with higher levels of menopause-related QOL. Analyses indicated that increases in physical activity and decreases in menopausal symptoms over the 2-year period were related to increases in physical self-worth (βs = .23 and −.52) and for symptoms also to decreased positive affect (β = −.47), and both physical self-worth (β = .34) and affect (β = .43) directly influenced enhancements in QOL (R2 = .775).
Conclusions
The findings support the position that physical activity effects on QOL are in part mediated by intermediate psychological outcomes and that physical activity can have long-term benefits for women undergoing the menopausal transition.
Keywords: Quality of Life, Menopause, Symptoms, Hot Flashes, Physical Activity, Affect, Self-Esteem
Although the menopausal transition is part of the normal aging process, the hormonal changes occurring at this stage of life alter the health-risk profile of women1–3 and manifest acutely in the form of vasomotor symptoms such as hot flashes and night sweats. The menopausal transition may also be a source of psychological distress or instability, although certain subgroups of women may be more vulnerable to such adverse outcomes than others4–6. Collectively, these adverse physical and mental health changes may negatively impact quality of life (QOL) as women transition through menopause. Whether quality of life is impacted during menopause however depends on multiple factors including symptoms and other physical health, psychosocial, lifestyle, and contextual variables7–9. Menopause has been found to have the most dramatic effect on QOL during the peri- and early-postmenopausal stage (especially in symptomatic women), although it has been suggested that this effect depends on the QOL measure used10.
Several reviews of existing measures have been published11–14 illustrating three general approaches to the assessment of QOL in the menopause-related literature: (1) to evaluate menopausal symptoms and infer QOL from them, (2) to focus on the assessment of functional/health status under the health-related quality of life (HRQL) umbrella term, or (3) to evaluate QOL at the psychological construct level (i.e., as a sense of well-being either at the global construct level or as perceptions of one’s life across different domains). There are obvious limitations associated with the first two approaches as both seem to imply that women experiencing menopausal symptoms or women with compromised functional/health status must also have poor QOL. It has been pointed out by others that both health status (objectively defined or perceived) and QOL represent two distinct constructs 15 and that even individuals with considerable physical or health limitations report high satisfaction with life 16. The menopausal transition is a normal developmental process in the lives of women and equating it with a disease state or pathology is inappropriate. Indeed, when QOL of life is assessed under the health-related QOL umbrella term, no observable adverse effects have generally been found in nationally representative data, once adjusted for health status and menopausal symptoms7, or in response to treatment such as hormone therapy 9, 17.
Far fewer studies have conceptualized QOL at the psychological construct level, viewing it as a generalized sense of well-being. The Utian Quality of Life Score (UQOL)18 is a recent attempt at constructing a QOL measure targeting domains of life that tend to suffer during menopausal transition and are distinct from menopausal symptoms. The scale has been used in several studies in a variety of contexts 19–22. In a previous study, we reported the effects of 4-month walking and yoga interventions and a control group on menopause-related QOL assessed by the UQOL measure 19. We found that both walking and yoga improved QOL, especially the health and emotional QOL domains for the walking and the sexual QOL domain for the yoga group. Regardless of treatment condition, women who reported reductions in menopausal symptoms across the intervention exhibited greater improvements in QOL as compared to women whose symptoms increased over that time. We observed the same pattern for other psychological outcomes including positive affect in the same study and demonstrated positive effects of the intervention on aspects of physical self-esteem in a separate study 23.
Although the general physical activity literature supports consistently positive effects of exercise and physical activity participation on psychological well-being and QOL in a variety of populations, few investigations of these effects exist in women undergoing the menopausal transition. There are several reviews of the general physical activity and QOL literature 24–27, altogether suggesting that physical activity may have both direct and indirect effect on QOL. Several physical activity outcomes have been identified as potential mediators of the physical activity and QOL relationship, including outcomes such as positive affect and self-esteem 28, but these have not been directly tested in women undergoing the menopausal transition. The purpose of this study was to examine the longitudinal associations among physical activity, symptoms, and menopause-related QOL in a sample of middle-aged women previously enrolled in a randomized controlled exercise trial. The women were contacted two years following the end of the trial and completed a mail-in survey. Using a covariance modeling framework, a longitudinal panel model was tested. It was hypothesized that over time physical activity effects on menopause-related QOL would be mediated by positive affect and physical self-worth (i.e., self-esteem specific to the physical domain). Additionally, menopausal symptoms were expected to be associated with menopause-related QOL both directly and indirectly through its effects on positive affect and physical self-worth.
