Vaccines are temperature-sensitive biological products. Exposure to heat shortens a vaccine's shelf life,1 while freezing vaccines that should not be frozen causes irreversible loss of potency.2 Therefore, maintaining vaccines inside the cold chain (ICC) is an essential part of a successful immunization program. However, in many developing countries in Asia, a cold-chain infrastructure is not available, especially in remote and rural areas.3,4 Some people live in remote areas of the western plateau of China, far away from the county health center, and there is no cold-chain infrastructure. As a strategy to extend vaccination coverage, some local health units have suggested winter delivery of vaccines, relying on ambient temperatures outside the cold chain (OCC).
Areas in China's western plateau at 3,000 to 5,000 meters above sea level have an extreme continental climate with long, cold winters. The mean temperature during November, December, and January (the coldest period) ranges from −22°C to −3°C, and the ambient temperature is sub-zero from the beginning of October to the end of April. For some health unit workers, the term “cold chain” implies, incorrectly, that avoiding heat is the sole objective, and that unduly low temperatures would not be an issue for vaccine storage and delivery.
We conducted the fieldwork for this survey in November 2007. Our objective was to investigate whether hepatitis B (HepB) and measles vaccines stored and transported in the winter were subjected to below freezing and/or high temperatures during the routine distribution of vaccines to peripheral health centers in remote regions of China's western plateau. We designed the study to assess the effects of exposure to temperatures OCC on vaccine potency.
MATERIALS AND METHODS
Transport and storage
Vaccines were produced by the Biological Product Corporation (BPC) and distributed in a refrigerated truck from the BPC depot to provincial stores, and from provincial stores to each district store. Cold rooms are available at the BPC depot and at the provincial stores, where vaccines are kept in a refrigerator—usually a special cryopreservation model. In temporary stores and during transport from district stores to immunization clinics (e.g., hospitals and health centers), vaccines are transported in non-refrigerated boxes by truck and by plane.
Study sites
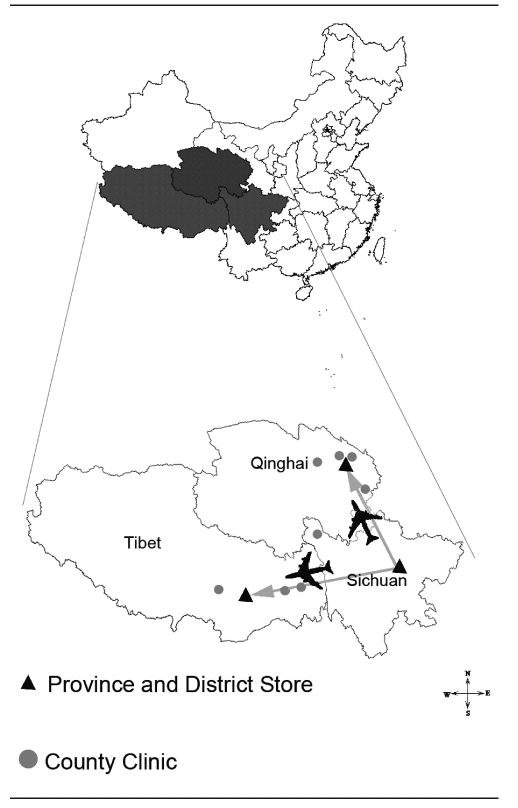
There are 27 immunization clinics in this remote area, and we selected eight clinics at random that were reasonably accessible and were considered to be representative of the plateau's geographic and economic diversity. We focused this study on the delivery routes from the provincial stores to the immunization clinics (Figure 1). The ambient temperatures in these remote areas during the study period (November 2007) ranged from −7.9°C to 12°C.
Figure 1.
Map of China showing the location of the routes used in a study of outside-the-cold-chain vaccine deliverya
aTransport from provincial store to provincial and district store was by air, whereas transport from stores to outstations was by road.
