Abstract
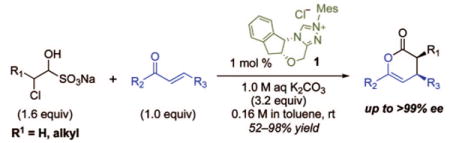
α-Chloroaldehyde bisulfite adducts were successfully employed in chiral NHC-catalyzed hetero-Diels–Alder reactions with various oxodienes under biphasic reaction conditions with high levels of enantioselectivity. This new protocol makes possible enantioselective additions from commercially available chloroacetaldehyde sodium bisulfite and demonstrates that this unique class of catalysts readily tolerates aqueous conditions.
Enantioselective hetero-Diels–Alder reactions provide a versatile and convergent approach to the construction of chiral heterocycles.1 In recent years, significant progress has been achieved in the development of efficient chiral metal and organic catalysts for enantioselective hetero-Diels–Alder reactions.2,3 Their great synthetic utility continues to drive further interest in improved protocols and expanded substrate scope. Of particular need are reactions in which the starting materials are readily accessible, and low catalyst loadings can be employed while still maintaining high levels of enantioselectivity.
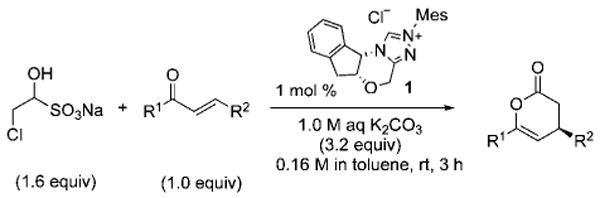
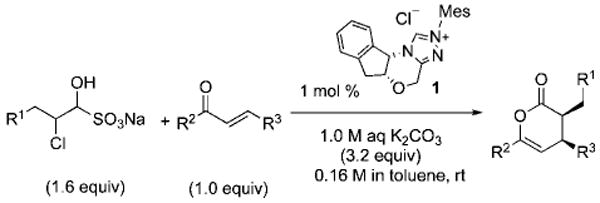
We have recently reported the first chiral N-heterocyclic carbene (NHC) catalyzed hetero-Diels–Alder reaction, via the catalytic generation of chiral enolates that serve as dienophiles.4,5 We have also extended the dienophile precursors from electron-deficient enals to α-chloroaldehydes,6 which can be readily prepared from the corresponding aldehydes.7 However, α-chloroaldehydes are sensitive to moisture and oxygen,8a and their preparation and storage require precautions. Furthermore, α-chloroacetaldehyde, which would provide entry into challenging but synthetically important enantioselective acetate additions,9 is difficult and unsafe to obtain in pure form.8b In this communication, we document efficient solutions to both of these challenges through the use of α-chloroaldehyde bisulfite salts under biphasic conditions (eq 1). This new protocol both extends the scope and operational simplicity of NHC-catalyzed enantioselective hetero-Diels–Alder reactions and demonstrates that this unique class of catalysts readily tolerates aqueous conditions.10
 |
(1) |
At the outset of our studies, we investigated a number of α-chloroaldehyde surrogates, including hemiacetals, bromopyruvic acid, and the α-chloroaldehyde bisulfite adducts. Among these starting materials, the α-chloroaldehyde bisulfite adducts were particularly attractive as they were bench-stable solids that could be readily prepared by the addition of aqueous sodium bisulfite to a solution of the appropriate aldehydes.11b Their use as substrates, however, would necessitate the use of aqueous conditions to decompose the bisulfite adducts to the corresponding aldehydes in the presence of the NHC catalyst.11,12
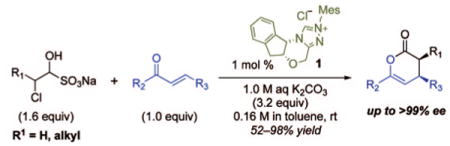
To test the feasibility of our approach, we selected inexpensive, commercially available chloroacetaldehyde sodium bisulfite 2 as a precursor of chloroacetaldehyde for the NHC-promoted reactions with two representative oxodienes: 4-oxoenoate 3a and β,γ-unsaturated α-ketoester 4a (Scheme 1). We screened a number of reaction conditions before selecting biphasic conditions employing 1.0 M K2CO3 as the inorganic base and EtOAc as the organic solvent for further development. These studies established the feasibility of using the bisulfite adducts as starting materials but confirmed our fears of epimerization. The use of 3a as the oxodiene provided product 5a only in 31% ee, while the use of 4a afforded adduct 6a in >99% ee.4b
Scheme 1.
