Abstract
Phobic responses are strong emotional reactions towards phobic objects, which can be described as a deficit in the automatic regulation of emotions. Difficulties in the voluntary cognitive control of these emotions suggest a further phobia-specific deficit in effortful emotion regulation mechanisms. The actual study is based on this emotion regulation conceptualization of specific phobias. The aim is to investigate the neural correlates of these two emotion regulation deficits in spider phobics. Sixteen spider phobic females participated in a functional magnetic resonance imaging (fMRI) study in which they were asked to voluntarily up- and down-regulate their emotions elicited by spider and generally aversive pictures with a reappraisal strategy. In line with the hypothesis concerning an automatic emotion regulation deficit, increased activity in the insula and reduced activity in the ventromedial prefrontal cortex was observed. Furthermore, phobia-specific effortful regulation within phobics was associated with altered activity in medial prefrontal cortex areas. Altogether, these results suggest that spider phobic subjects are indeed characterized by a deficit in the automatic as well as the effortful regulation of emotions elicited by phobic compared with aversive stimuli. These two forms of phobic emotion regulation deficits are associated with altered activity in different medial prefrontal cortex subregions.
Keywords: phobia, emotion regulation, medial prefrontal cortex, cognitive control, fMRI
INTRODUCTION
Recent research has emphasized the importance of emotion regulation for understanding the (neural) processes underlying mental disorders (Campbell-Sills and Barlow, 2007; Etkin and Wager, 2007; Gross and Thompson, 2007; Johnstone et al., 2007; Linehan et al., 2007). Also, in specific phobia strong emotional responses towards phobic objects can be described in terms of diminished down-regulation of emotions. Symptom provocation studies indicate increased neural activity related to phobic reactions in areas such as the amygdala, insula and dorsal anterior and mid-cingulate cortex (Carlsson et al., 2004, Etkin and Wager, 2007; Goossens et al., 2007; Hermann, et al., 2007; Schienle et al., 2005, 2007). There are also studies that show diminished responses in prefrontal cortex (PFC) areas (Johanson et al., 1998, 2006; Carlsson et al., 2004; Hermann et al., 2007; Schienle et al., 2007), e.g. in the medial prefrontal cortex. Such hypoactivations have also been observed in anxiety disorders other than specific phobias (Milad and Rauch, 2006, 2007a; Etkin and Wager, 2007). Emotion regulation studies indicate that these prefrontal cortex regions are involved in both effortful (dorsal PFC and anterior cingulate cortex regions) as well as automatic (ventral PFC regions) emotion regulation processes (Ochsner and Gross, 2005, 2007). Dorsolateral PFC regions which are important for executive and working memory processes as implicated in effortful emotion regulation (Phelps, 2006), have few direct reciprocal connections with emotional appraisal regions (Ochsner and Gross, 2007). So, effortful regulation of emotions is considered to influence brain mechanisms related to automatic regulation processes in order to reduce activity in emotional appraisal regions such as the amygdala (Phelps, 2006). Thus, previous results on the neural correlates of specific phobias might reflect a deficit in the automatic regulation of phobic responses as well as difficulties in the effortful cognitive control of these strong emotions (Hermann et al., 2007). Furthermore, individuals with specific phobias are characterized by irrational cognitions in phobic situations. Such catastrophizing thoughts as a form of effortful up-regulation of emotions might be an important factor in the maintenance of phobic disorders. The neural correlates of these processes in spider phobics have not been investigated as yet.
Moreover, several studies indicate that considering the form and time course of the blood oxygen level-dependent (BOLD) response in various brain regions is especially important in phobic compared with non-phobic responses and during the investigation of emotion regulation processes. Larson et al. (2006) reported that the amygdala response has a faster onset and earlier peak in phobics than in controls and also in response to phobic compared with other negative emotional stimuli. Another study concerning emotion regulation processes in healthy subjects (Goldin et al., 2008) showed that different emotion regulation strategies can be distinguished by the temporal dynamics of the underlying response. All in all, an appropriate model for the underlying hemodynamic response is crucial for studying the neural correlates of emotion regulation especially in specific phobia.
The purpose of this study was to examine the neural correlates of (i) (phobic) emotional responses, (ii) automatic emotional dysregulation in response to phobic compared with non-phobic emotional stimuli, (iii) effortful (phobic) emotion regulation and (iv) deficits in the effortful regulation of phobic compared with generally aversive emotional responses within spider phobic subjects. Therefore, spider phobic individuals were asked to up- and down-regulate their emotions elicited by phobic and generally aversive emotional pictures or to look at the pictures (phobic, aversive, neutral) and respond naturally. A control group was not examined as spider pictures are experienced as affectively neutral by healthy controls (Schienle et al., 2005) and are insofar no appropriate stimuli for the regulation processes investigated in this study.
Successful emotion induction should be reflected in enhanced activity in the amygdala, insula and dACC. Concerning an automatic regulation deficit of phobic compared with non-phobic emotional responses, reduced activity in ventromedial PFC (vmPFC) regions accompanied by higher activity in the amygdala, insula and dACC is assumed for looking at spider compared with aversive pictures. Furthermore, we hypothesize that dorsal and ventral PFC areas are involved in the effortful (up- and down-) regulation of emotional responses by modulating activity in structures like the insula and amygdala. A deficit in the down-regulation of phobic compared to generally aversive emotional reactions is expected to be related to diminished activity in the medial PFC in response to phobic compared with generally aversive stimuli, whereas easier up-regulation in response to phobic stimuli is assumed to more strongly activate the dACC, amygdala and insula accompanied by higher activity in regulatory medial PFC regions. To account for varying time courses and shapes of the BOLD response, we used finite impulse responses to model the underlying hemodynamic response without assuming a specific shape. We expect early and short-lasting responses in the amygdala whereas activity in regulatory prefrontal cortex areas is assumed to be more sustained.
