Abstract
Epidemiological studies demonstrate that a lower blood pressure and decline in blood pressure over months or years are associated with higher mortality in dialysis patients. In contrast, randomized controlled trials lack power to establish benefits of antihypertensive therapy. Patients on long-term dialysis participating in randomized controlled trials and receiving antihypertensive drug therapy were the subject of this meta-analysis. Outcomes assessed were the hazard ratio of cardiovascular events and all-cause mortality in treated group compared to controls. Among 1202 patients we identified in 5 studies, the overall benefit of antihypertensive therapy compared to control or placebo group had a combined hazard ratio for cardiovascular events of 0.69 (95% CI 0.56 to 0.84) using a fixed effects model and 0.62 (95% CI 0.44 to 0.88) using a random effects model. In a sensitivity analysis we found that the hypertensive group had a pooled hazard ratio of 0.49 (95% CI 0.35 to 0.67), but when normotensives were included in the trial lesser cardiovascular protection was seen (pooled hazard ratio of 0.86 (95% CI 0.67 to 1.12)). Test for herterogenity between hypertensive and “normotensive-included” groups was significant (p<0.006). Similar results were seen for risk ratio for death and cardiovascular events. There was evidence of publication bias based on Egger's test and funnel plot. Randomized trials suggest benefit of antihypertensive therapy among hemodialysis patients. Adequately powered randomized trials are required to confirm these observations especially among those with hypertension.
Keywords: Systematic review, cardiovascular events, reverse epidemiology, hypertension, hemodialysis, treatment
Introduction
Hypertension is the third most important cause of global burden of disease in the general population. It trails only childhood and maternal underweight and unsafe sex to account for 64 million disability adjusted life years and 4.4% of the global disease burden 1. This cardiovascular risk factor was first recognized in cohort studies 2 and later supported by clinical trials 3. The vexing observation made by epidemiological studies in hemodialysis patients suggest that low-- not high— blood pressure is associated with all-cause mortality 4-9. On the basis of this reverse epidemiology paradox, some have cautioned against lowering blood pressure in patients with hypertension who are on long-term hemodialysis 8.
In the last 5 years, several randomized trials have tested the notion whether antihypertensive therapy based on a variety of antihypertensive drugs including beta-blockers 10, ACE inhibitors 11 and angiotensin receptor blockers 12, 13 and dihydropyridine calcium channel blockers 14, 15 can prevent cardiovascular events. However, these trials have been small and effect size estimates have sometimes crossed the hazard ratio of 1 to yield statistically insignificant results.
Another important issue that has become evident is that blood pressures obtained before and after dialysis which are most often used for medical decision making may be of limited value in determining the true blood pressure as measured by interdialytic ambulatory blood pressure monitoring 16. Indeed, current studies often rely solely on blood pressures obtained in the dialysis unit 10-13. Since antihypertensive therapy on average lowers blood pressure in dialysis patients it may be better to examine the impact of antihypertensive therapy on outcomes rather than examining the extent of blood pressure lowering. Most patients who are treated with antihypertensive drugs have at least some degree of hypertension and in fact most studies deliberately, and rightly so, exclude patients with symptomatic hypotension or very low blood pressure 10-13. However, it is unclear whether the effect estimates may be influenced by inclusion of patients who are not hypertensive on hemodialysis as has been deliberately done in 3 studies 10-12.
The goal of this systematic review was to determine the presence and the magnitude of benefit in treating hemodialysis patients with antihypertensive drugs.
Methods
Data Sources
We searched the Pubmed (Jan 1996 to Oct 2008) database and The Cochrane Central Register of Controlled Trials (3rd Quarter 2008). The terms “hypertension” and “dialysis” were searched in the title, original title, abstract, MESH headings, heading words and keyword. The result was limited to randomized controlled trials using a highly sensitive filter17. A similar search was performed in EMBASE. To be included in this review, studies had to randomize hemodialysis patients to antihypertensive drugs regardless of the presence or absence of hypertension and reported cardiovascular and/or mortality outcomes. In addition to the above search, we manually reviewed references cited in the retrieved articles and review articles. We also searched the proceedings of the American Society of Nephrology and European Dialysis and Transplantation Association to retrieve unpublished studies.
