Abstract
Background: Moderate alcohol consumption is associated with a decreased risk of type 2 diabetes in the general population, but little is known about the effects in individuals at high risk of diabetes.
Objectives: The objectives were to determine associations between alcohol consumption and diabetes risk factors and whether alcohol consumption was a predictor of incident diabetes in individuals enrolled in the Diabetes Prevention Program (DPP).
Design: DPP participants (n = 3175) had impaired glucose tolerance (2-h glucose: 7.8–11.1 mmol/L), elevated fasting glucose (5.3–7.0 mmol/L), and a body mass index (in kg/m2) ≥24. Participants were randomly assigned to placebo, metformin, or lifestyle modification and were followed for a mean of 3.2 y. Alcohol intake was assessed at baseline and year 1 by using a semiquantitative food-frequency questionnaire. Diabetes was diagnosed by annual oral-glucose-tolerance testing and semiannual fasting plasma glucose measurement.
Results: Participants who reported higher alcohol consumption tended to be male, older, white, and less obese and to have a higher calorie intake and a higher HDL-cholesterol concentration. Higher alcohol consumption was associated with lower insulin secretion at any level of insulin sensitivity. We found lower incidence rates of diabetes with higher alcohol consumption in the metformin (P < 0.01 for trend) and lifestyle modification (P = 0.02 for trend) groups, which remained significant after adjustment for multiple baseline covariates. No similar association was observed in the placebo group.
Conclusions: Despite overall low rates of alcohol consumption, there was a reduced risk of incident diabetes in those who reported modest daily alcohol intake and were assigned to metformin or lifestyle modification. Moderate daily alcohol intake is associated with lower insulin secretion—an effect that warrants further investigation. This trial was registered at clinicaltrials.gov as NCT00038727.
INTRODUCTION
Diabetes mellitus is a common chronic condition that affects an estimated 20.8 million Americans (1). It is a major cause of premature mortality and morbidity because of cardiovascular, renal, ophthalmic, and neurologic diseases. Although a family history of diabetes is an established risk factor for type 2 diabetes, lifestyle factors also play an important etiologic role (2). For example, the rising incidence of diabetes in the United States has been largely attributed to physical inactivity and excess calorie intake, both of which can be modified to decrease the risk of diabetes (3).
Alcohol consumption is also prevalent in the United States: an estimated 126 million Americans, or ≈52% of those aged ≥12 y, currently drink alcohol (4). Cohort studies have shown associations between moderate alcohol consumption and a decreased risk of diabetes and coronary heart disease (5, 6). These findings are consistent with studies that have found greater insulin sensitivity among moderate drinkers than nondrinkers (7, 8). Effects of alcohol consumption on other aspects of glucose metabolism, including insulin secretion, have been less well studied. Furthermore, little is known about the effect of alcohol consumption on diabetes incidence and related metabolic indexes among individuals who are at high risk of diabetes or in conjunction with therapies that reduce this risk. Accordingly, we examined the associations of alcohol consumption with diabetes risk factors, diabetes incidence, insulin secretion, and insulin resistance using data from the Diabetes Prevention Program (DPP), a clinical trial designed to evaluate the efficacy of interventions to delay or prevent development of diabetes in high-risk individuals with impaired glucose tolerance, elevated fasting glucose, and overweight/obesity.
SUBJECTS AND METHODS
Study participants
The DPP is a randomized clinical trial that enrolled 3234 participants at 27 centers throughout the United States. The methods have been described in detail elsewhere (9), and the protocol is available at http://www.bsc.gwu.edu/dpp. Briefly, eligibility criteria included an age of ≥25 y, a body mass index (BMI; in kg/m2) of ≥24 (≥22 in Asians), a plasma glucose concentration of 5.3–7.0 mmol/L (95–125 mg/dL) in the fasting state (≤7.0 mmol/L in American Indian centers), and 7.8–11.1 mmol/L (140–199 mg/dL) 2 h after a 75-g oral glucose load. Because of the potential for lactic acidosis with metformin intake, individuals were ineligible for DPP if they had a history of excessive alcohol intake, as defined by one of the following factors: 1) average consumption of ≥3 alcoholic beverages/d in the past 12 mo, 2) consumption of ≥7 alcoholic beverages within a 24-h period in the past 12 mo, and 3) clinical assessment of alcohol dependence based on ≥2 positive responses to the CAGE questionnaire (10) or based on other evidence available to the clinic staff. Of >32,000 individuals screened for DPP eligibility, 114 were excluded because of excessive alcohol intake, and 20 were excluded because of a history of alcoholic hepatitis or pancreatitis.
On enrollment, participants were randomly assigned to 1 of 3 treatment arms: placebo, 850 mg metformin twice daily, or an intensive lifestyle modification program. The protocol was approved by the Institutional Review Boards for the protection of human subjects at each study site, and all participants provided written informed consent.
