SYNOPSIS
Objective
We obtained population estimates of the prevalence of lack of diagnostic follow-up after an abnormal prostate-specific antigen (PSA) result and assessed the role of sociodemographic, access, and risk perception factors on follow-up of abnormal tests.
Methods
We used data from the 2000 National Health Interview Survey cancer control supplement. For 3,310 men aged 40 or older with a PSA test, 463 men reported an abnormal PSA test. Outcomes were abnormal PSA and lack of diagnostic follow-up in the latter group. Covariates for logistic regression included sociodemographic variables (age, race/ethnicity, and education), access to care (health insurance and usual source), and risk of cancer (family history and perceived risk). Survey analysis procedures accounted for the complex survey design.
Results
Abnormal PSA results were associated with age, family history, and perceived risk of cancer. Approximately 15% of men with abnormal PSA tests reported no follow-up. The estimated number was 423,549 (95% confidence interval [CI] 317,755, 529,343). No follow-up was more likely in Hispanic men (odds ratio [OR] = 2.21, 95% CI 1.04, 4.70) and men without insurance (OR=6.56, 95% CI 2.02, 21.29), but less likely in men with a family history of prostate cancer or higher perceived risk of cancer.
Conclusions
Substantial numbers of men had no follow-up of abnormal PSA tests. Primary care physicians should assess continuity of care following abnormal PSA results. Data about prostate cancer screening and follow-up are needed to support clinical and policy decisions.
Despite controversy, screening for prostate cancer with the prostate-specific antigen (PSA) test and digital rectal examination is widespread. The controversy surrounding prostate cancer screening stems from important clinical uncertainty. With respect to prostate cancer screening, the evidence for treatment effectiveness on reducing mortality has not been clearly established,1 though mortality for prostate cancer has declined in recent years.2 Treatments may adversely affect quality of life by contributing to sexual, urinary, or bowel problems.3–5 Due to false positives and false negatives of the screening procedures, some men receive unnecessary invasive testing and cancer may be missed in others.6,7 There is also a flourishing body of research concerning the optimum threshold for follow-up of an abnormal or suspicious PSA test.7 Values higher than the traditional PSA cutoff of 4.0 nanograms/milliliter (ng/ml) frequently prompt more testing, which can include PSA retesting, or transrectral ultrasonography and prostate biopsy. In successful screening programs, the loop should be closed with follow-up of positive tests occurring promptly, as treatment is generally more effective in early disease stages.
Professional guidelines for prostate cancer screening vary, but agree that it is important to provide information to men about the advantages and disadvantages of screening so they can make informed choices that are consistent with their personal values and preferences.8–11 Screening recommendations frequently consider a man's age and health. For example, the U.S. Preventive Services Task Force recommends not screening men older than age 75,1 while other groups (e.g., the American Cancer Society) ask clinicians to consider the health status and life expectancy of men when making screening decisions.10 The vision of informed decision-making has emphasized processes occurring before the decision to be screened, but may also include actions taken in the wake of a positive screening result.
Because large numbers of men are being screened for prostate cancer,12,13 it is important to identify characteristics associated with diagnostic follow-up. Factors associated with an abnormal PSA result include both risk factors for the disease and for being tested, particularly regular testing, which gives more opportunities for a positive result.14 The most consistently identified risk factors for prostate cancer are race (African American), older age, and family history of the disease.15,16 Factors associated with regular testing include older age (65–79 years), higher education, health insurance coverage, usual source of care, family history of prostate cancer, both risk factors, and enhanced access to care. Evaluation studies of the PSA report abnormal results in 6% to 20% of men.6,17–22 Age is positively related to having an abnormal result.22,23 A community-based study found higher PSA levels in African American men at each age level.23
Little is known about what influences whether men with abnormal results receive follow-up, even from clinical trials but particularly for the general population. In the major U.S. trial, the Prostate, Lung, Colorectal, and Ovary Cancer Screening Trial (PLCO), 8% of men in the intervention arm at baseline had PSA results above the threshold of 4.0 ng/ml and were referred to their regular physician or health plan. Of that group, 41% had a biopsy, with more than half having a repeat PSA prior to biopsy. Biopsies were more common with higher PSA values and less common in men older than age 65 or with a history of prostate problems or prior negative biopsies.24 Participants in the PLCO are volunteers and, as such, differ from the general population in ways that are difficult to assess.
