Abstract
Background: Diet is a key component of a healthy lifestyle in the prevention of type 2 diabetes mellitus (T2DM). The role of long-chain omega-3 (n–3) fatty acids (LCFAs) in the development of T2DM remains unresolved.
Objective: We examined the association between dietary LCFAs and incidence of T2DM in 3 prospective cohorts of women and men.
Design: We followed 195,204 US adults (152,700 women and 42,504 men) without preexisting chronic disease at baseline for 14 to 18 y. Fish and LCFA intakes were assessed at baseline and updated at 4-y intervals by using a validated food-frequency questionnaire.
Results: During nearly 3 million person-years of follow-up, 9380 new cases of T2DM were documented. After adjustment for other dietary and lifestyle risk factors, LCFA intake was positively related to incidence of T2DM. The pooled multivariate relative risks in 3 cohorts across increasing quintiles of LCFAs were as follows: 1 (reference), 1.00 (95% CI: 0.91, 1.09), 1.05 (95% CI: 0.97, 1.13), 1.17 (95% CI: 1.07, 1.28), and 1.24 (95% CI: 1.09, 1.40) (P for trend < 0.001). Compared with those who consumed fish less than once per month, the relative risk of T2DM was 1.22 (95% CI: 1.08, 1.39) for women who consumed ≥5 servings fish/wk (P for trend <0.001).
Conclusions: We found no evidence that higher consumption of LCFAs and fish reduces the risk of T2DM. Instead, higher intakes may modestly increase the incidence of this disease. Given the beneficial effects of LCFA intake on many cardiovascular disease risk factors, the clinical relevance of this relation and its possible mechanisms require further investigation.
INTRODUCTION
Diet and lifestyle factors are central to the prevention of type 2 diabetes mellitus (T2DM). Previous studies have suggested that the consumption of specific dietary fats, particularly low omega-6 polyunsaturated fatty acids and high trans unsaturated fatty acids, increases the risk of T2DM, but the role of omega-3 fats remains unclear (1). Omega-3 fats (also known as n−3 fatty acids), particularly long-chain omega-3 fats from seafood sources, alter the expression of peroxisome proliferator–activator receptor genes, which are involved in signaling nutrition status (2), and of the production of inflammatory cytokines, which are associated with T2DM (3). These findings suggest that omega-3 fatty acids could lower the risk of T2DM (4).
In epidemiologic studies, intake of long-chain omega-3 fatty acids was associated with better glucose tolerance in some studies (5–7) but not in others (8, 9). Some intervention studies have found that omega-3 intake resulted in an increase in glycated hemoglobin (Hb A1C) (10, 11) and in fasting blood glucose (range: 2−25 mg/dL) (8, 11). Epidemiologic studies of the relation of long-chain fatty acid intake with T2DM have reported conflicting results (12–16). Furthermore, because studies have suggested that environmental contaminants such as dioxins (17), found in fish, might raise the risk of T2DM, the risks and benefits of fish intake remain controversial (18).
The aim of this study was to evaluate the relation between intake of omega-3 fatty acids and fish and the risk of T2DM in 3 large cohorts of women and men: the Nurses' Health Study (NHS), the Nurses' Health Study 2 (NHS2), and the Health Professionals Follow-Up Study (HPFS). Because inconsistent results from previous studies could be related to background differences in intake of α-linolenic acid (ALA), an omega-3 fatty acid from plant sources, we also investigated the interplay between long-chain omega-3 fatty acids and ALA in relation to T2DM risk.
SUBJECTS AND METHODS
Study population
We evaluated 3 separate large cohort studies. The NHS was established in 1976 with 121,700 female registered nurses aged 30–55 y at baseline; the NHS2 in 1989 with 116,609 female registered US nurses aged 26–46 y at baseline; and the HPFS in 1986 with 51,529 male US health care professionals aged 39–78 y at baseline. Follow-up questionnaires have been sent to all participants every 2 y to update information and identify newly diagnosed major illnesses. The vital status of nonrespondents was assessed on the basis of information from the National Death Index, next of kin, or the US postal system.
For this analysis, the follow-up period started in 1986 for NHS and HPFS and in 1991 for NHS2. We excluded participants if they had a history of T2DM, cancer, or cardiovascular disease at baseline or if they did not complete the baseline food-frequency questionnaire (FFQ), left >70 items blank, or had implausible reported total energy intakes (<600 kcal/d or >3500 kcal/d for women; <800 kcal/d and >4200 kcal/d for men). After exclusions, a total of 195,204 participants (61,031 in NHS, 91,669 in NHS2, and 42,504 in HPFS) were included in the present analysis.
Assessment of fish intake
We assessed diet using a detailed validated semiquantitative FFQ that included ≈120 items and was administered at 4-y intervals during the follow-up period (19). The questionnaire included 4 questions about seafood (with common units or portion sizes for each food): 1) dark-meat fish such as mackerel, salmon, sardines, bluefish, or swordfish (3–5 oz, 84–140 g); 2) canned tuna (3–4 oz, 84–112 g); 3) other fish (3–5 oz, 84–140 g); and 4) shrimp, lobster, or scallops as a main dish (3.5 oz, 98 g). For each item, each participant was asked how often, on average, he or she had consumed a given quantity during the previous year. Nine responses were possible, ranging from “almost never” to “6 or more per day.” The calculation of long-chain n−3 fatty acids was described in detail elsewhere (20). The average daily intake of other nutrients was calculated by multiplying the frequency of consumption of each item by its nutrient content per serving and totaling the nutrient intake for all food items. Fish intake was assessed by totaling the participant's response on 3 finfish items. To assess long-term dietary intake and to minimize misclassification, we calculated cumulative average intake such that the nutrient and fish intake in a given period was the average intake of previous and current periods (21).
The reproducibility and validity of the dietary questionnaires were assessed by comparing the data from the questionnaire with the data from two 1-wk dietary records, collected ≈6–8 mo apart (20, 22). The mean total fish intake was 3.7 servings/wk according to the questionnaire and 3.6 servings/wk according to two 1-wk dietary records [Spearman correlation coefficient (r) = 0.61, P < 0.001]. The correlations between 2 administrations of the FFQ 1 y apart were 0.54 for canned tuna, 0.63 for dark-meat fish, 0.48 for other fish, and 0.67 for shrimp, lobster, or scallops as a main dish. The calculated intake of the long-chain omega-3 fatty acid eicosapentaenoic acid (EPA) from the FFQ was also correlated with the fatty acid composition of adipose tissue (r = 0.47, P < 0.001) (23).