METHOD
Participants
The sample selection for the randomized controlled exercise trial and its results have been published previously 19, 23, 29. At study entry, the participants (N = 164) were sedentary or low active (i.e., exercising less than 2 times per week for 30 min or more at moderate intensity), middle-aged women (42–58 yrs.) experiencing menopausal symptoms (i.e., having experienced vasomotor symptoms such hot flashes or nights sweats in the last month). Additional inclusion criteria included no history of surgical menopause and no hormone therapy (HT) use in the last six months. The recruitment resulted in a sample of relatively healthy and primarily white women (83%), the majority of whom were married or in significant relationships (75%), had college education (64%), and above average income (67%). Based on self-reported menstrual bleeding patterns at baseline of the exercise trial 17% of women were categorized as pre-menopausal, 41% as peri-menopausal (28% in early and 23% in late peri-menopause), and 32% as post-menopausal (12% in early and 20% in late post-menopause). The majority of women in the sample were overweight or obese (70% of the women had BMI ≥ 25 kg/m2 with mean value of 29.67, SD = 7.06) and the overall retention rate in the trial was 90%. Out of the 164 women originally enrolled in the study, complete end-of-program data were available for 134 women, and 102 women agreed to take part in the two-year follow-up survey. Only 99 out of the 134 women ultimately returned their follow-up questionnaires, for an overall response rate of ~ 74%.
Measures
Background information
Basic demographic and health history information was collected including menopausal status which was assessed based on self-reported bleeding patterns and categorized according to the Stages of Reproductive Aging Workshop (STRAW) criteria 32 into pre-menopausal, early and late peri-menopausal, and early and late post-menopausal stages. Specifically, during initial telephone screening women were asked about their menstrual bleeding patterns and subsequently completed a questionnaire which presented several yes/no statements (e.g., I have had normal menses during the last 12 months) corresponding to the STRAW menopausal categories.
Menopausal symptoms
Menopausal symptoms were assessed by the Greene Climacteric Scale (GCS) 11. The GCS assesses the degree to which a woman is bothered by psychological (11 items), somatic (7 items), vasomotor (2 items), and sexual (1 item) symptoms on a 0–3 scale from not at all to extremely. Internal consistency of the scale was acceptable in the present study (α = .81–.86).
Physical activity and body mass index
Physical activity outside of the program was assessed by self-report utilizing the Aerobics Center Longitudinal Study Physical Activity Survey (ACLS) 31. The ACLS questionnaire assesses frequency, duration, and intensity of 14 different physical activities and allows for calculation of metabolic equivalents of energy expenditure in reported activities. Physical activity was entered into the model as weekly energy expenditure in leisure-time physical activity only (i.e., household and lawn work/gardening activities were excluded) and expressed in MET hours per week. In addition to physical activity, body mass index was computed from weight and height measured in the lab at the end of the trial and from self-reported weight and height in the follow-up survey.
Affect
Positive and negative affect was assessed using Affectometer 2 32. This is a 40-item self-report measure of general happiness based on the balance of positive and negative feelings in recent experience. There was a significant correlation between positive and negative affect at both time points (rs = −.78 and −.75 at the end of trial and 2-year follow-up, respectively), thus to avoid collinearity, only positive affect was included in the tested model. However, the pattern of relationships was very similar when the model was tested with negative affect instead. Internal consistencies of the scale at both time points exceeded .90.
Physical self-worth
The Physical Self-Worth scale (PSW) of the Physical Self-Perception Profile33 was used to assess self-esteem relative to the physical self. This scale is composed of six items assessing general feelings of pride, happiness, and satisfaction in the physical domain. Responses are indicated on a 4-point scale reflecting the degree to which each item was characteristic or true of them. Responses range from 1 (not at all true) to 4 (completely true), with a typical item being “I am extremely proud of who I am and what I can do physically.” Internal consistencies for the PSW in this study exceeded .80 at both time points.
Quality of life
The Utian Quality of Life Scale (UQOL)18 was used to assess menopause-specific QOL in this study. The scale has 23 items targeting occupational, health, emotional, and sexual QOL domains. Each question is answered on a 5-point Likert-type scale. A total score is computed by summing all domain scores, resulting in a possible score range of 0–115. Internal consistency of the total scale was acceptable in the present study (α = .85 − .86).
Demographic information was assessed at baseline of the randomized controlled trial and the remaining measures were administered again at the end of the trial and at two-year follow-up.
Procedure
All 164 women originally enrolled in the randomized controlled exercise trial were contacted two years following the end of the trial. All participants received a letter announcing the follow-up study and were contacted by telephone within two weeks of receiving the letter. The women who agreed to take part in the research were mailed a packet of questionnaires with pre-paid postage and self-addressed return envelope. An incentive to participate was offered in the form of a lottery for one of four $250 cash prizes. Participants were contacted at least three times by telephone with a reminder to complete the questionnaire and return it. The data were collected between January and November 2007 to correspond with the timing of women completing the intervention which took place in waves.