Assessment of alcohol consumption
Usual alcohol consumption over the past year was assessed at baseline and 1 y after randomization via an in-person interview by using a semiquantitative food-frequency questionnaire (FFQ) (11). This FFQ was previously used to assess alcohol consumption in epidemiologic studies (12). For each beverage type (beer, wine, and hard liquor), participants reported their consumption frequency and portion size. The 9 frequency response categories ranged from “never or less than once per month” to “6 or more times per day.” Portion sizes were reported as small, medium, or large compared with what other women or men of the same sex and age drank. These data were combined to yield 4 alcohol consumption categories: none (never or <1 drink/mo), ≥1 drink/mo to <1 drink/wk, 1 drink/wk to <1 drink/d, and ≥1 drink/d, in which one drink was defined as 12 g alcohol (13). During the study, participants in all 3 treatment arms were advised against “regular excessive drinking” (average consumption of ≥3 drinks/d) or “binge drinking” (≥7 drinks within a 24-h period). Participants in the lifestyle group were also instructed to consider alcohol as a source of calories. Because alcohol consumption did not change over the first year in any treatment group (11), baseline data were used for this analysis.
Outcome variables
The primary outcome was diabetes, diagnosed on the basis of an annual oral-glucose-tolerance test or a semiannual fasting plasma glucose test, according to American Diabetes Association criteria (14). Additional outcomes included change in insulin secretion and insulin resistance, both of which were determined at baseline and annually thereafter. Insulin secretion was calculated by using the corrected insulin response at 30 min (CIR30): insulin30min (μU/mL)/}glucose30min (mg/dL) × [glucose30min (mg/dL) – 70]} (15), which was then multiplied by a factor of 100. Insulin resistance was assessed by using the homeostasis model assessment of insulin resistance (HOMA-IR): (fasting insulin [μU/mL] × fasting glucose [mmol/L])/22.5 (16).
Statistical analysis
Descriptive statistics of baseline measurements and changes at year 1 were computed overall and by category of alcohol intake at baseline with chi-square tests used to test differences across alcohol categories for discrete variables and analysis of variance or covariance for continuous variables. Linear regression was used to estimate the relation between log-transformed insulin secretion (CIR30) and log-transformed insulin sensitivity (1/HOMA-IR) in each alcohol consumption category, based on the known nonlinear relation between these 2 variables (17, 18). The curves in Figure 1 were derived directly from the log-transformed data, which were then plotted as untransformed (re-exponentiated) curves. Cox regression models were used to assess the effect of baseline alcohol intake on the development of diabetes (19). Cox models were run separately for each treatment group adjusted for age, sex, self-reported ethnicity, C-reactive protein (CRP), weight, exercise, calorie intake, HOMA-IR, CIR30, and fasting plasma glucose. Subsequent models sequentially added the covariates of weight over time, HOMA-IR over time, CIR30 over time, calories over time, and exercise over time. Nominal P values are presented. P values for trends by increasing alcohol intake category were computed by entering the grouped alcohol intake variable into the models as a continuous variable. Similarly, we evaluated interactions between alcohol intake and treatment arm in a joint model with all 3 treatment arms and an interaction term between treatment (categorical) and alcohol intake group (continuous). The SAS analysis system (version 8.2) was used for all analyses (SAS Institute Inc, Cary, NC).
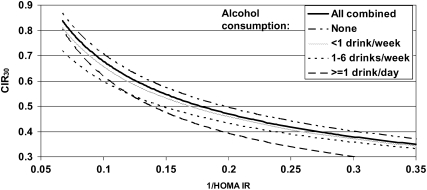
FIGURE 1.
Relation of insulin secretion (corrected insulin response at 30 min; CIR30) to insulin sensitivity [1/homeostasis model assessment of insulin resistance (HOMA-IR)] by baseline alcohol intake. Curves were derived from linear regression models between log-transformed insulin secretion (CIR30) and log-transformed insulin sensitivity (1/HOMA-IR) in each alcohol consumption category and were plotted as untransformed (re-exponentiated) curves. P < 0.001 for differences across alcohol consumption categories after adjustment for age and sex. P = 0.019 after adjustment for age, sex, race-ethnicity, BMI, exercise (metabolic equivalent task-hours/wk), and calorie intake (P = 0.049 excluding current smokers).
RESULTS
Participant characteristics
Baseline alcohol consumption data were available for 3175 of 3234 (98%) randomly assigned participants. Of these 3175 participants, 1772 (56%) reported no alcohol consumption over the past 12 mo, 608 (19%) reported consuming <1 drink/wk, 647 (20%) reported consuming 1–6 drinks/wk, and 148 (5%) reported consuming ≥1 drink/d. Wine consumption was reported by 32%, beer by 24%, and liquor by 20% of the cohort (some participants reported consuming more than one type). There were no differences in alcohol consumption by treatment arm.
Characteristics of the participants at baseline, by alcohol consumption category, for all treatment arms combined are shown in Table 1. Participants who reported higher levels of alcohol consumption were significantly older, less obese, and more likely to be male and white. They also had greater reported calorie intake, coffee and tea consumption, and levels of exercise; were more likely to report a current or past history of smoking; had higher HDL-cholesterol and fasting glucose concentrations; a lower HOMA-IR; and lower concentrations of fasting insulin, CIR30, and CRP. After adjustment for age, sex, and BMI, calorie intake (P = 0.0002), fat intake (P = 0.046), protein intake (P = 0.01), current smoking (P < 0.0001), coffee and tea consumption (P < 0.0001), HDL cholesterol (P < 0.0001), fasting insulin (P = 0.049), and CIR30 (P = 0.004) differed significantly by alcohol consumption category. Because the prevalence of smoking at baseline was so low, we evaluated its potential effect on the relation between alcohol intake and diabetes by conducting sensitivity analyses excluding these participants (see below).
TABLE 1.
