Abstract
The development of reward-based learning and decision-making, and the neural circuitry underlying these processes, appears to be influenced negatively by adverse child-rearing environments characterized by abuse and other forms of maltreatment. No research to-date has investigated whether normative variations in the child-rearing environment have effects on adolescent brain structure. We examined whether normative variations in maternal responses to adolescents’ positive affective behavior were associated with morphometric measures of the adolescents’ affective neural circuitry, namely the amygdala, orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC). Healthy adolescents (N = 113) participated in laboratory-based interaction tasks with their mothers, and underwent high-resolution (3T) structural magnetic resonance imaging (MRI). The mother–adolescent interactions included a pleasant event-planning interaction (EPI) and a conflictual problem-solving interaction (PSI). Adolescents, whose mothers displayed more punishing responses to their positive affective behavior during both tasks, and only during the PSI, had larger left dorsal ACC and bilateral OFC volumes, respectively. In addition, boys whose mothers evidenced this pattern of behavior during the EPI had larger right amygdala volumes. These results suggest that normative variations in maternal responses to affective behavior are associated with the structural characteristics of adolescents’ affective neural circuitry, which may have implications for the development of their social, cognitive and affective functioning.
Keywords: reward, neuroimaging, family, parenting, brain structure
The ability to evaluate and learn from the reward versus risk value of behavioral outcomes is a core component of healthy affective development, and contributes to adaptive decision-making and goal-directed behavior (Ernst and Paulus, 2005). Structures involved in these types of affective processes undergo significant change during adolescence (Giedd et al., 1999). These structures include the amygdala, orbitofrontal cortex (OFC), and anterior cingulate cortex (ACC), which comprise key afferent and efferent projections of a cortico-striato-midbrain pathway. The processing of reward-related information by this network impacts on dopaminergic cell function, which is synchronised in the striatum (Haber et al., 2006). The OFC and dorsal ACC (dACC), structures associated with a number of cognitive, social and emotional functions, are thought to be particularly important for the mediation of different aspects of reward-based decision-making. The OFC is involved in encoding and representing the reward value of stimuli, and in re-learning stimulus-reward value associations when reinforcement contingencies change (Kringelbach, 2005; Rolls, 2000). The dACC is involved in monitoring conflict between predicted and actual reward outcomes of behavior and using this information to modify subsequent behavior (Bush et al., 2002; Ullsperger and von Cramon, 2003; Holroyd and Coles, 2008). The amygdala and rostral/ventral ACC, which are believed to be involved in the detection and representation of affectively salient stimuli, relay information about emotional valence and salience for further processing (Haber et al., 2006).
During adolescence, there is a developmentally typical lag in maturation of cortical regions (particularly, those regions involved in the representation of reward values—OFC—and the use of those values to guide behavior—dACC) relative to limbic regions (particularly those regions, such as the amygdala and rostral/ventral ACC, involved in assigning emotional significance to sensory events). This lag has been suggested to underlie poor reward-related processing and decision-making (Spear, 2000; Ernst et al., 2006). Indeed, function of these cortical and limbic regions during reward-processing has been demonstrated as different in adolescence compared to adults (Bjork et al., 2004; Ernst et al., 2005; Galvan et al., 2006; Eshel et al., 2007). Relatedly, behavioral and emotional difficulties hypothesized to reflect disruptions in reward processing, such as depression and anxiety (Forbes et al., 2007), and risk-taking or reckless behaviors (Arnett, 1992), including gambling and substance abuse (Chambers and Potenza, 2003) increase during this developmental period.
Despite the significance of reward processing as a component of healthy adolescent development, and the evidence that neural development significantly contributes to this processing, there is little research investigating how important environmental processes may relate to reward-processing and associated neural structure during this time. There is some evidence that the family environment of young children may influence both the development of reward processing and its underlying neural circuitry. Most of the relevant studies involve childhood maltreatment or trauma, and demonstrate that these early experiences result in abnormalities in reward and punishment sensitivity, and difficulties in decision-making associated with risky behavior (Guyer et al., 2006). Early severe stress and maltreatment has also been associated with abnormal size of limbic brain regions, including the amygdala (Teicher et al., 2003), ventral and dorsal prefrontal cortical regions and corpus callosum (De Bellis et al., 2002; Richert et al., 2006), and abnormalities in activity in the prefrontal cortex (PFC, Teicher et al.), OFC and amygdala (Chugani, 1998).
Thus, accumulating evidence suggests that an adverse early environment may be associated with neural circuitry underlying reward-related processes and behaviors. However, no research to-date has investigated such associations in adolescence, when the neural circuitry underlying reward processing may be particularly vulnerable to environmental effects given that it is undergoing significant remodeling. Further, no research has investigated the association between normative variations in family environmental processes and reward-related neural circuitry. This is a significant omission given evidence that normative variations in family processes are salient in adolescence and have implications for emotional development and disorder (Denham et al., 1997; Sheeber et al., 2001; Morris et al., 2007).
Parental socialization of adolescent affective behavior may be important for the adolescents’ development of affective processing and behavior (and its underlying neural circuitry), as this type of parental behavior provides adolescents with important information about the consequences of their own affective behavior. There is much evidence that parental punishing (i.e. aggressive) responses to their child's affect are associated with adverse emotional and behavioral outcomes (Eisenberg et al., 1996, 1998). Whilst parental responses to child negative affect have received greater research attention (Eisenberg et al., 1996), the pertinence of examining the effects of parental responses to child positive affect has also been recognized. For example, parents’ attentiveness towards their children's positive affective displays has been related to children's emotional and social competence (Denham et al., 1997). Our previous work with the sample investigated in this study has shown that adolescents whose mothers respond in a punishing manner towards their positive affect during laboratory interaction tasks tend to display more emotionally dysregulated behaviors during these interactions, and report greater use of maladaptive emotion regulation strategies (Yap et al., 2008a). Such parental responses to children's positive affective behavior have also been associated with childhood depression (Messer and Gross, 1995). Further, parental responses to child positive affect may be particularly influential on the child's reward neural circuitry, given that this type of socialization is hypothesized to shape the child's ability to regulate their own positive affect (Yap et al., 2008a).