METHODS AND MATERIALS
Participants
Sixteen female patients with a DSM-IV (APA, 1994) diagnosis of specific phobia (animal type: spiders; 300.29) participated in an fMRI-study. Because of our specific interest in phobic vs non-phobic emotion regulation mechanisms within spider phobics and previously observed affectively neutral ratings of spider pictures in healthy controls (Schienle et al., 2005), preventing from a meaningful investigation of effortful regulation in healthy controls, we used a within-subject experimental design (without a control group). Participants were recruited by notices at the university campus and announcements in local newspapers and were paid 20€ for their participation. All of them were right handed. They gave written informed consent after the nature of the experiment had been explained to them. All procedures were conducted according to the Declaration of Helsinki. A diagnostic interview (Margraf, 1994) was completed in a first session to exclude subjects not meeting the criteria for a DSM-IV diagnosis of spider phobia. Current or past mental or neurological disorders and the use of psychotropic drugs were further exclusion criteria. As a further diagnostic tool the spider phobia questionnaire (SPQ; Klorman et al., 1974) was used (criterion: SPQ > 19; M = 22.79, s.d. = 1.72). Two participants were excluded from further analysis due to excessive head movements during scanning, leaving 14 of 16 subjects. The age of the subjects ranged from 19 to 26 years with a mean of 22.1 years (s.d. = 2.8 years).
Stimuli
The stimulus material consisted of 126 pictures that were taken from the International Affective Picture System (IAPS; Lang et al., 1997) and an additional picture set (Schienle et al., 2002), or were compiled by the authors. The spider category consisted of 54 pictures with different spiders in natural scenes or on several parts of human bodies. The aversive stimuli depicted aversive scenes (e.g. injuries, disgusting food), which are highly arousing. As neutral stimuli, scenes depicting objects or humans in different locations were chosen. In every category half of the pictures showed humans whereas the others did not.
Procedure
The study was conducted on 2 days. On the first day, the diagnostic interview was carried out and participants completed the SPQ. They also underwent an anatomical scan in order to get familiar with the MR scanner. The experiment was completed in a second session. The participants were given reappraisal instructions to down- or up-regulate the emotions elicited by the pictures or to look at the pictures and respond naturally. In the down-regulation condition, they were to imagine that the situation depicted was not real and that they were not personally involved. In order to up-regulate the negative emotions elicited by the pictures, they were instructed to imagine that it was a real situation in which they were personally involved. The instruction to look at the pictures meant to respond naturally without altering the appearing emotions and thoughts.
Emotion regulation instructions were given to the participants verbally and in written form outside the scanner. Understanding of the task was ensured by carrying out some practice trials with a clinical psychologist. After that, the subjects performed the regulation task (28 trials; pictures not used during the fMRI experiment) at an external computer to familiarize themselves with the task. During the experiment, the instruction was given for 1.5 s using a white arrow on a black screen pointing up, down or to both sides (left and right) indicating the three regulation instructions (up, down, look). After this, an aversive, a spider or a neutral picture was shown for 6 s, in response to which the subjects were asked to regulate their negative emotions or look at the picture for the entire time of presentation. Neutral stimuli were only presented in the ‘look’ condition. Thereafter, a rating screen appeared that requested participants to indicate the intensity of negative emotions experienced at the moment on a 7-point Likert scale (not at all—very strong). This scale disappeared after pressing a button or automatically 5 s after onset, even if no response had been given. Before the beginning of the next trial, a fixation cross appeared on the screen (mean duration: 2.5 s). The pictures in each category were randomly assigned to the regulation instructions (balanced for pictures depicting humans) and the sequence was also pseudo-randomized for each subject. In the scanner, the participants performed further practice trials (14 trials; ca. 3.5 min) before the experimental run (126 trials; ca. 32 min) was started. After scanning, subjects rated every picture on an external computer on the dimensions valence, arousal, fear and disgust (valence and arousal: self assessment manikin, Bradley and Lang, 1994; fear and disgust: 9-point Likert scales, not at all—very strong). In addition, they indicated the experienced regulation effort when performing the regulation on a 9-point Likert scale (no effort—strong effort).
Data analysis
Self-report data
Ratings were analyzed by computing repeated measures analyses of variance. For the post hoc t-tests, a Bonferroni correction was used to control for multiple comparisons. These statistical analyses were conducted using the general linear model procedure in SPSS 11.0.
fMRI data
Functional imaging data were acquired with a 1.5 T scanner with a standard head coil (Magnetom Symphony, Siemens, Erlangen, Germany). A total of 680 volumes was acquired during the experiment using a T2*-weighted gradient echo-planar imaging sequence with 30 slices covering the whole brain (slice thickness = 4 mm, 1 mm gap, descending, TE = 55 ms, TR = 2860 ms, flip angle = 90°, field of view = 192 mm × 192 mm, matrix size = 64 × 64). The orientation of the axial slices was parallel to the AC-PC line.
Pre-processing of the fMRI data was carried out with SPM2 and first- and second-level analysis with SPM5 (Wellcome Department of Cognitive Neurology). The first three volumes were discarded to allow for saturation effects. Data were realigned to the first image of the series, slice time corrected, normalized to the MNI-brain, and spatially smoothed with a Gaussian kernel of 8 mm FWHM. BOLD responses were modelled using finite impulse responses with a 8.58 s (3× TR) time window [2.86 s time bins (TB) = TR] for each regressor (look spider, down spider, up spider, look aversive, down aversive, up aversive, look neutral) starting 4s after picture onset (TB1-3; early, middle and late phase) to account for the lag of the BOLD response (see Eger et al., 2007). Modelling the complete BOLD response was not conducted to avoid model overfitting. Motion parameters were considered as covariates. Data were high-pass filtered (256 s) and corrected for autocorrelated errors [AR(1)]. Three contrast images per subject and condition were analyzed by means of a second level repeated measurement ANOVA. We computed t-contrasts between picture categories in the look condition (spider vs neutral, aversive vs neutral, spider vs aversive) and between instruction conditions (up vs look; down vs look) within each emotional picture category (spider, aversive) individually for each time bin (TB1-3). To test for the hypothesized interaction effects [contrast: spider(up–look)–aversive(up–look) masked inclusive (P < 0.05) by spider(up–look); contrast: aversive(down–look)–spider(down–look) masked inclusive (P < 0.05) by aversive(down–look)] we also computed t-tests for each time bin (TB1-3). As we were further interested in the association between symptom severity (SPQ) and activity during looking at spider compared with neutral or aversive pictures, we computed simple regression analyses in SPM5.
