Abstract
The present study examines the influence of induced affective state on performance monitoring. The electroencephalogram was recorded while human participants engaged in a speeded choice-reaction time task commonly used to examine performance monitoring processes. Prior to the experiment, participants were randomly allocated to receive either encouraging or derogatory feedback during task execution. Feedback was based on each participant's reaction times. Affective state was assessed prior and after the experiment with a state questionnaire. Although participants of both feedback groups loaded high on items describing tiredness in the state questionnaire, only those with derogatory feedback loaded higher on negative state items and lower on positive state items after completion of the experiment. The error-related negativity (ERN) as an index of performance monitoring was increased after derogatory feedback; this difference was not seen at the beginning of the experiment. Negative state correlated significantly with ERN amplitude. The error positivity, a later component following errors, did not differ between feedback groups. This study provides further evidence that changes in affective state influence how we monitor ongoing behavior.
Keywords: event-related brain potentials (ERPs), error-related negativity (ERN), error positivity (Pe), performance monitoring, emotion, feedback
INTRODUCTION
Many cognitive processes are modulated by affective state. Positive affect improves performance in a variety of cognitive activities, among them memory (Lee and Sternthal, 1999), problem solving (Isen et al., 1987; Isen, 2001), executive attention (Ashby et al., 2002) and decision making (Isen, 2001). Dreisbach and Goschke describe positive mood as a mediator between antagonistic constraints of cognitive control processes: Positive affect increases flexibility, but at the cost of increased distractibility (Dreisbach, 2006; Dreisbach and Goschke, 2004). Whereas it is well known that negative affect as it occurs in depression significantly impairs various aspects of cognition (Ravnkilde et al., 2002), laboratory studies on the impact of negative affect on cognitive performance are scarce. This is surprising, since negative affect might be of special biological importance, because it accompanies environmental events which might be harmful or threatening. Some research indicates that negative affect promotes more careful, risk-averse and analytic behavioral strategies (Park and Banaji, 2000; Bolte et al., 2003). In general, it can be said that there is no simple relation in that positive and negative affect show opposite effects on behavior. Moreover, results for the impact of negative affect on cognition are more equivocal (Mitchell and Phillips, 2007).
In the present investigation, we are interested in the effect of induced affect on performance monitoring. This might be a particularly fruitful area of research as there are well defined behavioral (e.g. post-error slowing) and neurophysiological markers available to characterize performance monitoring. Moreover, a specific aspect of performance monitoring, error detection, has been linked to the dopaminergic system, which is also thought to be amenable to affective manipulations. A neurophysiological marker of performance monitoring is the error-related negativity (ERN), a negative component in the event-related brain potential emerging directly after error commission (Falkenstein et al., 1991; Gehring et al., 1993). The ERN is observed with a frontocentral distribution and a peak-latency between 50–100 ms after response execution. Electrophysiological (Van Veen and Carter, 2002) and brain imaging techniques (Ridderinkhof et al., 2004) point to sources in the anterior cingulate cortex (ACC). Besides performance monitoring, the ACC is involved in a multitude of other tasks (Bush et al., 2000), among them the emotional evaluation of events (Luu et al., 2000).
In line with this, the ERN has been shown to be changed in patients with affective disorders. It is increased in patients suffering from major depression (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008), a condition associated with negative affect, with medication having a moderating effect on ERN amplitude (Schrijvers et al., 2008). The ERN is also increased in subjects with obsessive compulsive disorder (OCD) (Gehring et al., 2000; Hajcak and Simons, 2002; Münte et al., 2008; but see also Nieuwenhuis et al., 2005, for diverging results). Besides recurrent, unwanted thoughts and rituals, OCD is characterized by a high level of negative affect (Brown et al., 1998).
However, ERN-amplitude has also been shown to be modulated by induced changes in affective state in healthy subjects. Luu et al. (2000) were among the first relating trait and state measures to ERN amplitude. They found that negative affect goes along with increased ERN amplitude at the beginning of an experiment. An ERN relationship to trait, but not to state variables like anger, tension and fatigue has been reported by Tops et al. (2006). Although subjects scored higher on the post-experimental state variables tiredness and anger, this was not correlated to ERN amplitude. In addition, they did not find correlations for trait or state variables when analyzing the difference ERPs, based on correct response ERPs subtracted from the erroneous response ERPs (ΔERN). The authors interpret their findings of a correlation between trait variables (behavioral shame, agreeableness) with ERN amplitude as reflecting higher task engagement rather than changes in negative affect. The ERN is also increased in subjects scoring high on a trait scale for punishment sensitivity (Boksem et al., 2006a, 2006b), on a anxiety scale (Hajcak et al., 2003a) and on negative affect scales (Hajcak et al., 2004).
Taken together, there is good evidence that a long-lasting negative affect associated with psychiatric diseases or character traits goes along with an increased ERN amplitude. However, how short-term affective state changes interact with ERN amplitude is less clear. Findings of changed performance monitoring during affective state changes in normal participants would be important, as they would be able to explain performance alterations in related everyday situations. One study (Moser et al., 2005) investigated the he effects of fear induction on error processing and attentional allocation. Whereas ERPs indicated reductions in attentional allocation the ERN was not changed.
The ERN is typically followed by a component more positive for errors (error positivity, Pe). The Pe emerges around 200 ms after error commission and is slightly more posterior than the ERN (Falkenstein et al., 2000). It has been suggested that the Pe reflects post-error processing and is susceptible to error salience (Falkenstein et al., 2000). The relationship between Pe amplitude and state/trait measures is less clear. Tops et al. (2006) found no relationship between state and trait questionnaires and Pe amplitudes, whereas Hajcak and colleagues (2004) reported a smaller Pe for anxious subjects. There are no Pe-differences between depressed patients and controls (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008).
The present study was conducted to further examine affective influences on performance monitoring. Subjects were engaging in a speeded response task, while half of the subjects received derogative and half of the subjects received encouraging feedback. Feedback about an individual's performance has been shown to be an effective method to induce changes in affective states, especially for negative emotions (Westermann et al., 1996). Feedback was based on individual reaction times and provided after completion of 30 trials. State changes are assessed by a questionnaire administered before and after the experiment. Importantly, normal healthy participants, not pre-selected for trait negative affect, rather than extreme groups were exposed to the two feedback conditions.
