Abstract
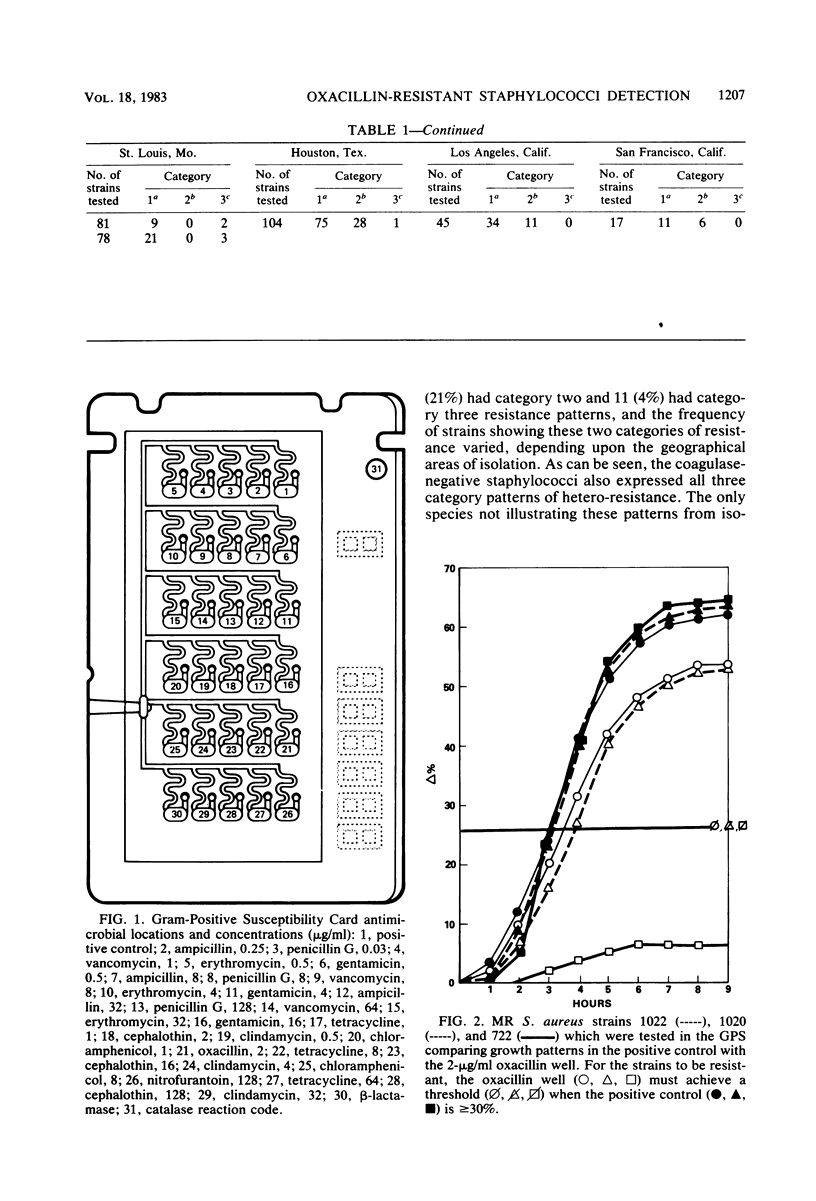
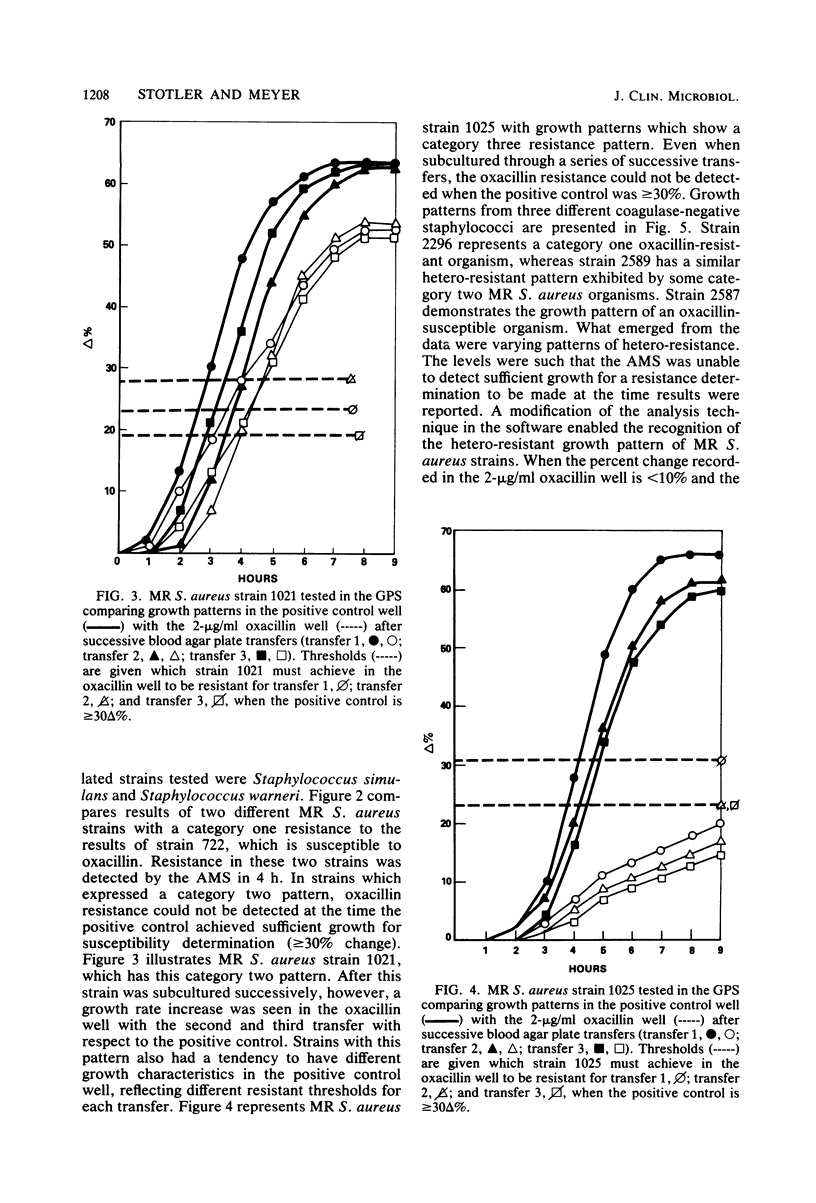
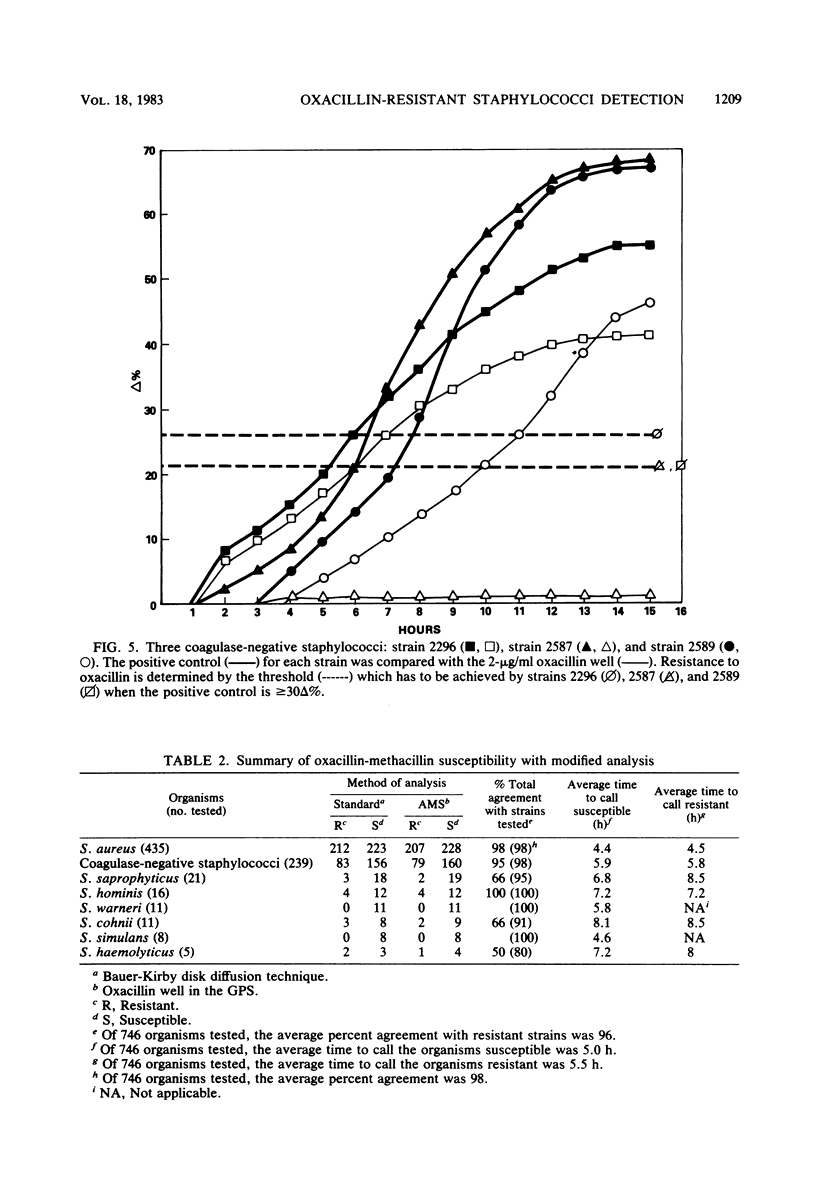
The AutoMicrobic system (Vitek Systems, Inc., Hazelwood, Mo.) is an automated instrument designed for rapid microbiological identification and susceptibility reporting in the clinical laboratory. The reliability of a rapid, automated approach to testing methicillin-resistant staphylococci was evaluated. To determine the accuracy in detecting oxacillin-methicillin resistance by the AutoMicrobic system, 746 staphylococci from seven different geographical areas were tested. Results were compared with the Bauer-Kirby agar disk diffusion technique as the reference method. Of the 304 staphylococci, 209 coagulase-positive and 95 coagulase-negative strains were resistant to oxacillin-methicillin. These organisms fell into three categories of resistance detection. The first category had resistance levels high enough for initial detection, the second category had low resistance levels requiring modified data analysis techniques for detection, and the third category had resistance levels too low for detection. Of the resistant strains tested, 21% showed a category two resistant growth pattern. Major errors, as a result of hetero-resistant growth patterns of the tested strains, were resolved by computer analysis of growth curves. These data analysis applications enabled detection of 96% of the oxacillin-methicillin-resistant organisms. Results for all resistant staphylococci tested were available in an average time of 5.5 h.