All data was abstracted with a standardized data collection form. From each article included we abstracted the study design, year, number of included patients, age, cardiovascular event rate and death rate, and treatment characteristics, including the type of drug and duration of use.
Statistical Analysis
Hazard ratios recorded in the reports were log transformed. The standard error of these log hazard ratios were calculated from the 95% confidence intervals. Using the inverse of the standard error of these hazard ratios we pooled the hazard ratios between studies with a fixed effects model. For the sake of comparison, random effects models are also reported. We used Forest plots to visualize the extent of variation between studies and the I2 statistic to quantify heterogeneity between studies. I2 values which range from 0% to 100% describe the proportion of variation in prevalence estimates that is due to between study variation rather than due to sampling error 18. We obtained the group-specific and overall I2 as standard output of the metan program. We conducted a sensitivity analysis to test the influence of hypertension status (studies with hypertensive patients only vs those studies which also included those with normotension) using the metan command of Stata 10.1 (Stata Corp, College Station, TX). Publication bias was tested with an Egger's test 19 and the funnel plot 20 using metabias and metafunnel programs respectively in Stata. Using the metainf program, sensitivity analysis was carried out by excluding one trial at a time from pooled effects to determine whether any one study was particularly influential.
Results
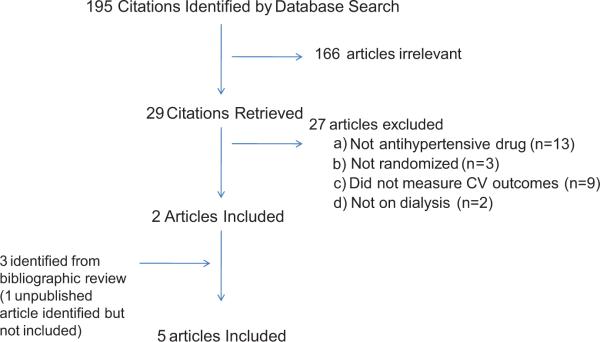
A total of 195 studies were obtained from my search of which 6 studies in adults on hemodialysis were initially identified for this analysis 10-15. One study published in abstract form several years ago was not included in this meta-analysis given that despite positive results the study had not been published. However, inclusion of this study did not materially influence the outcome of this meta-analysis. The causes of exclusion are shown in Figure 1.
Figure 1.
Inclusion and exclusion diagram for articles finally selected for meta-analysis
The total number of hemodialysis subjects from all studies was 1202 and range of subjects per study was 80 to 397. The study characteristics are shown in Table 1.
Table 1.