NHC-Catalyzed Hetero-Diels–Alder Reactions with Chloroaldehyde Sodium Bisulfite 2
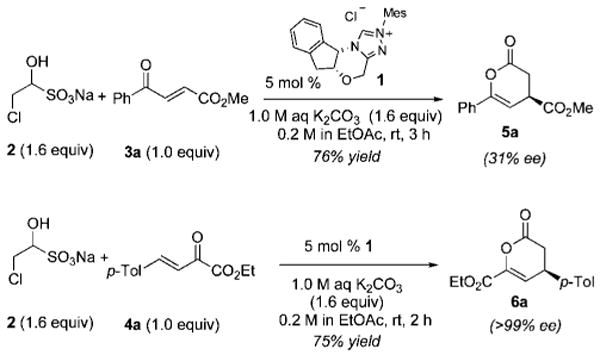
We reasoned from these results that the low enantioselectivity observed for 5a was due to epimerization of the annulation product. Notably, this product contains a readily epimerizable stereocenter by virtue of the β,γ-unsaturated ester and lacks adjacent substitution, which had been present in all of the examples from our prior work. To improve the enantiomeric excess of the product 5a, we investigated the effect of reaction conditions including inorganic bases and organic solvents (Table 1). Although the use of either a weaker base (NaHCO3, entries 1 and 2) or a nonpolar solvent (entry 3) suppressed epimerization, the combination of NaHCO3 and toluene afforded the product only in 75% ee. In contrast, those of 1.0 M K2CO3 and toluene minimized the epimerization (entries 4 and 5) at the expense of diminished yields. Some of the product loss was traced to hydrolysis, and improved yields could be obtained simply by employing the more stable ethyl ester (entries 6 and 7). Notably, these conditions also allowed us to lower the catalyst loading to 1 mol % of chiral triazolium precatalyst1.
Table 1.
Effect of Inorganic Bases and Organic Solvents on Epimerizationa
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | X | R | aqueous base | organic solvent | time/h | yield/%b | ee/%c |
| 1 | 5 | Me | 1.6 equiv of 1.0 M K2CO3 | 0.2 M EtOAc | 2.5 | 76 | 31 |
| 2 | 5 | Me | 1.6 equiv of 1.0 M NaHCO3 | 0.2 M EtOAc | 23.0 | 78 | 65 |
| 3 | 5 | Me | 1.6 equiv of 1.5 M NaHCO3 | 0.2 M toluene | 23.0 | 48 | 75 |
| 4 | 5 | Me | 1.6 equiv of 1.0 M K2CO3 | 0.2 M toluene | 2.5 | 40 | 99 |
| 5 | 5 | Me | 1.6 equiv of 1.0 M K2CO3 | 0.2 M toluene | 4.0 | 55 | 93 |
| 6 | 5 | Me | 1.6 equiv of 1.0 M K2CO3 | 0.2 M toluene | 6.0 | 62 | 86 |
| 7 | 1 | Et | 3.2 equiv of 1.0 M K2CO3 | 0.16 M toluene | 6.0 | 84 | 90 |
All reactions were performed with 1.6 equiv of 2 and 1.0 equiv of oxodiene.
Isolated yield after chromatography.
Determined by HPLC analysis.
The scope of these conditions for NHC-catalyzed Diels–Alder reactions with chloroacetaldehyde bisulfite salt 2 with various oxodiene substrates is shown in Table 2. This survey demonstrated that this biphasic process accommodated various substrates 3b–e (entries 1–4) including aromatic and aliphatic substitution. Except for entry 2, all of the substrates screened afforded the desired 4,6-disubstituted dihydropyran-2-ones with good enantioselectivity, albeit slightly diminished by epimerization. As expected, the β,γ-unsaturated α-ketoesters 4a and 4b furnished the corresponding products in good yields and with excellent enantioselectivity. The absolute configuration of the products was determined by single-crystal X-ray analysis of the product (R)-ethyl 6-(4-bromophenyl)-2-oxo-3,4-dihydro-2H-pyran-4-carboxylate (Table 2, entry 2).13 Interestingly, the sterically hindered 4-oxoenolate 7, which did not react with any α-chloroaldehydes in our previous investigations, afforded bicyclic product 8 with excellent enantioselectivity when catalyzed by 5 mol % of 1.
Table 2.
