Abstract
Individuals with Williams syndrome (WS), a genetically determined disorder, show relatively strong face-processing abilities despite poor visuospatial skills and depressed intellectual function. Interestingly, beginning early in childhood they also show an unusually high level of interest in face-to-face social interaction. We employed functional magnetic resonance imaging (fMRI) to investigate physiological responses in face-sensitive brain regions, including ventral occipito-temporal cortex and the amygdala, in this unique genetic disorder. Participants included 17 individuals with WS, 17 age- and gender-matched healthy adults (chronological age-matched controls, CA) and 17 typically developing 8- to 9-year-old children (developmental age controls, DA). While engaged in a face discrimination task, WS participants failed to recruit the amygdala, unlike both CA and DA controls. WS fMRI responses in ventral occipito-temporal cortex, however, were comparable to those of DA controls. Given the integral role of the amygdala in social behavior, the failure of WS participants to recruit this region during face processing may be a neural correlate of the abnormally high sociability that characterizes this disorder.
Keywords: Williams syndrome, face processing, amygdala, fMRI, social cognition
Williams syndrome (WS) is a neurodevelopmental disorder caused by a known chromosomal microdeletion (Ewart et al., 1993; Korenberg et al., 2000). Individuals with WS typically show mild-to-moderate mental retardation (Howlin et al., 1998), very poor spatial abilities, and comparatively strong language skills (Bellugi et al.,, 1999; Mervis et al., 2000). In addition, individuals with WS have been described as ‘hypersocial’, referring to their unique, highly affiliative and engaging social style (Jones et al., 2000). This remarkable cognitive and behavioral profile together with knowledge of the underlying genetic abnormality indicate that WS has strong potential for linking genes to human cognition (Bellugi and St George, 2001).
In addition to heightened sociability, individuals with WS possess a notable interest and skill in face processing, an essential aspect of most human social interactions. The WS predilection for faces is marked early in life by heightened interest in faces over other classes of stimuli (Laing et al., 2002). In later development, it is manifested as accuracy on face discrimination tasks that has been observed to fall above IQ-based expectations [e.g. Benton Face task performance at the level of chronological age (CA) controls; Bellugi et al., 1994], coupled with an irrepressible inclination to engage in social exchange (Bellugi and St George, 2001).
While face processing is generally considered to be a strength in WS, particularly when compared with other visuospatial functions, the integrity of the underlying neurocognitive mechanisms has been the source of considerable debate. Although some studies have suggested that individuals with WS employ the same visual processing mechanisms when discriminating faces (Tager-Flusberg et al., 2003), others have found evidence for impaired processing of certain types of facial information (i.e. configural information, considered essential for normal face processing; Maurer et al., 2002), challenging the idea that face processing is normal in WS (Deruelle et al., 1999; Karmiloff-Smith et al., 2004). Further, electrophysiological recordings show that the WS response to faces is atypical and abnormally modulated by changes in stimulus orientation (i.e. upright vs inverted faces; Mills et al., 2000; Grice et al., 2001). However, the few extant functional magnetic resonance imaging (fMRI) studies investigating face processing in WS have produced inconsistent findings in prototypical face-responsive brain regions. Face-related activation in ventral occipito-temporal cortex (including the fusiform gyrus), a region that is highly responsive to visual patterns such as faces (Haxby et al., 2002), has been reported to be normal in WS (Meyer-Lindenberg et al., 2004, 2005). In contrast, the amygdala, a limbic structure that guides socio-emotional behavior and plays a role in the perception of facial identity and emotion (Haxby et al., 2002; Adolphs and Spezio, 2006), is reported to be hypoactive in WS in response to negative facial affect (Meyer-Lindenberg et al., 2005). However, face gaze processing, which is also associated with activation in the amygdala (Kawashima et al., 1999), has been found to elicit a typical amygdala response in WS (Mobbs et al., 2004). Thus, few studies have provided information regarding the functional integrity of brain regions involved in face processing in WS, and those that do have differed substantially in the tasks utilized. Further, these studies have utilized stimuli with salient socio-emotional connotation (e.g. affective expressions, changes in gaze), limiting our ability to draw conclusions regarding the brain response to neutral faces in WS. In addition, several of these studies were based on WS individuals with normal-range IQ (i.e. nonretarded) (Meyer-Lindenberg et al., 2004, 2005), raising concern regarding whether these findings are typical of persons with WS. This leaves open questions about the neural systems underlying typical WS proficiency in processing basic facial identity.