The objectives of this study were to (1) obtain population estimates on the prevalence of men having no diagnostic follow-up after an abnormal PSA result and (2) assess the role of sociodemographic factors, access to care, and risk of cancer on follow-up. The cancer control supplements of the National Health Interview Survey (NHIS) have been important sources of data for examining behaviors and trends related to cancer prevention and control.25 The NHIS has been useful, even essential, in characterizing population screening patterns. The data on abnormal PSA test results are limited and require careful analysis and thoughtful interpretation.
METHODS
Conceptual framework
The outcome of diagnostic follow-up of an abnormal screening test is a multistep process. Consequently, different constructs may influence the phases of the process.
Risk factors associated with disease incidence are likely to be associated with a positive screening test. Variables identified as major risk factors for prostate cancer include age, family history of the disease, and being African American.15,16 The probability of an abnormal result may also be influenced by characteristics associated with screening, in particular with repeat screening. Ross et al.12 identified access and social resource indicators as predictive of having three or more PSA tests in five years.
Constructs thought to be associated with receipt of diagnostic follow-up also include access and social resource variables. In addition, perceived and objective disease risk may prompt action to specify a diagnosis and potentially seek treatment. Screening-related behaviors may also be associated with follow-up, but we did not include these variables in statistical models, as they may trail the initial abnormal test.
We used data from the 2000 NHIS cancer control supplement.26 The NHIS is a probability-based survey of the noninstitutionalized civilian population. Its complex sample design includes clustering, stratification, and oversampling of African American and Hispanic populations. One of the hallmarks of the NHIS is the use of computer-aided personal interviews conducted by highly trained Census Bureau interviewers. The instrument has a general component that collects information about health status and utilization for each family member, and extended interviews of a randomly selected adult and child.
The cancer control supplement is part of the detailed adult interview. Interviews are conducted in either English or Spanish. The response rate in 2000 was 89% for households and 83% for the detailed adult interview for a net or conditional response rate of 72%. The 3,310 men aged 40 or older who reported having the PSA test were asked additional questions about their experience with PSA testing and are included in our analysis. The PSA questions were asked only of men aged 40 or older.
Measures
Screening behaviors.
The questions about PSA tests begin by asking if the man had heard of the PSA test. If so, he is asked additional questions as follows: If he ever had a PSA test, the interval since his last PSA test, the number of tests in the past five years, and his age when he initiated testing. Men were not asked about the receipt of digital rectal examinations for prostate cancer screening. The outcome of abnormal PSA results was obtained by asking, “Have you ever had a PSA test where the results were not normal?” Diagnostic follow-up was assessed with the question, “Because of these results, what additional tests or surgery did you have?” Follow-up procedures included another PSA test, prostate ultrasound, prostate biopsy, or further imaging studies. Men who reported an abnormal PSA result and no additional tests or surgery were classified as having no follow-up. Men were also asked if subsequent tests indicated cancer. Having an abnormal result was not specifically defined, and there was no information about specific PSA values.
Sociodemographic and access variables.
Sociodemographic variables were age (stratified as 40–64 or ≥65 years) and educational attainment (less than high school, high school graduate or general equivalency diploma, and some college). Respondents could report multiple race categories and either Hispanic or non-Hispanic ethnicity. These were categorized as non-Hispanic black, Hispanic, or other (predominately non-Hispanic white). Access variables included health insurance (categorized as private, public, or none) and usual source of care. Personal health status was used as a summary measure of overall health condition. There has been extensive research concerning this variable, and it has been found to be correlated with many objective health measures, including mortality.27 Health status was categorized as excellent, very good, or good vs. fair or poor.
Risk of prostate cancer.
Objective risk of cancer was measured by whether a man had a first-degree relative (biological father, brother, or son) with prostate cancer. Subjective risk was measured by the perceived risk of getting cancer in the future. The question asked, “Would you say your risk of getting cancer in the future is low, medium, or high?” This response was categorized as high vs. medium or low.
Analysis
In bivariate analyses, we examined the relationship of abnormal result and receipt of follow-up with sociodemographic factors, access, cancer risk, and screening behaviors. Results are presented as percentages and 95% confidence intervals (CIs). All estimates were weighted for selection probabilities except where noted.
In the multivariable analyses, we used Hispanic ethnicity (Hispanic or non-Hispanic) instead of the combined race/ethnicity measure, and categorized health insurance as none vs. private or public health insurance. We fitted the model in segments, for sociodemographic, access, and cancer risk variables. If a variable had an association in the segment models at p<0.10, we included it in the final model for that dependent variable. We did not use variables related to screening behavior in the final models, as they may be associated with follow-up of the initial abnormal PSA test (i.e., be endogenous).
