Abstract
Individuals with familial Parkinson’s disease (PD) due to a monogenic defect can show considerable clinical and neuropathological variability. To identify factors underlying this variability, histopathological analysis was performed in two clinically different A53T α-synuclein heterozygotes from Family H, a multigenerational α-synuclein A53T kindred. To determine whether additional genetic factors could contribute to phenotypic variability, Family H and another multigenerational A53T kindred were analyzed for parkin polymorphisms. We identified a previously described variant in parkin exon 4 associated with increased PD risk (S167N). The two A53T heterozygotes had markedly different neuropathology and different parkin genotypes: A N167 homozygote had early onset rapidly progressive disease, early dementia, myoclonus and sleep disorder, while a S167 homozygote had late onset, slowly progressive disease and late dementia. Both had brainstem, cortical, and intraneuritic Lewy bodies (LB). The N167 individual had widespread cortical neurofibrillary degeneration, while the S167 individual had only medial temporal lobe neurofibrillary degeneration. The N167 individual had severe neuronal loss in CA2 associated with Lewy neurites (LN), while the S167 individual had severe neuronal loss in CA1 associated with TDP-43 immunoreactive neuronal inclusions. These findings implicate TDP-43 in the pathology of familial PD and suggest that parkin may act as a modifier of the A53T α-synuclein phenotype of familial PD. Furthermore, they suggest a mechanism by which a rare genetic variant that is associated with a minor increase of PD risk in the heterozygous state may, in the homozygous state, exacerbate a disease phenotype associated with a highly penetrant dominant allele.
Introduction
The molecular analysis of familial PD has identified point mutations and gene-copy-number abnormalities in multiple genes, including SNCA, PRKN, UCHL-1, DJ-1, PINK1, LRRK2, and MAPT (reviewed in ref. 35, 45). As might be expected, different mutations in a single gene exhibit clinical and neuropathological variability. For example, individuals with A53T [12, 16, 24, 25, 29, 39], E46K [46] or copy-number [13–14, 32] mutations at SNCA have different phenotypes. However, it is striking that a single mutation also shows variability in the age of disease onset, rate of disease progression and duration, and neuropathology. Here we identify a potential contribution to this variability.
We consider the SNCA A53T mutation, which shows variability both within and between kindreds. Neuropathological findings previously reported to be associated with the A53T α-synuclein mutation include neuronal loss and LBs in the substantia nigra (SN) and locus ceruleus (LC), also seen in sporadic PD, prominent α-synuclein-immunoreactive axonal spheroids, and α-synuclein and tau pathology in the neocortex, hippocampus, striatum, nucleus basalis of Meynert (nbM), midbrain and pons. Tau pathology had a α-synuclein-like distribution in the Contursi kindred, but was much less prominent and consisted of neuropil threads, spheroids and sparse neurofibrillary tangles (NFTs) [12, 25]. In an Australian A53T individual [39] (α-synuclein-, but not tauimmunostaining was assessed), NFTs and senile plaques (SP) were absent, but microvacuolation of the medial temporal cortex was prominent, a common finding in some sporadic cases of diffuse Lewy body disease [19].
Given the reported variability in neuropathology among individuals with α-synuclein mutations, we assessed the neuropathology in two clinically different members of Family H, a large Greek-American A53T kindred. Furthermore, since PRKN is implicated in PD pathogenesis and Parkin interacts biochemically with α-synuclein [5], we hypothesized that PRKN alleles may modify the A53T SNCA phenotype. We therefore assessed whether different PRKN genotypes are associated with phenotypic and neuropathological variability in Family H [28–29] and an unrelated Greek A53T kindred.
Materials and Methods
Samples were collected after informed consent was obtained under an approved Institutional Review Board protocol.
Molecular analyses
DNAs from Family H and HEL-1 members were screened for PRKN polymorphisms using single strand conformation polymorphism (SSCP) analysis. Polymorphic samples were sequenced in both directions using previously reported primers [4] and an ABI PRISM 377 sequencer.
Neuropathological methods
At autopsy brains were divided along the mid-sagittal plane, the right half frozen at −80°C and the left half fixed in 10% buffered formaldehyde. The brainstem was removed with a transverse cut rostral to the midbrain. The supratentorial tissue was cut in a coronal plane and the brainstem and cerebellum were cut in a traverse plane perpendicular to the long axis of the brainstem. Sections were obtained from the superior and middle frontal gyri, cingulate gyrus, inferior parietal and occipital cortex, superior and middle temporal gyri, basal ganglia and amygdala, hippocampus, thalamus and subthalamic nucleus, midbrain, pons, medulla, cerebellar vermis and dentate nucleus.
Paraffin-embedded sections were stained with hematoxylin and eosin (H&E), Bielschowsky silver stain, Luxol fast blue (LFB) and thioflavin-S. Sections were processed for immunohistochemistry with antibodies to α-synuclein (rabbit polyclonal; NACP [17] 1:3000), tau (mouse monoclonal IgG1; CP13; Peter Davies, Albert Einstein College of Medicine), glial fibrillary acidic protein (mouse monoclonal IgG1; GFAP; Biogenex), HLA-DR (mouse monoclonal IgG2b; LN3; ICN), pan-Aβ (mouse monoclonal IgG1; 33.1.1: Todd Golde, Mayo Clinic, Jacksonville) and TDP-43 (rabbit polyclonal; 1: 3000, ProteinTech, Chicago, IL).
Glass-mounted 5 µm thick tissue sections were immunostained using a DAKO-Autostainer (DAKO-Cytomaton, Carpinteria, CA). Deparaffinized and rehydrated sections were steamed in distilled water for 30 minutes for antigen retrieval. Subsequently, slides were placed in Tris-buffered saline-Tween (0.05 mol/L) and blocked for endogenous peroxidase activity with 0.03% hydrogen peroxide for 5 minutes. For α-synuclein immunostaining, sections were pretreated with 80% formic acid for 10 minutes. Slides were treated with 5% normal goat serum for 20 minutes, and incubated with polyclonal or monoclonal antibodies for 45 minutes. After washing the slides were treated for 2 hours with peroxidase-labeled polymer Envision-anti-rabbit (DAKO, Santa Barbara, CA) and signal detected with 3, 3-diaminobenzidine.
Results
Molecular genetic analysis for PRKN polymorphisms
We analyzed all PRKN exons and adjacent intron/exon boundaries in members of two A53T kindreds [Family H, n=32 (Fig. 1a) and HEL-1, n=10 (Fig. 1b)] and identified a previously described polymorphism in PRKN exon 4: a G601A transition that leads to a S167N missense mutation in Parkin. Sequencing of PRKN exon 4 in Family H (n=32) and HEL-1 (n=7) revealed that the S167N polymorphism was present only in one nuclear family of Family H: all other individuals were S167 (G/G at the nucleotide level). Figure 2 shows the transmission of PRKN alleles within this nuclear family. Individual V-16 is A/A and had a less severely affected G/A parent, IV-10. An A53T-positive, G/A half-sibling, V-18, was unaffected at the time, when he was ten years younger than the average age of disease onset for members of his generation.