We addressed this using fMRI, to assay the neural responses associated with a neutral face identity discrimination task in a representative WS sample. Two healthy control groups were included to enable both comparisons based on CA and developmental age (DA). As WS is a neurodevelopmental disorder associated with a genetic abnormality that is likely to alter the course of development from its very initial stages, the ability to draw developmentally based comparisons is critical for understanding these individuals (Karmiloff-Smith, 2007). Previous fMRI studies with WS have included only adult controls and therefore, have been unable to interpret their findings within a developmental context. In this study, we focused on ventral occipito-temporal cortex and the amygdala (findings from other regions are described elsewhere; Paul, 2007), two brain regions that act in concert to support the perceptual and social-affective demands of face processing (Haxby et al., 2002). Results from previous functional imaging studies, in the context of the unique behavioral profile of WS, inform hypotheses about the profile of response in these regions. Specifically, in response to face stimuli, dysfunction of the amygdala has been more prominent than for ventral occipito-temporal cortex; this mirrors the juxtaposition of relatively good face discrimination with atypical socio-emotional behaviors that is observed in WS. Together, these findings suggest that if differences are observed, the amygdala may show relatively more functional compromise than ventral occipito-temporal cortex.
METHODS AND MATERIALS
Participants
For WS participants, the diagnosis of WS was established by FISH (fluorescence in situ hybridization probes for the elastin gene on chromosome 7) and the presence of phenotypic features (criteria set forth by the American Academy of Pediatrics, 2001). The WS sample (N = 17, 10 females; M = 30.6 years) was representative with respect to overall cognitive ability, with mean IQ scores (WAIS-R/WISC-R) (Wechsler, 1974, 1981) falling within the typical WS range (Howlin et al., 1998; Searcy et al., 2004) (Table 1). As previously alluded to, a disparity between language and spatial ability is often observed in WS, and was also evident in this sample. This disparity is reflected in developmental age-equivalent estimates for vocabulary and visuospatial ability in the WS sample, which were based on two well-accepted developmental measures yielding age-equivalent scores [Peabody Picture Vocabulary Test [PPVT-III] (Beery, 1997) and the Developmental Test of Visuomotor Integration [VMI] (Dunn and Dunn, 1997). PPVT-III scores for the WS sample revealed a receptive vocabulary age-equivalent of 12.6 years while VMI scores revealed an age-equivalent of 5.9 years (Table 1).
Table 1.
Background data for WS participants
| Mean (s.d.) | Range | |
|---|---|---|
| CA of WS Sample (N = 17) | 30.6 (11.3) | 14.9–52.3 |
| Wechsler Full Scale IQa | 67.5 (10.0) | 47–82 |
| Wechsler Verbal IQa | 72.8 (7.8) | 59–89 |
| Wechsler Performance IQa | 64.4 (10.3) | 44–83 |
| PPVT-III (Verbal: Receptive Vocabulary) Age Equivalent | 12.6 (4.5) | 5.8–22 |
| VMI (Nonverbal: Figure Drawing) Age Equivalent | 5.9 (1.6) | 4.1–11.3 |
aWAIS-R in all but two cases (for these participants, the WISC-R was used). IQ scores are standard scores, which have a mean of 100 and a s.d. of 15.
Given the unique cognitive profile of WS, several factors were considered in the selection of comparison groups for the WS sample. In accordance with past functional imaging studies of WS (Meyer-Lindenberg et al., 2005), a group of neurologically normal adults matched participant-wise for age and gender were included to afford a conventional CA comparison (CA group; N = 17, M = 31.0 years, s.d. = 11.2). Mean ages of the WS and CA groups were not different [t(32) = 0.115, P > 0.5]. The developmental nature of the disorder raises the possibility that brain responses in WS that diverge from CA controls might be observed in younger, typical subjects who have not yet achieved the mature response profile. The addition of a younger, typically developing control group would thus allow responses of this type in the WS group to be more accurately characterized as immature, rather than aberrant. Foremost in determining the age of this control group was a consideration of performance on the experimental task. Specifically, past results (Paul et al., 2002) suggested that 8- to 9-year-old children would perform the face identity-matching task with accuracy comparable to the WS group, while possessing sufficient maturity to be able to complete the imaging study. Accordingly, the DA group consisted of typically developing 8- to 9-year-old children (M = 8.8 years, s.d. = 0.7; 17 typically developing children, 9 females). It is also noted that a CA of 8- to 9-year falls roughly within range of the mean visuospatial and language age-equivalent estimates for the WS group (5.9 and 12.6 years, respectively).
The study was approved by the Institutional Review Boards of the Salk Institute, the University of California, San Diego (UCSD) and San Diego State University (SDSU). Adult participants and parents/guardians of WS and child participants gave written informed consent; in addition, children and WS participants provided written assent to participate.