We conducted analysis using Stata® 9.2 software28 to take into account unequal sampling probabilities and the complex sample design. Results from the logistic regression analyses are reported as adjusted odds ratios (ORs) and 95% CIs. The study was approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston.
RESULTS
The analysis sample included 3,310 men aged 40 or older who reported ever having a PSA test. Abnormal results for any prior test were reported by 13.3% of tested men (95% CI 12.1, 14.6). Among 463 men with an abnormal result, 14.8% (95% CI 11.8, 18.5) reported no follow-up diagnostic procedures, for an estimated population total of 423,500 men (95% CI 317,800, 529,300). Another PSA test was reported by 44.3% (95% CI 38.8, 50.0), biopsy of the prostate by 59.2% (95% CI 54.1, 64.2), and ultrasound by 24.3% (95% CI 19.9, 29.4) of the men. The ultrasound procedures were likely in conjunction with a biopsy and may have been viewed by men as secondary to the biopsy. Multiple responses concerning procedures were possible so that the percentages for the follow-up procedures did not total 100.0%. Prostate cancer was reported by 46.4% (95% CI 41.3, 51.6) of the men.
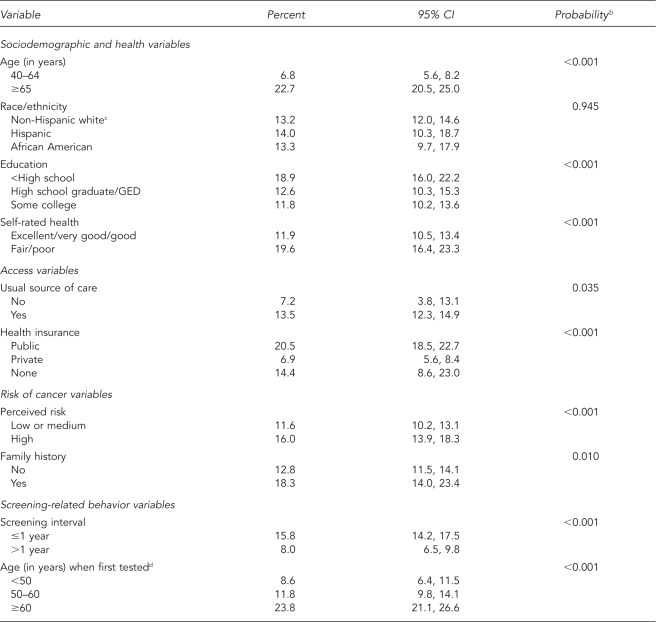
Among sociodemographic variables, abnormal PSA tests were more likely in older men, those with poorer health, and those with less than a high school education (Table 1). We found no correlation between race/ethnicity and abnormal PSA results. Abnormal PSA results were more likely in men with a usual source of care and less likely in men with private insurance. Men with a family history of prostate cancer or who perceived their risk of cancer as above average were more likely to have had an abnormal result. Men screened within the past year or those who initiated testing after age 60 were more likely to have an abnormal test. The association with age of initiation was likely related to when the PSA test was introduced into practice.
Table 1.
Report of any abnormal PSA test by sociodemographic, access, risk perception, and screening variablesa
aData source: the 2000 National Health Interview Survey
bFrom survey design-based version of Pearson's Chi-square test
cIncludes non-Hispanic other
dLimited to men ≥50 years of age
PSA = prostate-specific antigen
CI = confidence interval
GED = general equivalency diploma
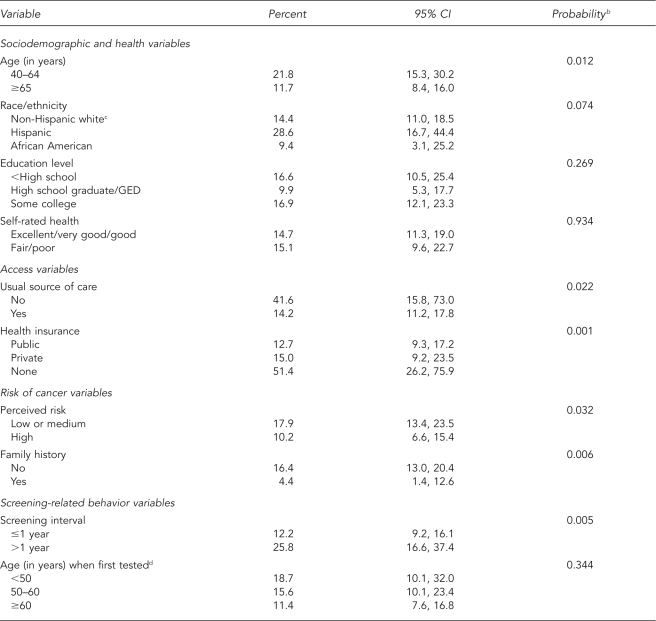
Among men with abnormal results (Table 2), younger men and Hispanic men were less likely to have follow-up, but the difference for Hispanic men was not statistically significant (p=0.07). Access to health care was influential: men with no usual source of care or without health insurance were less likely to report follow-up, though the 95% CIs were wide. Men with a family history of prostate cancer or with higher perceived risk of cancer were more likely to report diagnostic follow-up. We found an association between longer screening intervals and lack of follow-up.