NHC-Catalyzed Biphasic Diels–Alder Reactions of 2 with Different Oxodienesa
 | ||||||
|---|---|---|---|---|---|---|
| entry | R1= | R2= | t / h | product | yield / %b | ee / %c |
| 1 | Ph | C02Et 3b | 6.0 | 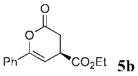 |
84 | 90 |
| 2 | p-Br-C6H4 | C02Et 3c | 2.5 | 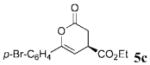 |
71 | 73 |
| 3 | p-OMe-C6H4 | C02Et 3d | 5.0 | 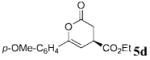 |
90 | 99 |
| 4 | c-Hex | C02Et 3e | 24.0 | 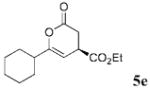 |
52 | 91 |
| 5 | C02Et | p-Tol 4a | 6.0 | 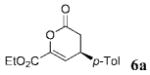 |
74 | 99 |
| 6 | C02Et | n-Pr 4b | 4.0 | 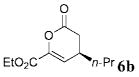 |
80 | >99 |
| 7[d] | 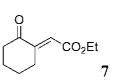 |
12.0 | 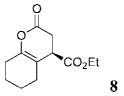 |
40 | >99 | |
All reactions were performed at 0.16 M in toluene with 1.6 equiv of chloroacetaldehyde sodium bisulfite 2, 1.0 equiv of oxodiene, 1 mol % of 1, and 3.2 equiv of 1.0 M aq K2CO3.
Isolated yield after chromatography.
Determined by HPLC or SFC analysis.
5 mol % of 1 was employed.
In addition to allowing direct use of the commercially available chloroacetaldehyde bisulfite adduct, we also sought to utilize these conditions to simplify the preparation, handling, and reactions of substituted α-chloroaldehydes. After some experimentation, we identified reliable conditions for the conversion of common α-chloroaldehydes to the bench-stable, solid bisulfite adducts (see Supporting Information).11b We were pleased to find that the reaction conditions optimized for the chloroacetaldehyde bisulfite salt were directly applicable to these substrates. Biphasic reactions with either substituted 4-oxoenolates (entries 1–7) or β,γ-unsaturated α-ketoesters (entry 8 and 9) provided the Diels–Alder products in good chemical yields and excellent enantioselectivity.14
In all cases, only 1 mol % of chiral triazolium precatalyst 1 was employed. The absolute configuration was determined by X-ray analysis of an enantiomerically pure sample of (3S,4S)-ethyl 3-benzyl-6-(4-bromophenyl)-2-oxo-3,4-dihydro-2H-pyran-4-carboxylate (Table 3, entry 2, see Supporting Information).15
Table 3.
NHC-Catalyzed Biphasic Diels–Alder Reactions of Chloroaldehyde Bisulfite Saltsa
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | R1= | R2= | R3= | t / h | product | yield / %b | ee / %c |
| 1 | Ph 9 | Ph | C02Et 3b | 6.0 | 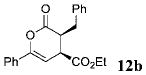 |
98 | 99 |
| 2 | Ph 9 | p-Br-C6H4 | C02Et 3c | 2.0 | 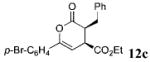 |
84 | >99 |
| 3 | Ph 9 | p-OMe-C6H4 | C02Et 3d | 15.0 | 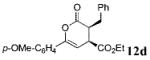 |
55 | >99 |
| 4 | Ph 9 | c-Hex | C02Et 3e | 10.0 | 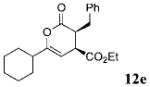 |
70 | 91 |
| 5 | Ph 9 | Me | C02Et 3f | 6.0 | 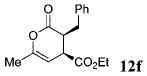 |
65 | 99 |
| 6 | c-Hex 10 | Ph | C02Et 3b | 15.5 |
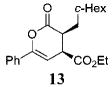
|
59 | >99 |
| 7 | n-C9H1911 | Ph | C02Et 3b | 4.5 |
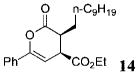
|
74 | >99 |
| 8 | Ph 9 | C02Et | p-Tol 4a | 5.5 |
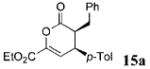
|
73 | >99 |
| 9 | Ph 9 | C02Et | n-Pr 4b | 5.0 |
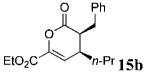
|
78 | >99 |
All reactions were performed at 0.16 M in toluene with 1.6 equiv of chloroaldehyde bisulfite adduct, 1.0 equiv of oxodiene, 1 mol % of 1, and 3.2 equiv of 1.0 M aq K2CO3. In all cases, only one diastereomer was detected in unpurified reaction mixtures.
Isolated yield after chromatography.
Determined by HPLC or SFC analysis.