The aim of this study was to examine whether maternal socialization of adolescent positive affect was associated with the morphology of brain structures hypothesized to be relevant to reward-processing, namely the amygdala, OFC and ACC. Although the striatum is a key region of the reward neural circuitry, we did not include it in our investigation due to methodological difficulties associated with its measurement. Currently, delineation protocols for regions of the striatum are still not well-validated, and reliable delineation (particularly of the nucleus accumbens) has been noted to be difficult (Ahsan et al., 2007). Building on our previous work in this sample, we investigated whether adolescent brain structure was related to maternal aggressive responses to adolescent positive affective behavior. We observed and coded the behavior of mother–adolescent dyads during structured laboratory interactions in order to investigate normative variations in maternal socialization of positive affective behavior. We also investigated gender differences in the associations, given data indicating that (i) boys and girls may respond differently to maternal socialization (Garside and Klimes-Dougan, 2002; Yap et al., 2008a), and (ii) gender differences exist in brain function and structure associated with affective processing and behavior (Giedd et al., 1997; Whittle et al., 2008a, 2008b).
METHODS
Participants
The sample consisted of 113 adolescents (51% male, M age 12.5 ± 0.5 years, range 11.4 − 13.6 years) recruited from schools across metropolitan Melbourne, Australia. Adolescents were recruited as part of a broader adolescent development study (Yap et al., 2008a) such that those with particularly high, and particularly low, temperamental risk for mental health problems were over-sampled. Using the Edinburgh Handedness Inventory (Oldfield, 1971), 106 students were identified as right-handed and eight as left-handed. No adolescent had a current or past case-level Axis I depressive, substance use, or eating disorder diagnosis, established using The Schedule for Affective Disorder and Schizophrenia for School-Aged Children: Epidemiologic Version (K-SADS-E, Orvaschel and Puig-Antich, 1994). Informed consent was obtained from each adolescent and parent, in accordance with the guidelines of the Human Research Ethics Committee of the University of Melbourne, Australia. Mothers were given $50 AUD cash and adolescents a $30 AUD shopping voucher as reimbursement for participation.
Family interactions
Procedure
Adolescents and their mothers participated in two 20-min family interactions: an Event-Planning Interaction (EPI) followed by a Problem-Solving Interaction (PSI), which were video recorded for coding purposes. Topics for the EPI and PSI were identified based on participant responses to the Pleasant Events Checklist (PEC, MacPhillamy and Lewinsohn, 1976) and the Issues Checklist (IC, Prinz et al., 1979), respectively. The PEC comprises 50 activities that people typically enjoy doing, such as ‘Taking a trip or vacation’, and ‘Snow skiing’. The IC comprises 44 topics about which adolescents and parents may have conflict, such as ‘lying’ and ‘talking back to parents’. Up to five activities that both mother and adolescent indicated to be very pleasant on the PEC were chosen for mother–adolescent dyads to plan during the EPI, and up to five issues that were reported to be conflictual on the IC were chosen for the dyads to resolve during the PSI.
As different interactional tasks elicit different types of interpersonal behaviors (Melby et al., 1995), we examined maternal socialization behaviors during both the EPI and the PSI. The EPI provides a sampling of behavior under conditions that are relatively low-stress and pull for positive affect. On the other hand, the PSI pulls for more negative affect and conflictual behavior and may require greater affect regulation on the part of the participants. Hence, the use of these two tasks enabled us to elicit a greater range of affect and to examine maternal behavior under conditions of high and low stress. The EPI was always conducted first because negative affective states have slower decay rates than positive states (Gilboa, 1994) and we did not want the affect generated by the PSI to influence the EPI. As expected, participants displayed more positive affect in the EPI and more negative affect in the PSI (Yap et al., In Press).
Observational coding of family interactions
The affective and verbal content of the interactions were coded using the Living in Family Environments (LIFE, Hops et al., 1995) coding system. The LIFE is an event-based coding system in which new codes are entered when participants’ affect or verbal content changes. LIFE codes were defined conceptually, but have an extensive validation history (Davis et al., 2000; Katz and Hunter, 2007; Sheeber et al., 2007; Whittle et al., 2008a; Yap et al., 2008a). The LIFE consists of 10 affect codes (e.g. contempt, neutral, happy) and 27 verbal content codes (e.g. validation, approve, provoke). Two composite constructs, derived from the individual affect and content codes, were used in the present study. Aggressive behavior includes all codes with contemptuous, angry and belligerent affect, as well as disapproving, threatening or argumentative statements with neutral affect. Positive Interpersonal behavior consists of statements made with happy, pleasant and caring affect as well as approving or affirming statements, and statements that serve to maintain the conversation. More detailed information about the LIFE system is presented in Hops et al. (1995).
All video recordings were coded by extensively trained observers blind to participant characteristics (e.g. symptomatology levels) and study hypotheses. Random pairs of observers were assigned to the interactions to minimize drift between any two observers. Approximately 20% of the interactions were coded by a second observer to provide an estimate of observer agreement. Kappa coefficients for the composite codes were: Aggressive = 0.77 and Positive Interpersonal = 0.89; these scores reflect good to excellent agreement (Fleiss, 1981).
Observational indices of maternal socialization were derived from these constructs. Allison-Liker Z-scores (Allison and Liker, 1982) for sequential relations indicate the extent to which a specified sequence of behavior occurs more or less often than would be expected as a function of the base rate of each behavior. Z-scores represent whether a particular antecedent behavior increases (positive z-score) or suppresses (negative z-score) the likelihood of a particular consequent behavior. Maternal ‘punishing’ of adolescent positive affect is indexed by a greater likelihood (i.e. more positive z-scores) of Aggressive maternal responses following adolescent positive interpersonal behavior.
Neuroimaging
Image acquisition
Magnetic resonance imaging (MRI) scans were performed on a 3 Tesla GE scanner at the Brain Research Institute, Austin and Repatriation Medical Centre, Melbourne, Australia, using a gradient echo volumetric acquisition sequence (repetition time = 36 ms; echo time =9 ms; flip angle = 35°, field of view = 20 cm2, pixel matrix = 410 × 410) to obtain 124 T1-weighted contiguous 1.5 mm-thick slices (voxel dimensions = 0.4883 × 0.4883 × 1.5 mm).