Baseline characteristics of Diabetes Prevention Program participants by category of alcohol consumption1
| Alcohol consumption |
||||
| Characteristic | None (n = 1772) | <1 drink/wk (n = 608) | 1–6 drinks/wk (n = 647) | ≥1 drink/d (n = 148) |
| Treatment arm [% (n)] | ||||
| Placebo | 57 (605) | 18 (191) | 21 (224) | 4 (44) |
| Metformin | 54 (575) | 20 (209) | 21 (220) | 5 (53) |
| Lifestyle | 56 (592) | 20 (208) | 19 (203) | 5 (51) |
| Age [% (n)]2 | ||||
| 25–44 y | 73 (715) | 13 (124) | 12 (122) | 2 (19) |
| 45–59 y | 50 (751) | 22 (336) | 23 (339) | 5 (69) |
| 60–85 y | 43 (277) | 22 (138) | 27 (171) | 9 (55) |
| Sex [% (n)]2 | ||||
| Men | 41 (425) | 19 (197) | 31 (316) | 9 (87) |
| Women | 63 (1347) | 19 (411) | 15 (331) | 3 (61) |
| Race-ethnicity [% (n)]2 | ||||
| White | 47 (830) | 22 (377) | 25 (436) | 6 (107) |
| African American | 62 (388) | 18 (114) | 17 (109) | 3 (17) |
| Hispanic | 63 (314) | 16 (81) | 17 (82) | 4 (19) |
| Asian | 73 (101) | 17 (23) | —3 | —3 |
| American Indian | 86 (139) | —3 | —3 | —3 |
| Weight (kg) | 94.7 ± 21.24 | 93.4 ± 18.9 | 94.3 ± 19.1 | 91.3 ± 17.1 |
| BMI (kg/m2)2 | 34.8 ± 7.1 | 33.6 ± 6.2 | 32.7 ± 5.9 | 31.6 ± 5.2 |
| Energy (kcal/d)2 | 2127 ± 1032 | 2046 ± 988 | 2144 ± 1056 | 2368 ± 1167 |
| Protein (g/d) | 88.1 ± 44.0 | 87.0 ± 43.1 | 90.8 ± 45.8 | 93.8 ± 47.1 |
| Carbohydrate (g/d) | 257.9 ± 122.8 | 248.7 ± 117.7 | 255.7 ± 126.8 | 266.9 ± 139.7 |
| Fat (g/d) | 83.5 ± 48.5 | 78.8 ± 46.3 | 81.6 ± 48.8 | 86.1 ± 52.4 |
| Coffee and tea [% (n)]2 | ||||
| None | 14 (243) | 6 (35) | 5 (31) | 5 (7) |
| >0 to ≤2 cups/d | 54 (949) | 49 (297) | 45 (284) | 33 (48) |
| >2 to ≤4 cups/d | 24 (422) | 34 (202) | 37 (237) | 48 (70) |
| >4 cups/d | 8 (136) | 11 (69) | 13 (86) | 14 (21) |
| Smoking [% (n)]2 | ||||
| Never | 66 (1176) | 55 (335) | 45 (290) | 39 (57) |
| Past | 28 (488) | 38 (229) | 46 (300) | 53 (78) |
| Current | 6 (108) | 7 (44) | 9 (57) | 9 (13) |
| Exercise (MET-h/wk)2 | 15.32 ± 27.4 | 17.1 ± 29.7 | 17.3 ± 17.5 | 22.4 ± 22.0 |
| HDL cholesterol (mmol/L)2 | 1.17 ± 0.30 | 1.18 ± 0.29 | 1.21 ± 0.33 | 1.24 ± 0.37 |
| LDL cholesterol (mmol/L) | 3.20 ± 0.84 | 3.26 ± 0.85 | 3.26 ± 0.85 | 3.26 ± 0.90 |
| Triglycerides (mmol/L) | 1.81 ± 1.09 | 1.90 ± 1.07 | 1.90 ± 1.08 | 1.86 ± 1.16 |
| Fasting glucose (mmol/L)2 | 5.88 ± 0.46 | 5.92 ± 0.46 | 5.96 ± 0.47 | 5.99 ± 0.48 |
| Glucose at 120 min (mmol/L) | 9.15 ± 0.96 | 9.17 ± 0.92 | 9.09 ± 0.92 | 9.07 ± 0.96 |
| Fasting insulin (pmol/L)2 | 194.5 ± 103.5 | 176.4 ± 97.2 | 172.2 ± 109.0 | 159.7 ± 91.0 |
| CIR30 [pmol/L/(mmol/L)2] × 100 | 0.68 ± 0.44 | 0.60 ± 0.41 | 0.56 ± 0.40 | 0.51 ± 0.33 |
| HOMA-IR (μU/mL · mmol/L)2 | 7.39 ± 4.11 | 6.73 ± 3.87 | 6.63 ± 4.37 | 6.16 ± 3.52 |
| CRP (μg/mL)2 | 0.64 ± 0.80 | 0.53 ± 0.50 | 0.52 ± 0.74 | 0.49 ± 0.69 |
Percentages should be summed across rows for treatment arm, age, sex, and race-ethnicity and should be summed within columns for coffee and tea and smoking; percentages may not add up to 100 because of rounding. MET, metabolic equivalent task; CIR30, corrected insulin response at 30 min; HOMA-IR, homeostasis model assessment of insulin resistance; CRP, C-reactive protein.