Fig. 1.
Family H and HEL-1 pedigrees. Filled squares/circles represent affected individuals with the α-synuclein A53T mutation. (a) Family H pedigree. The arrows point to IV-5, who is a α-synuclein A53T heterozygote and has S167 (wild-type) Parkin and to individual V-16, who is a α-synuclein A53T heterozygote and has N167 Parkin. (b) The HEL-1 pedigree.
Fig. 2.
Molecular analysis of PRKN alleles in Family H. The sequence traces of parkin exon 4 and the alleles present at parkin and α-synuclein loci in three members of in a nuclear family from Family H. Symbols are as in Figure 1. The filled lower quadrant in V-18 indicates α-synuclein A53T heterozygosity. The males all are heterozygous for the G209A (A53T) mutation in exon 4 of α-synuclein. Individual IV-10 is a heterozygote at PRKN (G/A, S167N), and developed symptoms at age 56. Individual V-16 is a PRKN homozygote (A/A, N167) and developed symptoms at age 29. Individual V-18 is a PRKN heterozygote (G/A, S167N) and remained asymptomatic at age 25.
As the G601A polymorphism introduces an AlwI restriction site, restriction fragment length polymorphism analysis was used to determine the frequency of the G/G, G/A and A/A genotypes in ethnically matched controls (n=62; 124 chromosomes). One was G/A while all others were G/G, suggesting that chromosomes with the A allele are uncommon in the Greek population and that the A/A genotype is rare. The allele and genotype frequencies of the S176 PRKN polymorphism in the two kindreds and in Greek population controls are shown in Table 1. The A/A genotype is present only in one individual, an individual within Family H. The A allele is present in only two members of Family H and in one individual from the control group. The frequency of the A/A genotype in control individuals is entirely consistent with findings from a meta-analysis described in the PDGene database (www.pdgene.org). This analysis indicated that the A/A genotype is very rare in the Caucasian population. In the studies included in the database involving 1078 Caucasian PD patients and 947 normal controls, the frequency of the A allele was 0.02. More notably, the frequency of the A/A genotype was 0.0. This is in contrast to the Asian population where the frequencies of the A allele and A/A genotype in PD cases (n=439) are 0.43 and 0.182, respectively, and in normal controls (n=498) are 0.38 and 0.164, respectively.
Table 1.
Allele and genotype frequencies of the G601A PRKN polymorphism.
| n | Allele G | Allele A | G/G | G/A | A/A | |
|---|---|---|---|---|---|---|
| Family H | 32 | 58 (91%) | 6 (9%) | 27 (84%) | 4 (12%) | 1 (3%) |
| HEL-1 | 7 | 14 (100%) | 0 (0%) | 14 (100%) | 0 (0%) | 0 (0%) |
| Controls | 62 | 61 (98%) | 1 (2%) | 61 (98%) | 1 (2%) | 0 (0) |
The clinical characteristics and mutational status are shown in Table 2.
Table 2.
Clinical Phenotype and Mutation Status of Affected Family H Individuals
| Family H Pedigree Number |
Age of Disease Onset |
Clinical Phenotype |
PRKN at 601 |
SNCA at 209 |
|---|---|---|---|---|
| IV-23 | 49 | Tremor, rigidity, bradykinesia, postural instability, late onset dementia |
G/G | G/A |
| IV-21 | 47 | Rigidity, bradykinesia, postural instability | G/G | G/A |
| IV-10 | 53 | Rigidity, bradykinesia, postural instability, late onset dementia |
G/A | G/A |
| IV-3 | 57 | Rigidity, bradykinesia, postural instability | G/G | G/A |
| IV-5* | 59 | Tremor, rigidity, bradykinesia, postural instability, orthostatic hypotension, late onset dementia; slowly progressive (12- year disease course) |
G/G | G/A |
| IV-7 | 56 | Rigidity, bradykinesia, postural instability | G/G | G/A |
| V-16* | 31 | Rigidity, bradykinesia, postural instability, early onset dementia, myoclonus, sleep disturbance; rapidly progressive (7-year disease course) |
A/A | G/A |
| V-39 | 37 | Rigidity, bradykinesia, postural instability | G/G | G/A |
Post mortem neuropathology
Clinical phenotypes of V-16 and IV-5
CLINICAL FEATURES OF V-16
Individual V-16 had the earliest disease onset in the kindred and also the most severe clinical phenotype. The initial presentation at age 31 included asymmetric rigidity, which a few months later was followed by bradykinesia and postural instability. The motor symptoms responded to levodopa therapy. Disease progression was rapid. Individual V-16 underwent bilateral pallidotomies with an interval of 1 year between the two sides. The first pallidotomy was performed approximately 4 years after the initial presentation with a good response of the motor symptoms. The second pallidotomy was performed 1 year later. The motor symptoms remained stable for approximately 1 year after the second pallidotomy, but then continued to progress. In contrast to other affected members of the kindred, cognitive impairment was an early feature and myoclonus, sleep disturbances and hypotensive episodes were also observed. The cognitive impairment continued to progress in parallel to the motor impairment. In the last year of his life, dementia became a prominent clinical feature, and he became mute during the last 6 months of life. In that interval, the motor symptoms also worsened rapidly with minimal response to levodopa. Death occurred at age 39 after a 7-year disease course.
CLINICAL FEATURES OF IV-5
Individual IV-5 had disease onset at age 59. The initial presentation included resting tremor and unilateral rigidity. Bradykinesia was noted within one year, and a diagnosis of PD was made. There was a good and lasting response to levodopa therapy. Within 3 years of disease onset, postural instability became more prominent and orthostatic hypotension was noted. In contrast to V-16, myoclonus and sleep disturbances were not observed. Also in contrast to V-16 where dementia was manifested early, individual IV-5 developed dementia late in the disease course. Disease progression was slow in comparison to individual V-16 and individual IV-5 died at age 71 after a 12-year disease course.
Neuropathological findings
NEUROPATHOLOGY OF V-16
The macroscopic evaluation was unremarkable without any significant focal atrophy. Coronal sections of the brain revealed a small cystic lesion in the ventral globus pallidus corresponding to the pallidotomy. There was mild diffuse thinning of the cortical ribbon and atrophy of the white matter. The ventricular system was mildly dilated. Transverse sections of the brainstem revealed marked loss of pigment in the SN and LC. The remainder of the brainstem and the cerebellum were unremarkable.