Table 2.
Lack of diagnostic follow-up in men with abnormal PSA result by sociodemographic, access, risk, and screening variablesa
aData source: the 2000 National Health Interview Survey
bFrom survey design-based version of Pearson's Chi-square test
cIncludes non-Hispanic other
dLimited to men ≥50 years of age
PSA = prostate-specific antigen
CI = confidence interval
GED = general equivalency diploma
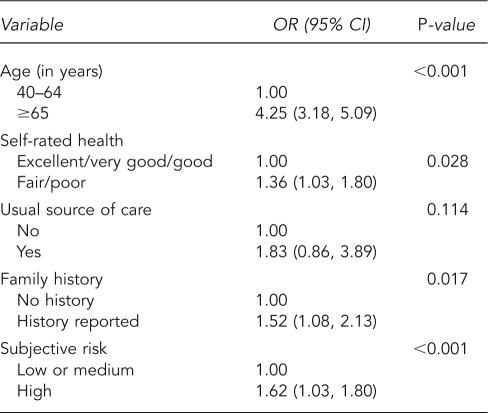
Table 3 presents ORs and 95% CIs for the logistic regression of abnormal test results. Older men and those with poorer health status were more likely to have an abnormal result. In addition, men with a family history of prostate cancer and men with a higher perceived risk of cancer were more likely to have abnormal results. We found the strongest association with age (OR=4.25, 95% CI 3.18, 5.09).
Table 3.
Weighted logistic regression for abnormal PSA resulta
aData source: the 2000 National Health Interview Survey
PSA = prostate-specific antigen
OR = odds ratio
CI = confidence interval
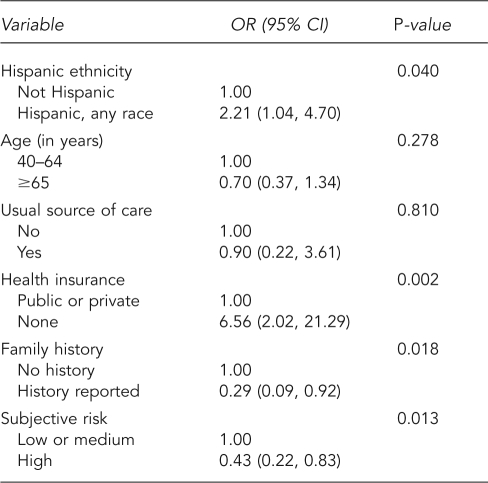
Table 4 summarizes analyses for lack of diagnostic follow-up. Lack of follow-up was more common in Hispanic men (OR=2.21, 95% CI 1.04, 4.70) and men with no health insurance (OR=6.56, 95% CI 2.02, 21.29). Men with a family history of prostate cancer or those with greater perceived risk of cancer were more likely to have diagnostic follow-up.
Table 4.
Weighted logistic regression for lack of follow-up of PSA test resultsa
aData source: the 2000 National Health Interview Survey
PSA = prostate-specific antigen
OR = odds ratio
CI = confidence interval
DISCUSSION
We found an association between age and a family history of prostate cancer—both risk factors for the disease—and abnormal test results. Although they have higher incidence rates for prostate cancer, African American men were not more likely to report a prior abnormal test result. Age and poorer health status were associated with increased likelihood of abnormal tests. A portion of this effect may be through more frequent physician visits, with better opportunity for testing.