P < 0.01 for chi-square test of discrete variables and ANOVA of continuous variables.
Data for groups with sample sizes <15 are not shown.
Mean ± SD (all such values).
Insulin secretion and insulin sensitivity are tightly linked metabolic variables; therefore, an analysis of insulin secretion relative to the prevailing insulin sensitivity is necessary to obtain an accurate assessment of β cell function. Across the 3 treatment arms combined, the relations at baseline between insulin sensitivity (1/HOMA-IR) and insulin secretion (CIR30) by alcohol consumption category are depicted in Figure 1. We found that at any level of insulin sensitivity, greater alcohol consumption was associated with lower levels of insulin secretion—a relation that remained highly significant after adjustment for age and sex (P < 0.001) and after further adjustment for race-ethnicity, BMI, exercise (metabolic equivalent task–hours/wk), and calorie intake (P = 0.019). This relation was unchanged after the relatively small number of participants who reported current smoking at baseline was excluded.
Diabetes incidence
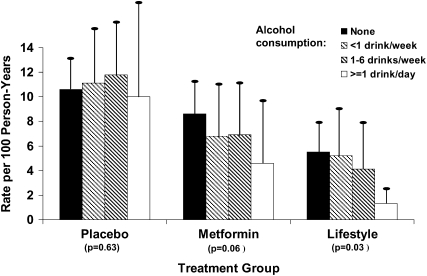
During an average follow-up of 3.2 y, the incidence of diabetes per 100 person-years was 10.8, 7.7, and 5.0 in the placebo, metformin, and lifestyle modification arms, respectively. The incidence of diabetes by alcohol consumption category, stratified by treatment arm, is presented in Figure 2. In both active treatment arms, overall diabetes incidence was lower among participants who consumed ≥1 drink/d than among those who consumed less alcohol. Hazard ratios for the development of diabetes are shown in Table 2. After adjustment for age, sex, self-reported ethnicity, baseline CRP, weight, exercise, calorie intake, HOMA-IR, CIR30, fasting plasma glucose, weight over time, HOMA-IR over time, CIR30 over time, calories over time, and exercise over time, higher levels of alcohol consumption were associated with decreased diabetes risk in the metformin (P = 0.01 for trend) and lifestyle modification (P = 0.02 for trend) arms. After adjustment for age, sex, self-reported ethnicity, baseline CRP, weight, exercise, calorie intake, HOMA-IR, CIR30, fasting plasma glucose, weight over time, HOMA-IR over time, CIR30 over time, calories over time, and exercise over time, higher levels of alcohol consumption were associated with decreased diabetes risk in the metformin (P = 0.001 for trend) and lifestyle modification (P = 0.016 for trend) arms. Sequential models with fewer covariates and additional adjustment for coffee and tea consumption did not substantially alter the hazard ratios. Similarly, the exclusion of current smokers did not appreciably alter these results. The interaction between treatment group and alcohol consumption category was significant for lifestyle (P = 0.01) and metformin (P = 0.04) compared with placebo.
FIGURE 2.
Unadjusted diabetes incidence rates by alcohol consumption category and treatment arm in the Diabetes Prevention Program. Vertical lines represent 1 SE. P values represent the unadjusted trend in diabetes incidence across alcohol consumption categories.
TABLE 2.
Adjusted diabetes hazard ratios (and 95% CIs) in Diabetes Prevention Program participants by alcohol consumption category and treatment arm
| Alcohol consumption |
|||||
| Treatment arm | None (n = 1772) | <1 drink/wk (n = 608) | 1–6 drinks/wk (n = 647) | ≥1 drink/d (n = 148) | P for trend |
| Interim model1 | |||||
| Placebo | 1 | 1.17 (0.85, 1.60) | 1.16 (0.85, 1.58) | 1.54 (0.56, 1.98) | 0.37 |
| Metformin | 1 | 0.71 (0.49, 1.03) | 0.69 (0.48, 0.99) | 0.51 (0.24, 1.12) | 0.011 |
| Lifestyle | 1 | 1.03 (0.67, 1.56) | 0.80 (0.50, 1.29) | 0.35 (0.08, 1.44) | 0.16 |
| Fully adjusted2 | |||||
| Placebo | 1 | 1.09 (0.79, 1.50) | 1.08 (0.79, 1.47) | 0.87 (0.47, 1.67) | 0.83 |
| Metformin3 | 1 | 0.83 (0.57, 1.22) | 0.58 (0.39, 0.84) | 0.46 (0.21, 1.01) | 0.001 |
| Lifestyle4 | 1 | 0.80 (0.52, 1.23) | 0.64 (0.40, 1.05) | 0.28 (0.07, 1.16) | 0.016 |
| Fully adjusted, excluding current smokers2 | |||||
| Placebo | 1 | 1.16 (0.83, 1.62) | 1.80 (0.77, 1.51) | 1.16 (0.60, 2.26) | 0.52 |
| Metformin3 | 1 | 0.82 (0.55, 1.23) | 0.60 (0.41, 0.90) | 0.44 (0.19, 1.03) | 0.003 |
| Lifestyle4 | 1 | 0.74 (0.46, 1.19) | 0.52 (0.31, 0.88) | 0.27 (0.07, 1.14) | 0.003 |
Based on Cox proportional hazards models adjusted for age, sex, self-reported ethnicity, weight, exercise, calorie intake, weight over time, calories over time, and exercise over time.