METHODS
Participants
Twenty-five right-handed women participated in the experiment. To keep comparability with previous research (Tops et al., 2006; Wiswede, 2007; Wiswede et al., 2009), the present study gathered data from female subjects only. ERP data were analyzed from 24 women (mean age 23.75 years, range 19–30 years). One subject was excluded, because it turned out after the examination that she had taken psychoactive medication. Subjects were allocated randomly to receive either encouraging or derogatory feedback based on their reaction times. There were 12 subjects in each feedback group. All participants had normal or corrected to normal vision, provided written informed consent according to the Declaration of Helsinki (Br Med J 1991; 302: 1194), and received either course credit or € 6.50 per hour following completion of the experiment. The study protocol was approved by the ethics committee of the University of Magdeburg.
Stimuli and procedure
The experiment was based on the flanker task (Eriksen and Eriksen, 1974). A trial consisted of the following sequence (timing is provided in brackets): fixation cross (600–800 ms, mean 700 ms), flanker stimulus until response. Flanker stimuli consisted of black capital letters (‘Courier new’ font) H or S presented in front of a gray background (128, 128, 128 in RGB color space). A congruent flanker string was either HHHHH or SSSSS; incongruent flanker strings were SSHSS or HHSHH. Flankers were presented in random order; there were 60% congruent and 40% incongruent trials. They covered 2.1° of visual angle. Subjects were asked to respond as fast and as correct as possible to the central letter of the flanker string. They responded with a left-hand key to the H and with a right-hand key to the S. There were 10 blocks of 210 trials each. A feedback screen was presented after every 30 trials. A set of 30 trials was defined as a sub-block. Thus, there were seven sub-blocks per block; during the entire experiment, subjects received 70 feedbacks.
Subjects received feedback based on the mean reaction time in the last-performed sub-block. Feedback was presented on the feedback screen at the end of each sub-block. If the mean RT of the last-performed sub-block N was faster than the mean RT of sub-block N-1, subjects in the encouraging feedback group received positively worded feedback on the screen, whereas subjects in the derogatory feedback group received no feedback (‘continue with mouse click’). If the mean RT of the current block was slower than the mean RT in the sub-block N-1, subjects in the encouraging feedback group received no feedback (‘continue with mouse click’), whereas subjects in the derogatory feedback group received negatively worded feedback on the screen. There were three levels of feedback for both groups, ranging from mild (‘good’ vs ‘not good’) over moderate (‘very good’ vs ‘bad’) to strong (‘brillant’ vs ‘very bad’). The feedback algorithm is described in Table 1.
Table 1.
Feedback given in the two feedback conditions
| Feedback | algorithm | Feedback group |
|
|---|---|---|---|
| Level | encouraging | derogatory | |
| Level 1 | RTN up to 20 ms faster than RTN-1: | ‘gut’ | ‘no feedback’ |
| Level 2 | RTN up to 40 ms faster than RTN-1: | ‘sehr gut’ | ‘no feedback’ |
| Level 3 | RTN more than 40 ms faster than RTN-1: | ‘hervorragend’ | ‘no feedback’ |
| Level 1 | RTN up to 20 ms slower than RTN-1: | ‘no feedback’ | ‘nicht gut’ |
| Level 2 | RTN up to 40 ms slower than RTN-1: | ‘no feedback’ | ‘schlecht’ |
| Level 3 | RTN more than 40 ms slower than RTN-1: | ‘no feedback’ | ‘sehr schlecht’ |
RT, mean reaction time for a sub-block; N, last-performed sub-block. German feedback words are translated as: ‘gut’, ‘good’; ‘sehr gut’, ‘very good’; ‘hervorragend’, ‘brilliant’; ‘nicht gut’, ‘not good’; ‘schlecht’, ‘bad’; ‘sehr schlecht’, ‘very bad’.
Feedback words were provided in a sentence, for example ‘Your reaction time in the last sub-block was bad’. If no feedback was given, the sentence ‘press the mouse button to continue’ was shown. Each feedback screen was terminated by a button press. Therefore, subjects were permitted to have a break if they omitted a button press after each feedback screen. This feedback algorithm allowed individually tailored feedback, which represents the actual performance of the subject. Although this means that not every subject received the same amount of mild, moderate and strong feedback, the algorithm assured that there were no large differences in feedback levels between both groups and fast and slow responders, as was confirmed by a post hoc analysis of the received feedback.
Questionnaire
Changes in ‘current feeling’ (German: ‘Momentanes Befinden’) were assessed with a questionnaire ‘Aktuelle Stimmungsskala’ (ASTS) administered before and after the experiment. The ASTS (Dalbert, 1992) is a shortened version of the ‘profile of mood state’ scale (POMS) (McNair et al., 1971). Non-English questionnaires derived from the POMS have previously been used to assess state changes in a similar performance monitoring experiment (Tops et al., 2006). The ASTS consists of 19 German adjectives, and subjects are required to estimate how well an adjective represents their current feelings on a scale ranging from 7 (very strong) to 1 (not at all). The items are summarized on five different scales, representing anger, sadness, hopelessness, positive mood and tiredness.
Data recording and analysis
Recordings were conducted in an electrically shielded recording chamber equipped with a Neuroscan EEG amplifier. Participants were seated in a comfortable chair at a distance of 80 cm to the screen. Stimuli were presented on a 19 in. analog monitor. Chamber illumination was slightly dimmed.
The electroencephalogram (EEG) was recorded from 29 positions including all 19 standard locations of the 10/20 system with tin electrodes mounted in an elastic cap relative to a reference electrode placed on the tip of the nose. Eye movements were recorded with electrodes affixed to the right and left external canthi [horizontal electrooculogram (hEOG), bipolar recording] and at the left and right orbital ridges [vertical electrooculogram (vEOG), bipolar recording]. Impedances of all electrodes were kept below 10 kΩ. Biosignals were amplified with a band-pass from 0.05 to 30 Hz and stored with a digitization rate of 250 Hz. Prior to ERP data analysis, all trials containing eye artifacts were corrected using blind component separation (Joyce et al., 2004). Artifacts on recording channels were rejected based on individual peak-to-peak amplitude criteria using a special purpose program with individual thresholds between 50 and 100 µV. ERPs were averaged for epochs of 1024 ms starting 100 ms prior to response execution. All ERP figures and all ERP statistics are based on unfiltered data (except bandpass from 0.05 to 30 Hz during recording).