Image pre-processing
Images were transferred to a SGI/Linux workstation for morphometric analysis. Image pre-processing was carried out using tools from the FMRIB software library (http://www.frmib.ox.ac.uk/fsl). Each 3D scan was stripped of all non-brain tissue (Smith, 2002), and aligned to the MNI 152 average template (six-parameter rigid body transform with trilinear interpolation) using FLIRT (Jenkinson and Smith, 2001). This registration served to align each image axially along the anterior commissure–posterior commissure (AC–PC) plane and sagittally along the interhemispheric fissure without any deformation. Images were re-sampled to 1 mm3.
Morphometric analysis
Regions of interest (ROIs) were defined and quantified based on previous techniques developed and published by the Melbourne Neuropsychiatry Centre (see below). All ROIs were traced using the software package ANALYZE (Mayo Clinic, Rochester, USA; http://www.mayo.edu/bir/). Brain tissue was segmented into grey matter, white matter, and cerebrospinal fluid using an automated algorithm, as implemented in FAST (Zhang et al., 2001). An estimate of whole brain volume (WBV) was obtained by summing grey and white matter pixel counts (i.e. WBV included cerebral gray and white matter, the cerebellum and brainstem, but not the ventricles, cisterns or cerebrospinal fluid). OFC and ACC estimates were based on gray matter pixel counts contained within the defined ROIs. Amygdala estimates were based on total voxels within the defined ROI.
Amygdala
The guidelines for tracing the amygdala were adapted from those described by Velakoulis and colleagues (Velakoulis et al., 1999, 2006). Adaptations, designed to maximize reliability, relate to marking the anterior boundary of the amygdala and the boundary between the amygdala and hippocampus. Amygdala boundaries were: posterior: appearance of amygdala gray matter above the temporal horn; superior-lateral: the thin strip of white matter that separates the amygdala from the claustrum and the tail of the caudate; medial: the angular bundle, which separates the amygdala from the entorhinal cortex; superior-medial: the semilunar gyrus; inferior: the hippocampus; inferior-lateral: the temporal lobe white matter and the extension of the temporal horn; anterior: the section posterior to the most posterior of either the point where the optic chiasm joins, or the point where the lateral sulcus closes to form the endorhinal sulcus. Watson et al.'s (1992) protocol was used to separate the amygdala from the hippocampus.
Orbitofrontal cortex
The boundaries of the OFC were based on a previously published method (Riffkin et al., 2005). A line through the AC–PC was used to define the superior boundary of the OFC. The posterior boundary was marked by a coronal plane passing through the most posterior aspect of the olfactory sulcus in each hemisphere. The inferior boundary was defined by the most inferior aspect of the cortex, the lateral boundary by the most lateral edge of the cortex and the medial boundary of each hemisphere by the longitudinal fissure. Our method included both the medial (gyrus rectus) and lateral aspects of the OFC in this measurement. All images were manually edited to eliminate subcortical tissue and artifacts related to the eye sockets and nasal bones.
Anterior cingulate cortex
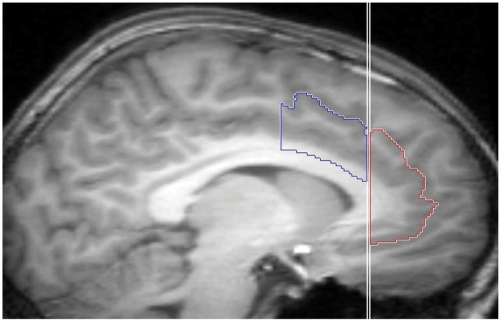
The boundaries of the ACC were based on a previously published method (Fornito et al., 2006). This protocol takes into account individual differences in morphology of the cingulate, paracingulate and superior rostral sulci in defining ACC volumes. The protocol divides the ACC into dorsal, rostral and ventral regions. For this study, four regions were delineated: left and right dorsal ACC (dACC), and left and right rostral/ventral ACC (r/vACC). These regions included cingulate gyrus gray matter, and when the paracingulate sulcus was present, gray matter within the paracingulate gyrus (when the paracingulate sulcus was absent, only gray matter on the upper bank of the cingulate sulcus was included). See Figure 1 for an example parcellation.
Fig. 1.
An example parcellation of the dorsal anterior cingulate cortex (dACC; blue) and the rostral/ventral anterior cingulate cortex (r/vACC; red). Note that because the paracingulate sulcus is present, region volumes included all grey matter within the cingulate and paracingulate gyri.
Statistical analysis
Interrater and intrarater reliabilities were assessed by means of the intraclass correlation coefficient (ICC; absolute agreement) using 15 brain images from a separate MRI database established specifically for this purpose. ICC values were acceptable for all ROIs (85% above 0.90 and none below 0.85).
All brain structural measures were corrected for whole brain size using a covariance adjustment method (Jack et al., 1989). Hypotheses were tested using a series of hierarchical linear regressions entering adolescent gender in Step 1, maternal socialization behavior (punishing responses) in Step 2, and socialization × gender in Step 3 (interaction terms were computed after centering all continuous variables). The outcome variable for each analysis was one of the brain structure measures (i.e. left and right amygdala, dACC, r/vACC, and OFC volumes). Separate regressions were run for the EPI and PSI; thus, 16 regressions (8 brain structures by 2 tasks) were performed. Significant gender interactions were followed by regression analyses for males and females separately.
OFC volumetric data was not available for one participant due to excessive artifact in the orbital region on the T1 image. Alison-Liker z-scores were unavailable for maternal punishing responses during the EPI for three participants, because these mothers did not display the responses of interest.
RESULTS
Table 1 shows means and s.d. for the volumes (relative to whole brain volume) of each brain ROI for males and females. There was a significant main effect for adolescent gender on the volumes of the left amygdala, right OFC and right r/vACC, with male gender predicting larger volumes.
Table 1.