Task
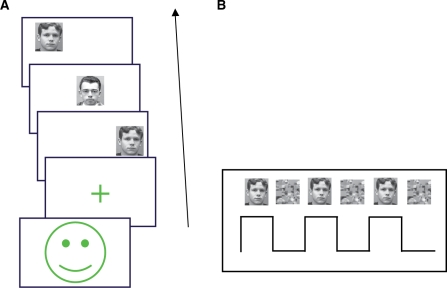
To obtain accurate measures of each participant's face-matching abilities, the task was administered in a quiet room, prior to the imaging session. The task (Figure 1A) required visual matching of facial identity over a series of three neutral expression stimuli. Stimuli were black and white photographs of male faces subtending 4.76° visual angle in the vertical dimension and 5.06° in the horizontal dimension, and appearing in one of the 12 possible positions on the screen. Photographs were obtained from the University of Essex face database (http://cswww.essex.ac.uk/mv/allfaces/index.html). Faces were presented against a uniform gray background, and lacked distinctive features (e.g. earrings, shadows, facial hair, glasses, skin markings) and any overt or identifiable affective expression. Trials were presented in blocks of six (6 blocks, 36 trials total). After a series of two reference stimuli (500 ms duration per stimulus), a third (test) stimulus appeared until the participant responded, or until 3500 ms elapsed. Participants indicated by pressing one of two buttons (‘yes’ or ‘no’) whether the identity of the test stimulus matched either of the two reference stimuli. Both accuracy (d′) and response times (RTs) were recorded.
Fig. 1.
Task. (A) Each block of six face identity-matching trials was preceded by a warning cue (green ‘smiley face’). For each trial, two face stimuli were presented in series, followed by a delay of either 500 or 1750 ms. Participants were then given 3500 ms to respond to the third face stimulus, indicating whether the identity matched either of those seen in the first two stimuli. A match was presented in half of the trials. Participants were encouraged to respond as quickly and accurately as possible. The first 18 trials used a 500 ms delay between the second reference stimulus and the test stimulus, in accordance with our previous face-processing study of WS (Paul et al., 2002). The second 18 trials used a 1750 ms delay, in line with the fMRI paradigm that has been successfully employed in the UCSD laboratory for several years (Passarotti et al., 2003; Stiles et al., 2006). Comparison of accuracy data from the two trial types across the three participant groups did not reveal a significant group × trial type interaction effect (P > 0.1). These data, therefore were collapsed over the two trial types in the present analyses. (B) Each 4 min, 50 s scanning run consisted of six 47.5 s blocks of face identity matching and control trials, in an interleaved fashion. Control trials followed the same presentation sequence as identity-matching trials. Control trial blocks were preceded by a distinct cue (an image of a black handprint, not shown), in order to warn participants as to which trial type would be shown in the upcoming task block.
Functional neuroimaging
Task
The behavioral task was adapted for block design fMRI (Figure 1B) and, based on preliminary data from 12 WS participants, modified slightly to promote optimal task performance (viz., increasing duration of the reference stimuli to 1000 ms and requiring a button press only for positive identity matches to avoid using two different buttons without direct visualization). During each fMRI run, interleaved blocks of five identity matching and five control trials were presented. Unlike the behavioral task, the third (test) stimulus was presented for a fixed duration of 3250 ms in order to achieve scanning runs of constant length. In the control condition three scrambled face images appeared sequentially, following the same timing parameters. Participants were instructed to wait for the third stimulus and then make a button press; no match/mismatch decision was required. Thus, although the control and experimental conditions involved a different response frequency, they were nonetheless comparable with regard both to their basic motoric and their low-level attention and sensory (i.e. detection and perception of a visual stimulus) requirements. All participants completed at least two task runs. WS participants were trained in a mock-scanner immediately prior to fMRI to improve compliance (e.g. reduce head and body motion) and ensure understanding of the task.
Image acquisition
Images were acquired on a Siemens 1.5-Tesla System according to a procedure described elsewhere (Passarotti et al., 2003). Functional images were acquired with a single-shot echo-planar (EPI) pulse sequence sensitive to blood oxygenation level-dependent (BOLD) contrast (FOV = 220 mm, TR = 2500 ms, TE = 40 ms, flip angle = 90°). Whole-head coverage was obtained with 27 5 mm slices (in-plane resolution 3.44 × 3.44 mm). Each fMRI run included 116 volumes. T1-weighted structural images were obtained using an MP-RAGE sequence (TR = 11.4 ms, TE = 4.4 ms, flip angle = 10°, resolution = 1 mm3; 180 sagittal slices).
FMRI data preprocessing
Image preprocessing was performed with algorithms developed at Washington University (A.Z.S., R.L.B. and others). Individual MP-RAGE images were registered (12-parameter affine transformation) to an atlas-representative target conforming to the atlas of Talairach and Tournoux (1988) as defined by the SN method of Lancaster et al. (1995). A study-specific atlas-representative target image was prepared from MP-RAGE data representing all three participant groups (20 WS, 10 CA adults and 10 DA children) using a previously described strategy (Buckner et al., 2004). This approach was adopted to minimize the influence of structural differences between groups on functional responses measured in atlas space. Structural differences between 8- to 9-year-old children and young adults are minor after 12-parameter affine transformation to a common atlas space (Burgund et al., 2002). Atlas transformation of the functional data was computed via each subject's MP-RAGE and combined with motion correction in one step to yield volumetric time series resampled to 3 mm3 voxels.