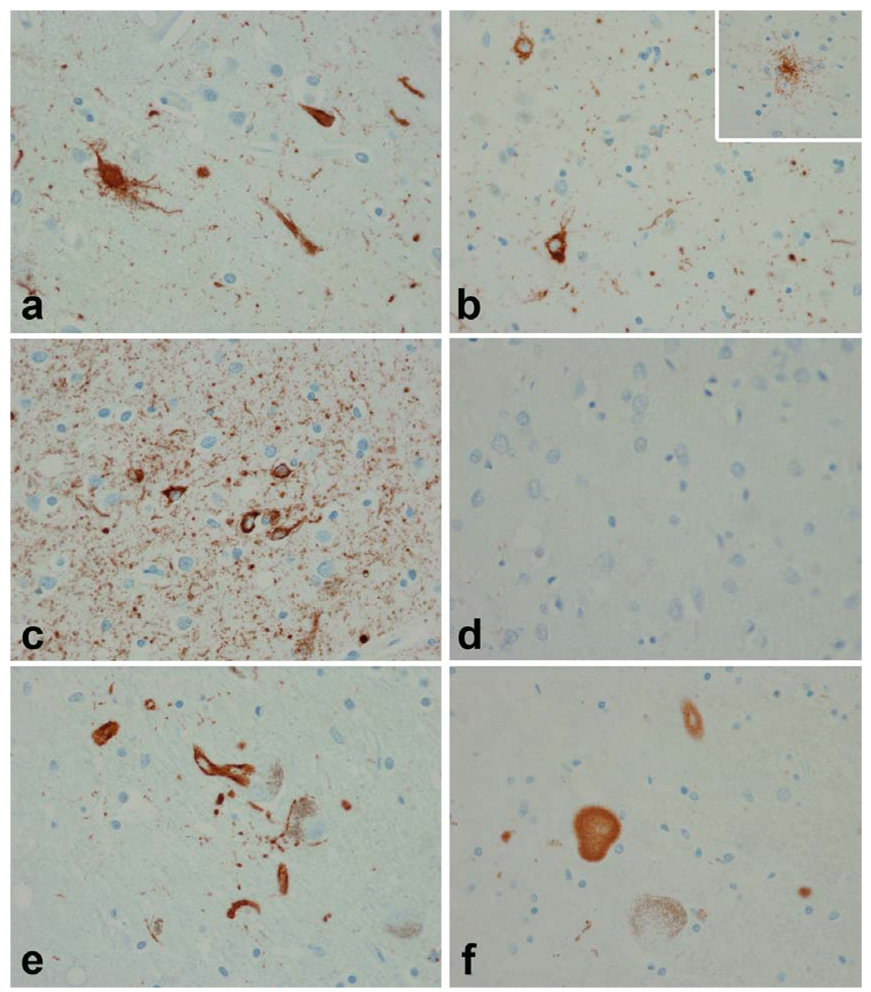
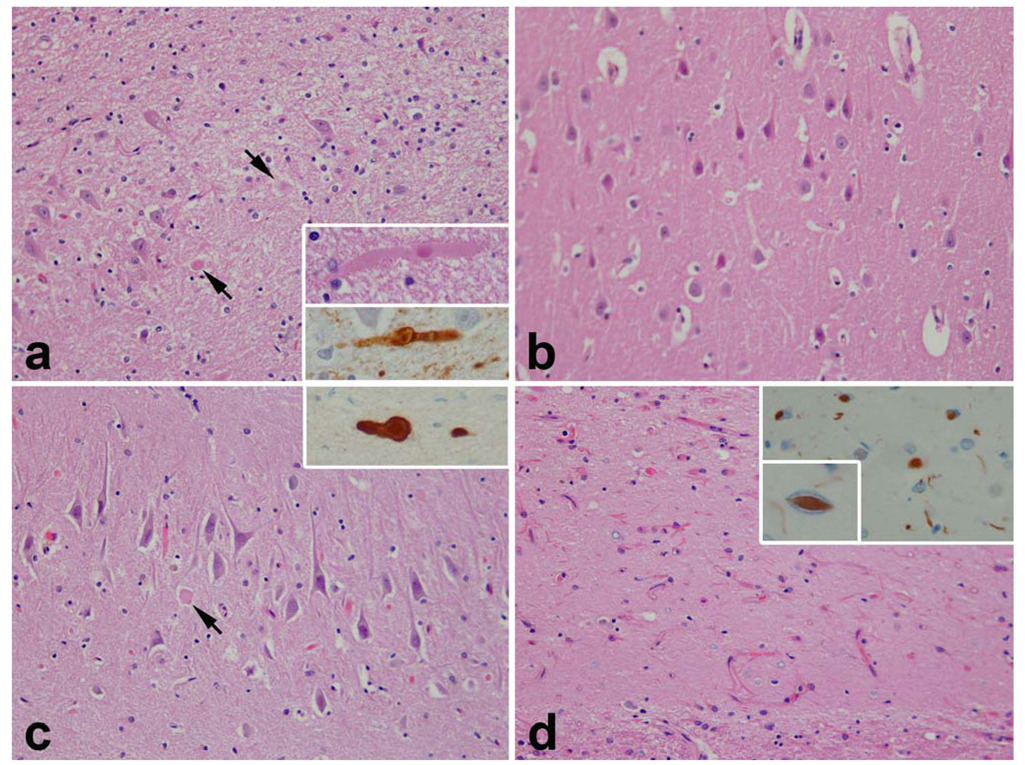
Histological evaluation revealed neuronal loss and rarefaction in the lower cortical layers and at the gray white junction, which was most clearly demonstrated using immunohistochemistry for GFAP and HLA-DR. There were α-synuclein-immunoreactive dystrophic axons and LN, but relatively few perikaryal LBs (Fig. 6). Cortical LBs, intraneuritic LBs and LN were most numerous in the limbic cortices, including the parahippocampal cortex, insular cortex and cingulate gyrus, but also notable in the superior temporal gyrus. The distribution of LB pathology was consistent with Braak stage 5 [6]. Sections for frontal and parietal lobes were minimally affected. In addition to neuronal loss and gliosis in lower cortical lamina, the limbic gray matter had striking spongiform changes. Sparse NFTs were noted in most cortical areas. The distribution was non-uniform with clusters of NFTs at the depths of sulci and in the superificial cortical layers (Fig. 5). In limbic areas, tau immunohistochemistry revealed neuropil threads associated with neurofibrillary degeneration that were most pronounced in cortical layers III and V (Fig. 5). The distribution and severity of the NFTs in this individual was consistent with Braak Stage IV.
Fig. 6.