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry A. L., Badal R. E. Reliability of the microdilution technic for detection of methicillin-resistant strains of staphylococcus aureus. Am J Clin Pathol. 1977 May;67(5):489–495. doi: 10.1093/ajcp/67.5.489. [DOI] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Boyce J. M., White R. L., Bonner M. C., Lockwood W. R. Reliability of the MS-2 system in detecting methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1982 Feb;15(2):220–225. doi: 10.1128/jcm.15.2.220-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canawati H. N., Sapico F. L., Montgomerie J. Z., Zucchero J. Temperature effect on cephalothin sensitivity of methicillin-resistant Staphylococcus aureus. Am J Clin Pathol. 1981 Mar;75(3):391–394. doi: 10.1093/ajcp/75.3.391. [DOI] [PubMed] [Google Scholar]
- Canawati H. N., Witte J. L., Sapico F. L. Temperature effect on the susceptibility of methicillin-resistant Staphylococcus aureus to four different cephalosporins. Antimicrob Agents Chemother. 1982 Jan;21(1):173–175. doi: 10.1128/aac.21.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J. R., Conley F. E., Cahall D. L. Methicillin-resistant Staphylococcus aureus susceptibility testing with Abbott MS-2 system. Antimicrob Agents Chemother. 1982 Apr;21(4):676–677. doi: 10.1128/aac.21.4.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary T. J., Maurer D. Methicillin-resistant Staphylococcus aureus susceptibility testing by an automated system, Autobac I. Antimicrob Agents Chemother. 