Means and s.d. of regional brain volumes (corrected for WBV)
| Brain measure | Full sample (N = 113) |
Males (N = 58) |
Females (N = 55) |
Gender difference | |||
|---|---|---|---|---|---|---|---|
| M | s.d. | M | s.d. | M | s.d. | t | |
| Left amygdala | 1882.30 | 228.10 | 1948.80 | 211.25 | 1812.17 | 225.93 | 3.322** |
| Right amygdala | 1813.67 | 237.83 | 1855.03 | 240.22 | 1770.06 | 229.41 | 1.778 |
| Left OFC | 19450.86 | 3292.53 | 19926.22 | 3502.15 | 18958.22 | 3013.10 | 1.566 |
| Right OFC | 20123.43 | 3352.77 | 20835.50 | 3572.08 | 19385.47 | 2963.85 | 2.334* |
| Left dACC | 4078.65 | 757.73 | 4084.58 | 813.45 | 4072.40 | 701.49 | 0.87 |
| Right dACC | 4163.72 | 727.00 | 4165.61 | 777.81 | 4161.74 | 676.25 | 0.29 |
| Left r/vACC | 6358.11 | 1587.31 | 6476.10 | 1592.88 | 6238.05 | 1586.64 | 0.803 |
| Right r/vACC | 6352.85 | 1510.72 | 6748.50 | 1526.17 | 5964.15 | 1402.38 | 2.843* |
*P < 0.05; **P < 0.005.
Maternal punishing responses to adolescent positive affect during the EPI were associated with adolescent left dACC volume (β = 0.195, t[109] = 2.060, P = 0.042, ΔR2 = 0.038). Specifically, adolescents whose mothers were more likely to punish their positive affective behavior had larger volumes of this structure. Maternal punishing responses to adolescent positive affect during the EPI also demonstrated a significant interaction effect with adolescent gender in predicting the volume of the right amygdala (β = 0.290, t[110] = 0.2.290, P = 0.027, ΔR2 = 0.044). Post hoc analyses revealed that boys whose mothers were more likely to punish their positive affective behavior had larger right amygdala volumes (β = 0.265, t[55] = 2.023, P = 0.048, R2 = .070), while there was no significant effect for females (β = −0.152, t[53] = −1.108, P = 0.273, R2 = .023).
Maternal punishing responses to adolescent positive affect during the PSI were associated with adolescent OFC and left dACC volumes. Specifically, adolescents whose mothers were more likely to punish their positive affective behavior had larger volumes of the left (β = 0.232, t[112] =2.507, P = 0.014, ΔR2 = 0.053) and right (β = 0.270, t[112] = 2.993, P = 0.003, ΔR2 = 0.072) OFC and left dACC (β = 0.222, t[112] = 2.377, P = 0.019, ΔR2 = 0.049). There were no other main effects of maternal socialization or interactive effects with gender (Ps = 0.108–0.984).
To examine the specificity of the obtained results, we conducted regression analyses for the left dACC, right amygdala, and left and right OFC, using as the following independent variables: the (i) frequency of maternal aggressive behavior during the EPI and the PSI (see Yap et al., 2008b,for details of these measures); and (ii) measures of maternal positive responding to adolescent aggressive behavior during the EPI and the PSI. These analyses were intended to clarify whether the observed volumetric enlargements of brain structures might be associated with maternal aversive responding in general (i), or maternal incongruent responding in general (ii). There were no significant effects for any of these behavioral independent variables (β = 0.003–0.0.112, P = 0.241 – 0.974, ΔR2 = <0.001–0.018).
DISCUSSION
Significant associations were found between observed maternal punishing responses to adolescents’ positive affective behaviors and the morphological characteristics of their affective neural circuitry. Specifically, adolescents whose mothers were more likely to punish (i.e. respond aggressively to) their displays of positive affect during a problem-solving task were found to have larger bilateral volumes of the OFC and left dACC. Adolescents exposed to this type of behavior during an event planning task were found to have larger volumes of the left dACC, and boys (but not girls) were also found to have larger right amygdala volumes. These associations were not explained by more general measures of maternal aversive behavior, or maternal incongruent responding to adolescent behavior. Our findings suggest that normative variations in maternal punishing responses to their adolescents’ positive affective behaviors may be associated with adolescent's reward-related behavior via their reward neural-circuitry.
No other study has examined neuroanatomical associations with normative variations in the family environment of adolescents, thus making comparisons with previous research difficult. However, our results may be compared with existing research examining neurobiological alterations associated with childhood maltreatment and abuse. Such research has implicated similar brain regions to this study. For example, adolescents with neglect- or abuse-related PTSD have been found to show volumetric enlargements in the ventral PFC (Richert et al., 2006). Also, post-institutionalized children have been found to have larger amygdala volumes, whereby amygdala size and function correspond to duration of institutional care (Eigsti et al., 2002). Whilst there have been reports of decreased size of both the PFC (De Bellis et al., 2002) and amygdala (Teicher et al., 2003) volume in individuals with a history of abuse and neglect, in the former case, the decrease in PFC volume was driven by white matter (whereas our result was restricted to gray matter), and in the latter case, participants were young adults, whereas our sample consisted exclusively of adolescents.
Because our data is cross-sectional, our findings provide no evidence for causality of relationships, however, there is some support from animal studies that the childhood care-giving environment may influence the development of affective neural circuitry. For example, manipulations to the maternal care-giving environment in animals appear to consistently affect functioning of the serotonin, norepinephrine, and dopamine systems, all of which function to mediate reward-related behaviors (Glaser, 2000). Maternal deprivation in non-human primates has also been found to result in lasting changes in prefrontal cortical anatomy (Cameron, 2004), and in gene expression in the amygdala (Sabatini et al., 2007). Although longitudinal work is required to examine the potential of causal connections in humans, in the following section we summarize functional and structural brain imaging research that provides a basis for plausible conjectures regarding the mechanisms by which maternal socialization behaviors may influence development of reward-related neural systems.
Mechanisms underlying relationships between maternal behavior and adolescent brain morphological characteristics
As described earlier, the OFC and dACC are involved in different aspects of reward-based learning and decision-making (Elliott et al., 2000; Rolls, 2000; Bush et al., 2002; Ridderinkhof et al., 2004; Alia-Klein et al., 2007; Holroyd and Coles, 2008). These structures are involved in processing related to both primary or natural reinforcers (e.g. pain or pleasant taste), and also more complex social reinforcement information, where the meaning of rewarding or punishing outcomes may be ambiguous and context-dependent, and prediction of reward must be inferred from ongoing trial and error (O'Doherty, 2007). Thus, it is possible that volumetric alterations of the OFC and dACC in adolescents whose mothers are more likely to punish their positive affective behaviors may be associated with the role of these regions in the processing of complex and possibly ambiguous reinforcement signals from mothers (given that one does not typically expect that positive behavior will elicit aggressive behavior).