ERPs were generated relative to a 100 ms pre-response baseline and referenced to an electrode placed on the tip of the nose. Consistent with previous research (Hajcak et al., 2005), only responses given within 200–800 ms after the flanker stimulus onset were included in data analysis. Statistical analysis was based on the within factor correctness (correct vs erroneous responses) and the group factor feedback (receiving either positive or derogatory feedback). The ERN was examined at its topographical maximum (averaged amplitude of electrodes FC1, FC2, Fz, Cz) and was quantified by a mean amplitude measure in a time window 0 to 80 ms after the erroneous (ERN) or the correct response. The later positivity following erroneous responses will be referred to as Pe. To keep results comparable to previous research (Hajcak et al., 2004), the Pe was defined as mean amplitude in a 200–400 ms time window. The Pe has been shown to be somewhat more posterior than the ERN (Falkenstein et al., 2000). Consistent with previous research (Tops et al., 2006), it will be analyzed on electrode Pz.
Correlations between questionnaire scores and ERP amplitudes were computed based on difference waves, the response-locked ERPs following correct responses were subtracted from response-locked erroneous responses. The difference wave will be referred to as ΔERN and ΔPe; the same time window as for ERN and Pe analysis were applied to ΔERN and ΔPe.
Behavioral responses (RT and error rates) were obtained and subjected to ANOVA statistics. In addition, post-error slowing (PES) was assessed. PES refers to the finding that responses directly following an erroneous response (post-error trials) are slower compared to trials that follow correct responses (post-correct trials; Hajcak et al., 2003b, 2004; Rabbitt, 1981, 2002). However, since responses for erroneous trials are usually faster than for correct trials, this effect could be caused by regression toward the mean. As fast responses are relatively rare, it is more likely that a fast response is succeeded by a slower response. Since errors are usually faster than correct responses, it is more likely that a relatively slow correct response follows. To distinguish between post-error effects caused by regression towards the mean from ‘pure’ error-induced RT slowing, a subset of correct trials was selected that matched the erroneous trials in terms of reaction time and total number (see Hajcak et al., 2003a, 2003b for a similar procedure). Thus, the selected correct trials belong to the faster responses among all of the correct trials. Reaction times of correct trials given directly after those response-matched correct trials (post-correct trials) and response times of correct responses given directly after an erroneous response (post-error trials) provide the basis for the computation of PES. However, one could argue that the above-described analysis of PES might include also responses which are correct just by chance, since premature responses or guesses are correct in 50% of the cases. In addition, one reviewer noted that there is a compatibility bias in errors vs hits (more congruent hits, more incongruent errors), which should be controlled for in PES analysis. Thus, for an additional analysis, a subset of congruent and incongruent correct trials was selected that matched the number of incongruent and congruent erroneous trials. As above, analysis was based on reaction time of succeeding correct trials.
Mood changes were analyzed with ANOVA based on the within subject factors mood (five levels, indicating the ASTS scales anger, sadness, hopelessness, positive mood and tiredness), PrePost (two levels, ASTS prior and after the experiment) and the group factor feedback.
RESULTS
Questionnaire
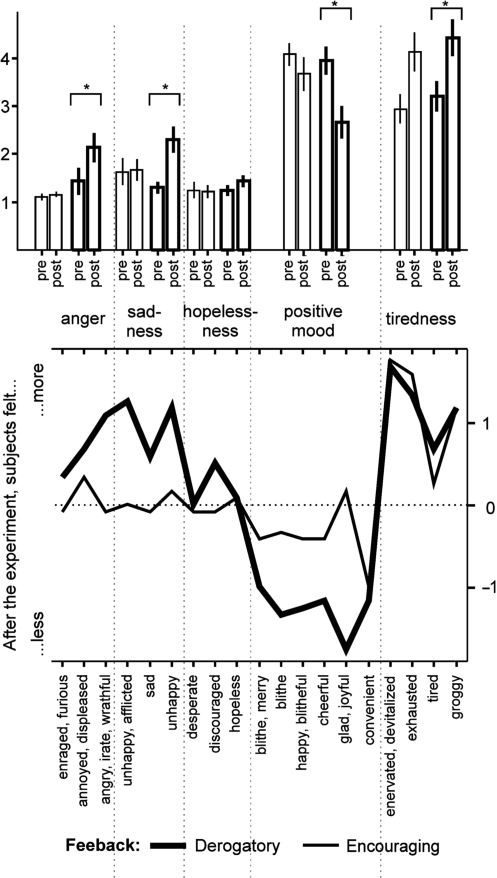
After the experiment, there were differences in current mood state, as indicated by a significant mood × PrePost × group interaction [F (4,88) = 6.3; P < 0.001, see Figure 1 and Table 2]. To get detailed information about the nature of mood changes induced by feedback procedure, statistics were conducted separately for each mood scale and feedback group. There were no differences between pre- and post-experimental ASTS scales in the encouraging feedback group, except on the scale tiredness, indicating that participants felt somewhat tired after experiment completion (paired sample t-test, Bonferroni-correction for multiple comparisons, t (anger, sadness, hopelessness, positive mood) < 0.04; t (tiredness) = −4.4; P < 0.001). The derogatory feedback group showed post-experimental mood changes on the scales anger (t = −3.2; P < 0.008), sadness (t = −3.5; P < 0.005) and positive mood (t = 5.2; P < 0.001). They were also more tired after the experiment (t = −4.4; P < 0.001). To summarize, the encouraging feedback group did not feel differently on scales indicating current positive and negative mood after the experiment. The derogatory feedback group showed increased negative and decreased positive mood after experiment completion.
Fig. 1.
Results of the ASTS questionnaire; feedback group is coded by line thickness. Upper part: scale level, as used for ANOVA. Items summarized according to the ASTS-manual. Asterisks indicate significant differences between pre- and post-test, P < 0.001. Lower part: item-level. ASTS-Scores are based on difference post-experimental ASTS minus pre-experimental ASTS results; positive values indicate that people loaded higher on this item after completion of the EEG experiment.
Table 2.