Study design
The methodology of this study was in line with the World Health Organization (WHO) protocol for temperature monitoring ICC and was similar to studies in Thailand and Papua New Guinea.5–7 The HepB vaccine is stable for up to four years at 2°C to 8°C, for months at 20°C to 25°C, for weeks at 37°C, and for days at 45°C. The HepB vaccine (Hissen Bio-Pharm INC, China) has a freezing point of −0.5°C and freezing it may cause a significant reduction of potency. We chose the HepB vaccine as a representative cold-sensitive vaccine.8 The measles vaccine in the dried form is not damaged by freezing and maintains at least the minimum level of potency for more than two years at 2°C to 8°C. The minimum required infectivity titer of the measles virus is retained for at least one month at 20°C to 25°C, and for at least one week at 37°C.1
We chose the measles vaccine (Shanghai Institute Biological Products, China) as a representative heat-sensitive vaccine.2 These vaccines are among those used routinely in China's immunization program. We monitored temperature with a computerized transit temperature data logger (Model TV-0050, Gemini Data Loggers [UK] Ltd.). This instrument, which has an operating range of −30°C to 50°C, was set to record temperatures at 30-minute intervals. Additionally, we used Freeze Watch™ indicators (Model 9805FW, 3M Health Care, St. Paul, Minnesota) to show whether temperatures at or below 0°C were encountered. Time/temperature indicators (Monitor Mark™ 9860C, 3M Health Care) were used as cold-chain monitors to indicate whether the temperature was above 10°C and, if so, for what length of time. Figures 2 and 3 show the typical changes of time/temperature indicators and Freeze Watch indicators.
Figure 2.
Time/temperature indicatorsa used in a study of outside-the-cold-chain vaccine delivery in remote regions of western China
aThe time/temperature indicator on the left is intact, while the indicator on the right shows a blue color produced in response to heat exposure for longer than 50 hours.
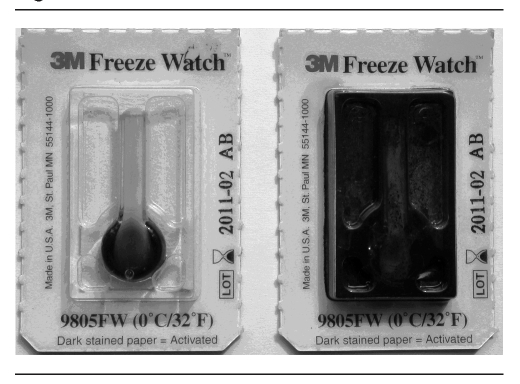
Figure 3.
Freeze Watch™ indicatorsa used in a study of outside-the-cold-chain vaccine delivery in remote regions of western China
aThe Freeze Watch indicator on the left is intact, while the indicator on the right has been burst by freezing.
Three instruments were placed in a box with the vaccines and sent under OCC conditions, together with other vaccines, on each of the selected distribution routes. The data logger started recording temperatures when the box entered the provincial store.
In the present study, we did not perform serological analysis for HepB surface antigen. We used the shake test to check for deterioration of the HepB vaccine. Whenever a Freeze Watch indicator registered that freezing had occurred, staff at the distribution level used the shake test on the HepB vaccine to determine whether it had been frozen. The shake test followed WHO guidelines.1 The suspect vial and one known to have been frozen (the control vial) were both shaken. Physical damage is indicated if the suspect vial has a rate of precipitation equal to or greater than that of the control.
After each box was delivered and had been kept at the immunization clinic, 48 vials of the measles vaccine were sent for potency testing. Fifty percent cell culture infectious dose (CCID50) assays were used in line with Chinese Biological Product Regulations.9 A 100-microliter (μl) sample of a suspension of Vero cells (Chinese Center for Disease Control and Prevention) at a density of 5×105 cells/milliliter was placed into a well of a 96-well culture plate (Corning, Corning, New York) in growth medium (modified 1640 medium [Hyclone, Logan, Utah], 5% volume-to-volume fetal bovine serum). A series of dilutions (10, 100, 1,000, and 10,000-fold) of the measles vaccine in sterile water was prepared. Cells were infected with 200μl of each dilution (12 wells per dilution). After eight days, the titers were calculated and expressed as log10 CCID50.