1978 May;13(5):837–841. doi: 10.1128/aac.13.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew W. L., Barry A. L., O'Toole R., Sherris J. C. Reliability of the Kirby-Bauer disc diffusion method for detecting methicillin-resistant strains of Staphylococcus aureus. Appl Microbiol. 1972 Aug;24(2):240–247. doi: 10.1128/am.24.2.240-247.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. C., Rosenblum E. D. Genetic behavior of the methicillin resistance determinant in Staphylococcus aureus. J Bacteriol. 1980 Dec;144(3):1200–1202. doi: 10.1128/jb.144.3.1200-1202.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Caruthers J. Q., Baker C. N. Effect of temperature on the in vitro susceptibility of Staphylococcus aureus to penicillinase-resistant penicillins. Antimicrob Agents Chemother. 1973 Sep;4(3):263–269. doi: 10.1128/aac.4.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornsberry C., Gavan T. L., Sherris J. C., Balows A., Matsen J. M., Sabath L. D., Schoenknecht F., Thrupp L. D., Washington J. A., 2nd Laboratory evaluation of a rapid, automatic susceptibility testing system: report of a collaborative study. Antimicrob Agents Chemother. 1975 Apr;7(4):466–480. doi: 10.1128/aac.7.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Nadakavukaren M. J. Methicillin-resistant septal peptidoglycan synthesis in a methicillin-resistant Staphylococcus aureus strain. Antimicrob Agents Chemother. 1983 Apr;23(4):610–613. doi: 10.1128/aac.23.4.610. [DOI] [PMC free article] [PubMed] [Google Scholar]