Characteristics of studies included in the meta analysis
| Author | Year | BP Medication |
Design | Exposure (mo) |
Nomotensives included |
Vintage (yrs) |
Age | N | BP baseline |
BP final | LV mass index (g/m2) |
Deaths | CV Events |
Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Zannad | 2006 | Fosinopril | DBRCT | 24 | Yes | 5.3 | 67 | 196 | 146/77 | 139/76 | 179 | 52 | 67 | LVH required for randomization. |
| Placebo | 4.4 | 67 | 201 | 145/77 | 143/74 | 169 | 49 | 60 | ||||||
| Takahashi | 2006 | Candesartan | PROBE | 36 (stopped early) | Yes | 2.74 | 60 | 43 | 153/82 | 149/80 | 143.3 | 0 | 7 | Excluded pts with CVD |
| Nothing | 19.4 avg exposure | 2.77 | 62 | 37 | 152/85 | 153/83 | 152.4 | 7 | 17 | Primary prevention trial | ||||
| Suzuki | 2008 | ARBs | Randomized open | 36 | No | 3.7 | 59 | 180 | 154/81 | 140/80 | 25 | 34 | Treatment and outcomes not masked | |
| Nothing | 3.7 | 60 | 180 | 156/82 | 140/78 | 38 | 59 | |||||||
| Cice | 2003 | Carvedilol | DBRCT for 12 mo, then open label | 24 | Yes | 7.1 | 55 | 58 | 134/75 | 120/70 | 30 | 17 | Dilated cardiomyopathy required | |
| Placebo | 6.8 | 55 | 56 | 135/75 | 135/76 | 41 | 39 | All pts on ACE inhibitors or ARBs | ||||||
| Tepel | 2008 | Amlodipine | DBRCT | 19 | No | 2.3 | 60 | 123 | 140/80 | 130/unchanged | 15 | 19 | ||
| Placebo | 1.9 | 62 | 128 | 141/80 | 140/unchanged | 22 | 32 |
DBRCT: double-blind randomized controlled trial. PROBE: Prospective randomized open-label blinded end-point, CV cardiovascular, CVD cardiovascular disease, LVH left ventricular hypertrophy, MAP Mean arterial pressure. ARBs angiotensin receptor blockers. NA not available.
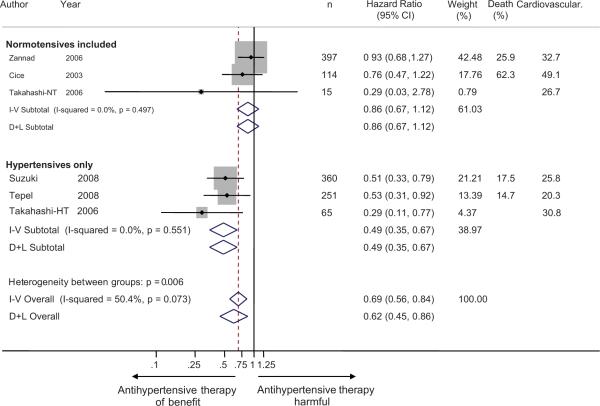
The hazard ratios for cardiovascular events from the individual studies ranged from 0.29 to 0.93 (Figure 2). No study had a point estimate that suggested harm with treatment. The overall benefit of antihypertensive therapy had a combined hazard ratio of 0.69 (95% CI 0.56 to 0.84) using an inverse-weighted fixed effects model and 0.62 (95% CI 0.44 to 0.88) using a random effects model. There was substantial heterogeneity between studies with respect to outcomes (I2 60.8%, p=0.037).
Figure 2.
Forest plot shows the hazard ratios of antihypertensive therapy on cardiovascular events. When studies were divided based on inclusion of normotensive subjects, it was found that those studies that included normotensive subjects did not consistently demonstrate cardiovascular protection, whereas those which included only hypertensive subjects provided significant protection. The test for interaction based on the grouping variable of presence or absence of normotension was significant (p=0.004). There was still significant heterogeneity between studies in hypertensive hemodialysis patients only. This may be due to study design. For example Takahashi et al studied primary prevention, whereas Suzuki and Tepel did not exclude patients with prior cardiovascular events.