α-Synuclein pathology in V-16 (a, c, e and g) and in IV-5 (b, d, f and h). The SN in both individuals shows marked neuronal loss. In V-16 axonal spheroids are evident (arrows). Classical LBs (insets in a and b) were uncommon in brainstem nuclei (inset in a -- locus ceruleus; inset in b -- oculomotor nucleus). Immunohistochemistry for α-synuclein shows prominent axonal spheroids in the SN in both individuals (c and d). The basal ganglia had prominent spheroids in V-16 (e). Glial cytoplasmic inclusions were prominent in IV-5 (f), but detected in both individuals (glial inclusions at higher magnification in V-16 -- inset in e -- and in IV-5 -- inset in f). Both individuals had cortical α-synuclein pathology most prominent in lower cortical layers, with a mixture of LN, dystrophic axons and cytoplasmic inclusions. Cortical pathology was worse in V-16 (g) than in IV-5 (h). (All figures ×400 original magnification)
Fig. 5.
Tau pathology in V-16 (a, c and e) and in IV-5 (b, d and f). Despite the young age of V-16 (35 years) there were NFTs and pretangles in the entorrhinal cortex (a) and striking tau pathology in neurons and neurites in the inferior temporal cortex (c). In IV-5 the tau pathology was sparse and limited to medial temporal lobe structures (b) and consistent with argyrophilic grain disease. Note the presence of pretangles and grains in the amygdala (b) as well as tau-positive ramified astrocytes typical of argyrophilic grain disease (inset in b). There was no tau pathology in the inferior temporal cortex (d). In both V-16 and IV-5 there was focal tau immunoreactivity in LBs and intraneuritic LBs (e and f). Intraneuritic LBs in SN show tau immunoreactivity at the periphery with pale or no staining at the center of the LB. (All figures ×400 original magnification)
The hippocampus had marked neuronal loss and gliosis in the CA2 sector, while CA1 and the subiculum were spared (Fig. 4). In CA2 there were a number of swollen dystrophic axons that were visible with routine histology and positive with α-synuclein immunohistochemistry (Fig. 4). There were also thinner, longer and more tortuous cell processes that were detected with α-synuclein immunohistochemistry and consistent with LN. Dystrophic axons were also present in the hippocampal endplate.
Fig. 4.
Hippocampal pathology in V-16 (a and b) and in IV-5 (c and d). Both individuals had focal neuronal loss and gliosis. In V-16 it was marked in CA2 (a) and associated with dystrophic axons (arrows; and at higher magnification in insets stained with H&E and α-synuclein), while CA1 was unremarkable (b). In IV-5 neuronal loss was minimal in CA2 (c) although there were a few dystrophic axons (arrow, and at higher magnification in inset stained with α-synuclein), but it was very severe in CA1 (d) and accompanied by TDP-43 immunoreactive neurites and neuronal cytoplasmic inclusions (inset) and lentiform intranuclear inclusions (small inset). TDP-43 images are from inferior temporal gyrus. (All figures ×400 original magnification)
The nbM had marked neuronal loss and many LBs and LN, but no NFTs. The hypothalamus also has many LBs and synuclein-immunoreactive dystrophic axons. There were sparse LBs in the amygdala, but far more numerous LN and intraneuritic LBs. The LBs and neurites were prominent in both cortical and basolateral regions of the amygdala.
The SN had marked neuronal loss with diffuse gliosis and sparse extra-neuronal neuromelanin (Fig. 6). There were sparse LBs in the few residual neurons and, numerous intraneuritic LBs. Synuclein-immunoreactive dystrophic axons were also present in the dorsal raphe, oculomotor nucleus and periaqueductal gray matter. The raphe nucleus, LC and reticular formation had mild neuronal loss and numerous intraneuritic LBs and many perikaryal LBs. Numerous intraneuritic LBs were present in the dorsal motor nucleus of the vagus and the medullary reticular formation.
NEUROPATHOLOGY OF IV-5
Macroscopic evaluation revealed moderate frontal, marked superior temporal gyrus and minimal medial temporal lobe atrophy (Fig. 3). Coronal sections revealed mild enlargement of the frontal and temporal horns of the lateral ventricle, thinning of the cortical ribbon in the medial temporal lobe and atrophy of the hippocampal formation and amygdala (Fig. 3). The basal ganglia and thalamus were grossly unremarkable. Transverse sections of the brainstem revealed marked pigment loss in the SN and LC (Fig 3). The cerebellum was unremarkable.
Fig. 3.
Macroscopic findings of individual IV-5. The external convexity shows focal atrophy of the superior temporal gyrus (STG, arrow). Coronal sections show medial temporal atrophy (MTA, arrow) and atrophy of the hippocampus consistent with hippocampal sclerosis (HpScl, arrow). Transverse sections of midbrain and pons show loss of neuromelanin pigmentation in the SN (arrow) and LC (arrow).
Microscopic evaluation revealed marked cortical atrophy and spongiosis in the superior temporal gyrus and medial temporal lobe and less atrophy in the cingulate, frontal and parietal cortices. The primary motor and visual cortices were unaffected. A few SP and mild focal amyloid angiopathy were detected with thioflavin-S fluorescent microscopy, but there were no neocortical NFTs. The hippocampus had severe neuronal loss and gliosis in area CA1 and the subiculum with relative sparing of area CA2 and the endplate, consistent with hippocampal sclerosis (Fig. 4). Neuronal loss and gliosis was also notable in the basolateral amygdala. A few α-synuclein-positive dystrophic axons were present in area CA2 (Fig 4).
Tau-immunostaining revealed isolated pretangles and grains in the endplate. The entorrhinal and perirhinal cortices exhibited atrophy, spongiosis and a few SP, as well as tau-immunoreactive pretangles, grains and neurites. In this individual, the distribution and severity of the NFTs was consistent with Braak Stage I. Pretangles, grains and tau-immunoreactive ramified astrocytes were also detected in the amygdala and were consistent with argyrophilic grain disease (AGD) (Fig. 5). The distribution of the pathology was consistent with AGD stage II according to Saito et al (2004) [36].
Immunostaining for TDP-43 revealed neuritic processes in superficial cortical layers of the temporal lobe, as well as pleomorphic (crescent shaped and Pick body like) neuronal cytoplasmic inclusions (Fig. 4). There were sparse lentiform-shaped, intranuclear inclusions (Fig. 4). Sparse granular TDP-43 neuronal cytoplasmic inclusions were also present in the neurons of the dentate fascia. These findings would be consistent with frontotemporal lobar degeneration type 1 [9, 27].