Based on Cox proportional hazards models adjusted for age, sex, self-reported ethnicity, baseline weight, exercise, calorie intake, C-reactive protein, homeostasis model assessment of insulin resistance, corrected insulin response at 30 min, fasting plasma glucose, weight over time, calories over time, exercise over time, homeostasis model assessment of insulin resistance over time, and corrected insulin response at 30 min over time.
Significant interaction between treatment group and alcohol consumption for metformin compared with placebo, P = 0.04
Significant interaction between treatment group and alcohol consumption for lifestyle compared with placebo, P = 0.01.
Changes in risk factors by treatment group and alcohol consumption category
To explore potential mechanisms for the effect of alcohol consumption on diabetes risk, we examined changes from baseline to year 1 in several diabetes risk factors (Table 3). In the lifestyle modification arm, participants with greater baseline alcohol intake lost significantly more weight at year 1 (P for trend = 0.02). However, there was no relation between alcohol intake and weight loss in the metformin or placebo arms. Higher baseline alcohol consumption was associated with increased exercise at year 1 in the metformin arm (P < 0.001) and to a lesser extent in the placebo arm (P = 0.03), but not in the lifestyle modification arm (P = 0.80), and with decreases in reported calorie intake in the lifestyle modification arm (P = 0.01) and metformin arm (P = 0.025).
TABLE 3.
Change in risk factors from baseline to year 1 in Diabetes Prevention Program participants by alcohol consumption category and treatment arm1
| Alcohol consumption |
||||||||||||
| Lifestyle |
Metformin |
Placebo |
||||||||||
| None (n = 592) | <1 drink/wk (n = 208) | 1–6 drinks/wk (n = 203) | ≥1 drink/d (n = 51) | None (n = 575) | <1 drink/wk (n = 209) | 1–6 drinks/ wk (n = 220) | ≥1 drink/d (n = 53) | None (n = 605) | <1 drink/wk (n = 191) | 1–6 drinks/wk (n = 224) | ≥1 drink/d (n = 44) | |
| Weight change (kg) | −6.42 ± 0.30 | −7.28 ± 0.46 | −7.11 ± 0.55 | −8.75 ± 0.912 | −2.85 ± 0.21 | −2.67 ± 0.32 | −2.24 ± 0.30 | −3.01 ± 0.59 | −0.22 ± 0.20 | −0.71 ± 0.36 | −0.68 ± 0.31 | −0.49 ± 0.57 |
| Exercise change (MET-h/wk) | 7.79 ± 1.13 | 7.41 ± 1.43 | 5.95 ± 1.12 | 8.44 ± 3.31 | 0.22 ± 0.97 | 1.76 ± 3.81 | 2.82 ± 1.00 | 9.91 ± 6.223 | 0.62 ± 1.61 | −0.45 ± 1.68 | 3.08 ± 1.54 | 0.83 ± 2.633 |
| Energy change (kcal) | −485 ± 35.8 | −372 ± 52.5 | −422 ± 59.8 | −464 ± 111.83 | −326 ± 32.3 | −270 ± 54.7 | −198 ± 48.8 | −466 ± 88.22 | −263 ± 39.8 | −264 ± 47.0 | −187 ± 55.8 | −345 ± 112.2 |
| HOMA-IR change (μU/mL · mmol/L) | −1.54 ± 0.199 | −1.82 ± 0.254 | −1.39 ± 0.345 | −0.333 ± 0.963 | −1.15 ± 0.166 | −0.841 ± 0.244 | −1.05 ± 0.299 | −1.66 ± 0.440 | 0.328 ± 0.230 | 0.811 ± 0.343 | 0.229 ± 0.286 | 0.461 ± 0.325 |
| CIR30 change [pmol/L/(mmol/L)2] × 100 | 0.014 ± 0.022 | 0.026 ± 0.024 | 0.033 ± 0.025 | 0.168 ± 0.199 | −0.007 ± 0.017 | 0.071 ± 0.043 | −0.050 ± 0.032 | −0.039 ± 0.0332 | 0.017 ± 0.020 | −0.020 ± 0.027 | 0.020 ± 0.028 | −0.040 ± 0.0362 |
All values are means ± SEs. CIR30, corrected insulin response at 30 min; MET, metabolic equivalent task; HOMA-IR, homeostasis model assessment of insulin resistance.
P for trend across alcohol groups: 2P< 0.05, 3P< 0.01.
There was no association between alcohol consumption category and change in insulin sensitivity (HOMA-IR) for any treatment arm. Likewise, we found no association between alcohol consumption and changes in insulin secretion (CIR30) over time, except for a modest decline (P = 0.03) among participants reporting higher levels of alcohol consumption (≥1 drink/wk) in the metformin arm only.
DISCUSSION
In this randomized clinical trial, which was designed to evaluate the efficacy of interventions to delay or prevent the development of diabetes in high-risk individuals, higher levels of alcohol consumption were associated with a reduced risk of developing diabetes, but only in those assigned to active treatment. Previous observational studies found that, compared with no alcohol use, moderate alcohol consumption (1–3 drinks/d) was associated with a 33–56% lower incidence of diabetes (5), although this association was not found in all racial-ethnic groups (20). In our cohort, we found a similar and highly significant reduction in diabetes risk associated with higher levels of alcohol consumption, but this effect was limited to the 2 active treatment arms. Of note, this effect of alcohol was clearly apparent despite the robust effect of the study interventions on diabetes incidence (3).