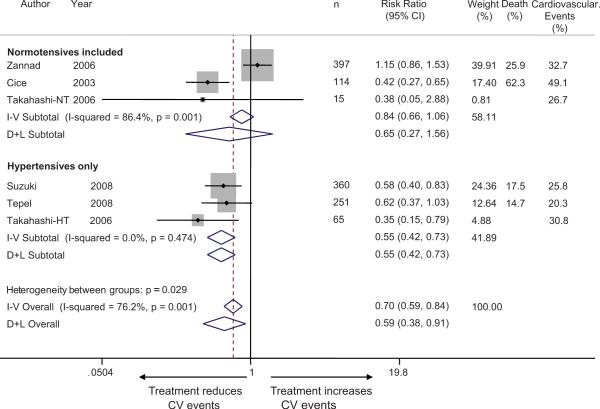
We also calculated the risk ratios for cardiovascular events from the individual studies which ranged from 0.35 to 1.15 (Figure 3). Based on data provided by the senior author, one study had an unadjusted risk ratio estimate that suggested harm with treatment 11. Takahashi et al at our request provided data on cardiovascular outcomes stratified by hypertension status, that is separately among 15 normotensive and 65 hypertensive patients. Among normotensives, 8 patients were allocated to control and 7 patients were allocated to candesartan groups. In patients without hypertension, 3/8 in the control group and 1/7 in the candesartan group experienced cardiovascular events (HR=0.288 [CI: 0.030-2.803], p=0.2834). In patients with hypertension, 14/29 in the control group and 6/36 in the candesartan group experienced cardiovascular events (HR=0.294 [0.112-0.766], p=0.0123).
Figure 3.
Forest plot shows the risk ratios of antihypertensive therapy on cardiovascular events. When studies were divided based on inclusion of normotensive subjects, it was found that those studies that included normotensive subjects did not consistently demonstrate cardiovascular protection, whereas those which included only hypertensive subjects provided significant protection. The test for interaction based on the grouping variable of presence or absence of normotension was significant (p=0.037). The heterogeneity between studies that included normotensive subjects may be due to study designs: Cice et al study was conducted in hemodialysis patients with dilated cardiomyopathy whereas Zannad et al study was conducted in hemodialysis patients with left ventricular hypertrophy and excluded patients with symptomatic heart failure.
The overall benefit of antihypertensive therapy had a combined unadjusted risk ratio of 0.70 (95% CI 0.59 to 0.84) using an inverse-weighted fixed effects model and 0.59 (95% CI 0.38 to 0.91) using a random effects model. There was substantial heterogeneity between studies with respect to outcomes (I2 76.2%, p=0.001).
The heterogeneity in hazard ratios is not unexpected given the differing study designs and populations. The heterogeneous design of these studies is evident from examination of Table 1. For example Zannad et al11 required the presence of left ventricular hypertrophy, Cice et al10 required symptomatic dilated cardiomyopathy and Takahashi et al12 absence of cardiovascular disease for participation in their trials. Zannad et al11 had 159/397 (40%) of the patients who were normotensive. Cice et al10 also had substantial but uncertain number of normotensive patients. When studies were divided based on inclusion of normotensive subjects in the randomized group there was considerable heterogeneity noted between groups (p=0.006 for hazard ratio and p=0.029 for risk ratio of cardiovascular events but p>0.2 for all-cause mortality). Whereas the hypertensive only group had a pooled hazard ratio of 0.49 (95% CI 0.35 to 0.67), the “normotensive included” group had a pooled hazard ratio of 0.86 (95% CI 0.67 to 1.12). Similarly the hypertensive group had a pooled risk ratio of cardiovascular events of 0.55 (95% CI 0.42 to 0.73), the “normotensive-included” group had a pooled risk ratio of 0.84(0.66 to 1.06).
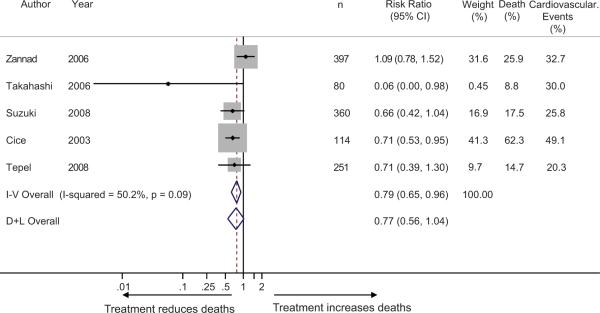
There was moderate heterogeneity in all-cause mortality between trials (I2 50.2%, p=0.09) but this was not explained (p>0.2 for group effect) by inclusion or exclusion of normotensive subjects (Figure 4). All cause mortality was reduced significantly when calculated by the fixed effects model (risk ratio 0.79 (95% CI 0.65 to 0.96) but not when estimated by the random effects model (risk ratio 0.77 (95% CI 0.56 to 1.04).