Statistical analysis
Demographic characteristics were compared between respondents and non-respondents using independent sample t-tests or chi-square tests in the case of categorical variables using the SPSS 16.0 statistical software package.
The data were subsequently analyzed using structural equation modeling (SEM) with the full-information maximum likelihood (FIML) estimator in Mplus 5.1 34. FIML was selected because there were missing data, and the full-information estimator is an optimal method for the treatment of missing data in SEM 35–37. For the 134 women who completed the psychosocial measures at the end of the 4-month trial, there was 4.5% of symptom and 5.2% of QOL data missing (representing women who chose to omit questions related to sexual symptoms and sexual well-being). At the two-year follow-up, the data were missing for 26.9% (positive affect), 28.4% (symptoms), 26.1% (physical self-worth and physical activity), and 31.3% (QOL) of the 134 women.
Subsequently, a longitudinal panel model hypothesizing relationships among physical activity, symptoms, positive affect, physical self-worth, and QOL was tested. A longitudinal panel analysis framework enables an examination of both cross-sectional and longitudinal relationships simultaneously. The longitudinal component of the analysis tests relationships among changes in variables over time while controlling for the initial values of study variables by way of estimating stability coefficients. The tested model specified hypothesized paths between: (a) changes in physical activity and symptoms and changes in physical self-worth and positive affect; (b) changes in physical self-worth and positive affect and changes in QOL; and (c) changes in symptoms and changes in QOL. In addition, stability coefficients 38 were calculated for each variable over time, correlations among exogenous variables were allowed, and uniquenesses among the endogenous variables were correlated.
Model-data fit was assessed using standard indices: the chi-square statistic 38; the standardized root mean square residual (SRMR)39 (the SRMR should approximate or be less than .08 for a good fitting model40); the root mean square error of approximation (RMSEA) (values approximating .06 or less are indicative of a close fit 41); comparative fit index (CFI) 42(the CFI should approximate 0.95 or greater for a good fitting model 40). All path coefficients and correlations are reported as standardized estimates.
RESULTS
Sample description
No statistically significant differences were detected in demographic characteristics between follow-up responders and non-responders. As with the original sample, the women who completed the follow-up were mostly white (87.9%), well-educated (68.7% with college or university degree), and with annual family income above $40,000 (79.8%). At the two-year follow-up, the distribution of self-reported menopausal status was as follows: 12% pre-menopausal, 11% early peri-menopausal; 22% late peri-menopausal, 17% early post-menopausal, 37% late post-menopausal.
Means and standard deviations for all variables across both time points are presented in Table 1. Bivariate correlations among all variables are also presented in Table 1.
Table 1.
Bivariate correlations among all variables at the end-of-trial and two-year follow-up
| Variable | Descriptives | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| End-of-trial assessment | M | SD | ||||||||||
| 1. UQOL | 82.51 | 12.48 | 1.00 | |||||||||
| 2. Physical self-worth | 13.11 | 3.95 | 0.47** | 1.00 | ||||||||
| 3. Positive affect | 72.73 | 12.33 | 0.72** | 0.38** | 1.00 | |||||||
| 4. Symptoms | 10.73 | 6.55 | −0.56** | −0.30** | −0.55** | 1.00 | ||||||
| 5. Physical activity | 7.28 | 8.87 | 0.17 | 0.19* | 0.21* | −0.09 | 1.00 | |||||
| 2-year follow-up assessment | ||||||||||||
| 6. UQOL | 81.12 | 13.97 | 0.59** | 0.47** | 0.42** | −0.24* | 0.06 | 1.00 | ||||
| 7. Physical self-worth | 12.70 | 4.14 | 0.31** | 0.72** | 0.15 | −0.15 | −0.03 | 0.66** | 1.00 | |||
| 8. Positive affect | 73.01 | 13.62 | 0.49** | 0.40** | 0.53** | −0.31** | 0.11 | 0.78** | 0.46** | 1.00 | ||
| 9. Symptoms | 12.80 | 7.21 | −0.37** | −0.37** | −0.39** | 0.51** | −0.13 | −0.65** | −0.49** | −0.62** | 1.00 | |
| 10. Physical activity | 11.03 | 10.78 | 0.29** | 0.11 | 0.19 | 0.02 | 0.35** | 0.36** | 0.27** | 0.31** | −0.28** | 1.00 |
Note. * p < .05 ** p < .01; UQOL = Utian Quality of Life Scale.