In summary, we have developed a catalytic enantioselective method for hetero-Diels–Alder reactions that employs easily available, bench-stable α-chloroaldehyde bisulfite salts as starting materials. This enantioselective, biphasic hetero-Diels–Alder reaction represents the first enantioselective NHC-catalyzed reaction that is demonstrably water-tolerant. It provides an innovative solution to enantioselective additions of acetate equivalents using the inexpensive, commercially available bisulfite adduct of chloroacetaldehyde, thereby interfacing with ongoing efforts to employ commodity chemicals in lieu of preactivated or protected reactants.16 This approach also extends the scope of enantioselective NHC-catalysis and provides a more convenient and operationally simple protocol for NHC-catalyzed Diels–Alder reactions.
Supplementary Material
Acknowledgments
Partial support for this work was provided by the National Science Foundation (CHE-0449587). Support of this research program by Amgen, AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly, Roche, and the NIH (GM-079339) is gratefully acknowledged. J.W.B. is a fellow of the Packard Foundation, the Beckman Foundation, the Sloan Foundation, and a Cottrell Scholar. We thank Justin Struble (University of Pennsylvania) for catalyst preparation.
Footnotes
Supporting Information Available: Full experimental procedures and characterization data and X-ray crystallographic data for 5c. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.For recent reviews, see: Boger DL, Weinreb SM. Hetero Diels–Alder Methodology in Organic Synthesis. Chapter 2 Academic Press; San Diego: 1987. Evans DA, Johnson JS. In: Comprehensive Asymmetric Catalysis. Jacobsen EN, Pfaltz A, Yamamoto H, editors. Vol. 3. Springer; New York: 1994. p. 1177.Corey EJ. Angew Chem, Int Ed. 2002;41:1650–1667. doi: 10.1002/1521-3773(20020517)41:10<1650::aid-anie1650>3.0.co;2-b.Nicolaou KC, Snyder SA, Montagnon T, Vassilikogiannakis G. Angew Chem, Int Ed. 2002;41:1668–1698. doi: 10.1002/1521-3773(20020517)41:10<1668::aid-anie1668>3.0.co;2-z.Jørgensen KA. Eur J Org Chem. 2004:2093–2102.
- 2.For selected references of metal-catalyzed, enantioselective hetero-Diels–Alder reactions, see: Evans DA, Miller SJ, Lectka T. J Am Chem Soc. 1993;115:6460–6461.Evans DA, Olhava EJ, Johnson JS, Janey JM. Angew Chem, Int Ed. 1998;37:3372–3375. doi: 10.1002/(SICI)1521-3773(19981231)37:24<3372::AID-ANIE3372>3.0.CO;2-K.Jarvo ER, Lawrence BM, Jacobsen EN. Angew Chem, Int Ed. 2005;44:6043–6046. doi: 10.1002/anie.200502176.Abraham CJ, Paull DH, Scerba MT, Grebinski JW, Lectka T. J Am Chem Soc. 2006;128:13370–13371. doi: 10.1021/ja065754d.Kawasaki M, Yamamoto H. J Am Chem Soc. 2006;128:16482–16483. doi: 10.1021/ja066726y.Esquivias J, Arrayas RG, Carretero JC. J Am Chem Soc. 2007;129:1480–1481. doi: 10.1021/ja0658766.Yu ZP, Liu XH, Dong ZH, Xie MS, Feng XM. Angew Chem, Int Ed. 2008;47:1308–1311. doi: 10.1002/anie.200704759.
- 3.For selected references of enantioselective hetero-Diels–Alder reactions catalyzed by organic catalysts, see: Itoh J, Fuchibe K, Akiyama T. Angew Chem, Int Ed. 2006;45:4796–4798. doi: 10.1002/anie.200601345.Akiyama T, Morita H, Fuchibe K. J Am Chem Soc. 2006;128:13070–13071. doi: 10.1021/ja064676r.Wang Y, Li H, Wang YQ, Liu Y, Foxman BM, Deng L. J Am Chem Soc. 2007;129:6364–6365. doi: 10.1021/ja070859h.Singh RP, Bartelson K, Wang Y, Su H, Lu XJ, Deng L. J Am Chem Soc. 2008;130:2422–2423. doi: 10.1021/ja078251w.
- 4.(a) He M, Struble JR, Bode JW. J Am Chem Soc. 2006;128:8418–8420. doi: 10.1021/ja062707c. [DOI] [PubMed] [Google Scholar]; (b) He M, Uc GJ, Bode JW. J Am Chem Soc. 2006;128:15088–15089. doi: 10.1021/ja066380r. [DOI] [PubMed] [Google Scholar]; For an intramolecular example of this reaction, see: Phillips EM, Wadamoto M, Chan A, Scheidt KA. Angew Chem, Int Ed. 2007;46:3107–3110. doi: 10.1002/anie.200605235.