The amygdala is thought to be important for responding to emotional signals in the environment and for stimulus-reward learning (Baxter and Murray, 2002). As such, it is possible that greater involvement of the amygdala (for boys only) in responding to salient emotional signals from mothers has influenced structural change in this region.
The precise mechanisms driving the associations reported here, and the reason as to why we found enlarged volumes associated with maternal punishment of positive affect is unclear. It is possible that our findings reflect use-dependent cortical development, resulting from high levels of activity during mother-adolescent interactions (Richert et al., 2006). Findings are consistent with the suggestion that early stress may activate the developing prefrontal cortex, altering its development and producing precocious maturation but blunted final capacity (Teicher et al., 2003). Given that adolescence is a period of marked neuroanatomical development (Giedd et al., 1999), it will be important for future work to assess the longitudinal nature of the associations reported here and their functional significance at different stages of development.
Implications for adolescent functioning
There is evidence in the literature that the structure of the OFC, dACC, and amygdala has functional significance (in normative populations) across a range of social, cognitive and affective domains. Indeed, our previous work with the same adolescent sample has shown volumetric measures of the OFC and amygdala to correlate with observed measures of adolescent dysphoric and aggressive behavior, respectively (Whittle et al., 2008a). Other work has shown OFC structure to be correlated with social cognition in early adolescents (Wood et al., 2008), and executive function in healthy adults (McCarley et al., 2007). Dorsal ACC structure in the left hemisphere specifically has been associated with executive functioning in healthy adults (Fornito et al., 2008), and both OFC and dACC structural measures have been correlated with measures of general intelligence in adolescents and young adults (Frangou et al., 2004). Amygdala structure has been associated with neurotic personality in adults (Omura et al., 2005). Thus, growing evidence suggests that the structural associations found in the present study may have significance for a number of aspects of behavior.
Given that the plasticity in parts of the prefrontal and limbic cortex during adolescence likely render it particularly vulnerable to environmental influence (Giedd et al., 1999), it is thus likely that alterations in brain structure during adolescence may have long-term implications for adult functioning. Longitudinal research is required to test whether the observed relationships between maternal socialization behaviors and adolescent brain structure have consequences for the adolescents’ cognitive, social and affective function in late adolescence and adulthood.
Gender
The relationship between amygdala volume and maternal punishing of positive affective behavior was apparent only in males. This finding adds to a growing body of literature showing gender differences in the neurobiological basis of affect and behavior (Wager et al., 2003; Whittle et al., 2008b). In particular, gender differences in amygdala function have been observed, whereby a number of studies report a greater right lateralized involvement in affective processing in males (Wager et al., 2003; Williams et al., 2005; Kilpatrick et al., 2006). It has been suggested that this may reflect a greater sympathetic or ‘fight-flight’ style of responding to the environment in males compared to females (Kilpatrick et al., 2006), and one that is more dynamic in detecting salient emotional environmental signals (Williams et al., 2005). The functional and behavioral relevance of both the gender difference and lateralization findings of the current study are unclear, although they suggest that the adolescent male amygdala (particularly in the right hemisphere) may be particularly sensitive to environmental perturbations.
Task-specific effects
Of note, we found that different brain structures were associated with maternal punishing of adolescent positive affective behaviors in the two different interaction tasks. Whereas the dACC was implicated in both tasks, OFC volume was associated with behavior only during the PSI. The PSI is designed to be particularly emotionally challenging for both mother and adolescent, such that it typically evokes higher frequency and duration of negative affects (Yap et al., 2008a). This task thus requires more frequent and effortful monitoring and regulation of behavior on the part of both mother and adolescent. Given that the OFC is thought to be one of the key prefrontal structures involved in the effortful regulation of affect and behavior (Eippert et al., 2007; Contreras et al., 2008; Whittle et al., 2008b), the size of this structure may be particularly associated with maternal behavior during the more challenging PSI task. Given the dearth of research on socialization of positive affect (especially in different affective contexts) and its associations with neuroanatomical development, this hypothesis is largely speculative and further research is needed to address these outstanding questions.
Limitations
It is important to note the caveats to our interpretation of these findings. First, it is impossible to determine causality regarding the cross-sectional relationships reported. Though we have, for heuristic purposes, speculated as to the potential mechanisms by which maternal behaviors may influence adolescent brain structure, it is also possible that other genetic, biological, and environmental factors contribute to the observed relationships (Glaser, 2000). In particular, adolescent affective behavior may mediate the relationship between maternal behavior and adolescent brain structure; however, there is no clarity about which adolescent behaviors would be most appropriate to examine, and any analyses attempting to include adolescent behavior in the models would be largely exploratory. We believe that our work represents an important first step towards understanding the nature of environmental influences on the development of adolescent reward-based function and neural circuitry, and one that provides the basis upon which to begin more detailed examinations of causal mechanisms. Future work that comprehensively examines these mechanisms is clearly warranted.
Second, our methodology assumes a degree of correspondence between maternal behaviors in the laboratory and those that have occurred in day-to-day interactions across development. Though there are undoubtedly differences between the laboratory and the real-world due to the social and physical constraints of the former, feedback has indicated that differences are primarily in the intensity of aversive behaviours, with family members’ reportedly muting their responses in the laboratory. Laboratory-based interactions have been shown to have good predictive and convergent validity with other measures of family processes, suggesting that they capture valid and important information regarding in vivo family interactions (Sheeber, 1998; Gardner, 2000). Further, although we do not have data relating to the test-retest reliability of the structured interactions used in our study, there is other evidence for remarkable stability over development when one examines rank levels on measures of relationship closeness, conflict, and autonomy variables across families (Sheeber et al., 1998). For example, in a longitudinal study of children between the ages of 6 and 18, Loeber et al. (2000) reported moderate to high levels retest stability (r's = 0.56–0.70) on variables including relationship quality, communication and supervision, with similar values emerging across ages.