Preprocessing of the fMRI data involved a series of steps to remove artifacts due to properties of the MR scanning system, as well as subject motion; these have been described in more detail elsewhere (Fox et al., 2005). Steps included (i) correction of central spike artifact caused by signal drift, (ii) compensation for asynchronous slice acquisition, (iii) elimination of systematic odd vs even slice intensity differences due to interleaved acquisition, (iv) six-parameter rigid body head motion correction within and across fMRI runs and (v) intensity scaling to a whole-brain mode value of 1000 to facilitate across-run and across-subject comparisons. Standardization of the mode intensity, rather than the mean intensity, is performed to circumvent difficulties in computing whole-brain mean intensity that stem from the vulnerability of the statistic to extreme values, and the challenge of reliably determining the location of the edge of brain (Ojemann et al., 1997). Atlas transformation of the functional data was computed via each subject's MP-RAGE. The final preprocessing step combined motion correction and atlas transformation, via matrix multiplication of the two types of transforms, to yield the resampled time series.
FMRI analyses
As a quality assurance step, the standard deviation of the signal over the course of each functional run was calculated. Functional runs with excessive variability (defined, according to generally expected values of signal change attributable to the BOLD response, as a mean whole brain standard deviation over a run >2.5%: four, one and zero runs in DA, CA and WS groups, respectively) were excluded. Although the amygdala is a structure that may be susceptible to imaging artifact (LaBar et al., 2001), the exclusion of fewer runs for excessive variability in the WS group (zero) than the CA (one) and DA (four) groups suggests that any diminishment of amygdala activation found in WS would not be due to greater variability as a result of greater motion artifact. Individual and group fMRI analyses were performed using AFNI (Cox, 1996). Data were spatially smoothed (Gaussian kernel at FWHM = 6.88 mm). Multiple regression analysis was performed assuming a canonical hemodynamic response function of the gamma type (Cohen, 1997). As the stimuli were presented according to a block design (Figure 1B), this gamma function was convolved with the stimulus time series to generate the regressor of interest. Six head motion correction parameters, as well as the global mean and linear drift, were included as nuisance regressors. Voxel-wise t-statistic images representing BOLD modulation attributable to task performance (i.e. task vs control) were computed based on the resulting regression coefficient, and converted to equivalently probable z-scores. These z-score images from each individual were submitted to a voxel-wise one-way ANOVA, in order to generate three pairwise group contrasts (WS vs CA, WS vs DA, CA vs DA). To focus the analyses on areas of difference among the groups, these three-group contrast images were each masked to include only voxels in which a main effect of group had been present at P < 0.01 per voxel (uncorrected) in the previous one-way ANOVA. The resulting functional maps then were corrected for multiple comparisons using the False Discovery Rate procedure (Genovese et al., 2002) to obtain an overall alpha level of 0.05. Foci including less than five contiguous voxels (<135 µl; two or fewer native-space voxels) were discounted.
RESULTS
Behavioral task
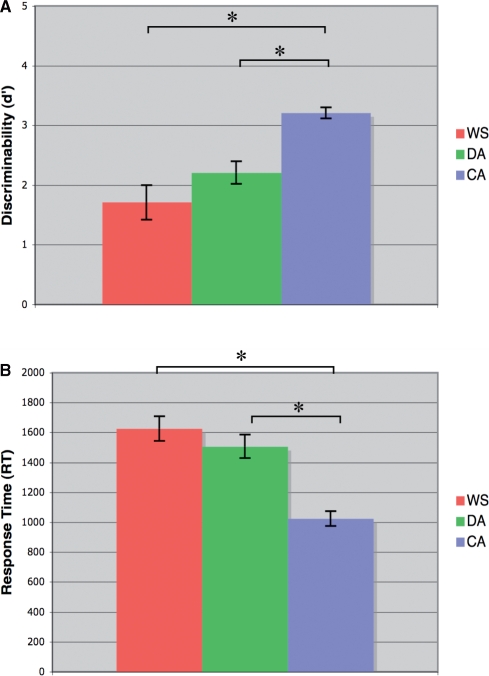
Due to equipment malfunction, behavioral session data were not available for five WS participants. One WS participant's data were excluded because he had difficulty in attending to the task during the behavioral session. Results (Figure 2) showed that the WS group was less accurate and slower than the CA group. No differences were found between the WS and DA groups for accuracy or RT.
Fig. 2.
Behavioral session results, face-matching task. (A) Accuracy. (B) RT. Results revealed an effect of group membership for both dependent variables [d′: F(2,42) = 17.39, *P < 0.001; RT: F(2,42) = 20.64, *P < 0.001]. Follow-up Tukey tests revealed the same pattern of results for both variables: both the WS and DA groups were outperformed by the CA group, with respect to both accuracy and RT (P < 0.001 for all t-test comparisons). The performances of the WS participants and DA controls, as expected, were not different (P > 0.2 for both d′ and RT).