Statistic images were thresholded using clusters determined by P < .05 (corrected for the whole brain, Worsley, 2001) for exploratory analyses and with P < 0.01 (uncorrected) for region of interest (ROI) analyses. Clusters of at least 10 contiguous voxels were considered in this analysis. Voxel intensities exceeding a significance threshold of P < 0.05 FWE-corrected (Worsley, 2001) were considered significant. ROIs were defined for the amygdala, insula and several prefrontal cortex regions [rostral anterior cingulate cortex (rACC), dorsal anterior and anterior mid-cingulate cortex (dACC), dorsolateral PFC (dlPFC), dorsomedial PFC (dmPFC), ventrolateral PFC (vlPFC) and ventromedial PFC including medial orbitofrontal cortex (vmPFC)]. ROI creation was based on the anatomical parcellation of the MNI brain as described by Tzourio-Mazoyer et al. (2002) and created with MARINA (Walter et al., 2003). For each specific contrast ROI analyses were done only for the hypothesized regions (e.g. spider—neutral: amygdala, insula, dACC).
RESULTS
Negative emotion experience
Subjective emotional experience
The negative emotion induction was successful as spider and aversive compared with neutral pictures led to stronger subjectively experienced negative emotions. This was indicated by higher online ratings of negative emotions, lower post scan valence and higher post scan arousal, disgust and fear ratings for each emotional picture category compared with the neutral category (pairwise t-tests: all P < 0.001; see Table 1).
Table 1.
Results of subjective ratings
| Online rating |
Post-scan ratings |
||||||
|---|---|---|---|---|---|---|---|
| Negative emotions (1–7) |
Regulation effort (1–9) |
Valence (1–9) |
Arousal (1–9) |
Fear (1–9) |
Disgust (1–9) |
||
| Picture category | Instruction | M (s.d) | M (s.d.) | M (s.d.) | M (s.d.) | M (s.d.) | M (s.d.) |
| Spider | Look | 5.52 (0.55) | – | ||||
| Down | 4.50 (0.61) | 6.50 (0.92) | 1.70 (0.58) | 7.30 (0.98) | 7.32 (1.08) | 7.55 (1.45) | |
| Up | 6.19 (0.51) | 2.50 (1.05) | |||||
| Aversive | Look | 4.10 (0.68) | – | ||||
| Down | 3.02 (0.61) | 3.95 (1.30) | 3.22 (0.50) | 5.00 (1.38) | 3.71 (1.56) | 4.66 (0.88) | |
| Up | 5.10 (0.68) | 4.08 (0.98) | |||||
| Neutral | Look | 1.18 (0.22) | – | 7.52 (1.13) | 1.07 (0.10) | 1.22 (0.34) | 1.07 (0.10) |
Post-scan ratings of regulation effort and valence, arousal, fear and disgust ratings for each picture category. Intensity of negative emotions after regulation (online).
Neural Responses (spider – neutral & aversive – neutral)
As expected, phobic stimuli evoked stronger activity in regions involved in the attentive processing of emotional stimuli (amygdala, insula, parietal and frontal cortex regions; Table 2). Furthermore, symptom severity was positively correlated with activity in the left and (marginally significant) with activity in the right amygdala during the middle phase (left: MNI: −24, −6, −18; t = 3.74; right: MNI: 24, −6, −12; t = 3.45; P < 0.08).
Table 2.
Looking at spider/aversive pictures compared with neutral pictures for the early, middle and late phase
| Early |
Middle |
Late |
|||||
|---|---|---|---|---|---|---|---|
| Region | Side | x y z | t | x y z | t | x y z | t |
| Look: Spider–Neutral | |||||||
| ROI analyses | |||||||
| Amygdala | L | – | −18 −3 −15 | 2.97 | − | ||
| dACC | L | −3 30 33 | 4.14 | −3 −15 39 | 3.85 | 0 27 33 | 4.40 |
| L | – | – | 0 3 27 | 3.24 | |||
| R | 3 −3 42 | 3.73 | 3 18 42 | 3.78 | 3 30 30 | 4.71 | |
| Insula | L | −39 0 3 | 4.30 | −39 12 −6 | 3.89 | – | |
| Exploratory analysis | |||||||
| Inferior Parietal gyrus | L | −39 −51 54 | 6.84 | −39 −51 57 | 5.85 | – | |
| Superior Parietal gyrus | R | 39 −48 60 | 5.65 | 42 −48 57 | 5.21 | – | |
| Supramarginal gyrus | L | −60 −30 39 | 5 37 | – | – | ||
| Look: Aversive–Neutral | |||||||
| ROI analyses | |||||||
| dACC | L | – | −9 24 30 | 3.59 | – | ||
| R | – | – | 3 30 36 | 4.22 | |||
| Exploratory analysis | |||||||
| Inferior Temporal gyrus | R | – | 54 −60 −9 | 7.48 | – | ||
| Supramarginal gyrus | R | – | 39 −36 42 | 5.94 | – | ||
| Inferior Occipital gyrus | R | – | 30 −93 −3 | 5.56 | – | ||
| Inferior Temporal gyrus | L | – | −42 −51 −18 | 5.37 | – | ||
| Middle frontal gyrus | R | – | – | 48 45 18 | 6.00 | ||
Side: hemisphere; L: left; R: right; x y z: MNI coordinates; t: maximum t-value.
Aversive pictures also led to stronger activation in several temporal, parietal and frontal cortex regions (see Table 2).
Automatic emotional dysregulation
Subjective emotional experience
According to our hypothesis of an automatic emotion regulation deficit in response to phobic compared to generally aversive material (within spider phobics) the intensity of negative emotions during simply looking at the pictures was higher for spider compared with aversive pictures (t-test: P < 0.001; see Table 1). The post scan valence, arousal, fear and disgust ratings (see Table 1) showed the same pattern of results with stronger negative emotions for spider compared with aversive pictures (pairwise t-tests: all P < 0.001).