Finally, only a few select brain regions were investigated based on extant evidence of their primary contribution to reward-processing. Note that hippocampal volumetric data for this sample is available as part of analyses associated with the wider goals of the research project. Although the hippocampus was not included in this paper as it was not a hypothesized region of interest, we did conduct analyses with this structure for completeness and to check the specificity of findings. These analyses revealed no significant relationships between left or right hippocampal volume and maternal punishing behavior during either interaction task. However, it is likely that many other brain regions are involved, and it will be important for future research to examine other brain structures, as well as other measures of brain function and connectivity. In particular, the nucleus accumbens and other striatal regions are especially relevant to reward functioning and the experience of positive affective states (Knutson et al., 2001; Berridge, 2003; Kringelbach and Rolls, 2004), though there are methodological difficulties associated with imaging this region in humans (Ahsan et al., 2007).
CONCLUSIONS
The results indicate that maternal behavior during mother–adolescent interactions is associated with adolescent brain structure. In particular, aggressive maternal responses to adolescent positive affective behaviors were related to volumetric measures of the adolescents’ OFC, dorsal ACC and amygdala, structures thought to be involved in reward-related decision-making and behavior. Observed gender differences suggest a male-specific sensitivity of the amygdala to maternal punishing of positive affective behavior. Together, these findings highlight the importance of considering the environment in the link between brain and behavior. Whereas previous literature has demonstrated marked effects of relatively severe forms of environmental stress, our results suggest that normative variations in the family environment may have significant effects on brain development. Further research is required to examine whether the associations between maternal behavior and adolescent neuroanatomy exist prospectively and if alterations in brain structure have long-term implications for development and functioning.
ACKNOWLEDGEMENT
We thank the Brain Research Institute for support in acquiring the neuroimaging data, and the Oregon Research Institute for its role in the coding of family interaction data. Neuroimaging analysis was facilitated by the Neuropsychiatry Imaging Laboratory managed by Ms. Bridget Soulsby at the Melbourne Neuropsychiatry Centre. ORYGEN Research Centre and the Colonial Foundation; Neurosciences Victoria; Australian Research Council Postdoctoral Fellowship (to S.W.); a Centre for Clinical Research Excellence Postdoctoral Fellowship (to M.B.H.Y.); National Health and Medical Research Council of Australia Program Grant I.D. 350241 (to M.Y.).
REFERENCES
- Ahsan RL, Allom R, Gousias LS, et al. Volumes, spatial extents and a probabilistic atlas of the human basal ganglia and thalamus. NeuroImage. 2007;38:261–70. doi: 10.1016/j.neuroimage.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Alia-Klein N, Goldstein RZ, Tornasi D, et al. What is in a word? No versus Yes differentially engage the lateral orbitofrontal cortex. Emotion. 2007;7:649–59. doi: 10.1037/1528-3542.7.3.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison PD, Liker JK. Analyzing sequential categorical data on dyadic interaction. Psychological Bulletin. 1982;91:393–403. [Google Scholar]
- Arnett J. Reckless behavior in adolescence – a developmental perspective. Developmental Review. 1992;12:339–73. [Google Scholar]
- Baxter MG, Murray EA. The amygdala and reward. Nature Reviews Neuroscience. 2002;3:563–73. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- Berridge KC. Pleasures of the brain. Brain and Cognition. 2003;52:106–128. doi: 10.1016/s0278-2626(03)00014-9. [DOI] [PubMed] [Google Scholar]
- Bjork JM, Knutson B, Fong GW, Caggiano DM, Bennett SM, Hommer DW. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. The Journal of Neuroscience. 2004;24:1793–802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Vogt BA, Holmes J, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:523–8. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron JL. NIMH Workshop on the Prevention of Depression in Children and Adolescents. Rockville, MD; 2004. The use of animal models for mechanistic and developmental studies. [Google Scholar]
- Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. Journal of Gambling Studies. 2003;19:53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chugani HT. A critical period of brain development: studies of cerebral glucose utilization with PET. Preventive Medicine. 1998;27:184–8. doi: 10.1006/pmed.1998.0274. [DOI] [PubMed] [Google Scholar]
- Contreras D, Catena A, Candido A, Perales JC, Maldonado A. The role of ventromedial prefrontal cortex in emotional decision-making. International Journal of Clinical and Health Psychology. 2008;8:285–313. [Google Scholar]
- Davis B, Sheeber L, Hops H, Tildesley E. Adolescent responses to depressive parental behaviors in problem-solving interactions: implications for depressive symptoms. Journal of Abnormal Child Psychology. 2000;28:451–465. doi: 10.1023/a:1005183622729. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Keshavan MS, Shifflett H, et al. Brain structures in pediatric maltreatment-related posttraumatic stress disorder: a sociodemographically matched study. Biological Psychiatry. 2002;52:1066–78. doi: 10.1016/s0006-3223(02)01459-2. [DOI] [PubMed] [Google Scholar]
- Denham SA, Mitchell-Copeland J, Strandberg K, Auerbach S, Blair K. Parental contributions to preschoolers’ emotional competence: direct and indirect effects. Motivation and Emotion. 1997;21:65–86. [Google Scholar]
- Eigsti IM, Munson SF, Tottenham N, Thomas KM, Durston S, Casey BJ. Neural and behavioral correlates of institutionalization. Journal of Cognitive Neuroscience. 2002;April, 2002; Suppl. S; meeting abstract B17:47–7. [Google Scholar]
- Eippert F, Veit R, Weiskopf N, Erb M, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–23. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Cumberland A, Spinrad TL. Parental socialization of emotion. Psychological Inquiry. 1998;9:241–73. doi: 10.1207/s15327965pli0904_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Murphy BC. Parents’ reactions to children's negative emotions: relations to children's social competence and comforting behavior. Child Development. 1996;67:2227–47. [PubMed] [Google Scholar]