The lack of an association of alcohol intake with diabetes incidence in the placebo group is unexplained, but may indicate that there is a synergistic effect of alcohol with both metformin and lifestyle modification. In the lifestyle modification arm, participants in the higher alcohol consumption categories lost more weight than the abstainers, which may partly explain the association between alcohol intake and lower diabetes risk. However, a lower diabetes risk with greater baseline alcohol consumption remained significant after adjustment for weight loss, which suggests that other mechanisms may also be involved. Furthermore, although participants in the metformin arm also lost weight, in this case weight loss did not differ by alcohol consumption category, which again supports the conclusion that other (as yet undefined) factors may play a role in mediating the association between alcohol and diabetes risk.
In a recent randomized clinical trial, moderate daily alcohol consumption (1 drink/d, or ≈13 g alcohol) lowered fasting plasma glucose (140–118 mg/dL) and glycated hemoglobin (7.4–7.1%) in patients with type 2 diabetes (21). Both metformin and lifestyle modification lowered fasting glucose in our cohort, as previously reported (16). The effects of alcohol and DPP interventions on fasting glucose concentrations may thus have been synergistic, resulting in greater diabetes prevention. Finally, compared with nondrinkers, the light and moderate alcohol consumers in the DPP cohort had slightly, but significantly, higher concentrations of fasting plasma glucose and a lower CIR30, both of which were potent predictors of diabetes risk in our cohort (17). However, the enhanced effectiveness of DPP interventions in those with higher alcohol intakes could be considered consistent with other observations that diabetes treatments have a greater affect on those with higher glycated hemoglobin (21, 22) or fasting plasma glucose (23) concentrations at baseline.
Although we found enhanced insulin sensitivity among moderate alcohol drinkers, a finding consistent with other studies (7, 8, 24–27) the effect was only of borderline significance after adjustment for age, sex, and BMI (P = 0.08). However, our finding that light-to-moderate alcohol consumption was associated with decreased insulin secretion independent of insulin sensitivity is noteworthy. Previous studies of the acute effects of alcohol consumption on insulin secretion are conflicting, with some (28), but not all (29), reporting a negative effect. A recent study by Risérus and Ingelsson (30) reported no association between chronic alcohol consumption and insulin secretion in a small cohort of elderly men. Our findings, however, are supported by data from a cohort of nondiabetic women, in which a dose-dependent inverse association between alcohol consumption and insulin secretion was observed (31). Nevertheless, whether the lower insulin secretion was due to an inhibitory effect of alcohol on β cell function enhanced suppression of hepatic glucose production or increased noninsulin-mediated glucose uptake in peripheral tissues is unclear. The lower baseline insulin secretion in the light-to-moderate alcohol groups did not result in any increase in diabetes risk and remains of uncertain significance. Furthermore, the decline in CIR30 over time observed among moderate alcohol consumers in the metformin arm was actually associated with lower diabetes risk in this group and thus could reflect a subtle improvement in hepatic insulin sensitivity.
In this study of high-risk individuals, we found that moderate drinkers had higher HDL cholesterol concentrations than did nondrinkers. This finding is consistent with previous reports (32–33) and supports a growing body of evidence that suggests that moderate daily alcohol consumption may reduce the risk of coronary heart disease (5, 34–35). Moderate drinking was also associated with lower C-reactive protein concentrations in our cohort, although this association was no longer significant after differences in age, sex, and BMI were controlled for.
Individuals in the highest category of alcohol consumption reported a significantly greater calorie intake than did nondrinkers—a difference that remained significant after age and sex were adjusted for. Despite the greater calorie intake, this group was less obese, an effect that was not fully explained by differences in reported physical activity. Others also observed this paradox (36), particularly in women, and it has been suggested that calories obtained from alcohol may be metabolized differently from other nutrients, which leads to increased thermogenesis and less fat storage (37). However, data from human studies to support this theory are limited, and this concept remains controversial (38). Furthermore, there are significant limitations to the accuracy of calorie intake data derived from FFQs (39).
This study had several strengths. Unlike many previous studies that have relied on self-report to detect incident diabetes (40–42), we performed oral-glucose-tolerance tests to diagnose diabetes on an annual basis. In addition, our enrollment strategy targeted an ethnically diverse study population, whereas much of the extant literature comes from studies of white or Asian individuals (5). This study also had several limitations. First, at baseline, the alcohol consumption categories differed in many important factors related to diabetes risk, including age, sex, BMI, fasting plasma glucose, and CIR30. Although our findings remained robust after adjustment for these variables, it remains possible that other nonmeasured factors (including consumption of other nutrients not analyzed here) confound interpretation of our results. In fact, it is likely that higher levels of alcohol consumption may be associated with other positive lifestyle behaviors relevant to diabetes risk. Second, as for most studies, we relied on self-report to assess alcohol consumption, which may have resulted in misclassification, and the FFQ used in DPP has not been specifically validated as an instrument to measure alcohol intake. However, the higher HDL-cholesterol concentrations among participants reporting greater levels of alcohol consumption suggest that our measurement of alcohol consumption was valid (32). Similarly, there was a trend toward higher baseline transaminase concentrations with increasing alcohol consumption category (P = 0.004 for ALT, P = 0.07 for AST). Third, although participants were not explicitly advised to abstain from drinking, alcohol consumption was low compared with that reported from other cohort studies, which may have limited our ability to discern diabetes risk across the spectrum of alcohol consumption, particularly in the placebo group. Finally, the study participants were a highly selected population of generally healthy subjects, which may limit the generalizability of our findings. However, because persons with impaired glucose tolerance, elevated fasting glucose concentrations, and overweight/obesity are at increased risk of developing diabetes, studying alcohol’s effect on diabetes risk in this population has substantial clinical relevance.