Nutrient intakes were adjusted for total energy intake by the residual approach (19). Intake of long-chain omega-3 fatty acids was primarily from fish (87% of the total intake) and secondarily from chicken (7%) and liver (2%), which is similar to sources in the US food supply data (24). Use of fish-oil supplements was assessed in 1988 (HPFS), 1990 (NHS), and 1991 (NHS2) and was infrequent in these cohorts, representing <2% of total cumulative person years. Estimated dietary EPA and docosahexaenoic acid (DHA) intakes included contributions from supplements.
Outcomes
Participants who reported having received a new diagnosis of T2DM in the biennial follow-up questionnaire were mailed a supplementary questionnaire regarding symptoms, diagnostic tests, and hypoglycemic therapy. Cases of T2DM were validated according to original National Diabetes Data Group criteria (25) by at least one of the following: classic symptoms plus a plasma glucose concentration of ≥140 mg/dL (7.8 mmol/L) in the fasting state or a randomly measured plasma glucose concentration of ≥200 mg/dL (11.1 mmol/L); in the absence of symptoms, ≥2 elevated plasma glucose concentrations on different occasions (≥140 mg/dL fasting or ≥200 mg/dL randomly measured, ≥ 200 mg/dL ≥2 h after an oral glucose challenge); or treatment with hypoglycemic medication (insulin or an oral hypoglycemic agent). The diagnostic criteria for T2DM changed in June 1996 such that the lower diagnostic cutoff for fasting glucose concentration (126 mg/dL) was considered the threshold for a diagnosis of T2DM (25). In a sample of NHS and HPFS participants, 98% and 97% of the self-reported T2DM cases documented by the supplementary questionnaire were confirmed by medical record review, respectively (26, 27).
Data analysis
We computed person-months of follow-up for each participant from baseline to the date of T2DM diagnosis, death from any cause, or the end of follow-up (June 2005 for NHS2, June 2004 for NHS, and January 2004 for HPFS). We divided the participants into 5 categories according to frequency of fish consumption (<1 time/mo, 1–3 times/mo, 1 time/wk, 2–4 times/wk, and ≥5 times/wk), quintiles, and 10 categories of omega-3 fatty acid intake by using predefined cutoffs across studies (by a 0.05-g/d increment) and omega-3 supplement intake. To test for a linear trend across categories of intake, the category medians were modeled as a continuous variable. Because diet could change because of a recent diagnosis of chronic disease, we stopped updating diet after development of cancer and heart disease. We also performed a sensitivity analysis using a 2-y lag between exposure and incidence of T2DM for each period, such that the long-chain omega-3 intake in 1986 was used to predict the risk of T2DM in 1988–1990 and so on.
The basic model for our analysis was the Cox proportional hazards model (SAS 2004; SAS Institute Inc, Cary, NC). We used the Anderson-Gill data structure, in which a new data record was created for each biennial questionnaire cycle in which the participant was at risk with covariates set to represent the value from the latest returned questionnaire to handle time-varying covariates efficiently (28). We adjusted for age and calendar time in all our models.
We checked for the heterogeneity of study results using the Cochran Q test (29) and pooled the results from NHS, NHS2, and HPFS using the logarithm of the relative risks (RRs) and corresponding SEs of each category using random-effects models that incorporate both a within-study and an additive between-study component of variance (30). We tested for interactions by calculating the cross-product of medians of quintiles of ALA and long-chain omega-3 fats as a continuous term for each cohort and pooling the coefficients to obtain a single degree of freedom (Wald test) for the quantitative interaction. Statistical significance was defined as α < 0.05. All analyses were performed by using SAS 9.1. The Institutional Review Board of Brigham and Women's Hospital approved the study.
RESULTS
During 2,954,078 person-years of follow-up among the 195,204 participants in 3 cohorts, we documented 9380 cases of T2DM (4159 in NHS, 2728 in NHS2, and 2493 in HPFS). At baseline, the participants with a higher long-chain omega-3 fatty acid consumption were more physically active, less likely to smoke, had higher consumption of alcohol, and lower consumption of trans fatty acids (Table 1).
TABLE 1.
Baseline characteristics of the study population by quintile (Q) of long-chain omega-3 fatty acid intake in 3 cohorts1
| NHS (1986) |
NHS2 (1991) |
HPFS (1986) |
|||||||
| Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | Q1 | Q3 | Q5 | |
| Median intake (g/d) | 0.06 | 0.18 | 0.49 | 0.06 | 0.15 | 0.36 | 0.09 | 0.28 | 0.62 |
| Age (y) | 51.9 ± 7.22 | 52.0 ± 7.1 | 52.2 ± 6.9 | 36.0 ± 4.6 | 36.1 ± 4.6 | 36.1 ± 4.6 | 53.4 ± 9.5 | 53.4 ± 9.4 | 53.5 ± 9.4 |
| Family history of DM (%) | 19 | 19 | 20 | 16 | 16 | 16 | 13 | 13 | 13 |
| Current smokers (%) | 23 | 22 | 18 | 12 | 12 | 12 | 12 | 10 | 8 |