Functional neuroimaging
FMRI task accuracy and RTs were similar to patterns of data collected during the behavioral session, confirming that all groups were engaged in the task during imaging. As expected, CA controls outperformed the two other groups with regard to performance accuracy. During the imaging session the performance of the DA group was better than the WS group (P < 0.001). Given that the trials presented were a subset of those included in the behavioral session, the slight decline in WS performance relative to the behavioral session is unlikely to be due to an inability to complete the task. RT was not different between WS and CA controls (P > 0.9). DA controls, however, were slower than both WS participants and CA controls (P < 0.03).
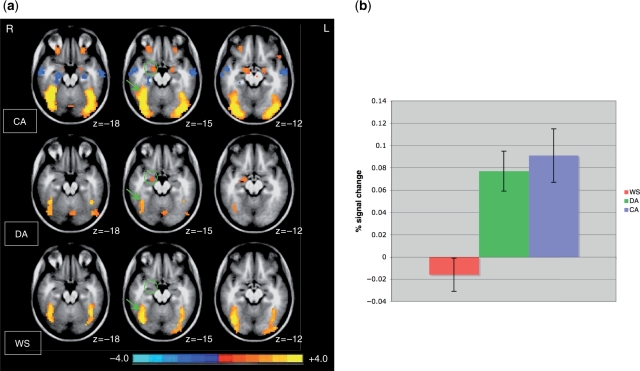
FMRI responses generally were similar in WS participants and DA controls, with both groups displaying less robust activations than CA controls. This pattern held in many ventral portions of occipito-temporal cortex as well (Figure 3), suggesting that the WS responses resembled an immature profile. However, the amygdala response in WS was distinctly abnormal (Figure 3), as both the DA and CA groups showed significant activation, while the WS group did not (see Supplementary Table 1 for a list of all brain regions displaying group differences). Although the current study focused on ventral occipito-temporal cortex and the amygdala, we note that no consistent differences between WS and controls (i.e. WS differing from both control groups) were found in prefrontal regions such as orbitofrontal cortex and medial prefrontal cortex, which are interconnected with the amygdala and play a role in social cognition and behavior (Stefanacci and Amaral, 2002). While differences in inferior frontal and dorsolateral prefrontal cortex were observed between WS and CA controls, unlike results from the amygdala, these differences were not observed when the WS group was compared with the younger DA controls. Thus, paralleling our findings in ventral occipito-temporal cortex, activation of these regions in WS is more consistent with an immature profile.
Fig. 3.
Activation in ventral occipito-temporal regions and the amygdala. (A) Mean z-score images depicting active voxels (P < 0.05, corrected) in CA-matched controls (top), developmental controls (middle) and WS (bottom). Data are displayed in radiological convention (right is on left). Green circles denote significant activation in both control groups in the right amygdalar region, absent in the WS group. Green arrows denote activation in all three groups in the fusiform gyrus, a region in ventral occipito-temporal cortex, i.e. highly sensitive to faces. Except for one focus in a more superior aspect of the fusiform gyrus, where the WS group showed more robust activation than both control groups [Talairach coordinates (38, –69, –9); % signal change = 0.21 for WS, 0.079 for DA controls and 0.13 for CA controls), most ventral occipito-temporal regions showed comparable levels of activation between the WS and DA groups. (B) Mean activation within a spherical region-of-interest (radius = 4.5 mm) placed within the right amygdalar region.
DISCUSSION
During face processing, the typically developing child controls (DA group) did not display the adult pattern of ventral occipito-temporal activation observed in the CA-matched controls (CA group). This difference is consistent with the idea that face processing normally follows a protracted developmental course (Chung and Thomson, 1995), and with recent functional imaging studies revealing age-related differences in activation of face-responsive regions (Aylward et al., 2005; Scherf et al., 2007; Grill-Spector et al., 2008). As the WS responses in this part of the brain resembled those of the DA group, this effect can be understood as reflecting developmental delay. The presence in WS of fMRI responses (albeit, immature) in cortical regions associated with face and object identification is consistent with the observation that these functions may be comparatively well-preserved (Bellugi et al., 1994).
While the DA and CA control groups alike showed robust activation in the amygdala, this response was absent in the WS group. In light of the relatively strong activation for faces in WS observed in ventral occipito-temporal cortex, which is heavily connected with the amygdala (Amaral and Price, 1984), the absence of amygdalar activation in this population is particularly striking. In addition to its importance in emotional processing, the amygdala is involved in detecting information that is socially or behaviorally relevant (Sander et al., 2003; Ousdal et al., 2008) and in regulating social approach behavior (Amaral, 2002). The amygdala normally shows heightened activity during discrimination of unfamiliar faces (Gobbini et al., 2004). Absent amygdala responses to faces in WS is therefore consistent with unusually positive approachability ratings of strangers’ faces that have also been observed in this population (Bellugi et al., 1999). A similar positive approachability bias has been seen in patients with amygdala damage (Adolphs et al., 1998). What is not yet clear, however, is whether the current amygdala finding represents merely a correlate of reduced vigilance when assessing unfamiliar faces, or a more primary deficit involving the amygdala.