Nearly 15% of men with abnormal PSA results reported no follow-up. Social resources and access variables were influential for receipt of follow-up, with lack of follow-up more common in men of Hispanic ethnicity and those who lacked health insurance. African American men were not disadvantaged relative to non-Hispanic white men, but Hispanic men were. Hispanic men have also been found to have lower awareness of the PSA test.29
In addition, we found a relation between both family history of prostate cancer and higher perceived risk of cancer—indicators of objective and subjective risk, respectively—and follow-up care. In prior research, family history but not perceived risk was related to use of screening.29 Physicians may be attuned to family history in framing their discussions and recommendations, but men may also be prompted by their subjective risk perceptions.30
The recent update by the U.S. Preventive Services Task Force has suggested that men older than 75 years of age not be screened for prostate cancer because the harms of screening and treatment for prostate cancer outweigh the benefits.1 The data examined in this study (from 2000) do not address whether physicians follow this recommendation. However, 45% of men older than 75 years of age reported a PSA test in the past year, as compared with 48% of men aged 65–74 years (data not shown), consistent with research showing that clinicians do not temper their screening recommendations based on advanced age or poor health.31 In addition, the prevalence of diagnostic follow-up was the same for these two age categories.
We were not able to specifically link information about discussions of PSA testing with men's physicians and their use of diagnostic follow-up, nor can we know about the content of the discussion.32,33 For example, some of the men may have decided not to obtain follow-up after a discussion of the nature of prostate cancer and the outcomes and side effects of treatment. We can report that men with abnormal test results and normal test reports had an equal likelihood of discussing the pros and cons with physicians.33
There are several reasons for men to not obtain follow-up care, ranging from psychosocial reasons of fear or denial to issues of access. Indeed, men may have discussed their options with their physician and decided not to obtain follow-up, because the survey questions do not identify men who discussed options following an abnormal test result with their physician. The screening setting may also influence receipt of follow-up. For example, PSA tests are often conducted outside ongoing primary care, at health fairs or similar events. Men who are screened in such settings may be more likely to lack health insurance or socioeconomic resources, and these test sites may lack support systems to facilitate follow-up care. Primary care physicians should consider asking men about PSA tests obtained in community settings. Despite these possible explanations for lack of follow-up, major screening initiatives such as the National Breast and Cervical Cancer Early Detection Program34 have identified follow-up and access to treatment as important unsolved issues. It is important to close the loop to ensure that screening services achieve proper evaluation of men with abnormal or suspicious results.
Limitations
Among the limits of this study is that it was difficult to fix the sequence of events in time, as all events were based on self-reports. For example, risk perceptions may have been heightened by the abnormal test result (though family history of prostate cancer generally would not be). In addition, we lacked information about what recommendations were conveyed to the man upon communication of the test result and concerning the effect on subsequent screening behavior.32
This study benefited from a nationally representative sample. Its detailed questions about screening initiation, frequency and recency of testing, experience with abnormal results, and receipt of diagnostic follow-up permitted construction of a retrospective account of screening and follow-up. As the number of men reporting abnormal PSA results was relatively small, it was more difficult to identify real effects in this subsample. The usual solution to this problem would be to aggregate data from several years, but the next cancer control supplement of the NHIS in 2005 did not ask about abnormal results or follow-up, nor are these questions included annually. Because of the public health significance of this question, we used this relatively small available sample. The analysis identified statistically significant effects, but the size of the effect could not be precisely fixed given the wide CIs. There may also have been smaller effects, which we were unable to identify.
CONCLUSIONS
Given the clinical and public health importance of prostate cancer, it is important to expand the amount and quality of relevant data about screening, treatment, and the experience of survivors. Common question areas in health surveys concern recency and frequency of testing, as well as age of initiation. Other important questions to ask concern men's awareness of prostate cancer screening tests, the discussion of advantages and disadvantages of the test, how test results are communicated to them, whether a physician had recommended the test, and in what type of setting the test was performed. It may be that our major national surveys were slow to include questions about prostate cancer screening because of clinical controversy regarding prostate cancer screening and its absence in the national health objectives. However, this investment in data collection is important to provide accurate information about prostate cancer screening and follow-up.