| BMI (kg/m2) | 24.6 ± 4.9 | 24.9 ± 4.9 | 24.9 ± 4.7 | 24.5 ± 5.4 | 24.7 ± 5.2 | 24.4 ± 5.1 | 24.9 ± 4.9 | 24.9 ± 4.7 | 24.7 ± 5.1 |
| Physical activity (METs/d) | 11.1 ± 16.5 | 13.9 ± 20.7 | 18.2 ± 24.3 | 17.8 ± 25.2 | 19.9 ± 25.7 | 25.5 ± 16.0 | 22.8 ± 75.7 | 26.0 ± 74.4 | 29.2 ± 65.0 |
| Caffeine intake (mg/d) | 305 ± 242 | 287 ± 226 | 267 ± 224 | 240 ± 229 | 246 ± 223 | 246 ± 219 | 269 ± 272 | 238 ± 233 | 218 ± 240 |
| Energy intake (kcal/d) | 1772 ± 537 | 1834 ± 575 | 1697 ± 515 | 1786 ± 565 | 1745 ± 544 | 1743 ± 530 | 1977 ± 617 | 2111 ± 592 | 1928 ± 612 |
| trans Fats (g/d) | 3.2 ± 1.0 | 2.9 ± 0.8 | 2.5 ± 0.8 | 3.6 ± 1.3 | 3.3 ± 1.1 | 2.8 ± 1.0 | 3.2 ± 1.2 | 2.9 ± 1.0 | 2.2 ± 1.0 |
| PUFAs (g/d) | 10.7 ± 3.0 | 10.8 ± 2.8 | 11.1 ± 2.8 | 11.0 ± 2.9 | 11.3 ± 2.6 | 11.3 ± 2.6 | 12.8 ± 3.6 | 13.1 ± 3.3 | 13.5 ± 3.5 |
| Saturated fats (g/d) | 22.2 ± 4.8 | 20.8 ± 4.2 | 18.7 ± 4.2 | 23.7 ± 5.3 | 22.6 ± 4.4 | 20.8 ± 4.5 | 26.8 ± 6.3 | 25.0 ± 5.6 | 21.4 ± 5.7 |
| Cereal fiber (g/d) | 4.3 ± 3.2 | 4.4 ± 3.0 | 4.5 ± 3.2 | 5.5 ± 3.2 | 5.6 ± 2.9 | 5.7 ± 3.1 | 5.6 ± 3.5 | 5.7 ± 3.6 | 6.1 ± 4.3 |
| Glycemic index | 53.1 ± 3.6 | 52.1 ± 3.4 | 50.8 ± 3.8 | 54.4 ± 3.4 | 53.9 ± 3.2 | 53.1 ± 3.4 | 53.7 ± 3.6 | 53.1 ± 3.4 | 52.4 ± 3.7 |
| Alcohol intake (g/d) | 5.3 ± 10.3 | 6.4 ± 10.9 | 6.4 ± 9.9 | 2.5 ± 6.1 | 2.8 ± 5.3 | 3.8 ± 6.4 | 11.0 ± 16.4 | 12.6 ± 16.1 | 10.6 ± 13.3 |
NHS, Nurses' Health Study; NHS2, Nurses' Health Study 2; HPFS, Health Professionals Follow-Up Study; DM, diabetes mellitus; PUFAs, polyunsaturated fatty acids; METs, metabolic equivalent tasks. The test for trend was significant (P < 0.05) for all variables except for BMI, smoking (NHS2), and alcohol (HPFS).
Mean ± SD (all such values).
In age-adjusted models, consumption of long-chain omega-3 fatty acids was not associated with risk of T2DM in NHS2 and HPFS and was weakly positively associated with risk of T2DM in NHS (Table 2). After adjustment for other lifestyle and dietary risk factors such as cereal fiber and glycemic index, a higher intake of omega-3 fatty acids was associated with increased risk of T2DM in all cohorts. In the final adjusted model, which included BMI in addition to diet and lifestyle variables (Table 2), the pooled RRs across increasing quintiles of long-chain omega-3 fatty acids were 1.00 (95% CI: 0.91, 1.09), 1.05 (95% CI: 0.97, 1.13), 1.17 (95% CI: 1.07, 1.28), and 1.24 (95% CI: 1.09, 1.40) (P for trend < 0.001). Omega-3 supplement use was not related to risk of T2DM (RR: 1.02; 95% CI: 0.93, 1.13). These results did not change when we excluded long-chain omega-3 fatty acids from supplements from total dietary long-chain omega-3 intake; when we adjusted for waist circumference, the ratio of polyunsaturated fat to saturated fat, processed meat and carbonated soft drink intakes in multivariate models; or when we used a 2-y lag period. We performed an analysis stratifying the study population by blood pressure (high compared with normal) and blood cholesterol (high compared with normal), and the results were similar to the original results.
TABLE 2.
Cumulative average long-chain omega-3 fatty acid intake and relative risk (RR) of diabetes mellitus in cohorts of women and men, by quintile (Q) of intake1
| Long-chain omega-3 fatty acid intake |
||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | P for trend | |
| NHS | ||||||
| Median intake (g/d) | 0.06 | 0.12 | 0.18 | 0.27 | 0.49 | |
| No. of cases | 748 | 802 | 883 | 833 | 893 | |
| Person-years | 194,684 | 212,125 | 207,877 | 202,705 | 204,417 | |
| Age-adjusted RR | 1 | 0.96 (0.87, 1.06) | 1.03 (0.94, 1.14) | 1.02 (0.92, 1.13) | 1.10 (0.99, 1.21) | 0.01 |
| Multivariate-adjusted RR | 1 | 1.00 (0.91, 1.11) | 1.12 (1.02, 1.24) | 1.17 (1.05, 1.29) | 1.23 (1.11, 1.37) | <0.001 |
| NHS2 | ||||||
| Median intake (g/d) | 0.06 | 0.10 | 0.15 | 0.22 | 0.36 | |
| No. of cases | 504 | 558 | 542 | 567 | 557 | |
| Person-years | 251,066 | 250,610 | 248,336 | 247,475 | 256,770 | |
| Age-adjusted RR | 1 | 1.04 (0.92, 1.17) | 1.01 (0.90, 1.14) | 1.01 (0.90, 1.14) | 1.04 (0.92, 1.17) | 0.07 |
| Multivariate-adjusted RR2 | 1 | 1.04 (0.92, 1.17) | 1.08 (0.95, 1.22) | 1.15 (1.02, 1.30) | 1.25 (1.10, 1.42) | <0.001 |
| HPFS | ||||||
| Median intake (g/d) | 0.09 | 0.18 | 0.28 | 0.39 | 0.62 | |
| No. of cases | 489 | 504 | 484 | 490 | 526 | |
| Person-years | 135,420 | 140,124 | 136,161 | 127,414 | 138,898 | |
| Age-adjusted RR | 1 | 0.98 (0.86, 1.11) | 0.92 (0.81, 1.04) | 1.03 (0.92, 1.17) | 0.96 (0.84, 1.08) | 0.73 |
| Multivariate-adjusted RR2 | 1 | 1.00 (0.88, 1.13) | 0.99 (0.87, 1.12) | 1.11 (0.98, 1.26) | 1.12 (0.98, 1.28) | 0.03 |
| Pooled analysis | ||||||
| No. of cases | 1741 | 1864 | 1909 | 1890 | 1976 | |
| Person-years | 581,170 | 602,859 | 592,374 | 577,594 | 600,085 | |
| Multivariate-adjusted RR2 | 1 | 1.00 (0.91, 1.09) | 1.05 (0.97, 1.13) | 1.17 (1.07, 1.28) | 1.24 (1.09, 1.40) | <0.001 |
NHS, Nurses' Health Study; NHS2, Nurses' Health Study 2; HPFS, Health Professionals Follow-Up Study.