In summary, we found that among high-risk individuals participating in the DPP, higher levels of alcohol consumption were associated with a decreased risk of developing diabetes among participants randomly assigned to the metformin and lifestyle modification arms. This suggests the potential benefits of alcohol use in preventing diabetes may be limited to those who are actively pursuing other therapies to reduce risk. Moderate alcohol intake was associated with decreased insulin secretion, independent of insulin sensitivity. The effect of chronic alcohol consumption on glucose metabolism, especially β cell function, warrants further investigation.
Supplementary Material
Acknowledgments
We thank the thousands of volunteers in this program for their devotion to the goal of diabetes prevention. McKesson BioServices Corp, Matthews Media Group, Inc, and the Henry M Jackson Foundation provided support services under subcontract with the Coordinating Center. Special thanks to William C Knowler for his valuable insights and contributions to this manuscript.
The authors’ responsibilities were as follows—JPC, SP, and AAH: study design and writing of the first draft of the manuscript; SLE: statistical analysis; and all authors: analysis and interpretation of the data and critical revision of the manuscript for important intellectual content. None of the authors had a personal or financial conflict of interest.
REFERENCES
- 1.Centers for Disease Control and Prevention National diabetes fact sheet: general information and national estimates on diabetes in the United States, 2005. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2005 [Google Scholar]
- 2.Rewers M, Hamman RF. Risk factors for non-insulin dependent diabetes. Diabetes in America. Bethesda, MD: National Institute of Diabetes and Digestive and Kidney Diseases, 1995:179–220 [Google Scholar]
- 3.Diabetes Prevention Program Research Group Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Substance Abuse and Mental Health Services Administration Results from the 2005 National Survey on Drug Use and Health: national findings. Rockville, MD: Office of Applied Studies, 2006 [Google Scholar]
- 5.Howard AA, Arnsten JH, Gourevitch MN. Effect of alcohol consumption in diabetes mellitus. A systematic review. Ann Intern Med 2004;140:211–9 [DOI] [PubMed] [Google Scholar]
- 6.Koppes LLJ, Dekker JM, Hendriks HFJ, Bouter LM, Heine RJ. Moderate alcohol consumption lowers the risk of type 2 diabetes. Diabetes Care 2005;28:719–25 [DOI] [PubMed] [Google Scholar]
- 7.Kiechl S, Willeit J, Poewe W, et al. Insulin sensitivity and regular alcohol consumption: large, prospective, cross sectional population study (Bruneck study). BMJ 1996;313:1040–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lazarus R, Sparrow D, Weiss ST. Alcohol intake and insulin levels: the Normative Aging Study. Am J Epidemiol 1997;145:909–16 [DOI] [PubMed] [Google Scholar]
- 9.The Diabetes Prevention Program Research Group The Diabetes Prevention Program: design and methods for a clinical trial in the prevention of type 2 diabetes. Diabetes Care 1999;22:623–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatry 1974;131:1121–3 [DOI] [PubMed] [Google Scholar]
- 11.Mayer-Davis EJ, Sparks KC, Hirst K, et al. Dietary intake in the Diabetes Prevention Program Cohort: baseline and 1-year post-randomization. Ann Epidemiol 2004;14:763–72 [DOI] [PubMed] [Google Scholar]
- 12.Cooper DE, Goff DC, Jr, Bell RA, Zaccaro D, Mayer-Davis EJ, Karter AJ. Is insulin sensitivity a causal intermediate in the relationship between alcohol consumption and carotid atherosclerosis?: the insulin resistance and atherosclerosis study. Diabetes Care 2002;25:1425–31 [DOI] [PubMed] [Google Scholar]
- 13.Dawson DA. Methodological issues in measuring alcohol use. Alcohol Res Health 2003;27:18–29 [PMC free article] [PubMed] [Google Scholar]
- 14.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97 [DOI] [PubMed] [Google Scholar]
- 15.Hanson RL, Pratley RE, Bogardus C, et al. Evaluation of simple indices of insulin sensitivity and insulin secretion for use in epidemiologic studies. Am J Epidemiol 2000;151:190–8 [DOI] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and b-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985;28:412–9 [DOI] [PubMed] [Google Scholar]
- 17.The Diabetes Prevention Program Research Group Role of insulin secretion and sensitivity in the evolution of type 2 diabetes in the Diabetes Prevention Program. Diabetes 2005;54:2404–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kahn SE, Prigeon RL, McCulloch DK, et al. Quantification of the relationship between insulin sensitivity and beta-cell function in human subjects. Evidence for a hyperbolic function. Diabetes 1993;42:1663–72 [DOI] [PubMed] [Google Scholar]