Figure 4.
Forest plot shows the risk ratios of antihypertensive therapy on all-cause mortality. The test for interaction based on the grouping variable of presence or absence of normotension was not significant (p>0.2). Risk ratio for all-cause mortality was moderately heterogeneous but showed protection with antihypertensive therapy with the fixed effects model only.
The Egger's publication bias plot showed bias with standardized effect size in hazard ratio of -4.04 (95% CI -8.20 to 0.11, p=0.05). The funnel plot indicates that studies which may have demonstrated increased hazards with low precision may not have been published (Figure 5).
Figure 5.
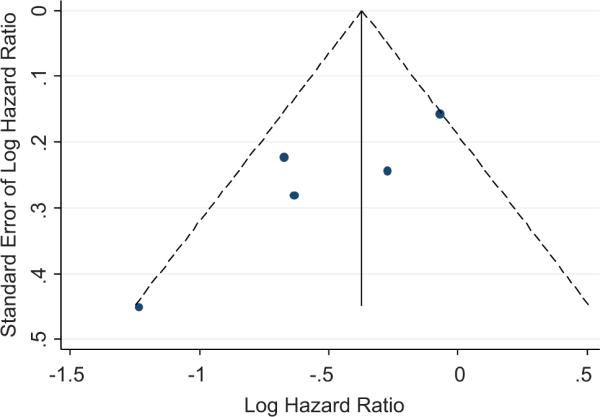
Funnel plot with pseudo 95% confidence intervals. Studies with low precision and high hazard ratios may have not have been published. The Egger's test showed evidence of publication bias.
Performing the meta-analysis after including the unpublished study15 did not materially alter the results. Sensitivity analysis to detect undue influence of any one study did not reveal the presence of any such evidence.
Discussion
In patients on hemodialysis cohort studies have nearly universally noted an increased risk of mortality with low or declining blood pressure thus calling into question the wisdom of lowering blood pressure in hemodialysis patients 4, 6-8. More recently Tentori et al reported that achieving the guideline recommended targets in hemodialysis patients was associated with increased mortality 21. However, some studies have suggested benefit of blood pressure lowering on longer-term follow up 22 which suggests that the instantaneous hazard of mortality may vary with time in this complex group of patients 23. However, most studies noted above did not distinguish between the benefits of deliberate lowering of blood pressure with antihypertensive drugs versus spontaneous lowering due to intercurrent illnesses 9, 24. Thus, the true benefit or risk of blood pressure lowering is uncertain in this group of patients.
Randomized controlled trials are the gold-standard to establish cause and effect relationships. However, when addressing the issue of hypertension in hemodialysis patients, these trials are small and often underpowered. Pooling these estimates may therefore yield insights that may offer evidence for controlling hypertension in this population with very high cardiovascular mortality. This meta-analysis pooled the results of 5 published to yield effect estimates that suggest benefit of blood pressure lowering. Repeating the meta-analysis after including the 1 unpublished trial15 did not materially change the results.
The major finding of this meta-analysis is that the overall benefit of antihypertensive therapy compared to control (or placebo) group reduced the combined hazard ratio for cardiovascular events by 31% using a fixed effects model and by 38% using a random effects model. There was substantial heterogeneity between studies with respect to outcomes (I2 60.8%, p=0.037). However, when studies were divided based on inclusion of normotensive subjects in the randomized group it explained most of between study variance. Heterogeneity between normotensive and hypertensive groups was highly statistically significant (p=0.006). Whereas the hypertensive group had a pooled hazard ratio of 0.49 (95% CI 0.35 to 0.67), the normotensive group had a pooled hazard ratio of 0.86 (95% CI 0.67 to 1.12). In fact, even all-cause mortality, an outcome most commonly measured in the observational studies, was not increased with treatment.