Neural responses (spider vs aversive)
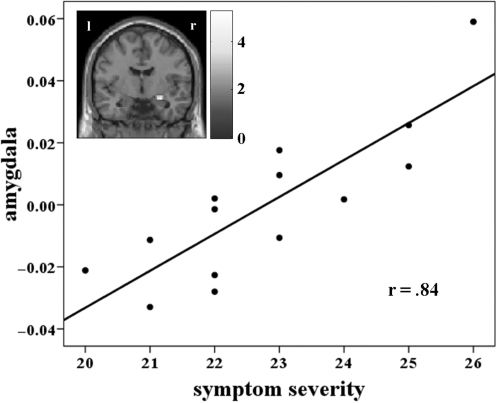
Responses to spider compared with aversive pictures during simply looking at them, led to stronger early activation in regions important for (visual) attention processes (right precuneus, inferior parietal cortex, right mid-cingulate cortex), in prefrontal control regions (left inferior frontal gyrus, dACC) and in the insula. During the middle phase significant activity was observed in the right mid-cingulate cortex, the bilateral superior temporal pole, the left supplementary motor area and the bilateral dACC (Table 3). Only the left insula showed a stable response during the early, middle and late processing whereas the expected amygdala activity could not be observed. However, we found a strong correlation between right amygdala activity and symptom severity (SPQ) during the early phase (MNI: 27, −6, −15; t = 5.29; r = 0.84) for spider compared with aversive pictures (Figure 1).
Table 3.
Looking at spider pictures compared with aversive pictures for the early, middle and late phase
| Early |
Middle |
Late |
|||||
|---|---|---|---|---|---|---|---|
| Region | Side | x y z | t | x y z | t | x y z | t |
| Look: Spider–Aversive | |||||||
| ROI analyses | |||||||
| dACC | L | −3 30 33 | 4.39 | −6 12 36 | 4.73 | – | |
| R | 3 −9 48 | 4.42 | 3 12 42 | 4.63 | – | ||
| Insula | L | −45 9 0 | 4.53 | −42 3 0 | 4.84 | −45 0 –3 | 3.60 |
| R | 36 −21 3 | 3.88 | – | – | |||
| Exploratory analysis | |||||||
| Precuneus | R | 9 −72 42 | 6.34 | – | – | ||
| Inferior parietal gyrus | L | −45 −51 54 | 5.45 | – | – | ||
| R | 51 −51 48 | 5.83 | – | – | |||
| Mid-cingulate cortex | R | 3 −27 30 | 5.68 | 0 −27 30 | 6.10 | – | |
| Inferior frontal gyrus | L | −48 9 0 | 5.47 | – | – | ||
| Superior temporal pole | L | – | −57 3 0 | 6.07 | – | ||
| R | – | 63 9 0 | 6.05 | – | |||
| Supplementary motor area | L | – | −3 −9 72 | 5.52 | – | ||
| Look: Aversive–Spider | |||||||
| ROI analyses | |||||||
| vmPFC | L | – | −3 45 −18 | 3.34a | −3 48 −18 | 4.91 | |
| R | – | – | 3 48 −18 | 4.16 | |||
Side: hemisphere; L: left; R: right; x y z: MNI coordinates; t: maximum t-value.
ap < .08.
Fig. 1.
Correlation between right amygdala activity (MNI: 27, −6, −15) and symptom severity for looking at spider compared with aversive pictures (early); bar displays t-values; threshold: P < 0.05.
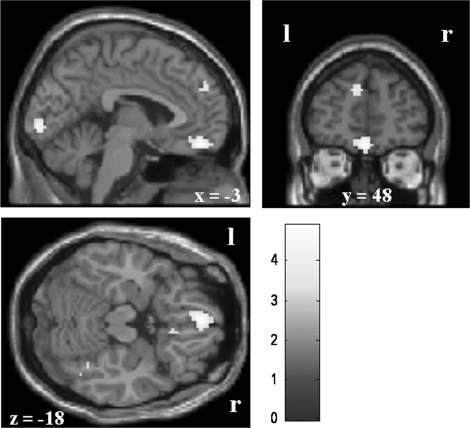
The hypothesized phobia material-specific automatic emotional dysregulation is further reflected in (marginally significant) diminished activity in ventromedial prefrontal cortex regions during the middle (left vmPFC, P < 0.08; Table 3 and Figure 2) and late processing phases (left and right vmPFC) when simply looking at spider compared with aversive pictures.
Fig. 2.
Phobia-specific diminished activity during looking at spider compared with aversive pictures in the vmPFC (late; left: MNI: −3, 48, −18; right: MNI: 3, 48, −18); bar displays t-values; threshold: P < 0.001.
Effortful emotion regulation
Subjective experience of emotional intensity and regulation effort
Online ratings of the intensity of negative emotions after regulation showed an interaction between picture category (spider and aversive) and regulation instruction [look, up and down; F(1.8, 23.5) = 11.23, P < 0.01; Table 1]. The regulation was successful in both the spider and aversive picture category and led to significant stronger emotions in response to phobic pictures compared with aversive pictures within each regulation instruction (all P < 0.001). However, in response to aversive pictures a stronger up-regulation (up > look) was observed compared with phobic pictures (P < 0.01).
The regulation effort showed an interaction between picture category and regulation instruction [F(1,13) = 64.66, P < 0.001]. In response to spider pictures regulation was associated with more effort for down-regulation compared with up-regulation (P < 0.001). However, in the aversive picture category up-regulation did not differ from down-regulation. A comparison of the emotional picture categories revealed higher effort in the spider category for down-regulation and lower effort for up-regulation of emotions (all P < 0.001).
Neural responses
(i) Down vs look
On the neural level down-regulation was associated with activity in reappraisal-related structures towards spider pictures (early: dlPFC and dmPFC; Table 4) and aversive pictures (early: right rACC; middle: right dlPFC, dmPFC, vmPFC, rACC, left vlPFC). A down-regulation of activity was found in the insula (early and late) and dACC (middle) for spider but not for aversive pictures.
Table 4.
Down-regulation compared with looking at pictures within the spider and the aversive category and for aversive—spider pictures for the early, middle and late phase
| Early |
Middle |
Late |
||||||
|---|---|---|---|---|---|---|---|---|
| Contrast | Region | Side | x y z | t | x y z | t | x y z | T |
| Down–Look | ||||||||
| Spider | dlPFC | R | 24 51 27 | 4.63 | – | – | ||
| dmPFC | R | 12 60 12 | 3.61 | – | – | |||
| Aversive | dlPFC | R | – | 27 60 24 | 4.18 | – | ||
| R | – | 42 33 45 | 4.09 | – | ||||
| dmPFC | R | – | 12 66 18 | 3.75 | – | |||
| vmPFC | R | – | 6 39 −6 | 3.66 | – | |||
| rACC | R | 12 39 9 | 3.56 | 3 39 −3 | 3.64 | – | ||
| vlPFC | L | – | −60 18 6 | 4.02 | – | |||
| Aversive–Spider | rACC | R | 12 39 9 | 3.62 | – | – | ||
| dmPFC | R | – | 9 69 18 | 3.69 | – | |||
| Look–Down | ||||||||
| Spider | Insula | L | −33 3 15 | 3.70 | – | – | ||
| dACC | L | – | −6 −6 48 | 3.83 | – | |||
| Insula | L | – | – | −45 0 −3 | 3.65 | |||
Exploratory analyses for all contrasts and the ROI analysis for Look–Up in the aversive category showed no significant results; side: hemisphere; L: left; R: right; x y z: MNI coordinates; t: maximum t-value.