The absence of amygdala activity during neutral face processing observed in the present study is consistent with Meyer-Lindenberg et al. (2005), who reported attenuated amygdala response in WS adults to threatening faces but elevated response to threatening scenes. These findings, in concert, suggest a disordered association between socio-emotional stimuli and the amygdala (rather than an absence of function). Moreover, Meyer-Lindenberg et al. (2005, 2006) have suggested that an atypical profile of amygdala responsivity in WS may reflect dysfunction in an amygdala-prefrontal cortex system crucial for the expression of appropriately modulated social behavior. Although the current study does not directly address amygdala-prefrontal connectivity, the pattern of activation in these two regions in WS was qualitatively different. Specifically, amygdala response differed from control participants of all ages, while prefrontal cortex activation was not different from child controls, raising the possibility that the nature of the involvement of these two regions in WS may differ. While the current findings are not incompatible with those of Meyer-Lindenberg and colleagues, particularly given fundamental differences in the experimental tasks (i.e. emotionally-valent vs neutral stimuli), the current findings do highlight the importance of considering activation differences in WS within a developmental context.
The amygdala is also thought to participate at an early developmental stage in the establishment of brain systems for face processing. As part of a fast subcortical pathway that includes the superior colliculus and the pulvinar (a thalamic structure that may contribute to the profound spatial deficits in WS; Eckert et al., 2006), the amygdala may be important to the human newborn's precocious ability to detect and orient to faces (Johnson, 2005). Thus, impairment of this structure could have far-reaching effects on the development of the adult ‘social brain’ network (Skuse, 2003). Our findings suggest that the amygdala is functional in the processing of social information, in this case faces, at least by middle childhood (seen in the robust amygdala response in the 8- to 9-year-old DA controls). In WS, however, genetic influences may impair amygdala function early in development, impacting the emergence of the ability to process information from faces. This may contribute to the complex constellation of social-cognitive and behavioral abnormalities observed during development in WS. Because of the dynamic nature of the developmental process, these characteristics themselves may interact in a complicated way (Karmiloff-Smith et al., 2004), to further influence what are ultimately observed as the very distinctive and intriguing features of the adult WS phenotype.
SUPPLEMENTARY DATA
Supplementary data are available at Scan online.
Conflict of Interest
None declared.
Acknowledgments
This research was supported in part by NIH Program Project HD33113 and a McDonnell Foundation Award to U.B., NIH grant NS06833 (M.E.R., A.Z.S.), NIH grant P50-NS22343 (J.S.) and an NIH Kirschstein Research Service Award (F31NS047086) to B.M.P. The authors wish to thank Fred Rose, Janet Shin, Nasim Bavar, Russ Hornbeck and Justin Vincent for technical assistance with this work, as well as Eric Fine, Yvonne Searcy and Silvia Paparello for helpful comments.
REFERENCES
- Adolphs R, Spezio M. Chapter 20: Role of the amygdala in processing visual social stimuli. Progress in Brain Research. 2006;156:363–78. doi: 10.1016/S0079-6123(06)56020-0. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature. 1998;393(6684):470–4. doi: 10.1038/30982. [DOI] [PubMed] [Google Scholar]
- Amaral DG. The primate amygdala and the neurobiology of social behavior: implications for understanding social anxiety. Biological Psychiatry. 2002;51(1):11–7. doi: 10.1016/s0006-3223(01)01307-5. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) The Journal of Comparative Neurology. 1984;230(4):465–96. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Aylward EH, Park JE, Field KM, et al. Brain activation during face perception: evidence of a developmental change. Journal of Cognitive Neuroscience. 2005;17(2):308–19. doi: 10.1162/0898929053124884. [DOI] [PubMed] [Google Scholar]
- Beery KE. The Beery–Buktenica Developmental Test of Visual-Motor Integration (VMI) 4th edn, Revised ed. Parsippany, NJ: Modern Curriculum Press; 1997. [Google Scholar]
- Bellugi U, Adolphs R, Cassady C, Chiles M. Towards the neural basis for hypersociability in a genetic syndrome. Neuroreport: For Rapid Communication of Neuroscience Research. 1999;10(8):1653–7. doi: 10.1097/00001756-199906030-00006. [DOI] [PubMed] [Google Scholar]
- Bellugi U, Lichtenberger L, Mills D, Galaburda A, Korenberg JR. Bridging cognition, the brain and molecular genetics: evidence from Williams syndrome. Trends in Neuroscience. 1999;22(5):197–207. doi: 10.1016/s0166-2236(99)01397-1. [DOI] [PubMed] [Google Scholar]