Results of the ASTS questionnaire; mean values for all five scales, see also Figure 1
| Both groups |
Encouraging |
Derogatory |
||||
|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | |
| Sadness | 1.5 | 2.0 | 1.6 | 1.7 | 1.3 | 2.3 |
| Hopelessness | 1.3 | 1.3 | 1.3 | 1.2 | 1.3 | 1.4 |
| Anger | 1.3 | 1.7 | 1.1 | 1.2 | 1.4 | 2.1 |
| Positive mood | 4.0 | 3.2 | 4.1 | 3.7 | 3.9 | 2.7 |
| Tiredness | 3.1 | 4.3 | 2.9 | 4.1 | 3.2 | 4.4 |
Behavioral data
Reaction times
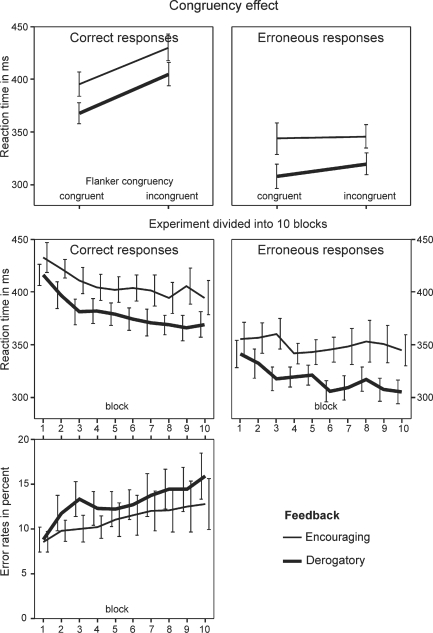
RT data were analyzed by ANOVA with factors correctness, congruency and feedback. Subjects were faster in giving erroneous compared to correct responses [correctness: F(1,22) = 89.4, P < 0.001]. Also, responses were faster for congruent than for incongruent flanker stimuli [congruency: F(1,22) = 76.2, P < 0.001]. The congruency effect was weaker for erroneous responses [congruency × correctness interaction; F(1,22) = 39.00; P < 0.001] and there was a tendency for subjects receiving derogatory feedback to respond faster [feedback F(1,22) = 3.16; P < 0.089]. When separate ANOVAs were computed for correct and erroneous responses, we obtained for the correct responses a main effect of congruency [F(1,22) = 212.5; P < 0.001] with the effects of feedback and the interaction between congruency × feedback were non-significant. For the erroneous responses a marginally significant main effect of congruency was obtained [F(1,22) = 4.1; P < 0.055, feedback and interaction ns). See Figure 2 and table 3.
Fig. 2.
Behavior data. Top row: reaction times divided by flanker congruency. Middle and bottom row: reaction times and error rates with the experiment divided into 10 blocks, each containing 210 trials. Error bars indicate ±1 standard error.
Table 3.
Reaction times in milliseconds
| Correctness | Congruency | Group |
|
|---|---|---|---|
| Derogatory | Encouraging | ||
| Correct | Congruent | 367 | 395 |
| Incongruent | 405 | 430 | |
| Error | Congruent | 308 | 343 |
| Incongruent | 319 | 345 | |
As feedback might affect behavior differently over the course of the session, the experiment was subdivided into 10 blocks, each containing seven sub-blocks of 210 trials. As shown in Figure 2, the derogatory feedback group was slightly but not significantly faster after receiving the initial seven feedback screens (first block); with this trend continuing during the entire experiment [ANOVA, as above, with additional factor block (10 levels); correctness × block: F(9,198) = 3.55; P < 0.001, feedback: F(1,22) = 3.45; P < 0.077]. Separate tests conducted for each block revealed group differences for erroneous responses in blocks 3, 6, 9 and 10 [t(22) > 2.09; P < 0.048].
Error rates
A similar ANOVA design (factors congruency, feedback) was applied to the error rates, which was found to be increased for incongruent flanker stimuli but was not modulated by the feedback manipulation [congruency: F(1,22) = 95.7; P < 0.001, feedback and interaction ns; encouraging/derogatory feedback, congruent 6.5/7.7%, incongruent 17.8/20.7%]. Dividing the experiment into 10 blocks did not change this pattern [block: F(9,198) = 61.7, P < 0.001; no significant effect of feedback or interaction].
Post-error slowing, response-matched data
Correct responses were faster when given after a correct trial than when given after an erroneous trial. This effect was consistently seen in both feedback groups (post correct trials, derogatory/encouraging feedback: 371 ms/392 ms; post-error trials, derogatory/encouraging feedback: 407 ms/431 ms). In an ANOVA with response (post-correct vs post-error) and feedback as factors a main effect of the former was obtained [F(1,22) = 76.4, P < 0.001; main effect of feedback and response × feedback interaction ns).
Post-error slowing, congruency-matched data
Equalizing the number of congruent and incongruent hits and errors did not change the PES pattern. Reaction time increased after erroneous responses; this effect was seen in both feedback groups (post-correct trials, derogatory/encouraging feedback: 378 ms/405 ms; post-error trials, derogatory/encouraging feedback: 407 ms/431 ms; response (post-correct vs post-error) F(1,22) = 29.8, P < 0.001; main effect of feedback and response × feedback interaction ns).
Response-locked ERPs
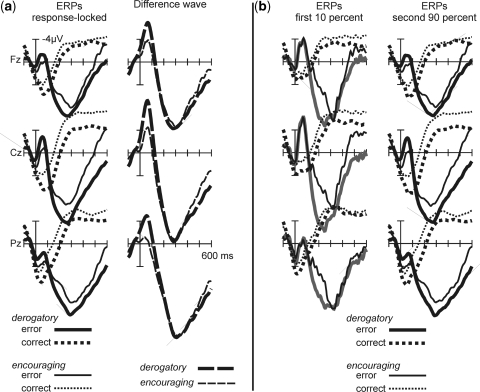
A typical ERN was seen for error trials compared to correct trials (Figure 3A). Visual inspection suggested that the ERN was more pronounced in the derogatory feedback group. This was corroborated by the statistical analysis (ANOVA with correctness and feedback as factors; correctness F(1,22) = 61.97; P < 0.001; correctness × feedback: F(1,22) = 9.38; P < 0.01). When separate tests were conducted for correct and erroneous responses, a reliable effect emerged for the error trials [t (22) = 2.13; P < 0.04] but not for the correct responses [t (22) = −1.49; ns, see Table 4 for amplitudes in microvolts). Thus, derogatory feedback resulted in a more negative ERN, but did not influence ERPs to correct responses.
Fig. 3.
(a) Response-locked ERPS on midline electrodes and difference waves (ΔERN; error minus correct). (b) Response-locked ERPs for the beginning of the experiment (first ten percent of errors) and the remaining experiment.
Table 4.