(ii) Up vs look
Up-regulation of emotional responses towards phobic pictures (up > look) was associated with activity in numerous emotional experience and regulation-related brain regions (early: right precentral gyrus, left supramarginal gyrus, left vmPFC, dACC, rACC, vlPFC, amygdala, insula; middle: dACC, insula, left vlPFC; Table 5). For aversive pictures a similar activation pattern (early: dACC, left rACC, vlPFC, amygdala, left insula; middle: left calcarine fissure, right superior temporal pole, right cerebellum, dACC, insula, left vlPFC) with additional significant activity in dorsolateral and dorsomedial PFC areas (early and middle phase) was observed (Table 5).
Table 5.
Up-regulation compared with looking at pictures within the spider and the aversive category and for spider—aversive pictures for the early, middle and late phase
| Early |
Middle |
Late |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Contrast | Region | Side | x y z | t | x y z | t | x y z | t | |
| Up–Look | |||||||||
| Spider | ROI analyses | ||||||||
| vmPFC | L | −12 36 −12 | 3.54 | – | – | ||||
| dACC | L | −3 18 30 | 4.64 | −3 15 33 | 4.83 | – | |||
| R | 6 12 39 | 4.36 | 6 12 33 | 4.64 | – | ||||
| rACC | L | 0 36 0 | 4.05 | – | – | ||||
| R | 3 36 −3 | 3.84 | – | – | |||||
| vlPFC | L | −42 9 18 | 4.63 | −48 6 6 | 5.00 | – | |||
| R | 48 12 0 | 3.88 | – | – | |||||
| R | 63 12 21 | 3.78 | – | – | |||||
| Amygdala | L | −27 −3 −18 | 3.27 | – | – | ||||
| R | 24 0 −15 | 3.89 | – | – | |||||
| Insula | L | −45 6 6 | 3.86 | −45 6 6 | 4.56 | – | |||
| R | 39 9 −9 | 4.60 | 36 9 3 | 3.79 | – | ||||
| R | – | 27 18 −21 | 3.79 | – | |||||
| Exploratory analysis | |||||||||
| Precentral gyrus | R | 24 −21 69 | 6.71 | – | – | ||||
| Supramarginal gyrus | L | −57 −36 24 | 5.15 | – | – | ||||
| Aversive | ROI analyses | ||||||||
| dlPFC | L | −12 57 30 | 4.54 | −21 69 9 | 5.13 | – | |||
| L | −33 15 54 | 4.05 | −36 18 51 | 3.93 | – | ||||
| L | −30 66 3 | 3.98 | – | – | |||||
| R | 24 51 36 | 4.28 | – | – | |||||
| dmPFC | L | −9 54 6 | 4.92 | −3 66 3 | 3.96 | – | |||
| R | 9 63 21 | 3.94 | – | – | |||||
| dACC | L | −6 9 39 | 4.03 | −3 18 33 | 3.59 | – | |||
| R | 12 9 36 | 4.57 | 3 15 42 | 3.85 | – | ||||
| rACC | L | −3 36 30 | 4.14 | – | – | ||||
| L | −3 51 3 | 3.86 | – | – | |||||
| vlPFC | L | −48 9 0 | 4.73 | −51 3 6 | 4.68 | – | |||
| R | 63 21 3 | 4.05 | – | – | |||||
| Amygdala | L | −30 3 −18 | 3.60 | – | – | ||||
| R | 30 3 −21 | 3.60 | – | – | |||||
| Insula | L | −45 9 −3 | 3.95 | −48 3 3 | 4.56 | – | |||
| R | – | 39 6 −6 | 3.91 | – | |||||
| Exploratory analysis | |||||||||
| Calcarine fissure | L | – | −6 −99 0 | 6.39 | – | ||||
| Superior temporal pole | R | – | 66 3 3 | 5.45 | – | ||||
| Cerebellum | R | – | 15 −90 −33 | 5.45 | – | ||||
| Spider–Aversive | ROI analyses | ||||||||
| rACC | L | 0 36 −3 | 3.66 | – | – | ||||
| R | 3 36 −3 | 3.48 | – | – | |||||
| Look–Up | |||||||||
| Spider | Exploratory analysis | ||||||||
| Inferior parietal gyrus | R | – | – | 39 −66 57 | 5.97 | ||||
The contrast up–look for aversive–spider pictures showed no significant exploratory results; side: hemisphere; L: left; R: right; x y z: MNI coordinates; t: maximum t-value.
Looking at spider pictures compared with up-regulating phobic responses resulted in stronger activity in the right inferior parietal cortex (late), whereas no differences were observed for aversive pictures.
(iii) Down—look (aversive—spider)
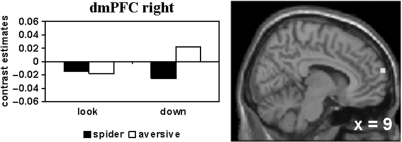
To figure out regulation deficits in response to phobic compared to generally aversive stimuli, regulatory activity was compared between the emotional picture categories (Tables 4 and 5). The deficit in the down-regulation of responses elicited by phobic material is reflected in decreased activity in the right rACC (early) and the right dmPFC (middle; Figure 3) when down-regulating phobic compared to generally aversive emotional responses within spider phobics.
Fig. 3.
Phobia-specific diminished activity during down-regulation of emotions [aversive (down–look)–spider (down–look) masked inclusive with aversive (down–look) at P < 0.05] in the right dmPFC (middle; MNI: 9, 69, 18); threshold: P < 0.001.