- Bellugi U, St George M, editors. Journey from Cognition to Brain to Gene: Perspectives from Williams syndrome. Cambridge, MA: The MIT Press; 2001. [Google Scholar]
- Bellugi U, Wang PP, Jernigan TL. Williams syndrome: an unusual neuropsychological profile. In: Broman SH, Grafman J, editors. Atypical Cognitive Deficits in Developmental Disorders: Implications for Brain Function. Hillsdale, NJ, England: Lawrence Erlbaum Associates, Inc.; 1994. pp. 23–56. [Google Scholar]
- Buckner RL, Head D, Parker J, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23(2):724–38. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Burgund ED, Kang HC, Kelly JE, et al. The feasibility of a common stereotactic space for children and adults in fMRI studies of development. Neuroimage. 2002;17(1):184–200. doi: 10.1006/nimg.2002.1174. [DOI] [PubMed] [Google Scholar]
- Chung MS, Thomson DM. Development of face recognition. British Journal of Psychology. 1995;86(1):55–87. doi: 10.1111/j.2044-8295.1995.tb02546.x. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6(2):93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29(3):162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Deruelle C, Mancini J, Livet MO, Casse-Perrot C, de Schonen S. Configural and local processing of faces in children with Williams syndrome. Brain and Cognition. 1999;41(3):276–98. doi: 10.1006/brcg.1999.1127. [DOI] [PubMed] [Google Scholar]
- Dunn LM, Dunn LM. Peabody Picture Vocabulary Test—Third Edition (PPVT-III) 3rd edn. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Eckert MA, Galaburda AM, Mills DL, et al. The neurobiology of Williams syndrome: cascading influences of visual system impairment? Cellular and Molecular Life Science. 2006;63(16):1867–75. doi: 10.1007/s00018-005-5553-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewart AK, Morris CA, Atkinson D, et al. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nature Genetics. 1993;5(1):11–6. doi: 10.1038/ng0993-11. [DOI] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Barch DM, Gusnard DA, Raichle ME. Transient BOLD responses at block transitions. Neuroimage. 2005;28(4):956–66. doi: 10.1016/j.neuroimage.2005.06.025. [DOI] [PubMed] [Google Scholar]
- Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Leibenluft E, Santiago N, Haxby JV. Social and emotional attachment in the neural representation of faces. Neuroimage. 2004;22(4):1628–35. doi: 10.1016/j.neuroimage.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Grice SJ, Spratling MW, Karmiloff-Smith A, et al. Disordered visual processing and oscillatory brain activity in autism and Williams syndrome. Neuroreport. 2001;12(12):2697–700. doi: 10.1097/00001756-200108280-00021. [DOI] [PubMed] [Google Scholar]
- Grill-Spector K, Golarai G, Gabrieli J. Developmental neuroimaging of the human ventral visual cortex. Trends in Cognitive Sciences. 2008;12(4):152–62. doi: 10.1016/j.tics.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, Gobbini MI. Human neural systems for face recognition and social communication. Biological Psychiatry. 2002;51(1):59–67. doi: 10.1016/s0006-3223(01)01330-0. [DOI] [PubMed] [Google Scholar]
- Howlin P, Davies M, Udwin O. Cognitive functioning in adults with Williams syndrome. Journal of Child Psychology and Psychiatry. 1998;39(2):183–9. [PubMed] [Google Scholar]
- Johnson MH. Subcortical face processing. Nature Reviews Neuroscience. 2005;6(10):766–74. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- Jones W, Bellugi U, Lai Z, et al. II. Hypersociability in Williams syndrome. Journal of Cognitive Neuroscience. 2000;12(Suppl 1):30–46. doi: 10.1162/089892900561968. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A. Atypical epigenesis. Developmental Science. 2007;10(1):84–8. doi: 10.1111/j.1467-7687.2007.00568.x. [DOI] [PubMed] [Google Scholar]
- Karmiloff-Smith A, Thomas M, Annaz D, et al. Exploring the Williams syndrome face-processing debate: the importance of building developmental trajectories. Journal of Child Psychology and Psychiatry. 2004;45(7):1258–74. doi: 10.1111/j.1469-7610.2004.00322.x. [DOI] [PubMed] [Google Scholar]
- Kawashima R, Sugiura M, Kato T, et al. The human amygdala plays an important role in gaze monitoring. A PET study. Brain. 1999;122(Pt 4):779–83. doi: 10.1093/brain/122.4.779. [DOI] [PubMed] [Google Scholar]
- Korenberg JR, Chen XN, Hirota H, et al. VI. Genome structure and cognitive map of Williams syndrome. Journal of Cognitive Neuroscience. 2000;12(Suppl 1):89–107. doi: 10.1162/089892900562002. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gitelman DR, Mesulam MM, Parrish TB. Impact of signal-to-noise on functional MRI of the human amygdala. Neuroreport. 2001;12(16):3461–4. doi: 10.1097/00001756-200111160-00017. [DOI] [PubMed] [Google Scholar]
- Laing E, Butterworth G, Ansari D, et al. Atypical development of language and social communication in toddlers with Williams syndrome. Developmental Science. 2002;5(2):233–46. [Google Scholar]