REFERENCES
- 1.U.S. Preventive Services Task Force. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:185–91. doi: 10.7326/0003-4819-149-3-200808050-00008. [DOI] [PubMed] [Google Scholar]
- 2.Ries LAG, Melbert D, Krapcho M, Stinchcomb DG, Howlader N, Horner MJ, et al., editors. SEER Cancer Statistics Review, 1975–2005. Bethesda (MD): National Cancer Institute; 2007. [Google Scholar]
- 3.Litwin MS, Hays RD, Fink A, Ganz PA, Leake B, Leach GE, et al. Quality-of-life outcomes in men treated for localized prostate cancer. JAMA. 1995;273:129–35. doi: 10.1001/jama.273.2.129. [DOI] [PubMed] [Google Scholar]
- 4.Potosky AL, Legler J, Albertsen PC, Stanford JL, Gilliland FD, Hamilton AS, et al. Health outcomes after prostatectomy or radiotherapy for prostate cancer: results from the Prostate Cancer Outcomes Study. J Natl Cancer Inst. 2000;92:1582–92. doi: 10.1093/jnci/92.19.1582. [DOI] [PubMed] [Google Scholar]
- 5.Stanford JL, Feng Z, Hamilton AS, Gilliland FD, Stephenson RA, Eley JW, et al. Urinary and sexual function after radical prostatectomy for clinically localized prostate cancer: the Prostate Cancer Outcomes Study. JAMA. 2000;283:354–60. doi: 10.1001/jama.283.3.354. [DOI] [PubMed] [Google Scholar]
- 6.Mandelson MT, Wagner EH, Thompson RS. PSA screening: a public health dilemma. Annu Rev Pub Health. 1995;16:283–306. doi: 10.1146/annurev.pu.16.050195.001435. [DOI] [PubMed] [Google Scholar]
- 7.Thompson IM, Pauler DK, Goodman PJ, Tangen CM, Lucia MS, Parnes HL, et al. Prevalence of prostate cancer among men with a prostate-specific antigen level < or = 4.0 ng per milliliter [published erratum appears in N Engl J Med 2004;351:1470] N Engl J Med. 2004;350:2239–46. doi: 10.1056/NEJMoa031918. [DOI] [PubMed] [Google Scholar]
- 8.American College of Physicians. Screening for prostate cancer. Ann Intern Med. 1997;126:480–4. [PubMed] [Google Scholar]
- 9.American Urological Association. Prostate-specific antigen (PSA) best practice policy. Oncology (Williston Park) 2000;14 267-72, 277-8, 280. [PubMed] [Google Scholar]
- 10.Smith RA, Mettlin CJ, Davis KJ, Eyre H. American Cancer Society guidelines for the early detection of cancer. CA Cancer J Clin. 2000;50:34–49. doi: 10.3322/canjclin.50.1.34. [DOI] [PubMed] [Google Scholar]
- 11.Zoorob R, Anderson R, Cefalu C, Sidani M. Cancer screening guidelines. Am Fam Physicians. 2001;63:1101–12. [PubMed] [Google Scholar]
- 12.Ross LE, Coates RC, Breen N, Uhler RJ, Potosky AL, Blackman D. Prostate-specific antigen test use reported in the National Health Interview Survey. Prev Med. 2004;38:732–44. doi: 10.1016/j.ypmed.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 13.Sirovich BE, Schwartz LM, Woloshin S. Screening men for prostate and colorectal cancer in the United States. Does practice reflect the evidence? JAMA. 2003;289:1414–20. doi: 10.1001/jama.289.11.1414. [DOI] [PubMed] [Google Scholar]
- 14.Elmore JG, Barton MB, Moceri VM, Polk S, Arena PJ, Fletcher SW. Ten-year risk of false positive screening mammograms and clinical breast examinations. N Engl J Med. 1998;338:1089–96. doi: 10.1056/NEJM199804163381601. [DOI] [PubMed] [Google Scholar]
- 15.Boyle P, Severi G, Giles GG. The epidemiology of prostate cancer. Urol Clin NA. 2003;30:209–17. doi: 10.1016/s0094-0143(02)00181-7. [DOI] [PubMed] [Google Scholar]
- 16.Crawford ED. Epidemiology of prostate cancer. Urology. 2003;62(6) Suppl 1:3–12. doi: 10.1016/j.urology.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Brindle LA, Oliver SE, Dedman D, Donovan JL, Neal DE, Hamdy FC, et al. Measuring the psychosocial impact of population-based prostate-specific antigen testing for prostate cancer in the UK. BJU Int. 2006;98:777–82. doi: 10.1111/j.1464-410X.2006.06401.x. [DOI] [PubMed] [Google Scholar]