ERN amplitudes in microvolts
| Group | Correctness | Amplitude | divided into two parts |
|
|---|---|---|---|---|
| 10% | 90% | |||
| Derogatory | Correct | 5.0 | 3.1 | 5.4 |
| Error | −1.1 | −2.4 | −0.9 | |
| Encouraging | Correct | 3.4 | 1.8 | 3.7 |
| Error | 0.8 | −2.2 | 1.1 | |
However, correct responses were given more slowly than erroneous responses and they vastly outnumber erroneous trials. To ensure that the null finding for correct responses is not due to these factors, an analysis was conducted including only a subset of correct trials, which matched the erroneous trials in terms of reaction time and total number. This analysis did not change the pattern described above [t-test on response-matched correct trials, t(22) = −1.2; P < 0.23; ns). See Hajcak et al. (2003a, 2004) for a similar procedure.1
To exclude the possibility that group differences in ERN amplitude were present prior to mood induction, data sets were split into two parts: the first part comprised the first 10% errors committed at the beginning of the experiment and the corresponding correct trials, the second part contained the remainder of the data.2 A clear ERN was present in both experimental parts which was modulated by feedback in the second but not in the first part (ANOVA, Factors part, correctness, feedback, interaction part × correctness × feedback, F(1,22) = 4.2; P < 0.05). This three-way interaction was decomposed by assessing feedback effects separately for the correct and erroneous response ERPs, and separately for first and second parts. Only the comparison for erroneous responses in the second part was significant [t (22) = 2.3; P < 0.03]. See Figure 3B, amplitudes in microvolts are given in Table 4.
Correlation of mood scales with ERN amplitude
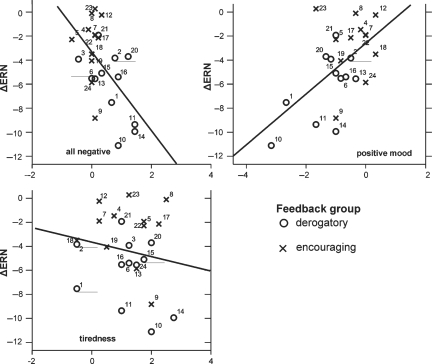
ΔERN amplitudes were correlated with the ASTS questionnaire scores (difference post-experimental minus pre-experimental ASTS, see Figure 4). There was a significant negative correlation for all scales indicating negative states (anger: r = −0.435; P < 0.034, sadness: r = −0.469; P < 0.02, hopelessness: r = −0.55; P < 0.001, average of all negative scales: r = −0.56; P < 0.004) and a positive correlation for the positive mood scale (r = 0.57; P < .004). The tiredness scale did not correlate significantly with ERN amplitude (r = −0.18; ns). Importantly, the positive mood scale did not correlate significantly with sadness and hopelessness. Thus, having more negative or less positive feelings after the experiment was associated with greater ΔERN amplitude.
Fig. 4.
Relationship between ΔERN amplitude (error minus correct, mean of electrodes Fz, CZ, FC1, FC2 in a time window 0–80 ms after response) and scales for negative (left site) and positive (right site) state. State measures are based on the difference pre-experimental ASTS minus post-experimental ASTS questionnaire. Numbers on the group symbols identify individual subjects.
Pe analysis
There was a clear difference between erroneous and correct responses in the Pe time window (200–400 ms, electrode Pz). This effect was analyzed by ANOVA (factors correctness, feedback). Importantly, no main effect of feedback was obtained [F(1,22) = 2.3; ns), whereas the effect of congruency was significant [F(1,22) = 79.6; P < 0.001; interaction ns; mean amplitudes: correct, encouraging/derogative feedback: −5.3/−3.2 µV; error, encouraging/derogative 9.2/11.3 µV). One could argue that the Pe amplitude might be influenced by differences already emerging in the ERN time window. Thus, the same analysis as above was conducted with amplitudes defined as (mean amplitude 200–400 ms) minus (mean amplitude 0–80 ms), which is the same as setting the baseline to the ERN time window. This analysis resulted in the same conclusion; feedback did not influence Pe amplitude [correctness: F(1,22) = 99.4; P < 0.001; feedback F(1,22) = 2.1; P < 0.16, interaction ns; correct, encouraging/derogative −9.6/−9.4 µV; error, encouraging/derogative 5.6/9.2 µV).
Correlation of mood scales with Pe amplitude
There was no significant correlation between ΔPe amplitude with any of the mood scales.
DISCUSSION
The present study provides evidence that induced negative affect is associated with greater amplitude of the ERN, a key marker of human performance monitoring. Affective state measured via a questionnaire administered prior and after the experiment demonstrated that negative but not positive emotions were amplified by feedback on task performance. Behavioral data showed a tendency towards a faster, but somewhat more erroneous response strategy when receiving derogatory feedback. The ERN modulation emerged only after receiving encouraging or derogatory feedback and was correlated to the degree of increased negative or decreased positive affective state.
Whereas negative affect was successfully induced by giving derogatory feedback, encouraging feedback did not elevate positive feelings. Indeed, even after receiving permanent encouraging feedback, subjects felt slightly more negative. Although this might appear surprising at first glance, it is in line with a meta analysis showing that common methods of mood induction, among them performance feedback, are much weaker in inducing positive relative to negative states (Westermann et al., 1996). Together with the increased tiredness caused by a demanding task, this might explain why subjects did not feel more positive even after repeated encouraging feedback.
Although the effect was not significant across all blocks, subjects in the derogatory feedback condition were somewhat faster on errors at the cost of a slightly increased error rate. Thus, there was a slight tendency in the derogatory feedback group to emphasize speed over accuracy. It is unlikely, however, that this explains the ERN differences, because previous research showed that the ERN is smaller rather than larger when speed is emphasized over accuracy (Gehring et al., 1993; Falkenstein et al., 2000). Likewise, a more impulsive strategy with premature responses leads to a decrease rather than increase of ERN amplitude (Pailing et al., 2002). Thus, it is unlikely that changes in response strategies rather than changes in affective state contributed to the ERN increase.
In addition to negative affect, derogatory feedback might also lead to increased motivation which in turn might have influenced ERN amplitude.3 A study by Boksem et al. (2006a, 2006b) manipulated motivation but not affect and might thus be revealing. In an extended Simon-Task, motivation was increased by providing extra-money for the participants exhibiting the best performance. Those who became faster at the cost of increased error rates did not show changes in ERN amplitude, whereas subjects who increased accuracy at the cost of performing more slowly increased ERN amplitude. On the behavioral level, this pattern resembles our derogatory (faster RT, higher error rate) and encouraging feedback (longer RT, lower error rate) group, respectively, although we note, again, that the differences between groups were non-significant for both, error-rates and reaction times. Moreover, the ERN in the current study is higher for subjects with faster reaction times (derogatory feedback group), whereas it was lower in the Boksem et al. (2006a, 2006b) study. This argues against a motivational explanation of the ERN changes observed in the present study.