- Elliott R, Dolan RJ, Frith CD. Dissociable functions in the medial and lateral orbitofrontal cortex: evidence from human neuroimaging studies. Cerebral Cortex. 2000;10:308–17. doi: 10.1093/cercor/10.3.308. [DOI] [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, et al. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–91. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Paulus MP. Neurobiology of decision making: a selective review from a neurocognitive and clinical perspective. Biological Psychiatry. 2005;58:597–604. doi: 10.1016/j.biopsych.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel N, Nelson EE, Blair RJ, Pine DS, Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–9. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleiss JL. Statistical Methods for Rates and Proportions. NY: Wiley; 1981. [Google Scholar]
- Forbes EE, Shaw DS, Dahl RE. Alterations in reward-related decision making in boys with recent and future depression. Biological Psychiatry. 2007;61:633–9. doi: 10.1016/j.biopsych.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Fornito A, Whittle S, Wood SJ, Velakoulis D, Pantelis C, Yucel M. The influence of sulcal variability on morphometry of the human anterior cingulate and paracingulate cortex. NeuroImage. 2006;33:843–54. doi: 10.1016/j.neuroimage.2006.06.061. [DOI] [PubMed] [Google Scholar]
- Fornito A, Wood SJ, Whittle S, et al. Variability of the paracingulate sulcus and morphometry of the medial frontal cortex: associations with cortical thickness, surface area, volume, and sulcal depth. Hum Brain Mapping. 2008;29:222–36. doi: 10.1002/hbm.20381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frangou S, Chitins X, Williams SCR. Mapping IQ and gray matter density in healthy young people. NeuroImage. 2004;23:800–5. doi: 10.1016/j.neuroimage.2004.05.027. [DOI] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, et al. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–92. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner F. Methodological issues in the direct observation of parent-child interaction: do observational findings reflect the natural behavior of participants? Clinical Child and Family Psychology Review. 2000;3:185. doi: 10.1023/a:1009503409699. [DOI] [PubMed] [Google Scholar]
- Garside RB, Klimes-Dougan B. Socialization of discrete negative emotions: Gender differences and links with psychological distress. Sex Roles. 2002;47:115–28. [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, et al. Brain development during childhood and adolescence: a longitudinal MRI study. Nature Neuroscience. 1999;2:861–3. doi: 10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuropsychopharmacology and Biological Psychiatry. 1997;21:1185–201. doi: 10.1016/s0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Gilboa E. Personality and the structure of affective responses. In: van Goozen SHM, Van de Poll NE, Sergeant JA, editors. Emotions: Essays on Emotion Theory. Hillsdale, New Jersey: Lawrence Erlbaum Associates; 1994. [Google Scholar]
- Glaser D. Child abuse and neglect and the brain: a review. Journal of Child Psychology and Psychiatry. 2000;41:97–116. [PubMed] [Google Scholar]
- Guyer AE, Kaufman J, Hodgdon HB, et al. Behavioral alterations in reward system function: the role of childhood maltreatment and psychopathology. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:1059–67. doi: 10.1097/01.chi.0000227882.50404.11. [DOI] [PubMed] [Google Scholar]
- Haber SN, Kim KS, Mailly P, Calzavara R. Reward-related cortical inputs define a large striatal region in primates that interface with associative cortical connections, providing a substrate for incentive-based learning. Journal of Neuroscience. 2006;26:8368–76. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles MGH. Dorsal anterior cingulate cortex integrates reinforcement history to guide voluntary behaviour. Cortex. 2008;44:548–59. doi: 10.1016/j.cortex.2007.08.013. [DOI] [PubMed] [Google Scholar]
- Hops H, Davis B, Longoria N. Methodological issues in direct observation – Illustrations with the Living in Familial Environments (LIFE) coding system. Journal of Clinical Child Psychology. 1995;24:193–203. [Google Scholar]
- Jack CR, Twomey CK, Zinsmeister AR, Sharborough FW, Petersen R, Cascino GD. Anterior temporal lobes and hippocampal formations: normative volumetric measurements from MR images in young adults. Radiology. 1989;172:549–554. doi: 10.1148/radiology.172.2.2748838. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith SM. A global optimisation method for robust affine registration of brain images. Med Image Analysis. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Katz LF, Hunter EC. Maternal meta-emotion philosophy and adolescent depressive symptomatology. Social Development. 2007;16:343–60. [Google Scholar]
- Kilpatrick LA, Zald DH, Pardo JV, Cahill LF. Sex-related differences in amygdala functional connectivity during resting conditions. NeuroImage. 2006;30:452–61. doi: 10.1016/j.neuroimage.2005.09.065. [DOI] [PubMed] [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21:RC159:1–5. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nature Reviews Neuroscience. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neurophysiology. Progress in Neurobiology. 2004;72:341–72. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Loeber R, Drinkwater M, Yin YM, Anderson SJ, Schmidt LC, Crawford A. Stability of family interaction from ages 6 to 18. Journal of Abnormal Child Psychology. 2000;28:353–69. doi: 10.1023/a:1005169026208. [DOI] [PubMed] [Google Scholar]
- MacPhillamy DJ, Lewinsohn PM. Manual for the Pleasant Events Schedule. Eugene, OR: University of Oregon; 1976. [Google Scholar]
- McCarley R, Nakamura M, Nestor PG, et al. Region-specific orbitofrontal volume deficit in schizophrenia and orbitofrontal volumetric association with IOWA gambling task in healthy population. Schizophrenia Bulletin. 2007;33:345–345. [Google Scholar]
- Melby JN, Ge X, Conger RD, Warner TD. The importance of task in evaluating positive marital interactions. Journal of Marriage & The Family. 1995;57:981–94. [Google Scholar]
- Messer SC, Gross AM. Childhood depression and family-interaction – a naturalistic observation study. Journal of Clinical Child Psychology. 1995;24:77–88. [Google Scholar]
- Morris AS, Silk JS, Steinberg L, Myers SS, Robinson LR. The role of the family context in the development of emotion regulation. Social Development. 2007;16:361–88. doi: 10.1111/j.1467-9507.2007.00389.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty JP. Linking Affect to Action: Critical Contributions of the Orbitofrontal Cortex. Vol. 1121. Annals of the New York Academy of Sciences; 2007. Lights, camembert, action! The role of human orbitofrontal cortex in a encoding stimuli, rewards, and choices; pp. 254–72. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh handedness inventory. Neuropsychologia. 1971;9:97–114. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Omura K, Constable RT, Canli T. Amygdala gray matter concentration is associated with extraversion and neuroticism. Neuroreport. 2005;16:1905–8. doi: 10.1097/01.wnr.0000186596.64458.76. [DOI] [PubMed] [Google Scholar]