α-Synuclein-immunostaining revealed pathology in all cortical sections examined, including typical cortical and intraneuritic LBs (Fig. 6). The latter were most numerous in areas with spongiosis and gliosis. Scattered α-synuclein-positive glial cytoplasmic inclusions were detected in the cortex and white matter and most prominent in the limbic cortices and the temporal lobe being worst in lower cortical layers (Fig. 6). The nbM had neuronal loss, a few NFTs and numerous LBs and LN, as well as α-synuclein-immunoreactive dystrophic axons. There were LBs in the hypothalamus. The amygdala had marked gliosis in the basolateral region, but less in the corticomedial region. Tau-positive grains and pretangles and α-synuclein-positive LBs and LN were noted in the amygdala. The basal ganglia had gliosis in the caudate nucleus and nucleus accumbens with TDP-43-positive neuronal inclusions. There were α-synuclein-positive axonal spheroids and glia in the globus pallidus, as well as dot-like and curvilinear neurites in the striatum. The SN had almost complete neuronal loss and gliosis with sparse extra-neuronal neuromelanin (Fig. 6). No LBs were detected in the few residual neurons, but LN and intraneuritic LBs were present (Fig. 6). In the brainstem, LBs and α-synuclein-positive glia were present in the ventral tegmental region and dorsal raphe. Many LBs were present in the oculomotor nucleus. The LC had neuronal loss and LBs in residual neurons. The pontine tegmentum had α-synuclein-positive glia and LN. There were many intraneuritic LBs in the dorsal motor nucleus of the vagus and the medullary reticular formation. Isolated neurons in the pontine base and the inferior olive had α-synuclein immunoreactivity. The distribution of LB pathology was consistent with Braak stage 6 [6].
Table 4 summarizes the neuropathological findings in V-16 and IV-5. Similarities included severe neuronal loss in the SN, LC and nbM. The most significant differences include differences in the number and location of the LBs, differential neuronal loss in the hippocampus (CA1 vs. CA2), and fewer NFTs in IV-5 despite older age and longer disease duration. There were also TDP-43 immunoreactive lesions similar to those found in frontotemporal lobar degeneration with ubiquitin inclusions (FTLD-U) in IV-5, but no such pathology was observed in V-16.
Discussion
This report describes two individuals from two nuclear families within an A53T α-synuclein kindred whose different clinical and neuropathological phenotypes are associated with different PRKN genotypes. The N167 Parkin genotype is associated with the most severe clinical phenotype seen in the kindred, whereas the S167 Parkin (a homozygote for the most common wild-type PRKN allele), is associated with a less severe phenotype. Neuropathological differences are also associated with PRKN status. In both cases, the neuropathology was characterized by LBs, and abundant intraneuritic LBs in the brainstem and cortex, seen previously in the Contursi kindred [12, 25]. Whereas in sporadic PD, α-synuclein-positive intraneuritic LBs are usually confined to discrete brain regions (e.g. nbM and dorsal motor nucleus of the vagus), they were unusually widespread in the individual with N167 Parkin. The areas that had intraneuritic LBs also showed marked neuronal loss and striking astrocytic gliosis and reactive microglia, especially in the CA2 hippocampal region and in the lower cortical layers, In contrast, cortical α-synuclein pathology in the S167 individual (wild-type PRKN) was more typical of diffuse Lewy body disease.
When the cellular pathology of A53T individuals with wild-type and N167 Parkin is compared, there is differential neuronal loss both in degree and spatial distribution, and in the presence of TDP-43 positive inclusions. In the A53T individual with wild-type Parkin, neuronal loss was present in area CA1 and the subiculum and resembled hippocampal sclerosis, which in the elderly is often associated with ubiquitin or TDP-43 positive neuronal inclusions [1–2], but also had frontotemporal cortical atrophy and with TDP-43 positive neuritic and intranuclear inclusions. TDP-43 is a conserved heterogeneous ribonucleoprotein associated with RNA and DNA binding, transcription repression, alternative splicing and higher-order arrangement of nuclear bodies [4, 7, 44]. Neuronal and glial inclusions containing TDP-43 have been reported in FTLD-U, amyotrophic lateral sclerosis (ALS) and some AD cases [3, 33]. It should be noted that LB pathology has been seen in families with progranulin mutations, a common cause of hereditary FTLD-U [8, 22, 26]. However, the clinical course of the S167 individual had no resemblance to FTLD-U. While it cannot be excluded that the TDP-43 detected in IV-5 may represent concurrent FTLD-U, this finding raises the possibility of a role of TDP-43 with familial Lewy body disorders, potentially analogous to the association of tau pathology in Lewy body disorders [15]. Interestingly, TDP-43 has been recently associated with Lewy body disorders [32]. We are currently assessing members of Family H for the presence of progranulin gene variants. Analysis of tau exons did not reveal any gene variants in Family H (data not shown)
These cases exhibit spatial specificity in hippocampal neuronal loss. In the S167 individual it resembled FTLD-U and spared CA2, while in the N167 individual, it was most marked in CA2, similar to that reported in kindreds with SNCA mutations (e.g. the Contursi kindred, DWD, personal observations) and triplications (Iowa kindred) [17]. It has been proposed that CA2 neuronal loss and gliosis in association with LBs is highly suggestive of SNCA mutation [13]. The CA2 region is vulnerable in sporadic PD and in diffuse Lewy body disease [11] but it is rarely associated with the extent seen in the N167 individual.
Despite an advanced age which would render AD-type pathology likely, this is not observed in the S167 individual. The tauopathy was mostly in the medial temporal lobe was consistent with argyrophilic grain disease, a common pathology with increasing age [41]. In contrast, the much younger N167 individual had significant tauopathy consistent with a Braak NFT stage IV. While it cannot be excluded that the significant cognitive impairment observed in this individual was associated with the bilateral pallidotomies, the degree of tauopathy observed would provide a more likely explanation for the cognitive impairment. Interestingly, some cases of autosomal recessive early onset PD due to PRKN mutations have also been described as having cortical tau pathology [30]. A minority of the LB and LN showed tau immunoreactivity in both individuals, a finding that has been noted in both familial and sporadic cases of Lewy body disease [12, 42]. Both had α-synuclein immunoreactive glial cytoplasmic inclusions that are also seen in other familial synucleinopathies and in sporadic PD [17, 21, 42]. In summary, though the neuropathological changes observed in the two individuals share some characteristics that are consistent with diffuse Lewy body disease (DLB), they exhibit distinguishing characteristics as well. The distinguishing characteristics include the spatial specificity of the hippocampal changes and the differential distribution of tau-positive changes. These make it difficult to assign a simple classification of either DLB, frontotemporal lobar degeneration (FTLD) or AGD, and raise the possibility of different underlying pathogenetic mechanisms.