Examination of the hypothesized relationships within the panel model
The initial test of the model indicated an acceptable fit (χ2 = 54.034, df = 22, p < 0.001; SRMR = 0.082, RMSEA = 0.104; CFI = 0.937). Although the value of the chi-square was statistically significant, the SRMR and CFI values approached good model-data fit 40. The model modification indices suggested the model can be improved by allowing physical self-worth residuals to co-vary across the time points. The adjusted model resulted in an improved model fit (χ2 = 36.547, df = 20, p < 0.05; SRMR = 0.059, RMSEA = 0.076; CFI = 0.969).
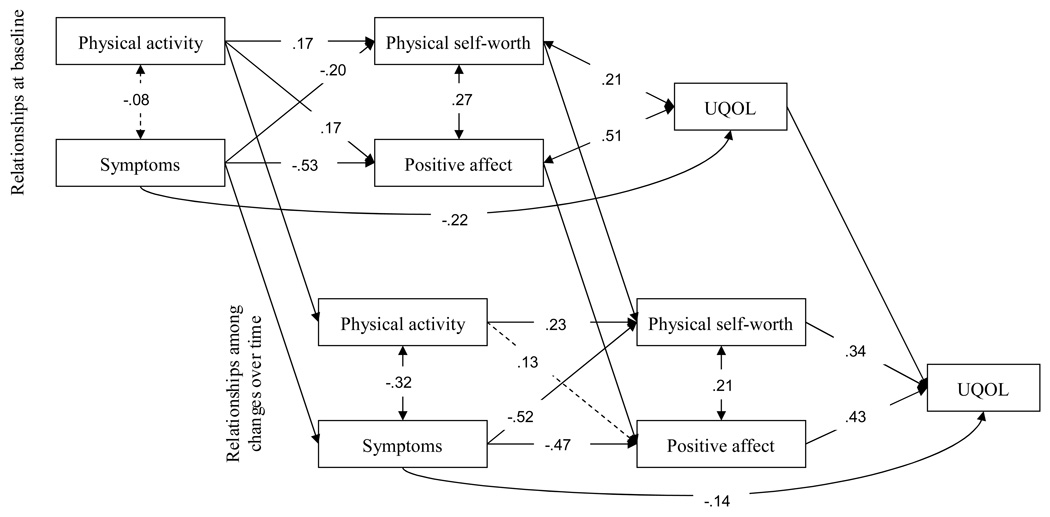
As can be seen in Figure 1, at baseline (the upper panel) there were statistically significant path coefficients (p < .05) for direct effects of physical activity (β = .17) and symptoms (β = −.20) on physical self-worth, and on positive affect (βs = .17 and −.53 for physical activity and symptoms, respectively). In turn, physical self-worth (β = .21) and positive affect (β = .51) were both significantly associated with menopause-related QOL. The relationships among model components over time (i.e., among changes in variables across the two-year period – see the lower panel of Figure 1) revealed a similar pattern. Women reporting greater increases in physical activity (β = .23) and smaller increases in symptoms (−.52) also reported greater increases in physical self-worth. Increases in symptoms were significantly associated with decreases in positive affect (−.47), and increases in both physical self-worth (.34) and positive affect (.43) in turn predicted more positive changes in menopause-related QOL. As noted, stability coefficients (i.e., relationships between the same constructs at the end of the trial and two-year follow-up) were also calculated for all constructs within the panel model. These were all significant but for clarity of presentation are not depicted in Figure 1. The statistical parameters of all paths in the longitudinal model are presented in Table 2.
Figure 1.
Panel model showing mediated effects of physical activity and symptoms on menopause-related quality of life at program end (upper panel) and in changes in these variables across the 2-year follow-up (lower panel). Solid lines represent significant parameter estimates, and dashed lines represent nonsignificant parameter estimates.
Table 2.
Longitudinal panel analysis results of the tested model
| Dependent Variable Predictor |
R2change | β | S.E. | Critical value |
p-value |
|---|---|---|---|---|---|
| Change in physical self-worth | |||||
| Baseline physical self-worth | 0.383 | 0.619 | 0.062 | 9.994 | 0.000 |
| Change in physical activity | 0.054 | 0.232 | 0.102 | 2.284 | 0.022 |
| Change in symptoms | 0.270 | −0.520 | 0.147 | −3.547 | 0.000 |
| Change in positive affect | |||||
| Baseline positive affect | 0.125 | 0.354 | 0.081 | 4.355 | 0.000 |
| Change in physical activity | 0.016 | 0.128 | 0.075 | 1.700 | 0.089 |
| Change in symptoms 0.217 | 0.217 | −0.466 | 0.076 | −6.117 | 0.000 |
| Change in quality of life | |||||
| Baseline quality of life | 0.069 | 0.243 | 0.062 | 3.953 | 0.000 |
| Change in physical self-worth | 0.112 | 0.335 | 0.059 | 5.655 | 0.000 |
| Change in positive affect | 0.181 | 0.426 | 0.068 | 6.244 | 0.000 |
| Change in symptoms | 0.020 | −0.144 | 0.068 | −2.110 | 0.035 |
Overall the model accounted for 77.5% of the variation in changes in menopause-related QOL. Finally, because participants were randomized to three different treatment groups during the original study, we reran the panel model analyses saturating the model for treatment condition. The fit of the model did not change (χ2 = 32.639, df = 16, p < 0.01; SRMR = 0.048, RMSEA = .080; CFI = 0.969) nor was there any change in the direction or the significance of any of the relationships reported above. The hypothesized model was also tested with body mass index (not reported here) but its inclusion failed to improve the model fit and did not change the overall direction of the tested relationships.
