Enantioenriched α-hydroxycarboxylic acid derivatives are not only present as subunits in a range of bioactive compounds, but they also serve as useful intermediates in organic chemistry.[1] Consequently, the development of efficient and versatile methods for their synthesis is an important objective. In the case of α-hydroxycarboxylic acid derivatives wherein the alcohol is tertiary, several catalytic asymmetric routes have been described, e.g., the cyanosilylation of ketones,[2,3] the addition of organometallic reagents to α-ketoesters,[2, 4 ] and the phase-transfer alkylation of oxazolidin-2,4-diones.[5] Nevertheless, the development of methods with broader scope is desirable.[6]
One potential route to enantioenriched α-hydroxycarboxylic acid derivatives is through the ring opening of a 1,2-oxazetidin-3-one, which might be accessed via a catalytic asymmetric [2+2] cycloaddition of a ketene with a nitroso compound (eq 1). However, there are only a handful of investigations of the cycloaddition itself,[7] and there have been no studies of catalysis of the transformation or of enantioselective variants.[8] In this report, we establish that such [2+2] cycloadditions are indeed subject to catalysis and that a planar-chiral DMAP derivative can provide the target 1,2-oxazetidin-3-ones in high ee.
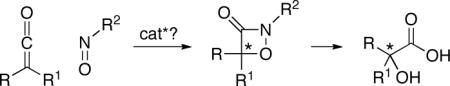 |
(1) |
In previous studies, we established that planar-chiral DMAP derivatives (e.g., 1) can serve as catalysts for [2+2] cycloadditions
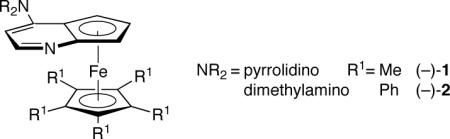 |
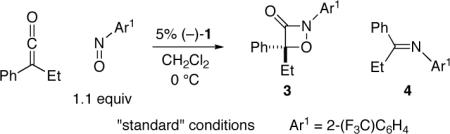
of ketenes with imines, aldehydes, and azo compounds.[9,10] In a preliminary investigation, we determined that 1 also catalyzes the cycloaddition of phenyl ethyl ketene with nitrosobenzene (eq 2).[11] However, a mixture of regioisomers is produced (as is often observed in the uncatalyzed process[12]) favoring the undesired heterocycle, which decarboxylates to afford an imine. In addition to being the minor product, the desired 1,2-oxazetidin-3-one is essentially racemic.
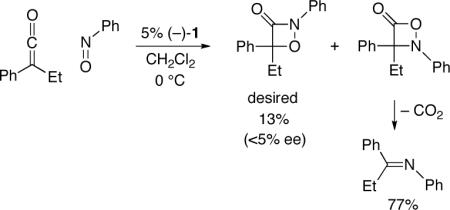 |
(2) |
Although we were pleased to determine that such [2+2] cycloadditions are amenable to catalysis, the regioselectivity and enantioselectivity were disappointing. In our earlier studies of [2+2] cycloadditions of ketenes with imines, aldehydes, and azo compounds, controlling the regioselectivity of the reaction had not been an issue.[9] Nevertheless, relative to enantioselectivity, this appeared to be the more tractable challenge to initially address.
For nucleophile-catalyzed reactions of ketenes with nitrosoarenes, the aromatic group provides a means to rationally modify the steric and electronic properties of the nitroso compound[13] so as to control the propensity of the presumed enolate intermediate to produce zwitterion A vs. B (Figure 1). In particular, we have determined that, by incorporating an electron-withdrawing substituent (CF3) in the ortho position of the aromatic ring, the regioselectivity for the nucleophile-catalyzed cycloaddition of phenyl ethyl ketene can be reversed (1:6→30:1; eq 2 vs. entry 1 of Table 1). Furthermore, the desired 1,2-oxazetidin-3-one is now formed with promising ee.
Figure 1.