Multivariate model adjusted for smoking (never, past, 1–14 cigarettes/d, 15–24 cigarettes/d, or >24 cigarettes/d); alcohol consumption (0, 0.1–4.9, 5.0–9.9, or ≥10 g/d); physical activity (quintiles; metabolic equivalent tasks/d); family history of diabetes mellitus; BMI (10 categories); intakes of saturated fat, trans fats, linolenic acid, linoleic acid, caffeine, and cereal fiber; glycemic index (all quintiles); and calories (quintiles). In the NHS, the multivariate models were also adjusted for menopausal status and postmenopausal hormone use. In the NHS2, the multivariate models were also adjusted for use of hormone replacement therapy (ever or never) and oral contraceptive use (never, past, or current).
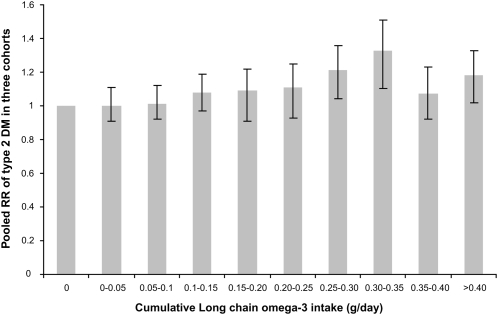
In a pooled analysis (Table 3, Figure 1), the multivariate RR of T2DM in the highest of 10 categories of cumulative long-chain omega-3 intake (>0.4 g/d) was 1.18 (95% CI: 1.02, 1.33) as compared with the lowest intake (0 g/d). The RR for each 0.3-g increment in intake of long-chain omega-3 intake was 1.11 (95% CI: 1.05, 1.16) in the NHS, 1.11 (95% CI: 1.06, 1.16) in the NHS2, and 1.08 (95% CI: 1.03, 1.13) in the HPFS. Overall, there was a dose-response relation between omega-3 intake and T2DM risk, although the highest risk was found in the eighth category and the risk was slightly reduced in the ninth and tenth category (Figure 1). The pooled RR for each 0.3-g increment in intake of long-chain omega-3 was 1.09 (95% CI: 1.06, 1.12). The relation of cumulative fish intake and T2DM in men and women was similar to that for marine omega-3 intake (Table 4). The RRs were 1.01 (95% CI: 0.92, 1.10) for fish consumption 1–3 times/mo, 1.05 (95% CI: 0.98, 1.14) for fish consumption 1 time/wk, 1.17 (95% CI: 1.07, 1.28) for fish consumption 2–4 times/wk, and 1.22 (95% CI: 1.08, 1.39) for fish consumption ≥5 times/wk (P for trend <0.001). The consumption of different types of fish items—canned tuna (RR >1/mo compared with <1 time/mo: 1.14, 95% CI: 1.08, 1.2); dark fish (RR >1/mo compared with <1 time/mo: 1.09, 95% CI: 1.01, 1.16); and shrimp, scallops, and lobster (RR >1 time/mo compared with <1 time/mo: 1.06, 95% CI: 0.97, 1.13)—was each related to a small increase in T2DM risk. Compared with other fish consumption <1 time/mo, the RR was 0.97 (95% CI: 0.92, 1.03) for 1–3 times/mo and 1.06 (95% CI: 0.99, 1.13) for >1 time/wk (data not shown).
TABLE 3.
Cumulative long-chain omega-3 fatty acid intake and relative risk (RR) of diabetes mellitus in cohorts of women and men1
| Long-chain omega-3 fatty acid intake |
|||||||||||
| 0 g/d | >0–0.05 g/d | >0.05–0.1 g/d | >0.1–0.15 g/d | >0.15–0.20 g/d | >0.20–0.25 g/d | >0.25–0.30 g/d | >0.30–0.35 g/d | >0.35–0.40 g/d | >0.40 g/d | P for trend | |
| NHS | |||||||||||
| No. of cases | 199 | 616 | 695 | 626 | 548 | 401 | 323 | 246 | 124 | 381 | |
| Person-years | 57,079 | 159,077 | 182,188 | 152,265 | 128,511 | 99,660 | 74,118 | 50,237 | 33,514 | 85,159 | |
| Multivariate-adjusted RR2 | 1 | 1.07 (0.91, 1.26) | 1.06 (0.91, 1.25) | 1.16 (0.98, 1.36) | 1.23 (1.05, 1.46) | 1.22 (1.03, 1.45) | 1.32 (1.10, 1.58) | 1.46 (1.21, 1.77) | 1.12 (0.89, 1.40) | 1.35 (1.13, 1.61) | <0.001 |
| NHS2 | |||||||||||
| No. of cases | 214 | 552 | 558 | 412 | 305 | 221 | 166 | 112 | 54 | 134 | |
| Person-years | 108,711 | 260,136 | 254,730 | 187,893 | 134,230 | 988,802 | 70,684 | 44,008 | 28,204 | 66,859 | |
| Multivariate-adjusted RR2 | 1 | 1.00 (0.86, 1.18) | 1.03 (0.88, 1.21) | 1.05 (0.89, 1.24) | 1.12 (0.94, 1.34) | 1.18 (0.98, 1.43) | 1.28 (1.04, 1.58) | 1.47 (1.17, 1.86) | 1.07 (0.79, 1.45) | 1.14 (0.91, 1.43) | 0.006 |
| HPFS | |||||||||||
| No. of cases | 98 | 225 | 283 | 259 | 228 | 252 | 226 | 208 | 155 | 559 | |
| Person-years | 28,012 | 59,984 | 79,702 | 69,957 | 64,280 | 69,200 | 65,326 | 51,510 | 42,185 | 147,861 | |
| Multivariate-adjusted RR2 | 1 | 0.90 (0.73, 1.10) | 0.92 (0.75, 1.12) | 1.00 (0.81, 1.23) | 0.90 (0.73, 1.10) | 0.91 (0.74, 1.13) | 1.02 (0.83, 1.26) | 1.08 (0.87, 1.36) | 1.01 (0.79, 1.28) | 1.05 (0.86, 1.29) | 0.04 |
NHS, Nurses' Health Study; NHS2, Nurses' Health Study 2; HPFS, Health Professionals Follow-Up Study.
Multivariate model adjusted for smoking (never, past, 1–14 cigarettes/d, 15–24 cigarettes/d, or >24 cigarettes/d); alcohol consumption (0, 0.1–4.9, 5.0–9.9, or ≥10 g/d); physical activity (quintiles; metabolic equivalent tasks/d); family history of diabetes mellitus; BMI (10 categories); intakes of saturated fat, trans fats, linolenic acid, linoleic acid, caffeine, and cereal fiber; glycemic index (all quintiles); and calories (quintiles). In the NHS, the multivariate models were also adjusted for menopausal status and postmenopausal hormone use. In the NHS2, the multivariate models were also adjusted for use of hormone replacement therapy (ever or never) and oral contraceptive use (never, past, or current).