The two studies which included normotensive patients had quite different study design compared to those that included only hypertensive patients. The study of Cice et al included symptomatic patients with dilated cardiomyopathy on hemodialysis to address the question whether exposure to carvedilol would reduce echocardiographic left ventricular dimensions at one year; the cardiovascular event rate was a secondary end-point 10. The study of Zannad et al also included those patients who did not become hypotensive on receiving lisinopril between 5 to 20 mg during a run-in period prior to double-blind randomization 11. Thus, the question Zannad et al addressed was whether high risk hemodialysis patients with left ventricular hypertrophy would benefit from ACE inhibition. Takahasi et al at our request, provided data stratified by hypertension status. Our meta-analysis raises the question that patients with hypertension on hemodialysis may benefit from blood pressure lowering unlike what is suggested by observational studies.
Drugs blocking the renin angiotensin system may have benefits beyond blood pressure lowering. Similarly, beta-blockers may have cardioprotective effects besides their effects on blood pressure lowering. Whether the benefits of the antihypertensive drugs used in hemodialysis patients were due to their blood pressure lowering effects or due to non-hemodynamic actions is difficult to ascertain because blood pressure was not carefully assessed by ambulatory or home blood pressure monitoring in any of the studies reported. Similarly, the definition of normotensive and hypertensive categories were as reported by the authors and not by rigorous assessment of interdialytic blood pressures.
A limitation of this meta-analysis is the presence of publication bias. As can be seen from the funnel plot (and supported by the Eggers test), low precision studies with effect estimates that did not show benefit were notably missing. This limitation can be overcome by designing well powered and executed randomized trials. The trials discussed in this review did not specifically target a lower blood pressure. Although lowering of blood pressure was seen in many trials we do not know the level to which blood pressure should be lowered to in hemodialysis patients. None of these trials utilized out-of-dialysis unit blood pressure monitoring which may be better to evaluate the extent of blood pressure lowering 16, 25. Whether the outcome benefits observed in this meta-analysis was due to blood pressure lowering or some non-hemodynamic effects of these drugs is also unclear. This meta-analysis suggests that the presence or absence of hypertension should be considered in designing future randomized trials. Given the limited number of studies, one cannot be certain whether normotensive patients will not derive benefits of antihypertensive therapy should a large trial be performed.
Perspective
The results of this meta-analysis may have therapeutic implications since patients with hypertension and hemodialysis may not be treated based on current observational studies. Our meta-analysis suggests that these concerns may be misplaced. When therapy is based blood pressures before and after hemodialysis treatment, it is possible that patients may be sub-optimally untreated or treated too aggressively. A simple yet effective strategy and one supported by the American Heart Association is to monitor home blood pressures to assess blood pressure control 26. Home blood pressure monitoring can improve achievement of blood pressure targets 27, have been directly—not inversely—associated with hard outcomes in hemodialysis patients 28 and in a clinical trial in hemodialysis patients associated with improved blood pressure control 29. Unfortunately, none of the randomized antihypertensive trials discussed in this review have utilized ambulatory or home blood pressure guided antihypertensive therapy. Future trials that utilize out-of-dialysis unit blood pressure monitoring to direct antihypertensive therapy may better demonstrate the benefit of lowering blood pressure in this high-risk population. The assessment of left ventricular mass and function will further refine cardiovascular risk assessment and the management of hypertension. Till these trials are done, collective evidence from randomized trials suggests that hypertension should be treated among hypertensive patients on hemodialysis.