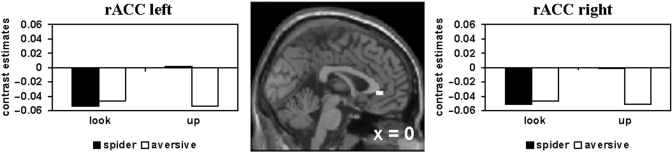
(iv) Up—look (spider—aversive)
Activation during up-regulation of phobic compared to generally aversive emotional responses (spider—aversive) was found in the left and right rACC (early phase; Figure 4).
Fig. 4.
Phobia-specific enhanced activity during up-regulation of emotions [spider (up–look)–aversive (up–look) masked inclusive with spider (up–look) at P < 0.05] in the rACC (early; left: MNI: 0, 36, −3; right: MNI: 3, 36, −3); threshold: P < 0.001.
DISCUSSION
This study was conducted to disentangle the neural correlates of automatic emotion regulation deficits and problems in the effortful regulation of phobic compared with non-phobic emotional responses in spider phobic subjects. Emotion induction was successful and led to stronger activity in several areas including the amygdala, insula and dACC. These regions have repeatedly been shown to be involved in the processing of phobic stimuli (Etkin and Wager, 2007).
Automatic emotional dysregulation
In accordance with an automatic regulation deficit of phobic compared to non-phobic emotional responses within phobics enhanced activity in the dACC and the left insula was observed for spider compared with aversive pictures. The dACC plays an important role in the expression of (conditioned) fear responses (Milad et al, 2007b; Quirk and Beer, 2006; Etkin and Wager, 2007) and in the up-regulation of amygdala activity (Quirk and Beer, 2006; Corcoran and Quirk, 2007). Interestingly, there was no significant amygdala activity in the ‘look’ condition for phobic compared with generally aversive pictures. However, the correlation between amygdala activity and symptom severity supports the idea of a hyperresponsive amygdala-centered fear network as a main feature of phobic disorders.
As expected, we found reduced activity in the vmPFC which is assumed to reflect a deficit in the automatic regulation of emotions in response to phobic stimulation (Hermann et al., 2007). This has also been observed in other anxiety disorders as, e.g. posttraumatic stress disorder (Etkin and Wager, 2007; Milad and Rauch, 2006, 2007a). The time course of neural activity in the vmPFC was characterized by a late deactivation towards spider stimuli compared with aversive stimuli. The finding of depressed activity in this region is known in both rat and human fear conditioning and extinction studies (Garcia et al.,1999, Milad et al., 2007c,Phelps et al., 2004). A bottom-up influence of the amygdala on the PFC is assumed to be related to this depressed activity in the vmPFC, although no significant amygdala activity for spider vs aversive pictures was found in this study. Milad et al. (2007c) supposed that such a signal depression might be needed to allow the expression of fear responses. Heterogenous previous results concerning vmPFC activity in specific phobia (Straube et al., 2006a; Hermann et al., 2007; Schienle et al., 2007) might partly be due to the specific time course of deactivation (late response) observed in this study. Previous studies modelled short responses (Dilger et al., 2003; Straube et al., 2006a) or defined other regions of interest (Straube et al., 2006b). However, studies showing diminished (vm)PFC activity used longer stimulus presentation times (Johanson et al., 1998, 2006; Carlsson et al., 2004; Hermann et al., 2007; Schienle et al., 2007). Hemodynamic modelling as done in the present study might therefore help to better understand the underlying brain mechanisms.
It is very important to emphasize that the observed differences in response to spider vs. aversive pictures were only found within a spider phobic group, and so do not necessarily be present in comparison to control subjects. Since our design does not allow for a meaningful examination of non-phobic subjects, due to the effortful regulation conditions in response to phobic stimuli, we need to restrict the interpretation of our results to individuals with spider phobia.
Effortful down-regulation of emotions
Down-regulation of emotional responses resulted in enhanced activity in dorsal PFC areas as seen in other emotion regulation studies (Ochsner et al., 2004; Ochsner and Gross, 2005, 2007; Phan et al., 2005; Goldin et al., 2008; Johnstone et al., 2007). Since the dlPFC has few direct connections to subcortical structures like the amygdala (Ochsner and Gross, 2007), it is assumed that the regulation of activity in these structures takes place by influencing (ventro)medial PFC areas (e.g. rACC), which in turn have reciprocal connections to these subcortical structures (Phelps, 2006; Urry et al., 2006; Johnstone et al., 2007). In line with this, activity in the vmPFC and rACC was observed during down-regulation of emotions in response to aversive pictures; yet, it was absent for down-regulation of phobic responses. However, activity in vlPFC regions was found for both picture categories and supports the importance of this region for the regulation of emotions (Lieberman et al., 2007). Regarding the role of the insula in the processing of emotions (Stark et al., 2007; Wager and Feldman Barrett, 2004), a reduction of insula activity during phobic down-regulation might be related to the attenuation of the emotional experience. This effect was also seen in the dACC, a region critically involved in the processing of phobogenic material in phobic subjects (Schienle et al., 2007; Etkin and Wager, 2007). In contradiction to our hypotheses, we did not find a down-regulation of activity in the amygdala. Furthermore, during down-regulation of emotions induced by aversive pictures no significant attenuation of activity in any brain region could be observed. This might be due to stronger evoked activity for spider vs. aversive pictures (as seen in the ‘look’ condition e.g. in the dACC and insula) resulting in a higher probability of significant down-regulation in these regions in the phobic category.
Diminished activity in the dmPFC during effortful down-regulation of phobic compared to non-phobic emotional responses within spider phobics is in accordance with previous results in blood-injection-injury phobic patients (Hermann et al., 2007). As this region is important in the effortful down-regulation of emotions (Ochsner and Gross, 2005, 2007; Goldin et al., 2008), the current results support the role of this region in a phobia-specific down-regulation deficit within spider phobics. In addition, we observed stronger activity in the right rACC for non-phobic down-regulation, a region involved in the automatic regulation of emotions (Ochsner and Gross, 2005). As effortful emotion regulation mechanisms are thought to modulate activity in subcortical structures by influencing neural systems involved in more automatic processes as, e.g extinction (Phelps, 2006), it is supposed that phobics are less capable in recruiting this brain region during down-regulation of emotions in response to phobic compared with generally aversive material. Regarding the timing differences, it is noticeable that dlPFC and dmPFC activity appears earlier during decreasing emotions in response to phobic than aversive stimuli. Phobic compared to non-phobic individuals are typically characterized by higher vigilance followed by stronger avoidance responses to phobic stimuli (Weierich et al., 2008). As importantly pointed out by a reviewer, the early phobic PFC activity might be associated with immediate cognitive avoidance in response to phobic stimuli, preventing the development of a full emotional response. According to the Emotional Processing Theory (Foa and Kozak, 1986; Foa et al., 2006) such cognitive avoidance interferes with a full activation of the fear response that is necessary for habituation during treatment. The down-regulation instruction might have triggered cognitive avoidance mechanisms in response to phobic material, resulting in diminished activity in the insula. This cognitive avoidance might in the long-term be associated with the maintenance of phobic fear.