FIGURE 1.
Long-chain omega-3 fatty acid intake and pooled multivariate relative risks (RR) of type 2 diabetes mellitus (DM) in cohorts of women and men. The values were adjusted for smoking (never, past, 1–14 cigarettes/d, 15–24 cigarettes/d, or >24 cigarettes/d); alcohol consumption (0, 0.1–4.9, 5.0–9.9, or ≥10 g/d); physical activity (quintiles; metabolic equivalent tasks/d); family history of diabetes mellitus; BMI (10 categories); intakes of saturated fat, trans fat, linolenic acid, linoleic acid, caffeine, and cereal fiber; glycemic index (all quintiles); and calories (quintiles). In the Nurses' Health Study, multivariate models were also adjusted for menopausal status and postmenopausal hormone use. In the Nurses' Health Study 2, multivariate models were also adjusted for use of hormone replacement therapy (ever or never) and oral contraceptive use (never, past, or current).
TABLE 4.
Cumulative average fish intake and relative risk (RR) of diabetes mellitus in cohorts of women and men1
| Fish intake |
||||||
| <1 time/mo | 1–3 times/mo | 1 time/wk | 2–4 times/wk | ≥5 times/wk | P for trend | |
| NHS | ||||||
| No. of cases | 205 | 495 | 2520 | 728 | 211 | |
| Person-years | 54,631 | 127,434 | 635,980 | 161,614 | 42,149 | |
| Age-adjusted RR | 1 | 1.02 (0.87, 1.20) | 1.03 (0.89, 1.19) | 1.15 (0.98, 1.34) | 1.31 (1.08, 1.59) | <0.001 |
| Multivariate-adjusted RR2 | 1 | 1.02 (0.87, 1.21) | 1.12 (0.97, 1.29) | 1.22 (1.04, 1.43) | 1.29 (1.05, 1.57) | <0.001 |
| NHS2 | ||||||
| No. of cases | 300 | 504 | 1566 | 294 | 64 | |
| Person-years | 135,920 | 246,152 | 735,493 | 113,843 | 228,488 | |
| Age-adjusted RR | 1 | 0.91 (0.79, 1.05) | 0.92 (0.81, 1.04) | 1.06 (0.90, 1.25) | 1.25 (0.95, 1.64) | 0.02 |
| Multivariate-adjusted RR2 | 1 | 0.97 (0.84, 1.12) | 1.06 (0.93, 1.20) | 1.20 (1.02, 1.43) | 1.32 (0.99, 1.74) | 0.002 |
| HPFS | ||||||
| No. of cases | 340 | 401 | 1122 | 447 | 183 | |
| Person-years | 89,392 | 107,896 | 317,972 | 116,465 | 46,292 | |
| Age-adjusted RR | 1 | 0.95 (0.82, 1.10) | 0.87 (0.77, 0.99) | 0.94 (0.81, 1.08) | 0.96 (0.80, 1.15) | 0.95 |
| Multivariate-adjusted RR2 | 1 | 1.00 (0.87, 1.16) | 0.99 (0.87, 1.12) | 1.10 (0.95, 1.28) | 1.16 (0.96, 1.41) | 0.03 |
| Pooled analysis | ||||||
| Cases | 845 | 1400 | 5208 | 1469 | 458 | |
| Person-years | 279,943 | 481,482 | 1,689,445 | 391,922 | 316,929 | |
| Multivariate-adjusted RR2 | 1 | 1.01 (0.92, 1.10) | 1.05 (0.98, 1.14) | 1.17 (1.07, 1.28) | 1.22(1.08, 1.39) | <0.001 |
NHS, Nurses' Health Study; NHS2, Nurses' Health Study 2; HPFS, Health Professionals Follow-Up Study.
Multivariate model adjusted for smoking (never, past, 1–14 cigarettes/d, 15–24 cigarettes/d, or >24 cigarettes/d); alcohol consumption (0, 0.1–4.9, 5.0–9.9, or ≥10 g/d); physical activity (quintiles; metabolic equivalent tasks/d); family history of diabetes mellitus; BMI (10 categories); intakes of saturated fat, trans fats, linolenic acid, linoleic acid, caffeine, and cereal fiber; glycemic index (all quintiles); and calories (quintiles). In the NHS, the multivariate models were also adjusted for menopausal status and postmenopausal hormone use. In the NHS2, the multivariate models were also adjusted for use of hormone replacement therapy (ever or never) and oral contraceptive use (never, past, or current).
We examined the joint consumption of long-chain omega-3 intake (EPA and DHA) and ALA by creating 25 categorical variables using quintiles of long-chain omega-3 and of ALA intakes (Table 5). A higher intake of long-chain omega-3 intake was associated with a higher risk of T2DM at all ALA intakes (P for interaction = 0.68). The relation of long-chain omega-3 intake with T2DM did not differ by intake of omega-6 fatty acids, cereal fiber, or physical activity (data not shown).
TABLE 5.
Pooled relative risks of diabetes mellitus by quintile (Q) of long-chain omega-3 fatty acid and α-linolenic acid intakes1
| Long-chain omega-3 fatty acid intake |
||||||
| Q1 | Q2 | Q3 | Q4 | Q5 | No. of cases | |
| α-Linolenic acid intake | ||||||
| Q1 | 1 | 1.03 (0.86, 1.22) | 1.06 (0.90, 1.24) | 1.24 (1.03, 1.46) | 1.24 (0.96, 1.50) | 1785 |
| Q2 | 1.04 (0.90, 1.21) | 1.04 (0.89, 1.21) | 1.29 (1.11, 1.49) | 1.14 (0.98, 1.33) | 1.24 (1.07, 1.45) | 1845 |
| Q3 | 1.07 (0.91, 1.24) | 1.09 (0.94, 1.27) | 1.13 (0.91, 1.33) | 1.15 (0.94, 1.34) | 1.31 (1.11, 1.52) | 1885 |
| Q4 | 1.09 (0.80, 1.31) | 1.04 (0.89, 1.21) | 1.07 (0.92, 1.25) | 1.24 (1.03, 1.45) | 1.29 (1.11, 1.50) | 1921 |
| Q5 | 1.05 (0.90, 1.23) | 1.12 (0.96, 1.13) | 1.08 (0.76, 1.32) | 1.28 (1.10, 1.48) | 1.24 (0.92, 1.47) | 1944 |
| No. of cases | 1559 | 1829 | 1975 | 1945 | 2072 | |
Multivariate models were adjusted for all variables as listed in Table 4. P for interaction = 0.68.