The transmission of an uncommon A601 parkin allele within a nuclear family of Family H produced a rare A/A homozygote with S167 parkin whose clinical course and neuropathological features were strikingly different from those of other A53T individuals. This inheritance pattern suggests a possible role for the S167N PRKN polymorphism in the mechanism(s) underlying disease severity in A53T-associated familial PD. Though there are conflicting reports regarding the association of PRKN polymorphisms with sporadic PD [43, 37], meta-analysis indicates that in contrast to other PRKN alleles, the S167N polymorphism confers a minor increase in PD risk (OR 1.13, 95% CI 0.9–1.41; www.pdgene.org). The underlying mechanism remains unclear, as substitution of Ser by Asn neither alters the secondary structure of Parkin nor modifies glycosylation, myristoylation or phosphorylation sites [37]. It may affect interactions between Parkin and α-synuclein or indirectly affect downstream processes upon which both impact [5, 23]. Recent studies have suggested that mutant α-synuclein that is more prone to aggregate is also more effective at stimulating Parkin accumulation and mislocalization [23, 40]. Therefore, even a small difference in the susceptibility of S167 and N167 Parkin to α-synuclein-stimulated Parkin accumulation and mislocalization could contribute to the disease phenotypes seen in individuals IV-5 and V-16. In a Drosophila PD model, parkin counteracts a behavioral phenotype and suppresses α-synuclein-mediated dopaminergic neuronal loss [20] suggesting that impairment of Parkin function may exacerbate a α-synuclein-associated phenotype. We are in the process of assessing the role of PRKN polymorphisms in a Drosophila model system.
Individuals IV-5 and V-16 could certainly exhibit additional genetic variation and also could have been exposed to different environmental factors that might have contributed to their clinical and neuropathological differences. The G209A SNCA allele that leads to the A53T form of alpha-synuclein has only been reported to date in Caucasian kindreds originating in Southern Italy and Southwestern Greece, and is itself is a rare allele in these populations. Since PRKN 601A/601A homozygotes are also rare in the Caucasian population, it is very unlikely that many individuals are both heterozygous for the G209A SNCA allele and homozygous for the 601A/601A PRKN genotype. This means that the genotype producing both A53T α-synuclein and N167 Parkin is likely to be very rare in the general PD population, making it difficult to ascertain whether the causal factor in the differing phenotypes of IV-5 and V-16 is the presence of N167 Parkin. Ongoing investigations in a model organism where reproducibility can be assessed should be helpful to test hypotheses about whether S167N Parkin or other genetic modifiers underlie the variability seen in familial PD phenotypes.
Genetic background and modifying genes have been shown to play a role on gene function in PD [10, 38] and other human diseases [18, 31]. It is possible that phenotypic variability observed in familial PD reflects genetic interactions arising from differences in genetic background. Rare genotypes that in a heterozygous state confer a minor increase in disease risk in sporadic PD (e.g., PRKN N167) may in a homozygous state exacerbate a disease phenotype due to a highly penetrant dominant allele (a genotype conferring a major increase in disease risk, e.g., SNCA A53T).
Table 3.
Summary of microscopic neuropathologic findings
| Individual IV-5 | Neuronal loss | LBs (×200) | NFTs (×400) | SPs (×100) |
|---|---|---|---|---|
| Mid-frontal cortex | ++ | 2–4 | 0 | 3–4 |
| Superior temporal cortex | +++ | 5–8 | 0 | 0 |
| Inferior parietal cortex | ++ | 1–3 | 0 | 0 |
| Cingulate gyrus | ++ | 6–8 | … | … |
| Parahippocampal gyrus | ++ | 4–8 | 0 | 0 |
| CA2/3 | + | … | 0–1 | 0 |
| CA1 | +++ | … | 0–1 | 0 |
| Endplate | + | … | 0 | 0 |
| Subiculum | + | … | 0–2 | 0 |
| Neuropathologic diagnosis: Diffuse Lewy body disease; argyrophilic grain disease (Braak stage I); TDP-43 proteinopathy consistent with frontotemporal lobar degeneration | ||||
| Individual V-16 | Neuronal loss | LBs (×200) | NFTs (×400) | SPs (×100) |
|---|---|---|---|---|
| Mid-frontal cortex | + | 0–1 | 1–5 | 0 |
| Superior temporal cortex | ++ | 1–2 | 1–3 | 0 |
| Inferior parietal | + | 0–1 | 0–1 | 0 |
| Cingulate gyrus | ++ | 2–3 | … | … |
| Parahippocampal gyrus | ++ | 3–5 | 0 | 0 |
| CA2/3 | +++ | … | 0–1 | 0 |
| CA1 | + | … | 0–1 | 0 |
| Endplate | + | … | 0 | 0 |
| Subiculum | + | … | 0–2 | 0 |
| Neuropathologic diagnosis: Diffuse Lewy body disease; tangle only pathology (Braak stage IV); status post: pallidotomy | ||||
Neuronal loss is subjectively assessed as + = mild, ++ = moderate or +++ = severe; values represent counts of the various lesions in representative low density and high density fields; LBs = Lewy bodies with α-synuclein immunohistochemistry; NFTs = neurofibrillary tangles, and SPs = senile plaques with thioflavin-S fluorescent microscopy. ·… = not assessed.
Acknowledgments
Supported by NIH grants P50-AG25711, P50-AG16574, P50-NS40256, P01-AG17216, P01-AG03949, R15-NS043162 and a seed grant to KM from the University of Nebraska Medical Center. The histological support of Virginia Phillips, Linda Rousseau and Monica Casey-Castanedes is greatly appreciated.