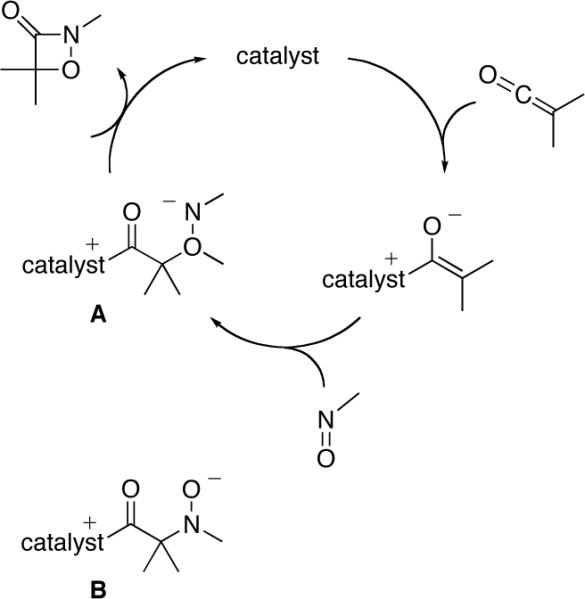
Possible mechanism for the nucleophile-catalyzed cycloaddition of a ketene with a nitrosoarene.
Table 1.
Nucleophile-catalyzed cycloaddition of a ketene with a nitrosoarene: Effect of reaction parameters.
 | ||||
|---|---|---|---|---|
| Entry | Change from the "standard" conditions | Yield of 3 (%)[a] | ee of 3 (%)[b] | Yield of 4 (%)[c] |
| 1 | none | 90 | 79 | 3 |
| 2 | no (−)-1 | 9 | - | 30 |
| 3 | 5% (−)-2, instead of (−)-1 | 16 | −22 | 33 |
| 4 | 5% (+)-5, instead of (−)-1 | 19 | −35 | 61 |
| 5 | 5% (+)-6, instead of (−)-1 | 33 | <2 | 17 |
| 6 | toluene, instead of CH2Cl2 | 89 | 66 | 2 |
| 7 | THF, instead of CH2Cl2 | 71 | 78 | 1 |
| 8 | −20 °C | 81 | 73 | 6 |
| 9 | r.t. | 89 | 76 | 4 |
Determined by 1H NMR spectroscopy versus an internal standard.
A negative ee value signifies that the opposite enantiomer of 3 is formed preferentially.
Yield of isolated product (average of two experiments).
The effects of certain reaction parameters are outlined in Table 1. In the absence of a catalyst, the undesired isomer is the major product (entry 2). An array of other catalysts, including a range of amines and phosphines, are less effective than 1 (e.g., entries 3−5). Cycloadditions in toluene and THF proceed with slightly diminished yield or ee than in CH2Cl2 (entries 6 and 7), as do reactions that are conducted at lower or higher temperature (entries 8 and 9).
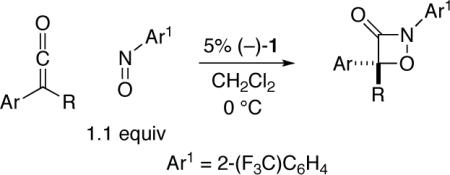
We have examined the scope of the catalytic asymmetric [2+2] cycloaddition of ketenes with a nitrosoarene (Table 2). In the case of aryl methyl ketenes wherein the aryl group is unhindered, poor enantioselectivity is observed (entries 1 and 2); otherwise, the 1,2-oxazetidin-3-ones are produced in good to excellent ee (entries 3−18).
Table 2.
Nucleophile-catalyzed asymmetric cycloaddition of ketenes with a nitrosoarene.
 | ||||
|---|---|---|---|---|
| Entry | Ar | R | Yield (%)[a] | ee (%) |
| 1 | Ph | Me | 72 | 13 |
| 2 | 4-(MeO)C6H4 | Me | 60 | 3 |
| 3 | o-tolyl | Me | 90 | 90 |
| 4 | 2-(MeO)C6H4 | Me | 92 | 97 |
| 5 | 2-BrC6H4 | Me | 93 | 94 |
| 6 | Ph | Et | 88 | 80 |
| 7 | m-tolyl | Et | 86 | 78 |
| 8 | 1-naphthyl | Et | 86 | 78 |
| 9 | o-tolyl | Et | 90 | 96 |
| 10 | 2-(MeO)C6H4 | Et | 93 (88[b]) | >98 (>98[b]) |
| 11 | Ph | CH2CH2i-Pr | 86 | 79 |
| 12 | Ph | i-Bu | 90 | 91 |
| 13 | Ph | i-Pr | 85 | 92 |
| 14 | 4-ClC6H4 | i-Pr | 84 | 92 |
| 15 | 4-(MeO)C6H4 | i-Pr | 81 | 90 |
| 16 | 3-thienyl | i-Pr | 78 | 84 |
| 17 | Ph | cyclopentyl | 81 | 91 |
| 18 | Ph | cyclohexyl | 84 | 93 |
All data are the average of two experiments.