Effortful up-regulation of emotions
As expected, the up-regulation of emotions led to increased activity in regions like the amygdala, insula and dACC. According to Larson et al. (2006), the amygdala had an early activation peak and did not show sustained activity. On the other hand insula and dACC showed longer lasting responses (early and middle phases) during up-regulation. The amygdala might therefore be necessary for the initiation but not the further monitoring of up-regulation of emotions. Further activity was found in ventral medial PFC regions (e.g. rACC), which are important in the regulation of activity in structures such as the amygdala.
The direct comparison of picture categories (spider vs. aversive) revealed a stronger involvement of the rACC in the up-regulation of emotional responses towards phobic compared with non-phobic material. As mentioned above, higher activity in rACC was also observed during down-regulation for aversive pictures. Thus, this region seems to be critical for effective emotion regulation because it shows stronger activity when emotion regulation can more easily be achieved. The activation pattern in the rACC could be related to the maintenance of phobic disorders; mainly, because of its importance in the regulation of e.g. amygdala activity (Quirk, 2007). This might occur because of a more automatic and easier up-regulation and a more difficult down-regulation of phobic compared with non-phobic emotional responses within spider phobics in the anticipatory, actual and late emotional processing in phobic situations. Diminished rACC activity is frequently found in posttraumatic stress disorder and is—within this disorder—associated with a reflexive emotion regulation deficit (Etkin and Wager, 2007; Rauch et al., 2006).
Emotion regulation conceptualization of specific phobias
Based on these results we propose a model that attempts to explain phobic disorders from an emotion regulation perspective. We assume that phobic compared to non-phobic emotional reactions within phobics are characterized by a deficient response in the neural mechanisms involved in automatic emotion regulation as reflected especially in depressed activity in the vmPFC. Anomalies in effortful emotion regulation mechanisms in response to phobic material might be associated with difficulties in the recruitment of the dmPFC and rACC during down-regulation. Enhanced activity in the rACC further seems to be related to a facilitated up-regulation of phobic emotional responses. Timing results further suggest that cognitive avoidance might play a role in phobic down-regulation, possibly leading to an immediate suppression of the emotional response rather than an objective evaluation of threat, as indicated by early prefrontal cortex and reduced early insula activity. One can say by summarizing that an easier up-regulation by catastrophizing thoughts and a reduced ability to voluntarily down-regulate these emotions might be an essential factor in the maintenance of phobic disorders. At this point it seems to be critical to emphasize that the results and conclusions of this study are restricted to emotional processes within spider phobic individuals. Due to the experimental design, a meaningful investigation of non-phobic subjects is not possible. So, the abovementioned deficits characterize emotional processing in response to phobic vs non-phobic emotional stimuli in phobics rather than differences between phobic and non-phobic persons. Future studies are needed to clarify the role of differential variables as for example the habitual use of reappraisal or suppression (John and Gross, 2007) in the investigation of the neural correlates and the treatment of phobic disorders.
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington, DC: American Psychiatric Press; 1994. [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: the Self-Assessment Manikin and the Semantic Differential. Journal of Behavior Therapy and Experimental Psychiatry. 1994;25:49–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH. Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: The Guilford Press; 2007. pp. 542–59. [Google Scholar]
- Carlsson K, Petersson KM, Lundqvist D, Karlsson A, Ingvar M, Öhman A. Fear and the amygdala: manipulation of awareness generates differential cerebral responses to phobic and fear-relevant (but nonfeared) stimuli. Emotion. 2004;4:340–53. doi: 10.1037/1528-3542.4.4.340. [DOI] [PubMed] [Google Scholar]
- Corcoran KA, Quirk GJ. Recalling safety: cooperative functions of the ventromedial prefrontal cortex and the hippocampus in extinction. CNS Spectrums. 2007;12:200–6. doi: 10.1017/s1092852900020915. [DOI] [PubMed] [Google Scholar]
- Dilger S, Straube T, Mentzel H-J, et al. Brain activation to phobia-related pictures in spider phobic humans: an event-related functional magnetic resonance imaging study. Neuroscience Letters. 2003;348:29–32. doi: 10.1016/s0304-3940(03)00647-5. [DOI] [PubMed] [Google Scholar]
- Eger E, Henson RN, Driver J, Dolan RJ. Mechanisms of top-down facilitation in perception of visual objects studied by FMRI. Cerebral Cortex. 2007;17:2123–33. doi: 10.1093/cercor/bhl119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, Wager TD. Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. American Journal of Psychiatry. 2007;164:1476–88. doi: 10.1176/appi.ajp.2007.07030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa EB, Huppert JD, Cahill SP. Emotional processing theory: an update. In: Rothbaum BO, editor. Pathological Anxiety: Emotional Processing in Etiology and Treatment. New York: The Guilford Press; 2006. pp. 3–24. [Google Scholar]
- Foa EB, Kozak MJ. Emotional processing of fear: exposure to corrective information. Psychological Bulletin. 1986;99:20–35. [PubMed] [Google Scholar]
- Garcia R, Vouimba RM, Baudry M, Thompson RF. The amygdala modulates prefrontal cortex activity relative to conditioned fear. Nature. 1999;402:294–6. doi: 10.1038/46286. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–86. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Schruers K, Peeters R, Griez E, Sunaert S. Visual presentation of phobic stimuli: amygdala activation via an extrageniculostriate pathway? Psychiatry Research. 2007;155:113–20. doi: 10.1016/j.pscychresns.2006.12.005. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: conceptual foundations. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: The Guilford Press; 2007. pp. 3–24. [Google Scholar]