References
- 1.Amador-Ortiz C, Ahmed Z, Zehr C, Dickson DW. Hippocampal sclerosis dementia differs from hippocampal sclerosis in frontal lobe degeneration. Acta Neuropathol. 2007;113:245–252. doi: 10.1007/s00401-006-0183-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amador-Ortiz C, Lin W-L, Ahmed Z, Personett D, Davies P, Duara R, Graff-Redford NR, Hutton ML, Dickson DW. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer's disease. Ann Neurol. 2007;61:435–445. doi: 10.1002/ana.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y, Oda T. TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun. 2006;351:602–611. doi: 10.1016/j.bbrc.2006.10.093. [DOI] [PubMed] [Google Scholar]
- 4.Ayala YM, Pantano S, D'Ambroglio A, Buratti E, Brindisi A, Marchetti C, Romano M, Baralle FE. Human, Drosophila and C.elegans TDP-43: Nucleic acid binding properties and splicing regulatory function. J Mol Biol. 2005;348:575–588. doi: 10.1016/j.jmb.2005.02.038. [DOI] [PubMed] [Google Scholar]
- 5.Baptista MJ, Cookson MR, Miller DW. Parkin and alpha-synuclein: opponent actions in the pathogenesis of Parkinson's disease. Neuroscientist. 2004;10:63–72. doi: 10.1177/1073858403260392. [DOI] [PubMed] [Google Scholar]
- 6.Braak H, Bohl JR, Müller CM, Rüb de Vos RAI, Del Tredici K. Stanley Fahn Lecture 2005: The staging procedure for the inclusion body pathology associated with Parkinson’s disease reconsidered. Mov Disord. 2005;21:2042–2051. doi: 10.1002/mds.21065. [DOI] [PubMed] [Google Scholar]
- 7.Buratti E, Dork T, Zuccato E, Pagani F, Romano M, Baralle FE. Nuclear factor TDP-43 and SR proteins promote in vitro and in vivo CFTR exon 9 skipping. EMBO J. 2001;20:1774–1784. doi: 10.1093/emboj/20.7.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns NJ, Neumann M, Bigio EH, Holm IE, Troost D, Hatanpaa KJ, Foong C, White CL, 3rd, Schneider JA, Kretzschmar HA, Carter D, Taylor-Reinwald L, Paulsmeyer K, Strider J, Gitcho M, Goate AM, Morris JC, Mishra M, Kwong LK, Stieber A, Xu Y, Forman MS, Trojanowski JQ, Lee VM, Mackenzie IR. TDP-43 in Familial and sporadic frontotemporal lobar degeneration with ubiquitin Inclusions. Am J Pathol. 2007;171:227–240. doi: 10.2353/ajpath.2007.070182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson Y, Kelly T, Mackenzie IRA, Pickering-Brown S, Du Plessis D, Neary D, Snowden JS, Mann DMA. Ubiquitinated pathological lesions in frontotemporal lobar degeneration contain TAR DNA-binding protein, TDP-43. Acta Neuropathol. 2007;113:521–533. doi: 10.1007/s00401-006-0189-y. [DOI] [PubMed] [Google Scholar]
- 10.DeStefano AL, Lew MF, Golbe LI, Mark MH, Lazzarini AM, Guttman M, Montgomery E, Waters CH, Singer C, Watts RL, Currie LJ, Wooten GF, Maher NE, Wilk JB, Sullivan KM, Slater KM, Saint-Hilaire MH, Feldman RG, Suchowersky O, Lafontaine AL, Labelle N, Growdon JH, Vieregge P, Pramstaller PP, Klein C, Hubble JP, Reider CR, Stacy M, MacDonald ME, Gusella JF, Myers RH. PARK3 influences age at onset in Parkinson disease: a genome scan in the GenePD study. Am J Hum Genet. 2002;70:1089–1095. doi: 10.1086/339814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickson DW, Ruan D, Crystal H, Mark MH, Davies P, Kress Y, Yen SH. Hippocampal degeneration differentiates diffuse Lewy body disease (DLBD) from Alzheimer's disease: light and electron microscopic immunocytochemistry of CA2-3 neurites specific to DLBD. Neurology. 1991;41:1402–1409. doi: 10.1212/wnl.41.9.1402. [DOI] [PubMed] [Google Scholar]
- 12.Duda JE, Giasson BI, Mabon ME, Miller DC, Golbe LI, Lee VM, Trojanowski JQ. Concurrence of alpha-synuclein and tau brain pathology in the Contursi kindred. Acta Neuropathol. 2002;104:7–11. doi: 10.1007/s00401-002-0563-3. [DOI] [PubMed] [Google Scholar]
- 13.Farrer M, Kachergus J, Forno L, Lincoln S, Wang DS, Hulihan M, Maraganore D, Gwinn-Hardy K, Wszolek ZK, Dickson DW, Langston JW. Comparison of kindreds with parkinsonism and alpha-synuclein genomic multiplications. Ann Neurol. 2004;55:174–179. doi: 10.1002/ana.10846. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs J, Nilsson C, Kachergus J, Munz M, Larsson EM, Schule B, Langston JW, Middleton FA, Ross OA, Hulihan M, Gasser T, Farrer MJ. Phenotypic variation in a large Swedish pedigree due to SNCA duplication and triplication. Neurology. 2007;68:916–922. doi: 10.1212/01.wnl.0000254458.17630.c5. [DOI] [PubMed] [Google Scholar]
- 15.Galpern WR, Lang AE. Interface between tauopathies and synucleinopathies: a tale of two proteins. Ann Neurol. 2006;59:449–458. doi: 10.1002/ana.20819. [DOI] [PubMed] [Google Scholar]
- 16.Golbe LI, Di Iorio G, Sanges G, Lazzarini AM, La Sala S, Bonavita V, Duvoisin RC. Clinical genetic analysis of Parkinson's disease in the Contursi kindred. Ann Neurol. 1996;40:767–775. doi: 10.1002/ana.410400513. [DOI] [PubMed] [Google Scholar]
- 17.Gwinn-Hardy K, Mehta ND, Farrer M, Maraganore D, Muenter M, Yen SH, Hardy J, Dickson DW. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary parkinsonism and dementia linked to chromosome 4p. Acta Neuropathol. 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 18.Haider NB, Ikeda A, Naggert JK, Nishina PM. Genetic modifiers of vision and hearing. Hum Mol Genet. 2002;11:1195–1206. doi: 10.1093/hmg/11.10.1195. [DOI] [PubMed] [Google Scholar]
- 19.Hansen LA, Masliah E, Terry RD, Mirra SS. A neuropathological subset of Alzheimer's disease with concomitant Lewy body disease and spongiform change. Acta Neuropathol. 1989;78:194–201. doi: 10.1007/BF00688209. [DOI] [PubMed] [Google Scholar]
- 20.Haywood AF, Staveley BE. Parkin counteracts symptoms in a Drosophila model of Parkinson's disease. BMC Neurosci. 2004;5:14. doi: 10.1186/1471-2202-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW. Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol. 2003;62:389–397. doi: 10.1093/jnen/62.4.389. [DOI] [PubMed] [Google Scholar]
- 22.Josephs KA, Ahmed Z, Katsuse O, Parisi JF, Boeve BF, Knoopman DS, Petersen RC, Davies P, Duara R, Graff-Redford NR, Uitti RJ, Rademakers R, Adamson J, Baker M, Hutton ML, Dickson DW. Neuropathologic features of frontotemporal lobar degeneration with ubiquitin-positive inclusions with progranulin gene (PGRN) mutations. J Neuropathol Exp Neurol. 2007;66:142–151. doi: 10.1097/nen.0b013e31803020cf. [DOI] [PubMed] [Google Scholar]