Yield of purified product.
Catalyst loading: 1%.
For example, for aryl methyl ketenes in which the aryl group is ortho-substituted, highly enantioenriched product is generated (entries 3−5). In the case of aryl ethyl ketenes, ∼80% ee is obtained with less hindered aryl substituents (entries 6−8), whereas very good enantioselectivity is observed with larger aromatic groups (entries 9 and 10). For o-anisyl ethyl ketene, use of a lower catalyst loading (1%) leads to only a small loss in yield (entry 10). Although the presence of a branch in the γ position of the alkyl group does not result in enhanced enantioselectivity (entry 11 vs. entry 6), a branch in the β position furnishes a significant increase in ee (entry 12). A range of aryl alkyl ketenes that bear a secondary alkyl substituent undergo cycloaddition with very good enantioselectivity (entries 13−18).[14]
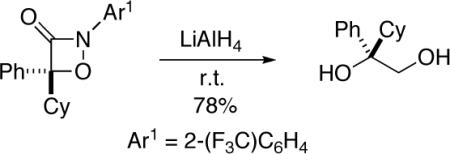
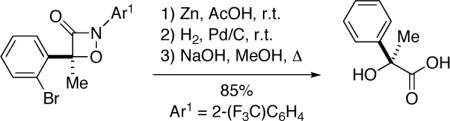
The enantioenriched 1,2-oxazetidin-3-ones can be converted into synthetically useful compounds such as 1,2-diols (eq 3) and α-hydroxycarboxylic acids (eq 4).[15] The sequence depicted in eq 4 illustrates a “work-around” for the poor enantioselectivity that is observed in the [2+2] cycloaddition of phenyl methyl ketene (entry 1 of Table 2): incorporation of the 2-bromo substituent leads to formation of the 1,2-oxazetidin-3-one in excellent yield and ee (entry 5 of Table 2), and the bromide “auxiliary” is then removed by hydrogenolysis.
 |
(3) |
 |
(4) |
In analogy to other [2+2] cycloadditions of ketenes that are catalyzed by planar-chiral DMAP derivatives,[9] we hypothesize that this new method for the asymmetric synthesis of 1,2-oxazetidin-3-ones proceeds via the pathway outlined in Figure 1. We have determined that the resting state of catalyst 1 during the reaction is the free catalyst, and that the rate law is first order in catalyst, ketene, and nitroso compound, which suggests that the addition of 1 to the ketene is not the turnover-limiting step of the catalytic cycle.[16]
In summary, we have provided the first examples of catalyzed cycloadditions of ketenes with nitroso compounds to form 1,2-oxazetidin-3-ones. Through the appropriate choice of chiral catalyst and nitroso compound, the synthesis of these intriguing heterocycles can now be achieved with very good regioselectivity and enantioselectivity. In addition to serving as potentially bioactive target molecules, 1,2-oxazetidin-3-ones can be transformed into other important classes of compounds, such as α-hydroxycarboxylic acid derivatives. The versatility of the present method compares favorably with other catalytic asymmetric approaches to the synthesis of α-hydroxycarboxylic acids that bear tertiary alcohols.
Footnotes
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences, grant R01-GM57034), the German Academic Exchange Service (postdoctoral fellowship for M.D.), Merck Research Laboratories, and Novartis.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.For some leading references, see: Coppola GM, Schuster HF. a-Hydroxy Acids in Enantioselective Synthesis. VCH; Weinheim: 1997. ;Gröger H. Adv. Synth. Catal. 2001;343:547–558.;Tao J, McGee K. Development of an Efficient Synthesis of Chiral 2-Hydroxy Acids. In: Blaser HU, Schmidt E, editors. Asymmetric Catalysis on Industrial Scale. Wiley–VCH; Weinheim: 2003. ; Chapter IV.1.