DISCUSSION
In this large prospective study of women and men, fish consumption was not associated with a decreased risk of T2DM. Instead, we observed a modest but significant positive relation between fish and omega-3 fatty acid consumption and incidence of T2DM after adjustment for established risk factors of T2DM (eg, physical activity and smoking) and dietary predictors of T2DM (eg, fiber, glycemic index, and trans and saturated fats). This relation did not change when we adjusted for BMI and waist circumference in the same model.
Few epidemiologic studies have examined the association between long-chain omega-3 fatty acid intake and risk of T2DM. In the Iowa Women's Study, the RR of T2DM was 1.11 (95% CI: 0.94, 1.30; P for trend = 0.14) in the highest compared with the lowest quintile of long-chain omega-3 intake (14). In the Atherosclerosis Risk in Communities Study, the phospholipid concentration of EPA and DHA was not significantly related to risk of T2DM (15). Another study, which used dietary records but also combined incident cases of impaired fasting glucose with T2DM, did not detect a significant relation (31). Limited power and the inability to control for important dietary risk factors such as cereal fiber could explain the lack of relation observed in studies that used biomarkers (15, 32, 33) and studies that collected dietary information using FFQ (32). Other possible reasons for differences between prior studies and our present results include our use of repeated measures of diet and lifestyle, longer follow-up, more detailed assessment of long-chain omega-3 intake (13), and better adjustment for confounders (16).
Observational studies and randomized controlled trials have consistently shown a benefit of fish consumption on risk of coronary heart disease (CHD) in healthy individuals, individuals with established CHD, or diabetic subjects (34, 35). However, the studies on long-chain omega-3 fatty acid consumption and markers of glucose metabolism are inconclusive. In several observational studies, intake of fish and omega-3 fatty acids was associated with better glucose tolerance (36–38). Other studies have found no effect (8, 39) or a slight deterioration (10, 40, 41). Several randomized controlled trials have evaluated the relation of omega-3 supplementation to glucose homeostasis. In a meta-analysis of 17 randomized controlled trials, the effects of omega-3 fatty acids were heterogeneous, inconsistent, and ranged from −29 to 25 mg/dL for fasting blood glucose and from −0.4% to 1.0% for Hb A1C (42). In the same review and in recent studies (9, 38), the effects of omega-3 fatty acids on fasting insulin concentrations were also found to be heterogeneous. In a meta-analysis of randomized trials restricted to individuals with T2DM, the pooled effect of omega-3 fatty acid supplementation was to increase fasting blood glucose by 5.87 mg/dL (95% CI: −0.15, 11.88) and increase Hb A1c by 0.21% (95% CI: −0.01, 0.44) (43).
It is unclear whether such increases in glucose concentrations are associated with changes in insulin resistance. In randomized controlled trials among patients with diabetes, omega-3 fatty acids did not affect glucose-stimulated plasma insulin responses during a hyperglycemic clamp (44) or fasting insulin (45). Additionally, omega-3 fatty acids are well-known to decrease triglyceride concentrations and increase HDL cholesterol concentrations (42). A protective effect of long-chain omega-3 fatty acids on insulin resistance and T2DM has been suggested based on their effects on peroxisome proliferators–activated transcription factor, inflammatory gene expression pathways (46), and inflammatory markers (3).
Omega-3 fatty acids may contribute to higher glucose concentrations through other mechanisms. Long-chain omega-3 can lower glucose utilization and increase glucagon-stimulated C-peptide (47) or could increase hepatic gluconeogenesis (10) by increasing uptake and oxidation of free fatty acids in liver (48) and lowering triacylglycerols (49). Thus, omega-3 fatty acid and fish consumption may increase the diagnosis of T2DM by increasing circulating concentrations of glucose, but without causing other adverse metabolic abnormalities (insulin resistance, high triglycerides, and low HDL cholesterol). Recent studies have also reported that toxins such as dioxins and methyl mercury may interrupt insulin signaling pathways (17, 50, 51). This suggests that our findings for omega-3 fatty acids could be related to confounding by other factors in fish, but evidence to support this hypothesis is limited and requires further investigation.
The strengths of our study included the large number of cases arising from ≈3 million person-years of follow-up that provided sufficient power to evaluate the association with T2DM over a wide range of dietary intakes of omega-3 fatty acids. The detailed, standardized and updated information on diet, physical activity, and BMI in 3 cohorts allowed us to minimize errors in measurement of long-chain omega-3 fatty acids and control for potential confounding factors in a great detail. The prospective design precludes recall bias, and the high rate of follow-up minimizes selection bias. Nonetheless, we cannot exclude measurement error due to self-reported diet, but this would lead to a lack of association rather than a modest positive association that was observed in our study. In addition, we could not assess the role of environmental contaminants found in some fish. Residual or unmeasured confounding remains a potential problem in our analyses, but fish intake is typically associated with a healthier diet and lifestyle and confounding by these factors would lead to an inverse association between fish and long-chain omega-3 fatty acid consumption and T2DM instead of a positive association. Higher rates of screening and surveillance among participants who have a healthier lifestyle associated with higher fish intake could also explain the observed positive relation. In addition, it is possible that people increase the consumption of fish after diagnosis of hypercholesterolemia or hypertension. However, our results did not change materially when we considered only “symptomatic” T2DM cases or restricted the analysis to participants without hypercholesterolemia or hypertension. Finally, because all of our participants are health professionals, the results of our study may not be generalizable to other populations. Given that we found similar associations across 3 independent cohorts, there is no reason to believe that the effects of omega-3 fatty acid intake on T2DM risk would differ substantially among different populations.
In summary, this prospective analysis does not support the hypothesis that long-term dietary intake of long-chain omega 3 fatty acids decreases the risk of T2DM. In contrast, higher fish and long-chain omega-3 fatty acid consumption appears to be associated with a modestly but significantly higher incidence of T2DM. Given the beneficial effects of fish and omega-3 fatty acids on multiple risk factors associated with diabetes, including triglycerides, HDL cholesterol, blood pressure, and inflammation, and on CHD, the major sequelae of diabetes, the clinical relevance of this relation and its possible mechanisms require further investigation.