DISCUSSION
Physical activity has been shown to have a positive impact on quality of life independent of age, activity and health status 25, 27, 43, 44. Relative to menopausal women, the role of physical activity in enhancing QOL however remains understudied. This study examined the longitudinal associations among physical activity, menopausal symptoms, physical self-worth, positive affect, and menopause-related QOL in a sample of women previously enrolled in a randomized controlled exercise trial. The proposed mediational model was posited on the premise that physical activity affects QOL indirectly through its effects on more proximal physical activity outcomes rather than directly. Support for this approach has been previously demonstrated in both cross-sectional and longitudinal investigations. McAuley et al. 44, 45 have shown both cross-sectionally and longitudinally that in older women physical activity influences global QOL indirectly via the mediation of physical and mental health status and self-efficacy. Another longitudinal study of older adults 28 demonstrated that increasing physical activity improves physical self-worth and positive affect, and that the improvements in affect lead to improvements in QOL. The results were similar in this study, where increases in physical activity were associated with enhancements in menopause-related QOL indirectly via its effects on physical self-worth and positive affect, providing support for our previous cross-sectional findings46.
Interestingly, changes in menopausal symptoms were significantly associated with changes in physical activity across the two-year period and directly associated with menopause-related QOL. It should be pointed out that the measure of menopausal symptoms used in this study reflects an aggregate value for several categories of symptoms (psychological, somatic, vasomotor, sexual). The meditational model fit reasonably well when the analysis was repeated for each symptom category individually (χ2 = 21.901–37.910, SRMR = .052–.070, RMSEA = .026–.079; CFI = .967–.996), with the pattern or relationships largely unchanged. Women who increased their physical activity over time reported decreases in somatic (β = −.21) and psychological (β = −.32) symptoms and there was a trend for a modest reduction in vasomotor symptoms (β = −.12). The literature on the direct relationship between physical activity and vasomotor symptoms in particular has remained controversial, although few longitudinal and experimental investigations of this relationship exist 47. More importantly, very little systematic examination has taken place relative to the effects of chronic exercise adaptations (e.g., improved fitness, reduced body fat, or changing hormonal profiles) on symptoms such as hot flashes, warranting further research in this area.
Clearly, the physical activity – QOL relationship during the menopausal transition is complex and may involve a number of alternative mechanisms. This study emphasized affective and cognitive mechanisms, however, given the well-supported neuroendocrine 48–50, body composition, thermoregulation, and mood 51 effects of exercise and physical activity, a combination of a number of different biopsychosocial factors should be considered in future research. To effectively examine the underlying biopsychosocial mechanisms in a longitudinal context, more sophisticated statistical techniques such as latent variable modeling or latent growth curve analyses may be necessary. Such techniques would provide more powerful tests of the structural relations among various theoretical constructs by allowing for simultaneous comparisons of several competing theoretical models without measurement error.
This study also points out the inadequacy of the symptomatology approach to QOL inference. Although it may be justified to use a measure of symptoms as a QOL proxy in special populations of women (e.g., clinical samples of women seeking care for the management of severe menopausal symptoms that greatly interfere with their daily functioning), it is inappropriate to infer a one-to-one association between reported symptoms and QOL in the general population of women. The direct associations between changes in symptoms and changes in menopause-related QOL were modest and explained only a small portion of variance, clearly indicating that other factors are at play. Presumably, a woman can still perceive her life as being fulfilling and satisfying when experiencing frequent or bothersome hot flashes, for example.