Acknowledgments
Source of Funding Supported by a grant from the NIH: 5RO1-DK062030-05
Footnotes
Conflict of Interest None
Reference List
- 1.Ezzati M, Lopez AD, Rodgers A, Vander HS, Murray CJ. Selected major risk factors and global and regional burden of disease. Lancet. 2002;360:1347–1360. doi: 10.1016/S0140-6736(02)11403-6. [DOI] [PubMed] [Google Scholar]
- 2.Dawber TR, Moore FE, Mann GV. Coronary heart disease in the Framingham study. Am J Public Health Nations Health. 1957;47:4–24. doi: 10.2105/ajph.47.4_pt_2.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360:1903–1913. doi: 10.1016/s0140-6736(02)11911-8. [DOI] [PubMed] [Google Scholar]
- 4.Zager PG, Nikolic J, Brown RH, Campbell MA, Hunt WC, Peterson D, Van Stone J, Levey A, Meyer KB, Klag MJ, Johnson HK, Clark E, Sadler JH, Teredesai P. “U” curve association of blood pressure and mortality in hemodialysis patients. Kidney Int. 1998;54:561–569. doi: 10.1046/j.1523-1755.1998.00005.x. [DOI] [PubMed] [Google Scholar]
- 5.Kalantar-Zadeh K, Kilpatrick RD, McAllister CJ, Greenland S, Kopple JD. Reverse epidemiology of hypertension and cardiovascular death in the hemodialysis population: the 58th annual fall conference and scientific sessions. Hypertension. 2005;45:811–817. doi: 10.1161/01.HYP.0000154895.18269.67. [DOI] [PubMed] [Google Scholar]
- 6.Port FK, Hulbert-Shearon TE, Wolfe RA, Bloembergen WE, Golper TA, Agodoa LY, Young EW. Predialysis blood pressure and mortality risk in a national sample of maintenance hemodialysis patients. Am J Kidney Dis. 1999;33:507–517. doi: 10.1016/s0272-6386(99)70188-5. [DOI] [PubMed] [Google Scholar]
- 7.Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, Johnson HK, Zager PG. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70:1858–1865. doi: 10.1038/sj.ki.5001868. [DOI] [PubMed] [Google Scholar]
- 8.Li Z, Lacson E, Jr, Lowrie EG, Ofsthun NJ, Kuhlmann MK, Lazarus JM, Levin NW. The epidemiology of systolic blood pressure and death risk in hemodialysis patients. Am J Kidney Dis. 2006;48:606–615. doi: 10.1053/j.ajkd.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Agarwal R. Hypertension and survival in chronic hemodialysis patients-Past lessons and future opportunities. Kidney Int. 2005;67:1–13. doi: 10.1111/j.1523-1755.2005.00050.x. [DOI] [PubMed] [Google Scholar]
- 10.Cice G, Ferrara L, D'Andrea A, D'Isa S, Di BA, Cittadini A, Russo PE, Golino P, Calabro R. Carvedilol increases two-year survivalin dialysis patients with dilated cardiomyopathy: a prospective, placebo-controlled trial. J Am Coll Cardiol. 2003;41:1438–1444. doi: 10.1016/s0735-1097(03)00241-9. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F, Kessler M, Lehert P, Grunfeld JP, Thuilliez C, Leizorovicz A, Lechat P. Prevention of cardiovascular events in end-stage renal disease: Results of a randomized trial of fosinopril and implications for future studies. Kidney Int. 2006;70:1318–1324. doi: 10.1038/sj.ki.5001657. [DOI] [PubMed] [Google Scholar]
- 12.Takahashi A, Takase H, Toriyama T, Sugiura T, Kurita Y, Ueda R, Dohi Y. Candesartan, an angiotensin II type-1 receptor blocker, reduces cardiovascular events in patients on chronic haemodialysis--a randomized study. Nephrol Dial Transplant. 2006;21:2507–2512. doi: 10.1093/ndt/gfl293. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki H, Kanno Y, Sugahara S, Ikeda N, Shoda J, Takenaka T, Inoue T, Araki R. Effect of angiotensin receptor blockers on cardiovascular events in patients undergoing hemodialysis: an open-label randomized controlled trial. Am J Kidney Dis. 2008;52:501–506. doi: 10.1053/j.ajkd.2008.04.031. [DOI] [PubMed] [Google Scholar]