Tops and colleagues (2006) reported ERN modification by trait variables. The present study extends those findings in that we included an experimental procedure to induce short-term state changes. In contrast to Tops et al. (2006), we found that an ERN increase goes along with less positive or more negative states, even when the correlation is based on ΔERN values. Differences in general emotional states might explain the divergent finding, since it is reasonable to assume that subjects in Tops et al. felt less negative after the experiment compared to the derogatory feedback subjects of the present study. Thus, our data show that besides task engagement and traits, the ERN also reflects short-term changes in negative affect.
Taken together, the present results are best explained by a modulation of the ERN by negative affect. Whereas the present results demonstrate the role of short term changes of affect, previous investigations have shown an ERN increase with long-term changes of affect, e.g. with depression (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008), obsessive compulsive disorder (Gehring et al., 2000; Hajcak and Simons, 2002), and increased trait anxiety (Hajcak et al., 2003a, 2004). In addition, ultra-short affective changes might also influence the ERN as demonstrated by trial by trial amplitude changes as a function of whether a flanker stimulus was preceded by a emotionally negative or positive picture (Wiswede et al., 2009).
Here, we report increased negativity after erroneous, but not after correct responses. Moreover, derogative feedback appears to result in a more positive component following hits, but this effect was not confirmed statistically. This is in contrast to previous research, which described an increased negativity also after correct responses in subjects high in trait negative affect (Hajcak et al., 2003a, 2004). This has been interpreted as ‘increased engagement of the response-monitoring system, evident on both correct and erroneous trials (Hajcak et al., 2004, p.196)’. Increased negativity after erroneous and correct responses was also reported when the flanker stimulus was preceded by an unpleasant picture (Wiswede et al., 2009), but not in subjects with depression relative to control subjects (Holmes and Pizzagalli, 2008). Thus, our null finding is hard to integrate into existing findings and needs further replication.
What could be the mechanism by which induced negative affect influences the ERN as the neural index of performance monitoring. Both, the ERN and affective changes have been associated with the neuromodulatory transmitter dopamine. For the ERN, Holroyd and Coles (2002) propose that performance monitoring is integrated into a system for reinforcement learning and that the ERN is generated by comparing the given response with a prediction provided by the basal ganglia. Error commission causes a negative reinforcement learning signal, which is coded by a phasic decrease in dopaminergic activity of mesencephalic structures. This, in turn, results in decreased disinhibition of the ACC and increased ERN amplitude. Several pieces of evidence support this notion including (i) a decrease of the ERN in Parkinson's disease in which dopaminergic neurotransmission is compromised (Falkenstein et al., 2001; Willemsen et al., 2008), (ii) a modulation of ERN amplitude by dopaminergic polymorphisms (Krämer et al., 2007), (iii) the presence of an ERN-like response in intracranial recordings from the Nucleus accumbens, a key dopaminergic structure (Münte et al., 2008), (iv) a decrease of the ERN after the administration of dopamine receptor blocking drugs (Zirnheld et al., 2004; de Bruijn et al., 2006) and (v) an increase of the ERN-amplitude after dopaminergic agents (de Bruijn et al., 2004). On the other hand, changes in affect have been proposed to lead to changes in dopaminergic tone in the same structures even though the evidence for a role of increased dopamine in positive affect has been much better substantiated (Ashby et al., 1999) than a possible decrease of dopamine as underlying negative affect (but see, for example, Lieberman, 2006). Affect-related tonic and error-related phasic changes in dopaminergic activity might thus interact to yield an increased ERN in the derogatory feedback condition. On the basis of our current results, we can not rule out the involvement of other neurotransmitter systems, however. For example, negative affect may lead to a higher arousal level mediated by noradrenaline. Moreover, an increase of noradrenergic tone induced by the presynaptic alpha-2 antagonist yohimbine has been shown to increase the ERN (Riba et al., 2005).
The present study did not find affect-related differences in the Pe time window, neither for correct nor for incorrect trials. In contrast to that, Hajcak et al. (2004) report that trait negative affect goes along with a general amplitude decrease around 200–400 ms, seen for correct and for incorrect trials. They suggest that subjects high in negative affect might be less aware of their errors or might find their errors less salient. However, we regard this interpretation as counterintuitive, since literature describes a relationship between anxiety and perfectionism, which is accompanied by overt critical self-evaluation (Kawamura et al., 2001; Flett et al., 1994). Thus, errors should be more rather than less salient to subjects high in negative affect. In addition, earlier research (Hajcak et al., 2003b) showed that error trials with smaller Pe are associated with decrease in post-error slowing. If there is a relationship between negative affect, decreased Pe amplitude and error salience, one would also expect decreased post error slowing in subjects high in negative affect. This was not the case in Hajcak et al. (2004). Taken together, we suggest that there is no direct relationship to trait nor state negative affect and Pe amplitude. The differences between the present findings and Hajcak et al. (2004) might also be methodological. It is conceivable that Pe differences are still influenced by effects seen in the ERN time window. Additional Pe analysis based on Pe amplitude minus ERN amplitude, as done in the current study, might abolish group differences. In addition, visual inspection of the Pe group differences in Hajcak et al. (2004) reveal that they were almost absent on parietal electrode Pz. Since the Pe tends to have a more posterior distribution than the ERN (Falkenstein et al., 2000, Tops et al., 2006), analysis on more parietal electrodes could integrate contradicting findings. Changed Pe amplitudes have also not been found in subjects with depression (Chiu and Deldin, 2007; Holmes and Pizzagalli, 2008), unless not treated with benzodiazepines (Schrijvers et al., 2008).
To summarize, our findings show a robust influence of induced short term negative affect on the amplitude of the ERN. Altered action monitoring processes might therefore underlie affect-related changes in performance.
Acknowledgments
We thank two anonymous reviewers for excellent suggestions. This article is supported by grants from the DFG (MU1311/11-3, SFB 779-A5 to T.F.M.); also supported by the BMBF (contract 01GO0202). D.W. was a fellow of the Hanse-Wissenschaftskolleg (Hanse Institute for Advanced Study) in Delmenhorst while writing this article. Thanks to Nadine Strien and Peggy Tausche for support in data collection.