- 2.For an overview, see: Riant O, Hannedouche J. Org. Biomol. Chem. 2007;5:873–888. doi: 10.1039/b617746h..
- 3.For a pioneering example, see: Hamashima Y, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2000;122:7412–7413..
- 4.For two seminal studies, see: DiMauro EF, Kozlowski MC. J. Am. Chem. Soc. 2002;124:12668–12669. doi: 10.1021/ja026498h.;Funabashi K, Jachmann M, Kanai M, Shibasaki M. Angew. Chem. Int. Ed. 2003;42:5489–5492. doi: 10.1002/anie.200351650..
- 5.Ooi T, Fukumoto K, Maruoka K. Angew. Chem. Int. Ed. 2006;45:3839–3842. doi: 10.1002/anie.200600470. [DOI] [PubMed] [Google Scholar]
- 6.For example, previously described catalytic asymmetric processes do not generally provide access to enantioenriched α-hydroxycarboxylic acids wherein the tertiary alcohol bears a secondary alkyl substituent or a hindered aromatic group (see Table 2).
- 7.a Staudinger H, Jelagin S. Chem. Ber. 1911;44:365–374. [Google Scholar]; b Makarov SP, Shpanskii VA, Ginsburg VA, Shchekotikhin AI;, Filatov AS, Martynova LL, Pavlovskaya IV, Golovaneva AF, Yakubovich AY. Dokl. Akad. Nauk SSSR. 1962;142:596–599. [Google Scholar]; c Kresze G, Trede A. Tetrahedron. 1963;19:133–136. [Google Scholar]; d Kerber RC, Cann MC. J. Org. Chem. 1974;39:2552–2558. [Google Scholar]; e Moderhack D, Stolz K. Chem. Zeit. 1990;114:5–7. [Google Scholar]
- 8.To the best of our knowledge, there are no reports of the synthesis of an enantioenriched 1,2-oxazetidin-3-one. This heterocycle has a structural similarity to a β-lactam and to cycloserine.
- 9.a Lee EC, Hodous BL, Bergin E, Shih C, Fu GC. J. Am. Chem. Soc. 2005;127:11586–11587. doi: 10.1021/ja052058p. Imines. [DOI] [PubMed] [Google Scholar]; Hodous BL, Fu GC. J. Am. Chem. Soc. 2002;124:1578–1579. doi: 10.1021/ja012427r. [DOI] [PubMed] [Google Scholar]; b Wilson JE, Fu GC. Angew. Chem. Int. Ed. 2004;43:6358–6360. doi: 10.1002/anie.200460698. Aldehydes. [DOI] [PubMed] [Google Scholar]; c Berlin JM, Fu GC. Angew. Chem. Int. Ed. 2008;47:7048–7050. doi: 10.1002/anie.200802439. Azo compounds. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.For excellent overviews of ketene chemistry, including investigations by others of catalytic asymmetric [2+2] cycloadditions, see: Tidwell TT. Eur. J. Org. Chem. 2006:563–576.;Orr RK, Calter MA. Tetrahedron. 2003;59:3545–3565..
- 11.The 1,2-oxazetidin-3-one is the major product of the uncatalyzed process.
- 12.For a discussion, see Reference 7d.
- 13.For uncatalyzed [2+2] cycloadditions of ketenes with nitrosoarenes, the electronic nature of the nitrosoarene can have a moderate impact upon product distribution (Reference 7d).
- 14.When conducted on a 5-mmol scale (2% catalyst), the cycloaddition depicted in entry 10 of Table 2 proceeds in 96% yield (1.6 g of product) and 98% ee.
- 15.Ring openings of 1,2-oxazetidin-3-ones (with Ar1 ≠ 2-(F3C)C6H4) by LiAlH4 and Zn/AcOH have previously been described (Reference 7c). Additional derivatizations are described in the Supporting Information.
- 16.We do not observe a non-linear effect: product ee correlates linearly with catalyst ee.