- Lancaster JL, Glass TG, Lankipalli BR, Downs H, Mayberg H, Fox PT. A modality-independent approach to spatial normalization of tomographic images of the human brain. Human Brain Mapping. 1995;3(3):209–23. [Google Scholar]
- Maurer D, Grand RL, Mondloch CJ. The many faces of configural processing. Trends in Cognitive Sciences. 2002;6(6):255–60. doi: 10.1016/s1364-6613(02)01903-4. [DOI] [PubMed] [Google Scholar]
- Mervis CB, Robinson BF, Bertrand J, Morris CA, Klein-Tasman BP, Armstrong SC. The Williams syndrome cognitive profile. Brain and Cognition. 2000;44(3):604–28. doi: 10.1006/brcg.2000.1232. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Hariri AR, Munoz KE, et al. Neural correlates of genetically abnormal social cognition in Williams syndrome. Nature Neuroscience. 2005;8(8):991–3. doi: 10.1038/nn1494. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Kohn P, Mervis CB, et al. Neural basis of genetically determined visuospatial construction deficit in Williams syndrome. Neuron. 2004;43(5):623–31. doi: 10.1016/j.neuron.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Mervis CB, Berman KF. Neural mechanisms in Williams syndrome: a unique window to genetic influences on cognition and behaviour. Nature Reviews Neuroscience. 2006;7(5):380–93. doi: 10.1038/nrn1906. [DOI] [PubMed] [Google Scholar]
- Mills DL, Alvarez TD, St George M, Appelbaum LG, Bellugi U, Neville H. III. Electrophysiological studies of face processing in Williams syndrome. Journal of Cognitive Neuroscience. 2000;12(Suppl 16):47–64. doi: 10.1162/089892900561977. [DOI] [PubMed] [Google Scholar]
- Mobbs D, Garrett AS, Menon V, Rose FE, Bellugi U, Reiss AL. Anomalous brain activation during face and gaze processing in Williams syndrome. Neurology. 2004;62(11):2070–6. doi: 10.1212/01.wnl.0000129536.95274.dc. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility artifacts. Neuroimage. 1997;6(3):156–67. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Ousdal OT, Jensen J, Server A, Hariri AR, Nakstad PH, Andreassen OA. The human amygdala is involved in general behavioral relevance detection: evidence from an event-related functional magnetic resonance imaging Go-NoGo task. Neuroscience. 2008;156(3):450–5. doi: 10.1016/j.neuroscience.2008.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passarotti AM, Paul BM, Bussiere JR, Buxton RB, Wong EC, Stiles J. The development of face and location processing: an fMRI study. Developmental Science. 2003;6(1):100–17. [Google Scholar]
- Paul BM. Unpublished Doctoral Dissertation. San Diego: University of California; 2007. How We Come to Process ‘What’ and ‘Where’ in Our Visual Environment: Insights From Typical and Atypical Developmental Populations. [Google Scholar]
- Paul BM, Stiles J, Passarotti A, Bavar N, Bellugi U. Face and place processing in Williams syndrome: evidence for a dorsal–ventral dissociation. Neuroreport. 2002;13(9):1115–9. doi: 10.1097/00001756-200207020-00009. [DOI] [PubMed] [Google Scholar]
- Sander D, Grafman J, Zalla T. The human amygdala: an evolved system for relevance detection. Reviews in the Neurosciences. 2003;14(4):303–16. doi: 10.1515/revneuro.2003.14.4.303. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Behrmann M, Humphreys K, Luna B. Visual category-selectivity for faces, places and objects emerges along different developmental trajectories. Developmental Science. 2007;10(4):F15–30. doi: 10.1111/j.1467-7687.2007.00595.x. [DOI] [PubMed] [Google Scholar]
- Searcy YM, Lincoln AJ, Rose FE, Klima ES, Bavar N, Korenberg JR. The relationship between age and IQ in adults with Williams syndrome. American Journal of Mental Retardation. 2004;109(3):231–6. doi: 10.1352/0895-8017(2004)109<231:TRBAAI>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Skuse D. Fear recognition and the neural basis of social cognition. Child and Adolescent Mental Health. 2003;8(2):50–60. doi: 10.1111/1475-3588.00047. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. The Journal of Comparative Neurology. 2002;451(4):301–23. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Stiles J, Paul B, Hesselink J. Spatial cognitive development following early focal brain injury: evidence for adaptive change in brain and cognition. In: Munakata Y, Johnson MH, editors. Processes of Change in Brain and Cognitive Development: Attention and Performance XXI. Oxford: Oxford University Press; 2006. pp. 535–61. [Google Scholar]
- Tager-Flusberg H, Plesa-Skwerer D, Faja S, Joseph RM. People with Williams syndrome process faces holistically. Cognition. 2003;89(1):11–24. doi: 10.1016/s0010-0277(03)00049-0. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar Stereotaxic Atlas of the Human Brain. New York: Thieme; 1988. [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children—Revised. San Antonio, TX: The Psychological Corporation; 1974. [Google Scholar]
- Wechsler D. San Antonio, TX: The Psychological Corporation; 1981. Wechsler Adult Intelligence Scale—Revised. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.