Acknowledgments
The authors' responsibilities were as follows—MK, FBH, WCW, and DS: study concept and design; FBH, WCW, JEM, and DS: acquisition of data; MK, FBH, WCW, JEM, and DM: analysis and interpretation of data; MK, FBH, WCW, DM, and DS: draft of the manuscript; MK, JEM, WCW, FBH, DM, and DS: critical revision of the manuscript for important intellectual content; MK, DS, and FBH: statistical expertise; FBH and WCW: obtained funding and study supervision; FBH: administrative, technical, or material support; and MK and FBH: had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. None of the authors had a conflict of interest.
REFERENCES
- 1.Hu FB, van Dam RM, Liu S. Diet and risk of type II diabetes: the role of types of fat and carbohydrate. Diabetologia 2001;44:805–17 [DOI] [PubMed] [Google Scholar]
- 2.Steerenberg PA, Beekhof PK, Feskens EJ, Lips CJ, Hoppener JW, Beems RB. Long-term effect of fish oil diet on basal and stimulated plasma glucose and insulin levels in ob/ob mice. Diabetes Nutr Metab 2002;15:205–14 [PubMed] [Google Scholar]
- 3.Lopez-Garcia E, Schulze MB, Manson JE, et al. Consumption of (n−3) fatty acids is related to plasma biomarkers of inflammation and endothelial activation in women. J Nutr 2004;134:1806–11 [DOI] [PubMed] [Google Scholar]
- 4.Joint WHO/FAO expert consultation Diet, nutrition and the prevention of chronic diseases. Geneva, Switzerland: World Health Organization, 2003 [Google Scholar]
- 5.Adler AI, Boyko EJ, Schraer CD, Murphy NJ. Lower prevalence of impaired glucose tolerance and diabetes associated with daily seal oil or salmon consumption among Alaska Natives. Diabetes Care 1994;17:1498–501 [DOI] [PubMed] [Google Scholar]
- 6.Feskens EJ, Bowles CH, Kromhout D. Inverse association between fish intake and risk of glucose intolerance in normoglycemic elderly men and women. Diabetes Care 1991;14:935–41 [DOI] [PubMed] [Google Scholar]
- 7.Fasching P, Ratheiser K, Waldhausl W, et al. Metabolic effects of fish-oil supplementation in patients with impaired glucose tolerance. Diabetes 1991;40:583–9 [DOI] [PubMed] [Google Scholar]
- 8.Toft I, Bonaa KH, Ingebretsen OC, Nordoy A, Jenssen T. Effects of n−3 polyunsaturated fatty acids on glucose homeostasis and blood pressure in essential hypertension. A randomized, controlled trial. Ann Intern Med 1995;123:911–8 [DOI] [PubMed] [Google Scholar]
- 9.Giacco R, Cuomo V, Vessby B, et al. Fish oil, insulin sensitivity, insulin secretion and glucose tolerance in healthy people: is there any effect of fish oil supplementation in relation to the type of background diet and habitual dietary intake of n−6 and n−3 fatty acids? Nutr Metab Cardiovasc Dis 2007;17:572–80 [DOI] [PubMed] [Google Scholar]
- 10.Woodman RJ, Mori TA, Burke V, Puddey IB, Watts GF, Beilin LJ. Effects of purified eicosapentaenoic and docosahexaenoic acids on glycemic control, blood pressure, and serum lipids in type 2 diabetic patients with treated hypertension. Am J Clin Nutr 2002;76:1007–15 [DOI] [PubMed] [Google Scholar]
- 11.Sirtori CR, Crepaldi G, Manzato E, et al. One-year treatment with ethyl esters of n−3 fatty acids in patients with hypertriglyceridemia and glucose intolerance: reduced triglyceridemia, total cholesterol and increased HDL-C without glycemic alterations. Atherosclerosis 1998;137:419–27 [DOI] [PubMed] [Google Scholar]
- 12.Nkondjock A, Receveur O. Fish-seafood consumption, obesity, and risk of type 2 diabetes: an ecological study. Diabetes Metab 2003;29:635–42 [DOI] [PubMed] [Google Scholar]
- 13.Salmeron J, Hu FB, Manson JE, et al. Dietary fat intake and risk of type 2 diabetes in women. Am J Clin Nutr 2001;73:1019–26 [DOI] [PubMed] [Google Scholar]
- 14.Meyer KA, Kushi LH, Jacobs DR, Jr, Folsom AR. Dietary fat and incidence of type 2 diabetes in older Iowa women. Diabetes Care 2001;24:1528–35 [DOI] [PubMed] [Google Scholar]
- 15.Wang L, Folsom AR, Zheng ZJ, Pankow JS, Eckfeldt JH, ARIC Study Investigators Plasma fatty acid composition and incidence of diabetes in middle-aged adults: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr 2003;78:91–8 [DOI] [PubMed] [Google Scholar]
- 16.van Dam RM, Willett WC, Rimm EB, Stampfer MJ, Hu FB. Dietary fat and meat intake in relation to risk of type 2 diabetes in men. Diabetes Care 2002;25:417–24 [DOI] [PubMed] [Google Scholar]
- 17.Lee DH, Lee IK, Song K, et al. A strong dose-response relation between serum concentrations of persistent organic pollutants and diabetes: results from the National Health and Examination Survey 1999-2002. Diabetes Care 2006;29:1638–44 [DOI] [PubMed] [Google Scholar]
- 18.Mozaffarian D, Rimm EB. Fish intake, contaminants, and human health: evaluating the risks and the benefits. JAMA 2006;296:1885–99 [DOI] [PubMed] [Google Scholar]
- 19.Willett WC. Nutritional epidemiology. 2nd ed.New York, NY: Oxford University Press, 1999 [Google Scholar]
- 20.Hu FB, Bronner L, Willett WC, et al. Fish and omega-3 fatty acid intake and risk of coronary heart disease in women. JAMA 2002;287:1815–21 [DOI] [PubMed] [Google Scholar]
- 21.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9 [DOI] [PubMed] [Google Scholar]
- 22.Feskanich D, Rimm EB, Giovannucci EL, et al. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc 1993;93:790–6 [DOI] [PubMed] [Google Scholar]
- 23.Hunter DJ, Rimm EB, Sacks FM, et al. Comparison of measures of fatty acid intake by subcutaneous fat aspirate, food frequency questionnaire, and diet records in a free-living population of US men. Am J Epidemiol 1992;135:418–27 [DOI] [PubMed] [Google Scholar]