We used the student's t-test for independent samples to identify statistically significant differences of mean virus titer between ICC (provincial stores) and OCC vaccines. We conducted statistical analysis using SPSS® Version 12.0,10 and we set the level of statistical significance at p<0.05.
Health worker cold-chain knowledge survey
Before the study began, we visited the health centers and used a standard questionnaire to ask health workers at all levels a series of questions about their knowledge, attitude, and practice regarding the cold chain. The questions and topics referred to in the interview followed WHO guidelines for cold-chain personnel.5
RESULTS
Temperature record
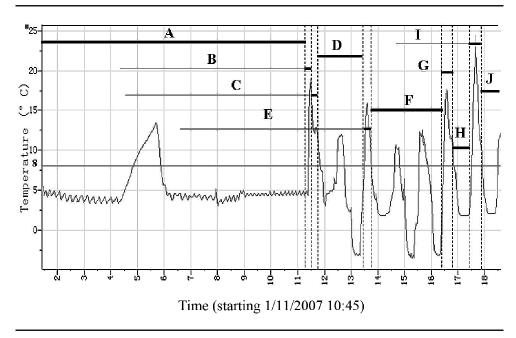
One automatic data logger failed for unknown reasons as soon as the vaccines left the provincial health center. Therefore, we had only seven routes with complete records. The median time was 224.8 hours ICC in provincial stores, and 187.6 hours OCC in temporary storage and transit. The recorded temperatures for each logger during each phase of the journey to the periphery health centers ranged from −5.5°C to 24.4°C, with a mean temperature of 5.4°C. An example of the data logger output for a single route is shown in Figure 4.
Figure 4.
Example of temperature record provided by the data logger for vaccine delivery during one route, outside the cold chain, in remote regions of western China
Legend: A = provincial store; B = transport by plane; C = transport by truck; D = district temporary store; E = transport by truck; F = county temporary store; G = transport by truck; H = another county temporary store; I = transport by truck; J = health center
Exposure to heat and freezing
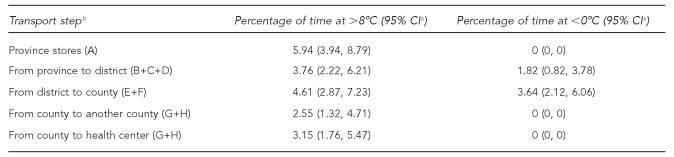
At the provincial store, the temperature was above 8°C for 24.5 hours due to a faulty refrigerator or a power failure. We recorded a temperature above 24°C for at least one of the four routes. In one case, it was known that the vaccines had been carried in the cabin of the truck. Temperatures are high under direct sunlight at noon in this region of the plateau, which is when the vaccines were in transit. Moreover, air-conditioning might also have played an important role. According to the data loggers, the temperature was above 8°C for 18.5% to 26.7% of the monitoring period for all seven routes, and for three routes these temperatures were maintained for more than 80 hours. Temperatures higher than 8°C were recorded during transport from province to district (31.3% of transit and storage time), from district to county (29.2% of transit and storage time), and from county to health center (43.6% of transit and storage time).
Of the seven routes for which records were complete, all had at least one hourly temperature record below −0.5°C, which is the freezing point of the HepB vaccine. These temperatures were recorded between 2.9% and 12.9% of the time spent under OCC storage. Temperatures below −0.5°C were recorded in district stores (18.5% of storage time) and in county stores (23.3% of storage time), and were observed for periods of 81 to 153 hours, with a median time of 112.1 hours. For all seven data loggers, there was a drastic drop in temperature after they left the airport and were transported by truck to a district store. Mostly, the temperature dropped to −3°C, but a temperature of −5.5°C was recorded for one route. The lowest temperatures were recorded in stores late at night and lasted for seven hours (Table 1).
Table 1.