The study’s results must be interpreted in the light of its limitations. The follow-up survey response rate, although in line with other survey research, was less than optimal and subsequently a significant portion of the data had to be estimated, which may have biased our results away from the null hypothesis. Physical activity was measured only by self-report and included estimates for leisure-time activities only, without corroboration of other physical parameters such as fitness or body composition. The inclusion of body mass index in the model however did not improve the model fit and had little impact on the structural relationships. Although objective physical activity monitoring was included for a sub-sample of the follow-up respondents, the data were not available at both time points and could not be entered into the model. The correlation between self-reported energy expenditure and objectively measured physical activity in this study was moderate (rs = .40–.54 for activity of different intensities) but corresponds with values reported in the general physical activity literature. Finally, the model tested did not consider the influence of other potential confounding factors and the sample was rather homogeneous. Thus, caution must be applied when generalizing these results.
CONCLUSION
The results of this study indicate that increasing physical activity may enhance menopause-related quality of life, albeit indirectly via its effects on physical self-worth and menopausal symptoms. When designing physical activity interventions, researchers and practitioners should incorporate strategies that help enhance women’s physical self-perceptions and optimize symptom management as ways to maximize improvements in quality of life. This is the first study to demonstrate such relationships longitudinally in menopausal women, awaiting further corroboration with more diverse samples of menopausal women.
Acknowledgment
The project described was supported by Grant Number K 12HD055882, "Career Development Program in Women's Health Research at Penn State," from the National Institute of Child Health and Human Development (PI: Elavsky) and the National Institute on Aging under Award No. AG12113 (PI: McAuley). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Child Health and Human Development or the National Institutes of Health.
The author would like to acknowledge Dr. Edward McAuley who assisted with the statistical analysis and provided feedback on the draft of the manuscript.
REFERENCES
- 1.Homko CJ, Trout K. Women and diabetes. Nurs Clin N Am. 2006;41:549–565. doi: 10.1016/j.cnur.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 2.Beaufrere B, Morio B. Fat and protein redistribution with aging: metabolic considerations. Eur J Clin Nutr. 2000;54 Suppl 3:S48–S53. doi: 10.1038/sj.ejcn.1601025. [DOI] [PubMed] [Google Scholar]
- 3.Carey DG, Jenkins AB, Campbell LV, Freund J, Chisholm DJ. Abdominal fat and insulin resistance in normal and overweight women: Direct measurements reveal a strong relationship in subjects at both low and high risk of NIDDM. Diabetes. 1996;45(5):633–638. doi: 10.2337/diab.45.5.633. [DOI] [PubMed] [Google Scholar]
- 4.Joffe H, Hall JE, Soares CN, et al. Vasomotor symptoms are associated with depression in perimenopausal women seeking primary care. Menopause. 2002;9(6):392–398. doi: 10.1097/00042192-200211000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Schilling C, Gallicchio L, Miller SR, Langenberg P, Zacur H, Flaws JA. Relation of body mass and sex steroid hormone levels to hot flushes in a sample of midlife women. Climacteric. 2007;10(1):27–37. doi: 10.1080/13697130601164755. [DOI] [PubMed] [Google Scholar]
- 6.Whiteman MK, Staropoli CA, Langenberg PW, McCarter RJ, Kjerulff KH, Flaws JA. Smoking, body mass, and hot flashes in midlife women. Obstet Gynecol. 2003;101(2):264–272. doi: 10.1016/s0029-7844(02)02593-0. [DOI] [PubMed] [Google Scholar]
- 7.Avis NE, Ory MG, Mathews KA, Shocken M, Bromberger J, Colvin A. Health-related quality of life in a multiethnic sample of middle-aged women. Medical Care. 2003;41(11):1262–1276. doi: 10.1097/01.MLR.0000093479.39115.AF. [DOI] [PubMed] [Google Scholar]
- 8.Dennerstein L, Helmes E. The menopausal transition and quality of life: Methodologic issues. Quality of Life Research. 2000;9(6) Suppl S:721–731. [Google Scholar]