- 5.For other recent work on enantioselective NHC-catalysis, see: Chiang PC, Kaeobamrung J, Bode JW. J Am Chem Soc. 2007;129:3520–3521. doi: 10.1021/ja0705543.He M, Bode JW. J Am Chem Soc. 2008;130:418–419. doi: 10.1021/ja0778592.Phillips EM, Reynolds TE, Scheidt KA. J Am Chem Soc. 2008;130:2416–2417. doi: 10.1021/ja710521m.Zhang YR, He L, Wu X, Shao PL, Ye S. Org Lett. 2008;10:277–280. doi: 10.1021/ol702759b.
- 6.For the use of α-functionalized aldehydes in NHC-catalysis, see: Chow KYK, Bode JW. J Am Chem Soc. 2004;126:8126–8127. doi: 10.1021/ja047407e.Reynolds NT, Read de Alaniz J, Rovis T. J Am Chem Soc. 2004;126:9518–9519. doi: 10.1021/ja046991o.Reynolds NT, Rovis T. J Am Chem Soc. 2005;127:16406–16407. doi: 10.1021/ja055918a.
- 7.Halland N, Braunton A, Bachmann S, Marigo M, Jørgensen KA. J Am Chem Soc. 2004;126:4790–4791. doi: 10.1021/ja049231m. [DOI] [PubMed] [Google Scholar]
- 8.(a) De Kimpe N, Verhé R. The Chemistry of α-Haloketones, α-Haloaldehydes, and α-Haloimines. Chapter 3 John Wiley & Sons; New York: 1988. [Google Scholar]; (b) House HO, Jones VK, Frank GA. J Org Chem. 1964;29:3327–3333. [Google Scholar]; (c) Kraus GA, Gottschalk P. J Org Chem. 1983;48:2111–2112. [Google Scholar]
- 9.(a) Hayashi Y, Itoh T, Aratake S, Ishikawa H. Angew Chem, Int Ed. 2008;47:2082–2084. doi: 10.1002/anie.200704870. [DOI] [PubMed] [Google Scholar]; (b) Yang JW, Chandler C, Stadler M, Kampen DB. Nature. 2008;452:453–455. doi: 10.1038/nature06740. [DOI] [PubMed] [Google Scholar]; (c) Alcaide B, Almendros P. Angew Chem, Int Ed. 2008;47:4632–4634. doi: 10.1002/anie.200801231. [DOI] [PubMed] [Google Scholar]; (d) García-García P, Ladépêche A, Halder RB List. Angew Chem, Int Ed. 2008;47:4719–4721. doi: 10.1002/anie.200800847. [DOI] [PubMed] [Google Scholar]; (e) Hayashi Y, Itoh T, Ohkubo M, Ishikawa H. Angew Chem, Int Ed. 2008;47:4722–4724. doi: 10.1002/anie.200801130. [DOI] [PubMed] [Google Scholar]
- 10.For a recent survey of organic reactions under aqueous conditions: Li CJ. Chem Rev. 2005;105:3095–3165. doi: 10.1021/cr030009u.
- 11.For recent references of use of aldehyde bisulfite adducts, see: Wuts PGM, Bergh CL. Tetrahedron Lett. 1986;27:3995–3998.Seki M, Hatsuda M, Yoshida S. Tetrahedron Lett. 2004;45:6579–6581.Seki M, Hatsuda M, Mori Y, Yoshida S, Yamada S, Shimizu T. Chem–Eur J. 2004;10:6102–6110. doi: 10.1002/chem.200400733.
- 12.We have previously shown that achiral, imidazolium-derived NHC catalysts can be employed for a different class of annulation reactions in the presence of protic solvents: Sohn SS, Rosen EL, Bode JW. J Am Chem Soc. 2004;126:14370–14371. doi: 10.1021/ja044714b.He M, Bode JW. Org Lett. 2005;7:3131–3134. doi: 10.1021/ol051234w.
- 13.A sample with 93% ee was prepared for the X-ray analysis, although in low yield. CCDC 689207 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- 14.These conditions eliminated epimerization and gave diastereoselectivities superior to the conditions in ref 4b.
- 15.CCDC 689208 contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif
- 16.(a) Lyothier I, Defieber C, Carreira EM. Angew Chem, Int Ed. 2006;45:6204–6207. doi: 10.1002/anie.200602408. [DOI] [PubMed] [Google Scholar]; (b) Defieber C, Ariger MA, Moriel P, Carreira EM. Angew Chem, Int Ed. 2007;46:3139–3143. doi: 10.1002/anie.200700159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