- Hermann A, Schäfer A, Walter B, Stark R, Vaitl D, Schienle A. Diminished medial prefrontal cortex activity in blood-injection-injury phobia. Biological Psychology. 2007;75:124–30. doi: 10.1016/j.biopsycho.2007.01.002. [DOI] [PubMed] [Google Scholar]
- Johanson A, Risberg J, Tucker DM, Gustafson L. Changes in frontal lobe activity with cognitive therapy for spider phobia. Applied Neuropsychology. 2006;13:34–41. doi: 10.1207/s15324826an1301_5. [DOI] [PubMed] [Google Scholar]
- Johanson A, Gustafson L, Passant U, et al. Brain function in spider phobia. Psychiatry Research. 1998;84:101–11. doi: 10.1016/s0925-4927(98)00051-1. [DOI] [PubMed] [Google Scholar]
- John O, Gross JJ. Individual differences in emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: The Guilford Press; 2007. pp. 351–72. [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. Journal of Neuroscience. 2007;27:8877–84. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klorman R, Weerts T, Hastings J, Melamed B, Lang P. Psychometric description of some fear-specific questionnaires. Behavior Therapy. 1974;5:401–9. [Google Scholar]
- Lang PJ, Bradley M, Cuthbert B. International Affective Picture System. Center for Research in Psychophysiology, University of Florida Gainesville; 1997. [Google Scholar]
- Larson CL, Schaefer HS, Siegle GJ, Jackson CAB, Anderle MJ, Davidson RJ. Fear is fast in phobic individuals: amygdala activation in response to fear-relevant stimuli. Biological Psychiatry. 2006;60:410–7. doi: 10.1016/j.biopsych.2006.03.079. [DOI] [PubMed] [Google Scholar]
- Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Baldin MW. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychological Science. 2007;18:421–8. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- Linehan MM, Bohus M, Lynch TR. Dialectical behavior therapy for pervasive emotion dysregulation: theoretical and practical underpinnings. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: The Guilford Press; 2007. pp. 581–605. [Google Scholar]
- Margraf J. Berlin. Heidelberg: Springer; 1994. Diagnostic short-interview for mental disorders. Mini DIPS [Diagnostisches Kurz-Interview bei psychischen Störungen. Mini DIPS] [Google Scholar]
- Milad MR, Rauch SL. The orbitofrontal cortex and anxiety disorders. In: Zald DH, Rauch SL, editors. The Orbitofrontal Cortex. Oxford: Oxford University Press; 2006. pp. 523–44. [Google Scholar]
- Milad MR, Rauch SL. The role of the orbitofrontal cortex in anxiety disorders. Annals of the New York Academy of Sciences. 2007a;1121:546–61. doi: 10.1196/annals.1401.006. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ, Pitman RK, Orr SP, Fischl B, Rauch SL. A role for the human dorsal anterior cingulate cortex in fear expression. Biological Psychiatry. 2007b;62:1191–4. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- Milad MR, Wright CI, Orr SP, Pitman RK, Quirk GJ, Rauch SL. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biological Psychiatry. 2007c;62:446–54. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–9. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The neural architecture of emotion regulation. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: The Guilford Press; 2007. pp. 87–109. [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, et al. For better or for worse: neural systems supporting the cognitive down- and up-regulation of negative emotion. Neuroimage. 2004;23:483–99. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–9. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA. Emotion and cognition: insights from studies of the human amygdala. Annual Review of Psychology. 2006;57:27–53. doi: 10.1146/annurev.psych.56.091103.070234. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Quirk GJ. Prefrontal-amygdala interactions in the regulation of fear. In: Gross JJ, editor. Handbook of Emotion Regulation. New York: The Guilford Press; 2007. pp. 27–46. [Google Scholar]
- Quirk GJ, Beer JS. Prefrontal involvement in the regulation of emotion: convergence of rat and human studies. Current Opinion in Neurobiology. 2006;16:723–7. doi: 10.1016/j.conb.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research–past, present, and future. Biologial Psychiatry. 2006;60:376–82. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Walter B, Stark R, Vaitl D. Brain activation of spider phobics towards disorder-relevant, generally disgust- and fear-inducing pictures. Neuroscience Letters. 2005;388:1–6. doi: 10.1016/j.neulet.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schäfer A, Hermann A, Rohrmann S, Vaitl D. Symptom provocation and reduction in patients suffering from spider phobia: an fMRI study on exposure therapy. European Archives of Psychiatry and Clinical Neuroscience. 2007;257:486–93. doi: 10.1007/s00406-007-0754-y. [DOI] [PubMed] [Google Scholar]
- Schienle A, Stark R, Walter B, et al. The insula is not specifically involved in disgust processing: an fMRI study. Neuroreport. 2002;13:2023–6. doi: 10.1097/00001756-200211150-00006. [DOI] [PubMed] [Google Scholar]
- Stark R, Zimmermann M, Kagerer S, et al. Hemodynamic brain correlates of disgust and fear ratings. Neuroimage. 2007;37:663–73. doi: 10.1016/j.neuroimage.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Straube T, Mentzel H-J, Miltner WHR. Neural mechanisms of automatic and direct processing of phobogenic stimuli in specific phobia. Biological Psychiatry. 2006a;59:162–70. doi: 10.1016/j.biopsych.2005.06.013. [DOI] [PubMed] [Google Scholar]
- Straube T, Glauer M, Dilger S, Mentzel H-J, Miltner WHR. Effects of cognitive-behavioral therapy on brain activation in specific phobia. Neuroimage. 2006b;29:125–35. doi: 10.1016/j.neuroimage.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, et al. Automated anatomical labelling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15:273–89. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience. 2006;26:4415–25. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Feldman Barrett L. From affect to control: Functional specialization of the insula in motivation and regulation. 2004 www.apa.org/PsychExtra.
- Walter B, Blecker C, Kirsch P, et al. MARINA: an easy to use tool for the creation of Masks for Region of Interest Analyses. Neuroimage. 2003;19 CD-ROM. [Google Scholar]
- Weierich MR, Treat TA, Hollingworth A. Theories and measurement of visual attentional processing in anxiety. Cognition and Emotion. 2008;22:985–1018. [Google Scholar]
- Worsley KJ. Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM, editors. Functional MRI: An Introduction to Methods. Oxford: Oxford University Press; 2001. pp. 251–70. [Google Scholar]