Footnotes
One reviewer suggested to analyze flanker congruency effects as well. We reanalyzed response-locked ERP data, including the factor congruency. This did not change the general pattern (congruency, congruency × correctness or congruency × feedback ns).
One could argue that it would be better to split the experiment before subjects received the first feedback. However, this was not possible here because none of the subjects committed enough errors during the first 30 trials to generate meaningful ERPs. Errors were not distributed equally across the experiment; some subjects performed better at the beginning, others at the end, with a slight tendency for increased error rates during the course of the experiment. Thus, splitting the experiment after a fixed set of feedback screens would include too few errors in subjects performing well at the beginning of the experiment. Thus, splitting the experiment after 10% of the errors assured a sufficient amount of errors in the ERP. However, this splitting procedure caused a different number of feedback screens included in the first 10% of errors. Average number of feedback screens given during the first 10% of errors: 8.9 out of 70; min 2, max 15; no differences between feedback groups.
Whereas the two feedback schemes were designed to be symmetrical, it is conceivable that the impact of the two feedback modes on participants’ motivation to change their behavior might have been different. For example, the word carrying ‘mild’ feedback in the encouraging and derogatory conditions were ‘good’ and ‘not good’, respectively. The incentive to improve performance might thus have been higher in the derogatory group. Also, the derogatory feedback might be considered to carry more important information than the encouraging feedback.
REFERENCES
- Ashby GF, Isen AM, Turken AU. A neuropsychological theory of positive affect and its influence on cognition. Psychological Review. 1999;106:529–50. doi: 10.1037/0033-295x.106.3.529. [DOI] [PubMed] [Google Scholar]
- Ashby GF, Valentin VV, Turken U. The effects of positive affect and arousal on working memory and executive attention. In: Moore SC, Oaksford M, editors. Emotional Cognition: From brain to Behaviour. Amsterdam: Benjamins; 2002. pp. 245–87. [Google Scholar]
- Boksem MAS, Meijman TF, Lorist MM. Mental fatigue, motivation and action monitoring. Biological Psychology. 2006a;72:123–32. doi: 10.1016/j.biopsycho.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Boksem MAS, Tops M, Wester AE, Meijman TF, Lorist MM. Error-related ERP components and individual differences in punishment and reward sensitivity. Brain Research. 2006b;1101:92–101. doi: 10.1016/j.brainres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- Bolte A, Goschke T, Kuhl J. Emotion and intuition: effects of positive and negative mood on implicit judgments of semantic coherence. Psychological Science. 2003;14:416–21. doi: 10.1111/1467-9280.01456. [DOI] [PubMed] [Google Scholar]
- Brown TA, Chorpita BF, Barlow DH. Structural relationships among dimensions of the DSM-IV anxiety and mood disorders and dimensions of negative affect, positive affect, and autonomic arousal. Journal of Abnormal Psychology. 1998;107:179–92. doi: 10.1037//0021-843x.107.2.179. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–22. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Chiu PH, Deldin PJ. Neural evidence for enhanced error detection in major depressive disorder. American Journal of Psychiatry. 2007;164:608–16. doi: 10.1176/ajp.2007.164.4.608. [DOI] [PubMed] [Google Scholar]
- Dalbert C. Subjektives Wohlbefinden junger Erwachsener: Theoretische und empirische Analysen der Struktur und Stabilität [Subjective well-being of young adults: theoretical and empirical analyses of structure and stability] Zeitschrift für Differentielle und Diagnostische Psychologie. 1992;13:207–220. [Google Scholar]
- de Bruijn E.RA, Hulstijn W, Verkes RJ, Ruigt GSF, Sabbe BGC. Drug-induced stimulation and suppression of action monitoring in healthy volunteers. Psychopharmacology. 2004;177:151–60. doi: 10.1007/s00213-004-1915-6. [DOI] [PubMed] [Google Scholar]
- de Bruijn ERA, Sabbe BGC, Hulstijn W, Ruigt GSF, Verkes RJ. Effects of antipsychotic and antidepressant drugs on action monitoring in healthy volunteers. Brain Research. 2006;1105:122–9. doi: 10.1016/j.brainres.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Dreisbach G. How positive affect modulates cognitive control: the costs and benefits of reduced maintenance capability. Brain and Cognition. 2006;60:11–9. doi: 10.1016/j.bandc.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Dreisbach G, Goschke T. How positive affect modulates cognitive control: reduced perseveration at the cost of increased distractibility. Journal of Experimental Psychology: Learning, Memory and Cognition. 2004;30:343–53. doi: 10.1037/0278-7393.30.2.343. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception and Psychophysics. 1974;16:143–9. [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J, Blanke L. Effects of crossmodal divided attention on late ERP components. II. Error processing in choice reaction tasks. Electroencephalography and Clinical Neurophysiology. 1991;78:447–55. doi: 10.1016/0013-4694(91)90062-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biological Psychology. 2000;51:87–107. doi: 10.1016/s0301-0511(99)00031-9. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hielscher H, Dziobek I, et al. Action monitoring, error detection, and the basal ganglia: an ERP study. Neuroreport. 2001;12:157–61. doi: 10.1097/00001756-200101220-00039. [DOI] [PubMed] [Google Scholar]
- Flett GL, Hewitt PL, Endler NS, Tassone C. Perfectionism and components of state and trait anxiety. Current Psychology. 1994;13(4):326–50. [Google Scholar]
- Gehring WJ, Goss B, Coles M. GH, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychological Science. 1993;4:385–90. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychological Science. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Anxiety and error-related brain activity. Biological Psychology. 2003a;64:77–90. doi: 10.1016/s0301-0511(03)00103-0. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. To err is autonomic: error-related brain potentials, ANS activity, and post-error compensatory behavior. Psychophysiology. 2003b;40:895–903. doi: 10.1111/1469-8986.00107. [DOI] [PubMed] [Google Scholar]