- 9.Hlatky MA, Boothroyd D, Vittinghoff E, Sharp P, Whooley MA. Quality-of-life and depressive symptoms in postmenopausal women after receiving hormone therapy: Results from the Heart and Estrogen/Progestin Replacement Study (HERS) trial. Jama. 2002;287(5):591–597. doi: 10.1001/jama.287.5.591. [DOI] [PubMed] [Google Scholar]
- 10.Utian W. Quality of life (QOL) in menopause. Maturitas. 2007;57(1):100–102. doi: 10.1016/j.maturitas.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 11.Greene JG. Constructing a standard climacteric scale. Maturitas. 1998;29(1):25–31. doi: 10.1016/s0378-5122(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 12.Schneider HPG. The quality of life in the post-menopausal woman. Best Pract Res Clin Obstet Gynaecol. 2002;16(3):395–409. doi: 10.1053/beog.2002.0289. [DOI] [PubMed] [Google Scholar]
- 13.Schneider HPG, Schultz-Zehden B, Rosemeier HP, et al. Assessing well-being in menopausal women. In: Studd J, editor. The Management of the Menopause - the Millenium Review 2000. New York, London: Parthenon Publishing; 2000. pp. 11–19. [Google Scholar]
- 14.Zollner YF, Acquadro C, Schaefer M. Literature review of instruments to assess health-related quality of life during and after menopause. Qual Life Res. 2005;14(2):309–327. doi: 10.1007/s11136-004-0688-z. [DOI] [PubMed] [Google Scholar]
- 15.Smith KW, Avis NE, Assmann SF. Distinguishing between quality of life and health status in quality of life research: a meta-analysis. Qual Life Res. 1999;8(5):447–459. doi: 10.1023/a:1008928518577. [DOI] [PubMed] [Google Scholar]
- 16.Thomas DR. The critical link between health-related quality of life and age-related changes in physical activity and nutrition. J Gerontol A Biol Sci Med Sci. 2001;56(10):M599–M602. doi: 10.1093/gerona/56.10.m599. [DOI] [PubMed] [Google Scholar]
- 17.Brunner RL, Gass M, Aragaki A, et al. Effects of conjugated equine estrogen on health-related quality of life in postmenopausal women with hysterectomy: results from the Women's Health Initiative Randomized Clinical Trial. Arch Intern Med. 2005;165(17):1976–1986. doi: 10.1001/archinte.165.17.1976. [DOI] [PubMed] [Google Scholar]
- 18.Utian W, Janata JW, Kingsberg SA, Schluchter M, Hamilton JC. The Utian Quality of Life (UQOL) Scale: development and validation of an instrument to quantify quality of life through and beyond menopause. Menopause. 2002;9(6):402–410. doi: 10.1097/00042192-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 19.Elavsky S, McAuley E. Physical activity and mental health outcomes during menopause: A randomized controlled trial. Ann Behav Med. 2007;33(2):132–142. doi: 10.1007/BF02879894. [DOI] [PubMed] [Google Scholar]
- 20.Koundi KL, Christodoulakos GE, Lambrinoudaki IV, et al. Quality of life and psychological symptoms in Greek postmenopausal women: association with hormone therapy. Gynecol Endocrinol. 2006;22(12):660–668. doi: 10.1080/09513590601010557. [DOI] [PubMed] [Google Scholar]
- 21.Smith AJ, Hall DR, Grové D. Postmenopausal hormone therapy and quality of life. Int J Gynaecol Obstet. 2006;95(3):267–271. doi: 10.1016/j.ijgo.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 22.Utian WH, Janata JW, Barbier S, Rosen AS, Mayer MH, Taylor MB. Effect of raloxifene on quality of life: a prospective study using the Utian Quality of Life (UQOL) Scale. Menopause. 2004;11(3):275–280. doi: 10.1097/01.gme.0000109295.37664.0e. [DOI] [PubMed] [Google Scholar]
- 23.Elavsky S, McAuley E. Exercise and self-esteem in menopausal women: A randomized controlled trial involving walking and yoga. Am J Health Promot. 2007;22(2):83–92. doi: 10.4278/0890-1171-22.2.83. [DOI] [PubMed] [Google Scholar]
- 24.McAuley E, Elavsky S. Physical activity, aging, and quality of life: Implications for measurement. In: Zhu W, Chodzko-Zajko W, editors. Measurement Issues in Aging and Physical Activity. Champaign, IL: Human Kinetics; 2006. pp. 57–68. [Google Scholar]
- 25.Rejeski W, Mihalko SL. Physical activity and quality of life in older adults. J Gerontol A Biol Sci Med Sci. 2001;56A(11SpecIssue):23–35. doi: 10.1093/gerona/56.suppl_2.23. [DOI] [PubMed] [Google Scholar]
- 26.Schechtman KB, Ory MG FICSIT group. The effects of exercise on the quality of life of frail older adults: a preplanned meta-analysis of the FICSIT trials. Ann Behav Med. 2001;23(3):186–197. doi: 10.1207/S15324796ABM2303_6. [DOI] [PubMed] [Google Scholar]
- 27.Spirduso WW, Cronin DL. Exercise dose-response effects on quality of life and independent living in older adults. Med Sci Sports Exerc. 2001;33(6 Suppl):S598–S608. doi: 10.1097/00005768-200106001-00028. discussion S609-510. [DOI] [PubMed] [Google Scholar]