Percentage of time inside the cold chain and outside the cold chain during transport of vaccines in remote regions of western Chinaa
aThe data shown are those inside-the-cold-chain and outside-the-cold-chain vaccines that were exposed below 0°C and above 8°C.
bA = provincial store; B = transport by plane; C = transport by truck; D = district temporary store; E = transport by truck; F = county temporary store; G = transport by truck; H = another county temporary store
CI = confidence interval
In total, 20 Freeze Watch indicators and 48 time/temperature indicators registered freezing and heat exposure, respectively, and there was no discrepancy between the data acquired by Freeze Watch activation, time/temperature indicators, and the data loggers.
Potency of vaccines
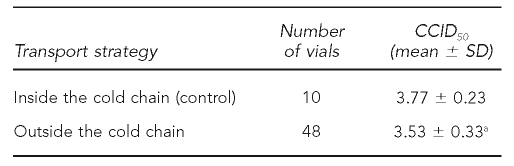
The 48 vials of measles vaccines were all tested for potency because of the considerable exposure to heat and freezing during transit. In three vials, potency was decreased to about 3.0 log10 and showed vaccine failure. Moreover, the titer of most vaccines was decreased compared with control vaccines that had been kept ICC in provincial stores (Table 2). Freeze Watch indicators registered freezing conditions in the district temporary store, but all of the HepB vials passed the shake test.
Table 2.
Virus titers of measles vaccine on Vero cells for different transport strategies in remote regions of western China
ap<0.05 vs. control
CCID50 = 50% cell culture infectious dose
SD = standard deviation
Health worker interviews
We conducted interviews with 32 health officers and workers involved in the study. At the province level, all health workers understood that maintaining the correct temperature of the cold chain was important to prevent both heating and freezing of the samples. At the district level, a small number of staff did not know that vaccines were harmed by freezing. The situation was worse in the peripheral health centers. We found that health staff, especially in the outlying health centers, had limited knowledge about the cold chain, such as the appropriate temperature during storage, or the equipment and processes needed for reliable temperature recording. Approximately half of these health workers presumed that avoiding heat was the only objective of the cold chain, and most of the health staff did not know that vaccines could be damaged by freezing, which is similar to the findings of studies in Bolivia.11 Individual staff members believed that a vaccine was still usable after freezing and thawing. The lack of knowledge of the cold chain was almost certainly due to inadequate staff training, likely as the result of an insufficient budget and insufficient time spent on education.
DISCUSSION
Maintenance of the cold chain during transport and storage is vital for vaccination effectiveness. In many developing countries, population vaccination is impractical because of the difficulty of keeping vaccines at the manufacturer's recommended temperature.12–16 In attempts to achieve better coverage of vaccination, Indonesia, Vietnam, and the Hunan area of China have successfully implemented an OCC strategy for delivery of the HepB vaccine when labeled vaccine vial monitors (VVMs) are monitored.3,17–19 However, the OCC transport strategy does not appear to be appropriate for some areas and some seasons. In Mongolia, even with ICC delivery of HepB vaccine, failure to administer the vaccine during the winter might have had an important role in the high prevalence of hepatitis B virus infection and the low rate of vaccine-induced immunity in rural areas.12,13 In some remote areas of China, the term “cold chain” is understood incorrectly; many assume that avoiding heat is the sole requirement. Vaccines have been transported OCC for a long time during the winter as part of the immunization program in China. However, there was no in-depth evaluation of the strategy prior to the present study.
The results of this study have shown that exposure to temperatures below 0°C and above 8°C were commonplace in the OCC winter delivery strategy in some remote areas of western China, and the potency of vaccines was decreased as a result. In this study, exposure to temperatures above 8°C exceeded 80 hours within an OCC period of 173.5 hours. In four exceptional cases, a temperature of 24°C was recorded for a period of one hour. The WHO estimates that measles vaccine kept at 22°C to 25°C will remain potent for four weeks.1 In the present study, however, the potency of the measles vaccine was decreased after OCC transport. We speculate that certain aspects of the harsh climate of the study area in the winter, such as large temperature differences, strong radiation, low atmospheric pressure, and hypoxia, might have contributed to the results, and further studies are needed to assess the impact of these factors.