- 19.Cox DR. Regression models and life tables. J R Stat Soc Series B 1972;34:187–220 [Google Scholar]
- 20.Saremi A, Hanson RL, Tulloch-Reid M, Williams DE, Knowler WC. Alcohol consumption predicts hypertension but not diabetes. J Stud Alcohol 2004;65:184–90 [DOI] [PubMed] [Google Scholar]
- 21.Shai I, Wainstein J, Harman-Boehm I, et al. Glycemic effects of moderate alcohol intake among patients with type 2 diabetes: a multicenter, randomized, clinical intervention trial. Diabetes Care 2007;30:3011–6 [DOI] [PubMed] [Google Scholar]
- 22.Sigurdardottir AK, Jonsdottir H, Benediktsson R. Outcomes of educational interventions in type 2 diabetes: WEKA data-mining analysis. Patient Educ Couns 2007;67:21–31 [DOI] [PubMed] [Google Scholar]
- 23.DeFronzo RA, Goodman AM. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. The Multicenter Metformin Study Group. N Engl J Med 1995;333:541–9 [DOI] [PubMed] [Google Scholar]
- 24.Facchini F, Chen YD, Reaven GM. Light-to-moderate alcohol intake is associated with enhanced insulin sensitivity. Diabetes Care 1994;17:115–9 [DOI] [PubMed] [Google Scholar]
- 25.Davies MJ, Baer DJ, Judd JT, Brown ED, Campbell WS, Taylor PR. Effects of moderate alcohol intake on fasting insulin and glucose concentrations and insulin sensitivity in postmenopausal women: a randomized controlled trial. JAMA 2002;287:2559–62 [DOI] [PubMed] [Google Scholar]
- 26.Kroenke CH, Chu NF, Rifai N, et al. A cross-sectional study of alcohol consumption patterns and biologic markers of glycemic control among 459 women. Diabetes Care 2003;26:1971–8 [DOI] [PubMed] [Google Scholar]
- 27.Meyer KA, Conigrave KM, Chu NF, et al. Alcohol consumption patterns and HbA1c, C-peptide and insulin concentrations in men. J Am Coll Nutr 2003;22:185–94 [DOI] [PubMed] [Google Scholar]
- 28.Flanagan DEH, Pratt E, Murphy J, et al. Alcohol consumption alters insulin secretion and cardiac autonomic activity. Eur J Clin Invest 2002;32:187–92 [DOI] [PubMed] [Google Scholar]
- 29.Avogaro A, Watanabe RM, Gottardo L, de Kreutzenberg S, Tiengo A, Pacini G. Glucose tolerance during moderate alcohol intake: insights on insulin action from glucose/lactate dynamics. J Clin Endocrinol Metab 2002;87:1233–8 [DOI] [PubMed] [Google Scholar]
- 30.Risérus U, Ingelsson E. Alcohol intake, insulin resistance, and abdominal obesity in elderly men. Obesity (Silver Spring) 2007;15:1766–73 [DOI] [PubMed] [Google Scholar]
- 31.Greenfield JR, Samaras K, Jenkins AB, Kelly PJ, Spector TD, Campbell LV. Moderate alcohol consumption, estrogen replacement therapy, and physical activity are associated with increased insulin sensitivity. Diabetes Care 2003;26:2734–40 [DOI] [PubMed] [Google Scholar]
- 32.Hulley SB, Gordon S. Alcohol and high-density lipoprotein cholesterol: causal inference from diverse study designs. Circulation 1981;64(suppl 3):57–63 [PubMed] [Google Scholar]
- 33.Rimm EB, Moats S. Alcohol and coronary heart disease: drinking patterns and mediators of effect. Ann Epidemiol 2007;17:S3–7 [Google Scholar]
- 34.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation 2003;107:443–7 [DOI] [PubMed] [Google Scholar]
- 35.Maclure M. Demonstration of deductive meta-analysis: ethanol intake and risk of myocardial infarction. Epidemiol Rev 1993;15:328–51 [DOI] [PubMed] [Google Scholar]
- 36.Colditz GA, Giovannucci R, Rimm EB, et al. Alcohol intake in relationship to diet and obesity in women and men. Am J Clin Nutr 1991;54:49–55 [DOI] [PubMed] [Google Scholar]
- 37.McCarty MF. The insulin sensitizing activity of moderate alcohol consumption may promote leanness in women. Med Hypotheses 2000;54:794–7 [DOI] [PubMed] [Google Scholar]
- 38.Buemann B, Astrup A. How does the body deal with energy from alcohol? Nutrition 2001;17:638–41 [DOI] [PubMed] [Google Scholar]
- 39.Blanton CA, Moshfegh AJ, Baer DJ, Kretsch MJ. The USDA Automated Multiple-Pass Method accurately estimates group total energy and nutrient intake. J Nutr 2006;136:2594–9 [DOI] [PubMed] [Google Scholar]
- 40.Conigrave KM, Hu B, Camargo CA, Stampfer M, Willett W, Rimm E. A prospective study of drinking patterns in relation to risk of type 2 diabetes among men. Diabetes 2001;50:2390–5 [DOI] [PubMed] [Google Scholar]
- 41.Ajani UA, Hennekens C, Spelsberg A, Manson J. Alcohol consumption and risk of type 2 diabetes mellitus among US male physicians. Arch Intern Med 2000;160:1025–30 [DOI] [PubMed] [Google Scholar]
- 42.Wannamethee SG, Shaper A, Perry I, Alberti KGMM. Alcohol consumption and the incidence of type II diabetes. J Epidemiol Community Health 2002;56:542–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.