- 23.Kawahara K, Hashimoto M, Bar-On P, Ho GJ, Crews L, Mizuno H, Rockenstein E, Imam SZ, Masliah E. α-Synuclein aggregates interfere with Parkin solubility and distribution: Role in the pathogenesis of Parkinson’s disease. J Biol Chem. 2008;283:6979–6987. doi: 10.1074/jbc.M710418200. [DOI] [PubMed] [Google Scholar]
- 24.Kotzbauer PT, Giasson BI, Kravitz AV, Golbe LI, Mark MH, Trojanowski JQ, Lee VM. Fibrillization of alpha-synuclein and tau in familial Parkinson's disease caused by the A53T alpha-synuclein mutation. Exp Neurol. 2004;187:279–288. doi: 10.1016/j.expneurol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 25.Langston JW, Sastry S, Chan P, Forno LS, Bolin LM, Di Monte DA. Novel alpha-synuclein-immunoreactive proteins in brain samples from the Contursi kindred, Parkinson's, and Alzheimer's disease. Exp Neurol. 1998;154:684–690. doi: 10.1006/exnr.1998.6975. [DOI] [PubMed] [Google Scholar]
- 26.Leverenz JB, Yu CE, Montine TJ, Steinbart E, Bekris LM, Zabetian C, Kwong LK, Lee VM, Schellenberg GD, Bird TD. A novel progranulin mutation associated with variable clinical presentation and tau, TDP43 and alpha-synuclein pathology. Brain. 2007;130:1360–1374. doi: 10.1093/brain/awm069. [DOI] [PubMed] [Google Scholar]
- 27.Mackenzie IRA, Baborie A, Pickering-Brown S, Du Plessis D, Jaros E, Perry RH, Neary D, Snowden JS, Mann DMA. Heterogeneity of ubiquitin pathology in frontotemporal lobar degeneration: classification and relation to clinical phenotype. Acta Neuropathol. 2006;112:539–549. doi: 10.1007/s00401-006-0138-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Markopoulou K, Wszolek ZK, Pfeiffer RF. A Greek-American kindred with autosomal dominant, levodopa-responsive parkinsonism and anticipation. Ann Neurol. 1995;38:373–378. doi: 10.1002/ana.410380306. [DOI] [PubMed] [Google Scholar]
- 29.Markopoulou K, Wszolek ZK, Pfeiffer RF, Chase BA. Reduced expression of the G209A alpha-synuclein allele in familial Parkinsonism. Ann Neurol. 1999;6:374–381. doi: 10.1002/1531-8249(199909)46:3<374::aid-ana13>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 30.Mori H, Hattori N, Mizuno Y. Genotype-phenotype correlation: familial Parkinson disease. Neuropathology. 2003;23:90–94. doi: 10.1046/j.1440-1789.2003.00476.x. [DOI] [PubMed] [Google Scholar]
- 31.Nadeau JH. Modifier genes in mice and humans. Nat Rev Genet. 2001;2:165–174. doi: 10.1038/35056009. [DOI] [PubMed] [Google Scholar]
- 32.Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, Arnold SE, Siderowf A, Grossman M, Leverenz JB, Woltjer R, Lopez OL, Hamilton R, Tsuang DW, Galasko D, Masliah E, Kaye J, Clark CM, Montine TJ, Lee VM, Trojanowski JQ. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta Neuropathol. 2007;114:221–229. doi: 10.1007/s00401-007-0261-2. [DOI] [PubMed] [Google Scholar]
- 33.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM, McCluskey LF, Miller BL, Masliah E, McKenzie TR, Feldman H, Feiden W, Kretzschmar HA, Trojanowski JQ, Lee VM. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. [DOI] [PubMed] [Google Scholar]
- 34.Nishioka K, Hayashi S, Farrer MJ, Singleton AB, Yoshino H, Imai H, Kitami T, Sato K, Kuroda R, Tomiyama H, Mizoguchi K, Murata M, Toda T, Imoto I, Inazawa J, Mizuno Y, Hattori N. Clinical heterogeneity of alpha-synuclein gene duplication in Parkinson's disease. Ann Neurol. 2006;59:298–309. doi: 10.1002/ana.20753. [DOI] [PubMed] [Google Scholar]
- 35.Pittman AM, Fung HC, de Silva R. Untangling the tau gene association with neurodegenerative disorders. Hum Mol Genet. 2006;15:R188–R195. doi: 10.1093/hmg/ddl190. [DOI] [PubMed] [Google Scholar]
- 36.Saito Y, Ruberu NN, Sawabe M, Arai T, Tanaka N, Kakuta Y, Yamanouchi H, Murayama S. Staging of argyrophilic grains: an age-associated tauopathy. J Neuropathol Exp Neurol. 2004;63:911–918. doi: 10.1093/jnen/63.9.911. [DOI] [PubMed] [Google Scholar]
- 37.Satoh J, Kuroda Y. Association of codon 167 Ser/Asn heterozygosity in the parkin gene with sporadic Parkinson's disease. Neuroreport. 1999;10:2735–2739. doi: 10.1097/00001756-199909090-00008. [DOI] [PubMed] [Google Scholar]
- 38.Scott WK, Nance MA, Watts RL, Hubble JP, Koller WC, Lyons K, Pahwa R, Stern MB, Colcher A, Hiner BC, Jankovic J, Ondo WG, Allen FH, Jr, Goetz CG, Small GW, Masterman D, Mastaglia F, Laing NG, Stajich JM, Slotterbeck B, Booze MW, Ribble RC, Rampersaud E, West SG, Gibson RA, Middleton LT, Roses AD, Haines JL, Scott BL, Vance JM, Pericak-Vance MA. Complete genomic screen in Parkinson disease: evidence for multiple genes. JAMA. 2001;286:2239–2244. doi: 10.1001/jama.286.18.2239. [DOI] [PubMed] [Google Scholar]
- 39.Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr alpha-synuclein mutation. Ann Neurol. 2001;49:313–319. [PubMed] [Google Scholar]
- 40.Springer W, Hoppe T, Schmidt E, Baumeister R. A Caenorhabditis elegans Parkin mutant with altered solubility couples alpha-synuclein aggregation to proteotoxic stress. Hum Mol Genet. 2005;14:3407–3423. doi: 10.1093/hmg/ddi371. [DOI] [PubMed] [Google Scholar]
- 41.Togo T, Cookson N, Dickson DW. Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol. 2002;12:45–52. doi: 10.1111/j.1750-3639.2002.tb00421.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wakabayashi K, Hayashi S, Yoshimoto M, Kudo H, Takahashi H. NACP/alpha-synuclein-positive filamentous inclusions in astrocytes and oligodendrocytes of Parkinson's disease brains. Acta Neuropathol. 2000;99:14–20. doi: 10.1007/pl00007400. [DOI] [PubMed] [Google Scholar]
- 43.Wang M, Hattori N, Matsumine H, Kobayashi T, Yoshino M, Morioka A, Kitada T, Asakawa S, Minoshima S, Shimizu N, Mizuno Y. Polymorphism in the parkin gene in sporadic Parkinson's disease. Ann Neurol. 1999;45:655–658. doi: 10.1002/1531-8249(199905)45:5<655::aid-ana15>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 44.Wang IF, Reddy NM, Shen CKJ. Higher order arrangement of the eukaryotic nuclear bodies. PNAS. 2002;99:13583–13588. doi: 10.1073/pnas.212483099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wood-Kaczmar A, Gandhi S, Wood NW. Understanding the molecular causes of Parkinson's disease. Trends Mol Med. 2006;12:521–528. doi: 10.1016/j.molmed.2006.09.007. [DOI] [PubMed] [Google Scholar]
- 46.Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, Vidal L, Hoenicka J, Rodriguez O, Atares B, Llorens V, Gomez Tortosa E, del Ser T, Munoz DG, de Yebenes JG. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]