- Orvaschel H, Puig-Antich . Schedule for Affective Disorder and Schizophrenia for School Age Children: Epidemiologic Version. 1994. Unpublished Manual. [Google Scholar]
- Prinz RJ, Foster SL, Kent RN, O'Leary KD. Multivariate assessment of conflict in distressed and nondistressed mother-adolescent dyads. Journal of Applied Behavioral Analysis. 1979;12:691–700. doi: 10.1901/jaba.1979.12-691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert KA, Carrion VG, Karchemskiy A, Reiss AL. Regional differences of the prefrontal cortex in pediatric PTSD: an MRI study. Depression and Anxiety. 2006;23:17–25. doi: 10.1002/da.20131. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, van den Wildenberg WPM, Segalowitz SJ, Carter CS. Neurocognitive mechanisms of cognitive control: the role of prefrontal cortex in action selection, response inhibition, performance monitoring, and reward-based learning. Brain and Cognition. 2004;56:129–40. doi: 10.1016/j.bandc.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Riffkin J, Yucel M, Maruff P, et al. A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Research-Neuroimaging. 2005;138:99–113. doi: 10.1016/j.pscychresns.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Rolls ET. The orbitofrontal cortex and reward. Cerebral Cortex. 2000;10:284–94. doi: 10.1093/cercor/10.3.284. [DOI] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, Lewis DA, Levitt P, Cameron JL, Mirnics K. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. Journal of Neuroscience. 2007;27:3295–304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeber L. Family relationships of depressed adolescents: a multimethod assessment. Journal of Clinical Child Psychology. 1998;27:268. doi: 10.1207/s15374424jccp2703_4. [DOI] [PubMed] [Google Scholar]
- Sheeber L, Davis B, Leve C, Hops H, Tildesley E. Adolescents' relationships with their mothers and fathers: associations with depressive disorder and subdiagnostic symptomatology. Journal of Abnormal Psychology. 2007;116:144–54. doi: 10.1037/0021-843X.116.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeber L, Hops H, Andrews J, Alpert T, Davis B. Interactional processes in families with depressed and non-depressed adolescents: reinforcement of depressive behavior. Behaviour Research and Therapy. 1998;36:417–27. doi: 10.1016/s0005-7967(97)10030-4. [DOI] [PubMed] [Google Scholar]
- Sheeber L, Hops H, Davis B. Family processes in adolescent depression. Clinical Child & Family Psychology Review. 2001;4:19–35. doi: 10.1023/a:1009524626436. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapping. 2002;17:143–55. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP. The adolescent brain and age-related behavioral manifestations. Neuroscience and Biobehavioral Reviews. 2000;24:417–63. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP, Kim DM. The neurobiological consequences of early stress and childhood maltreatment. Neuroscience and Biobehavioral Reviews. 2003;27:33–44. doi: 10.1016/s0149-7634(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Ito Y, Glod CA, Andersen SL, Dumont N, Ackerman E. Preliminary evidence for abnormal cortical development in physically and sexually abused children using EEG coherence and MRI. Annals of the New York Academy of Sciences. 1997;821:160–75. doi: 10.1111/j.1749-6632.1997.tb48277.x. [DOI] [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. Journal of Neuroscience. 2003;23:4308–14. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velakoulis D, Pantelis C, McGorry PD, et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia – A high-resolution magnetic resonance imaging study. Archives of General Psychiatry. 1999;56:133–41. doi: 10.1001/archpsyc.56.2.133. [DOI] [PubMed] [Google Scholar]
- Velakoulis D, Wood SJ, Wong MTH, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis – a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Archives of General Psychiatry. 2006;63:139–49. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- Wager TD, Phan KL, Liberzon I, Taylor SF. Valence, gender, and lateralization of functional brain anatomy in emotion: a meta-analysis of findings from neuroimaging. NeuroImage. 2003;19:513–31. doi: 10.1016/s1053-8119(03)00078-8. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic-resonance-imaging. Neurology. 1992;42:1743–50. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Whittle S, Yap MBH, Yucel M, et al. Prefrontal and amygdala volumes are related to adolescents’ affective behaviors during parent-adolescent interactions. Proceedings of National Academy of Sciences United States of America. 2008a;105:3652–7. doi: 10.1073/pnas.0709815105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, Yücel M, Fornito A, et al. Neuroanatomical correlates of temperament in early adolescents. Journal of American Academy of Child Adolescent Psychiatry. 2008b;47:682–93. doi: 10.1097/CHI.0b013e31816bffca. [DOI] [PubMed] [Google Scholar]
- Williams LM, Barton MJ, Kemp AH, et al. Distinct amygdala-autonomic arousal profiles in response to fear signals in healthy males and females. NeuroImage. 2005;28:618–26. doi: 10.1016/j.neuroimage.2005.06.035. [DOI] [PubMed] [Google Scholar]
- Wood JL, Murko V, Nopoulos P. Ventral frontal cortex in children: morphology, social cognition and femininity/masculinity. Social Cognitive and Affective Neuroscience. 2008;3:168–76. doi: 10.1093/scan/nsn010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap MBH, Allen NB, Ladouceur C. Maternal socialization of positive affect: The impact of "dampening" on adolescent emotion regulation and depressive symptomatology. Child Development. 2008a;79:1416–32. doi: 10.1111/j.1467-8624.2008.01196.x. [DOI] [PubMed] [Google Scholar]
- Yap MBH, Allen NB, O'Shea M, Di Parsia P, Simmons JG, Sheeber L. Development & Psychopathology. Early adolescents' temperament, emotion regulation during mother-child interactions and depressive symptomatology. [DOI] [PubMed] [Google Scholar]
- Yap MBH, Whittle S, Yücel M, et al. Parenting experiences interact with brain structure to predict depressive symptoms in adolescents. Archives of General Psychiatry. 2008b;65:1377–85. doi: 10.1001/archpsyc.65.12.1377. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Transactions of Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]