The vaccines were exposed to temperatures below −0.5°C for more than 15 hours on all seven transport routes, and the lowest temperature recorded was −5.5°C. As a result, it was expected that the potency of the HepB vaccine would be impaired. However, shake tests conducted by local staff failed to identify any vaccine damage. A possible explanation is that freezing almost always occurred at night, but the shake tests were conducted in the daytime and the frozen vaccines thawed during the test.
Another possible explanation is that the HepB vials were small and had large labels, making it difficult to see any precipitation, and the staff conducting the shake test had only limited experience. While the shake test is useful, it lacks the precision demanded for scientific assessment of vaccines.20
Freeze Watch indicators were not used in China's cold-chain monitoring before 2007. In this study, the indicators were consistent with the data logger and identified freezing conditions in all seven routes, showing that Freeze Watch indicators are effective in safeguarding HepB vaccine during transport and storage. At present, policy changes at the global and country level are needed to prioritize the prevention of freezing in the cold chain.21 The routine use of Freeze Watch monitors is helpful for preventing vaccines from freezing in the cold chain. In this study, time/temperature indicators were used to monitor vaccine exposure to heat, which is useful, but the range of time and temperature indicated is too narrow to monitor the cold chain successfully. In line with the WHO report,22 we would use VVMs in any follow-up study.
CONCLUSIONS
The results of this study show that there are some failings in the current vaccine delivery strategy in remote areas of China, which could prevent the immunization program's success. The OCC vaccine delivery strategy is not suitable for China's western plateau in winter and cannot replace the vaccine cold-chain system. Maintenance of the cold chain during transportation and storage is needed to guarantee the effectiveness of vaccination in remote areas. As soon as the results of this study were available, several areas began to establish their own cold-chain systems. Particular emphasis has been placed on the freezing problem in cold-chain training and education. Electronic devices for continuous measurement of temperature are available to accompany vaccines during transport and storage as part of a comprehensive supervision plan, according to the WHO protocol for temperature monitoring.5 At the same time, VVMs and Freeze Watch are routinely used to monitor the temperature of the cold chain. Exposure of vaccines to heat and freezing can be avoided only if an effective cold-chain system is in place.
At present, the control of communicable diseases has placed ever-increasing demands for a comprehensive vaccination program in China. Surveillance of vaccine use, government investment in the vaccine cold chain, and the timely exchange of related information are all required for a successful vaccination program. This study is the first to evaluate the effectiveness of the vaccine delivery strategy in remote areas of China, and provides important information for future intervention planning in this area.
Acknowledgments
The authors thank the staff of the Regional Offices for Disease Prevention and Control, and the staff of all the study sites, without whose efforts the project could not have been completed.
REFERENCES
- 1.Galazka A, Milstien J, Zaffran M. Thermostability of vaccines: global programme for vaccines and immunization (GPV) Geneva: World Health Organization; 1998. [Google Scholar]
- 2.Diminsky D, Moav N, Gorecki M, Barenholz Y. Physical, chemical and immunological stability of CHO-derived hepatitis B surface antigen (HBsAg) particles. Vaccine. 1999;18:3–17. doi: 10.1016/s0264-410x(99)00149-8. [DOI] [PubMed] [Google Scholar]