- 14.Tepel M, Hopfenmueller W, Scholze A, Maier A, Zidek W. Effect of amlodipine on cardiovascular events in hypertensive haemodialysis patients. Nephrol Dial Transplant. 2008;23:3605–3612. doi: 10.1093/ndt/gfn304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakao N, Takeshi U, Atsushi T, Takada M, Kimura G. Effects of blood pressure lowering and antihypertensive drug class on cardiovascular events of hemodialysis patients: results from hemodialysis angiotensin-inhibition rescue trial. Nephrol Dial Transplant. 2008;18:711. [Google Scholar]
- 16.Agarwal R, Peixoto AJ, Santos SF, Zoccali C. Pre and post dialysis blood pressures are imprecise estimates of interdialytic ambulatory blood pressure. Clin J Am Soc Nephrol. 2006;1:389–398. doi: 10.2215/CJN.01891105. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.0 [updated February 2008] The Cochrane Collaboration; 2008. Available at: http://www.cochrane-handbook.org. Accessed December 10, 2008. [Google Scholar]
- 18.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Egger M, Smith GD. Bias in location and selection of studies. BMJ. 1998;316:61–66. doi: 10.1136/bmj.316.7124.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M, Davey SG, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fagard RH, Cornelissen VA. Incidence of cardiovascular events in white-coat, masked and sustained hypertension versus true normotension: a meta-analysis. J Hypertens. 2007;25:2193–2198. doi: 10.1097/HJH.0b013e3282ef6185. [DOI] [PubMed] [Google Scholar]
- 22.Mazzuchi N, Carbonell E, Fernandez-Cean J. Importance of blood pressure control in hemodialysis patient survival. Kidney Int. 2000;58:2147–2154. doi: 10.1111/j.1523-1755.2000.00388.x. [DOI] [PubMed] [Google Scholar]
- 23.Tentori F, Hunt WC, Rohrscheib M, Zhu M, Stidley CA, Servilla K, Miskulin D, Meyer KB, Bedrick EJ, Johnson HK, Zager PG. Which targets in clinical practice guidelines are associated with improved survival in a large dialysis organization? J Am Soc Nephrol. 2007;18:2377–2384. doi: 10.1681/ASN.2006111250. [DOI] [PubMed] [Google Scholar]
- 24.Agarwal R. Exploring the paradoxical relationship of hypertension with mortality in chronic hemodialysis. Hemodialysis Int. 2004;8:207–213. doi: 10.1111/j.1492-7535.2004.01097.x. [DOI] [PubMed] [Google Scholar]
- 25.Agarwal R, Andersen MJ, Light RP. Location Not Quantity of Blood Pressure Measurements Predicts Mortality in Hemodialysis Patients. Am J Nephrol. 2007;28:210–217. doi: 10.1159/000110090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pickering TG, Miller NH, Ogedegbe G, Krakoff LR, Artinian NT, Goff D. Call to action on use and reimbursement for home blood pressure monitoring: a joint scientific statement from the American Heart Association, American Society Of Hypertension, and Preventive Cardiovascular Nurses Association. Hypertension. 2008;52:10–29. doi: 10.1161/HYPERTENSIONAHA.107.189010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ. 2004;329:145. doi: 10.1136/bmj.38121.684410.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alborzi P, Patel N, Agarwal R. Home blood pressures are of greater prognostic value than hemodialysis unit recordings. Clin J Am Soc Nephrol. 2007;2:1228–1234. doi: 10.2215/CJN.02250507. [DOI] [PubMed] [Google Scholar]
- 29.Kauric-Klein Z, Artinian N. Improving blood pressure control in hypertensive hemodialysis patients. CANNT J. 2007;17:24–26. [PubMed] [Google Scholar]