- 18.Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Comparison of digital rectal exam and serum prostate-specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994;151:1283–90. doi: 10.1016/s0022-5347(17)35233-3. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman RM, Blume P, Gilliland F. Prostate-specific antigen testing practices and outcomes. J Gen Intern Med. 1998;13:106–10. doi: 10.1046/j.1525-1497.1998.00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maattanen L, Auvinen A, Stenman UH, Rannikko S, Tammela T, Aro J, et al. European randomized study of prostate cancer screening: first-year results of the Finnish trial. Br J Cancer. 1999;79:1210–4. doi: 10.1038/sj.bjc.6690194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ritchie JP, Catalona WJ, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Effect of patient age on early detection of prostate cancer with serum prostate-specific antigen and digital rectal exam. Urology. 1993;42:365–74. doi: 10.1016/0090-4295(93)90359-i. [DOI] [PubMed] [Google Scholar]
- 22.Saraiya M, Kottiri BJ, Leadbetter S, Blackman D, Thompson T, McKenna MT, et al. Total and percent free prostate-specific antigen levels among U.S. men, 2001–2002. Cancer Epidemiol Biomarkers Prev. 2005;14:2178–82. doi: 10.1158/1055-9965.EPI-05-0206. [DOI] [PubMed] [Google Scholar]
- 23.deAntoni EP, Crawford ED, Oesterling JE, Ross CA, Berger ER, McLeod DG, et al. Age- and race-specific reference ranges for prostate-specific antigen from a large community-based study. Urology. 1996;48:234–9. doi: 10.1016/s0090-4295(96)00091-x. [DOI] [PubMed] [Google Scholar]
- 24.Andriole GL, Levin DL, Crawford ED, Gelmannn EP, Pinsky PF, Chia D, et al. Prostate cancer screening in the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial: findings from the initial screening round of a randomized trial. J Natl Cancer Inst. 2005;97:433–8. doi: 10.1093/jnci/dji065. [DOI] [PubMed] [Google Scholar]
- 25.Swan J, Breen N, Coates RJ, Rimer BK, Lee NC. Progress in cancer screening practices in the United States. Cancer. 2003;97:1528–40. doi: 10.1002/cncr.11208. [DOI] [PubMed] [Google Scholar]
- 26.National Center for Health Statistics (US) National Health Interview Survey, 2000. Machine-readable data file and documentation. Hyattsville (MD): National Center for Health Statistics; 2002. [Google Scholar]
- 27.Idler EL, Angel RJ. Self-rated health and mortality in the NHANES-I Epidemiologic Follow-up Study. Am J Public Health. 1990;80:446–52. doi: 10.2105/ajph.80.4.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.StataCorp. Stata®. Version 9.2 for Windows. College Station (TX): StataCorp.; 2001. [Google Scholar]
- 29.McFall SL. Use and awareness of prostate specific antigen tests and race/ethnicity. J Urology. 2007;177:1475–80. doi: 10.1016/j.juro.2006.11.096. [DOI] [PubMed] [Google Scholar]
- 30.Yabroff KR, Breen N, Vernon SW, Meissner HI, Freedman AN, Ballard-Barbash R. What factors are associated with diagnostic follow-up after abnormal mammograms? Findings from a U.S. national survey. Cancer Epidemiol Biomarkers Prev. 2004;13:723–32. [PubMed] [Google Scholar]
- 31.Walter LC, Bertenthal D, Lindquist K, Konety BR. PSA screening among elderly men with limited life expectancies. JAMA. 2006;296:2336–42. doi: 10.1001/jama.296.19.2336. [DOI] [PubMed] [Google Scholar]
- 32.McFall SL. U.S. men discussing prostate-specific antigen tests with a physician. Ann Fam Med. 2006;4:433–6. doi: 10.1370/afm.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han PK, Coates RJ, Uhler RJ, Breen N. Decision making in prostate-specific screening. National Health Interview Survey, 2000. Am J Prev Med. 2006;30:394–404. doi: 10.1016/j.amepre.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Lantz PM, Richardson LC, Sever LE, Macklem DJ, Hare ML, Orians CE, et al. Mass screening in low-income populations: the challenge of securing diagnostic and treatment services in a national cancer screening program. J Health Polit Policy Law. 2000;25:451–71. doi: 10.1215/03616878-25-3-451. [DOI] [PubMed] [Google Scholar]