- 3.Wang L, Li J, Chen H, Li F, Armstrong GL, Nelson C, et al. Hepatitis B vaccination of newborn infants in rural China: evaluation of a village-based, out-of-cold-chain delivery strategy. Bull World Health Organ. 2007;85:688–94. doi: 10.2471/BLT.06.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Samant Y, Lanjewar H, Block L, Parker D, Stein B, Tomar G. Relationship between vaccine vial monitors and cold chain infrastructure in rural districts of India. Rural and Remote Health. 2007;7:617. online. [PubMed] [Google Scholar]
- 5.World Health Organization. Study protocol for temperature monitoring in the vaccine cold chain. Geneva: WHO; 2005. [Google Scholar]
- 6.Techathawat S, Varinsathien P, Rasdjarmrearnsook A, Tharmaphornpilas P. Exposure to heat and freezing in the vaccine cold chain in Thailand. Vaccine. 2007;25:1328–33. doi: 10.1016/j.vaccine.2006.09.092. [DOI] [PubMed] [Google Scholar]
- 7.Wirkas T, Toikilik S, Miller N, Morgan C, Clements CJ. A vaccine cold chain freezing study in PNG highlights technology needs for hot climate countries. Vaccine. 2007;25:691–7. doi: 10.1016/j.vaccine.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 8.Hollinger FB, Liang TJ. Hepatitis B virus. In: Knipe DM, Howley PM, editors. Fields virology. 4th ed. Philadelphia: Lippincott-Raven; 2001. pp. 2971–3036. [Google Scholar]
- 9.Biological Product Standardization Council of China, editors. Chinese biological product regulations. Chemical Industry Publications. 2000:135–6. [Google Scholar]
- 10.SPSS Inc. SPSS®: Version 12.0 for Windows. Chicago: SPSS Inc.; 2004. [Google Scholar]
- 11.Nelson C, Froes P, Dyck AM, Chavarría J, Boda E, Coca A, et al. Monitoring temperatures in the vaccine cold chain in Bolivia. Vaccine. 2007;25:433–7. doi: 10.1016/j.vaccine.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 12.Edstam JS, Dulmaa N, Tsendjav O, Dambasuren B, Densmaa B. Exposure of hepatitis B vaccine to freezing temperatures during transport to rural health centers in Mongolia. Prev Med. 2004;39:384–8. doi: 10.1016/j.ypmed.2004.01.029. [DOI] [PubMed] [Google Scholar]
- 13.Davaalkham D, Ojima T, Wiersma S, Lkhagvasuren T, Nymadawa P, Uehara R, et al. Evidence based public health policy and practice: administration of hepatitis B vaccine in winter as a significant predictor of the poor effectiveness of vaccination in rural Mongolia: evidence from a nationwide survey. J. Epidemiol Community Health. 2007;61:578–84. doi: 10.1136/jech.2006.051375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanjeet K, Lye MS, Sinniah M, Schnur A. Evaluation of cold chain monitoring in Kelantan, Malaysia. Bull World Health Organ. 1996;74:391–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Jain R, Sahu AK, Tewari S, Malik N, Singh S, Khare S, et al. Cold chain monitoring of OPV at transit levels in India: correlation of VVM and potency status. Biologicals. 2003;31:237–44. doi: 10.1016/s1045-1056(03)00061-7. [DOI] [PubMed] [Google Scholar]
- 16.Kaipilyawar SB, Laxminarayan J. Andhra Pradesh cold chain system marching beyond routine to obtain WHO accreditation. Indian J Public Health. 2004;48:57–9. [PubMed] [Google Scholar]
- 17.Nelson CM, Wibisono H, Purwanto H, Mansyur I, Moniaga V, Widjaya A. Hepatitis B vaccine freezing in the Indonesian cold chain: evidence and solutions. Bull World Health Organ. 2004;82:99–105. [PMC free article] [PubMed] [Google Scholar]
- 18.Hipgrave DB, Tran TN, Huong VM, Dat DT, Nga NT, Long HT, et al. Immunogenicity of a locally produced hepatitis B vaccine with the birth dose stored outside the cold chain in rural Vietnam. Am J Trop Med Hyg. 2006;74:255–60. [PubMed] [Google Scholar]
- 19.Hipgrave DB, Maynard JE, Biggs BA. Improving birth dose coverage of hepatitis B vaccine. Bull World Health Organ. 2006;84:65–71. doi: 10.2471/blt.04.017426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimayuga R, Scheifele D, Bell A. Effects of freezing on DPT and DPT-IPV vaccines, adsorbed. Can Commun Dis Rep. 1995;21:101–3. [PubMed] [Google Scholar]
- 21.Matthias DM, Robertson J, Garrison MM, Newland S, Nelson C. Freezing temperatures in the vaccine cold chain: a systematic literature review. Vaccine. 2007;25:3980–6. doi: 10.1016/j.vaccine.2007.02.052. [DOI] [PubMed] [Google Scholar]
- 22.World Health Organization. Temperature monitors for vaccines and the cold chain. Geneva: WHO; 1999. [Google Scholar]