- 28.Elavsky S, McAuley E, Motl RW, et al. Physical activity enhances long-term quality of life in older adults: efficacy, esteem, and affective influences. Ann Behav Med. 2005;30(2):138–145. doi: 10.1207/s15324796abm3002_6. [DOI] [PubMed] [Google Scholar]
- 29.Elavsky S, McAuley E. Lack of perceived sleep improvement after 4-month structured exercise programsQ. Menopause. 2007;14(3):535–540. doi: 10.1097/01.gme.0000243568.70946.d4. [DOI] [PubMed] [Google Scholar]
- 30.Soules MR, Sherman S, Parrott E, et al. Executive summary: stages of reproductive aging workshop (STRAW) Fertil Steril. 2001;76(5):874–878. doi: 10.1016/s0015-0282(01)02909-0. [DOI] [PubMed] [Google Scholar]
- 31.Kohl HW, Blair RS, Paffenbarger J, Macera CA, Kronenfeld JJ. A mail survey of physical activity habits as related to measured physical fitness. Am J Epidemiol. 1988;127:1228–1239. doi: 10.1093/oxfordjournals.aje.a114915. [DOI] [PubMed] [Google Scholar]
- 32.Kammann R, Flett R. Affectometer 2: a scale to measure current level of general happiness. Aust J Psychol. 1983;35:259–265. [Google Scholar]
- 33.Fox KR, Corbin CB. The Physical Self-Perception Profile: Development and preliminary validation. J Sport Exerc Psychol. 1989;11:408–430. [Google Scholar]
- 34.Mplus [computer program]. Version 3.0. Los Angeles: Muthen & Muthen; 1998–2004. [Google Scholar]
- 35.Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced Structural Equation Modeling: Issues and Techniques. Mahwah, NJ: Lawrence Erlbaum Associates; 1996. pp. 243–278. [Google Scholar]
- 36.Enders CK. The impact of nonnormality on full information maximum-likelihood estimation for structural equation models with missing data. Psychol Methods. 2001;6:352–370. [PubMed] [Google Scholar]
- 37.Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–457. [PubMed] [Google Scholar]
- 38.Kessler RC, Greenberg DF. Linear Panel Analysis. Models of Quantitative Change. New York: Academic Press; 1981. [Google Scholar]
- 39.Bollen KA. Structural Equations With Latent Variables. New York: John Wiley & Sons; 1989. [Google Scholar]
- 40.Hu L, Bentler PM. Cutoff criteria for fit indices in covariance structure analysis: conventional versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- 41.Browne MW, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, editors. Testing structural equation models. Newbury Park, CA: Sage; 1993. pp. 136–162. [Google Scholar]
- 42.Bentler PM. Comparative fix indexes in structural models. Psychol Bull. 1990;107:238–246. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- 43.Courneya KS, Friedenreich CM. Physical exercise and quality of life following cancer diagnosis: a literature review. Ann Behav Med. 1999;21(2):171–179. doi: 10.1007/BF02908298. [DOI] [PubMed] [Google Scholar]
- 44.McAuley E, Doerksen SE, Morris KS, et al. Physical activity and quality of life in older women: A longitudinal investigation. Ann Behav Med. doi: 10.1007/s12160-008-9036-9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.McAuley E, Konopack JF, Motl RW, Morris KS, Doerksen SE, Rosengren KR. Physical activity and quality of life in older adults: influence of health status and self-efficacy. Ann Behav Med. 2006;31(1):99–103. doi: 10.1207/s15324796abm3101_14. [DOI] [PubMed] [Google Scholar]
- 46.Elavsky S, McAuley E. Physical activity, symptoms, esteem, and life satisfaction during menopause. Maturitas. 2005;52:374–385. doi: 10.1016/j.maturitas.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 47.Daley A, MacArthur C, Mutrie N, Stokes-Lampard H. Exercise for vasomotor menopausal symptoms. Cochrane Database Syst Rev. 2007;17(4):CD006108. doi: 10.1002/14651858.CD006108.pub2. [DOI] [PubMed] [Google Scholar]
- 48.Chaouloff F. Physical exercise and brain monoamines: a review. Acta Physiol Scand. 1989;137:1–13. doi: 10.1111/j.1748-1716.1989.tb08715.x. [DOI] [PubMed] [Google Scholar]
- 49.Weicker H, Struder HK. Influence of exercise on serotonergic neuromodulation in the brain. Amino Acids. 2001;20:35–47. doi: 10.1007/s007260170064. [DOI] [PubMed] [Google Scholar]
- 50.Janal MN, Colt EW, Clark WC, Glusman M. Pain sensitivity, mood and plasma endocrine levels in man following long-distance running: effects of naloxone. Pain. 1984;19:13–25. doi: 10.1016/0304-3959(84)90061-7. [DOI] [PubMed] [Google Scholar]
- 51.Sternfeld B, Marcus R. Exercise. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: biology and pathobiology. Academic Press; 2000. pp. 495–508. [Google Scholar]