- Hajcak G, McDonald N, Simons RF. Error-related psychophysiology and negative affect. Brain and Cognition. 2004;56:189–97. doi: 10.1016/j.bandc.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Moser JS, Yeung N, Simons RF. On the ERN and the significance of errors. Psychophysiology. 2005;42:151–60. doi: 10.1111/j.1469-8986.2005.00270.x. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Research. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Holmes AJ, Pizzagalli DA. Spatiotemporal dynamics of error processing dysfunctions in major depressive disorder. Archives of General Psychiatry. 2008;65(2):179–88. doi: 10.1001/archgenpsychiatry.2007.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holroyd CB, Coles M.GH. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychological Review. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Isen AM. An influence of positive affect on decision making in complex situations: Theoretical issues with practical implications. Journal of Consumer Psychology. 2001;11:75–85. [Google Scholar]
- Isen AM, Daubman KA, Nowicki GP. Positive affect facilitates creative problem solving. Journal of Personality and Social Psychology. 1987;52:1122–31. doi: 10.1037//0022-3514.52.6.1122. [DOI] [PubMed] [Google Scholar]
- Joyce CA, Gorodnitsky IF, Kutas M. Automatic removal of eye movement and blink artifacts from EEG data using blind component separation. Psychophysiology. 2004;41:313–25. doi: 10.1111/j.1469-8986.2003.00141.x. [DOI] [PubMed] [Google Scholar]
- Kawamura KY, Hunt SL, Frost RO, DiBartolo PM. Perfectionism, anxiety, and depression: are the relationships independent? Cognitive Therapy and Research. 2001;25(3):291–301. [Google Scholar]
- Krämer UM, Cunillera T, Càmara E, et al. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. Journal of Neuroscience. 2007;27:14190–8. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AY, Sternthal B. The effects of positive mood on memory. Journal of Consumer Research. 1999;26:115–27. [Google Scholar]
- Lieberman A. Depression in Parkinson's disease—a review. Acta Neurologica Scandinavica. 2006;113:1–8. doi: 10.1111/j.1600-0404.2006.00536.x. [DOI] [PubMed] [Google Scholar]
- Luu P, Collins P, Tucker DM. Mood, personality, and self-monitoring: negative affect and emotionality in relation to frontal lobe mechanisms of error monitoring. Journal of Experimental Psychology: General. 2000;129:43–60. doi: 10.1037//0096-3445.129.1.43. [DOI] [PubMed] [Google Scholar]
- McNair DM, Lorr M, Doppleman LF. EITS – Manual for Profile of Mood States. San Diego, California: Educational and Industrial Testing Service; 1971. [Google Scholar]
- Mitchell RL, Phillips LH. The psychological, neurochemical and functional neuroanatomical mediators of the effects of positive and negative mood on executive functions. Neuropsychologia. 2007;45:617–29. doi: 10.1016/j.neuropsychologia.2006.06.030. [DOI] [PubMed] [Google Scholar]
- Moser JS, Hajcak G, Simons RF. The effects of fear on performance monitoring and attentional allocation. Psychophysiology. 2005;42:261–8. doi: 10.1111/j.1469-8986.2005.00290.x. [DOI] [PubMed] [Google Scholar]
- Münte TF, Heldmann M, Hinrichs H, et al. Nucleus accumbens is involved in human action monitoring: evidence from invasive electrophysiological recordings. Frontiers in Human Neuroscience. 2008;1:11. doi: 10.3389/neuro.09.011.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Nielen MM, Mol N, Hajcak G, Veltman DJ. Performance monitoring in obsessive-compulsive disorder. Psychiatry Research. 2005;13(4):111–22. doi: 10.1016/j.psychres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002;39:198–206. doi: 10.1017/S0048577202010247. [DOI] [PubMed] [Google Scholar]
- Park J, Banaji MR. Mood and heuristics: the influence of happy and sad states on sensitivity and bias in stereotyping. Journal of Personality and Social Psychology. 2000;78:1005–23. doi: 10.1037//0022-3514.78.6.1005. [DOI] [PubMed] [Google Scholar]
- Rabbitt PMA. Sequential reactions. In: Holding D, editor. Human Skills. New York: Wiley; 1981. pp. 153–75. [Google Scholar]
- Rabbitt PMA. Consciousness is slower than you think. The Quarterly Journal of Experimental Psychology: Section A: Human Experimental Psychology. 2002;55:1081–92. doi: 10.1080/02724980244000080. [DOI] [PubMed] [Google Scholar]
- Ravnkilde B, Videbech P, Clemmensen K, Egander A, Rasmussen NA, Rosenberg R. Cognitive deficits in major depression. Scandinavian Journal of Psychology. 2002;43:239–51. doi: 10.1111/1467-9450.00292. [DOI] [PubMed] [Google Scholar]
- Riba J, Rodríguez-Fornells A, Morte A, Münte TF, Barbanoj MJ. Noradrenergic stimulation enhances human action monitoring. Journal of Neuroscience. 2005;25:4370–4. doi: 10.1523/JNEUROSCI.4437-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Schrijvers D, de Bruijn E.RA, Maas Y, De Grave C, Sabbe B.GC, Hulstijn W. Action monitoring in major depressive disorder with psychomotor retardation. Cortex. 2008;44(5):569–79. doi: 10.1016/j.cortex.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Tops M, Boksem M.AS, Wester AE, Lorist MM, Meijman TF. Task engagement and the relationships between the error-related negativity, agreeableness, behavioral shame proneness and cortisol. Psychoneuroendocrinology. 2006;31:847–58. doi: 10.1016/j.psyneuen.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Westermann R, Spies K, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: a meta-analysis. European Journal of Social Psychology. 1996;26:557–80. [Google Scholar]
- Willemssen R, Müller T, Schwarz M, Hohnsbein J, Falkenstein M. Error processing in patients with Parkinson's disease: the influence of medication state. Journal of Neural Transmission. 2008;115:461–8. doi: 10.1007/s00702-007-0842-1. [DOI] [PubMed] [Google Scholar]
- Wiswede D. Emotional Modulation of Memory Encoding and Performance Monitoring. Göttingen: Sierke; 2007. [Google Scholar]
- Wiswede D, Münte TF, Goschke T, Rüsseler J. Modulation of the error-related negativity by induction of short-term negative affect. Neuropsychologia. 2009;47(1):83–90. doi: 10.1016/j.neuropsychologia.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Zirnheld PJ, Carroll CA, Kieffaber PD, O'Donnell BF, Shekhar A, Hetrick WP. Haloperidol impairs learning and error-related negativity in humans. Journal of Cognitive Neuroscience. 2004;16:1098–112. doi: 10.1162/0898929041502779. [DOI] [PubMed] [Google Scholar]