- 24.Raper NR, Cronin FJ, Exler J. Omega-3 fatty acid content of the US food supply. J Am Coll Nutr 1992;11:304–8 [DOI] [PubMed] [Google Scholar]
- 25.Anonymous Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 1997;20:1183–97 [DOI] [PubMed] [Google Scholar]
- 26.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med 2001;161:1542–8 [DOI] [PubMed] [Google Scholar]
- 27.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non-insulin-dependent diabetes mellitus in women. Lancet 1991;338:774–8 [DOI] [PubMed] [Google Scholar]
- 28.Therneau TM. Extending the Cox model. Anonymous proceedings of the First Seattle Symposium in Biostatistics. New York, NY: Springer-Verlag, 1997 [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58 [DOI] [PubMed] [Google Scholar]
- 30.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88 [DOI] [PubMed] [Google Scholar]
- 31.Laaksonen DE, Lakka TA, Lakka HM, et al. Serum fatty acid composition predicts development of impaired fasting glycaemia and diabetes in middle-aged men. Diabet Med 2002;19:456–64 [DOI] [PubMed] [Google Scholar]
- 32.Hodge AM, English DR, O'Dea K, et al. Plasma phospholipid and dietary fatty acids as predictors of type 2 diabetes: interpreting the role of linoleic acid. Am J Clin Nutr 2007;86:189–97 [DOI] [PubMed] [Google Scholar]
- 33.Vessby B, Aro A, Skarfors E, Berglund L, Salminen I, Lithell H. The risk to develop NIDDM is related to the fatty acid composition of the serum cholesterol esters. Diabetes 1994;43:1353–7 [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama M, Origasa H, Matsuzaki M, et al. Effects of eicosapentaenoic acid on major coronary events in hypercholesterolaemic patients (JELIS): a randomised open-label, blinded endpoint analysis. Lancet 2007;369:1090–8 [DOI] [PubMed] [Google Scholar]
- 35.Daviglus ML, Stamler J, Orencia AJ, et al. Fish consumption and the 30-year risk of fatal myocardial infarction. N Engl J Med 1997;336:1046–53 [DOI] [PubMed] [Google Scholar]
- 36.Ouellet V, Marois J, Weisnagel SJ, Jacques H. Dietary cod protein improves insulin sensitivity in insulin-resistant men and women: a randomized controlled trial. Diabetes Care 2007;30:2816–21 [DOI] [PubMed] [Google Scholar]
- 37.Feskens EJ, Virtanen SM, Rasanen L, et al. Dietary factors determining diabetes and impaired glucose tolerance: a 20-year follow-up of the Finnish and Dutch cohorts of the Seven Countries Study. Diabetes Care 1995;18:1104–12 [DOI] [PubMed] [Google Scholar]
- 38.Ebbesson SO, Tejero ME, Nobmann ED, et al. Fatty acid consumption and metabolic syndrome components: the GOCADAN study. J Cardiometab Syndr 2007;2:244–9 [DOI] [PubMed] [Google Scholar]
- 39.Eritsland J, Seljeflot I, Abdelnoor M, Arnesen H, Torjesen PA. Long-term effects of n−3 fatty acids on serum lipids and glycaemic control. Scand J Clin Lab Invest 1994;54:273–80 [DOI] [PubMed] [Google Scholar]
- 40.Hendra TJ, Britton ME, Roper DR, et al. Effects of fish oil supplements in NIDDM subjects. Controlled study. Diabetes Care 1990;13:821–9 [DOI] [PubMed] [Google Scholar]
- 41.Borkman M, Chisholm DJ, Furler SM, et al. Effects of fish oil supplementation on glucose and lipid metabolism in NIDDM. Diabetes 1989;38:1314–9 [DOI] [PubMed] [Google Scholar]
- 42.Balk E, Chung M, Lichtenstein A, et al. Effects of omega-3 fatty acids on cardiovascular risk factors and intermediate markers of cardiovascular disease. Evid Rep Technol Assess (Summ) 2004;93:1–6 [PMC free article] [PubMed] [Google Scholar]
- 43.MacLean CH, Mojica WA, Morton SC, et al. Effects of omega-3 fatty acids on lipids and glycemic control in type II diabetes and the metabolic syndrome and on inflammatory bowel disease, rheumatoid arthritis, renal disease, systemic lupus erythematosus, and osteoporosis. Evid Rep Technol Assess (Summ) 2004;89:1–4 [PMC free article] [PubMed] [Google Scholar]
- 44.Annuzzi G, Rivellese A, Capaldo B, et al. A controlled study on the effects of n−3 fatty acids on lipid and glucose metabolism in non-insulin-dependent diabetic patients. Atherosclerosis 1991;87:65–73 [DOI] [PubMed] [Google Scholar]
- 45.Dunstan DW, Mori TA, Puddey IB, et al. The independent and combined effects of aerobic exercise and dietary fish intake on serum lipids and glycemic control in NIDDM. A randomized controlled study. Diabetes Care 1997;20:913–21 [DOI] [PubMed] [Google Scholar]
- 46.Deckelbaum RJ, Worgall TS, Seo T. n−3 fatty acids and gene expression. Am J Clin Nutr 2006;83:1520S–5S [DOI] [PubMed] [Google Scholar]
- 47.Mostad IL, Bjerve KS, Bjorgaas MR, Lydersen S, Grill V. Effects of n−3 fatty acids in subjects with type 2 diabetes: reduction of insulin sensitivity and time-dependent alteration from carbohydrate to fat oxidation. Am J Clin Nutr 2006;84:540–50 [DOI] [PubMed] [Google Scholar]
- 48.Puhakainen I, Ahola I, Yki-Jarvinen H. Dietary supplementation with n−3 fatty acids increases gluconeogenesis from glycerol but not hepatic glucose production in patients with non- insulin-dependent diabetes mellitus. Am J Clin Nutr 1995;61:121–6 [DOI] [PubMed] [Google Scholar]
- 49.Carpentier YA, Portois L, Malaisse WJ. n−3 fatty acids and the metabolic syndrome. Am J Clin Nutr 2006;83(suppl):1499S–504S [DOI] [PubMed] [Google Scholar]
- 50.Hubert HB, Feinleib M, McNamara PM, Castelli WP. Obesity as an independent risk factor for cardiovascular disease: a 26-year follow-up of participants in the Framingham Heart Study. Circulation 1983;67:968–77 [DOI] [PubMed] [Google Scholar]
- 51.Chen YW, Huang CF, Tsai KS, et al. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes 2006;55:1614–24 [DOI] [PubMed] [Google Scholar]