Abstract
Butyrate, one of the SCFA, promotes the development of the intestinal barrier. However, the molecular mechanisms underlying the butyrate regulation of the intestinal barrier are unknown. To test the hypothesis that the effect of butyrate on the intestinal barrier is mediated by the regulation of the assembly of tight junctions involving the activation of the AMP-activated protein kinase (AMPK), we determined the effect of butyrate on the intestinal barrier by measuring the transepithelial electrical resistance (TER) and inulin permeability in a Caco-2 cell monolayer model. We further used a calcium switch assay to study the assembly of epithelial tight junctions and determined the effect of butyrate on the assembly of epithelial tight junctions and AMPK activity. We demonstrated that the butyrate treatment increased AMPK activity and accelerated the assembly of tight junctions as shown by the reorganization of tight junction proteins, as well as the development of TER. AMPK activity was also upregulated by butyrate during calcium switch-induced tight junction assembly. Compound C, a specific AMPK inhibitor, inhibited the butyrate-induced activation of AMPK. The facilitating effect of butyrate on the increases in TER in standard culture media, as well as after calcium switch, was abolished by compound C. We conclude that butyrate enhances the intestinal barrier by regulating the assembly of tight junctions. This dynamic process is mediated by the activation of AMPK. These results suggest an intriguing link between SCFA and the intracellular energy sensor for the development of the intestinal barrier.
Introduction
Normal intestinal function depends on the establishment and maintenance of a mucosal barrier that is lined by a monolayer of intestinal epithelial cells. The intestinal epithelium undergoes marked structural and functional changes during development (1,2). The intestinal barrier may not be completely developed at birth, particularly in preterm infants (3). It has been well established that postnatal maturation of the intestinal barrier occurs coincident with increasing enteral feeding and establishment of normal intestinal bacterial colonization (4,5). The importance of normal bacterial colonization in the development and maintenance of the intestinal barrier is further supported by the observations that the gastrointestinal tract gene expression profile and intestinal barrier development are altered by early treatment with broad-spectrum antibiotics or withholding enteral feeding (6,7).
SCFA, mainly acetic acid, butyric acid, and propionic acid, are the products of the bacterial fermentation of undigested carbohydrates in the intestine (8,9). Production of SCFA in the intestinal lumen is important for normal intestinal biology such as energy salvage and the absorption of water and salt in the colon. Furthermore, SCFA may be an important factor linking postnatal gastrointestinal adaptation and maturation with normal bacterial colonization (10–12). Using an in vitro model of the intestinal epithelial barrier with Caco-2 cells grown in a trans-well system, we, as well as others, have demonstrated that SCFA, especially butyrate, in physiological concentrations, can enhance intestinal barrier function as measured by increases in transepithelial electrical resistance (TER)6 and decreases in inulin permeability (13,14). However, the molecular mechanisms underlying this effect remain to be elucidated.
One major component of the intestinal barrier is the formation of tight junctions between epithelial cells (15,16). The effect of butyrate on the epithelial barrier may be mediated by regulation of the tight junction proteins. Recent studies demonstrated that the regulation of tight junction assembly involves activation of the AMP-activated protein kinase (AMPK). The tight junction assembly is impaired when AMPK activity is downregulated by a genetic manipulation strategy (17,18). AMPK is a serine/threonine kinase that is evolutionarily conserved from yeast to mammals. The binding of AMP to AMPK allows it to be phosphorylated on Thr-172, resulting in its activation. In contrast, the binding of ATP prevents its activation. Normally, AMPK functions as a cellular fuel gauge that regulates metabolic pathways in glucose and fatty acid metabolism and protein synthesis (19). The aim of the present study was to test the hypothesis that the effect of butyrate on the intestinal barrier is mediated by the regulation of the assembly of tight junctions involving the activation of the AMPK. We examined the effect of butyrate at physiological concentrations on the assembly of tight junction proteins in a well-established Caco-2 cell monolayer model and found that the butyrate treatment increased AMPK activity and accelerated the assembly of tight junctions. Our study provides some evidence suggesting an intriguing link between SCFA and the intracellular energy sensor for the development and maintenance of the intestinal barrier.
Materials and Methods
Cell culture.
Caco-2, a human colonic epithelial cell line, was obtained from the American Type Culture Collection and maintained routinely in 100- × 15-mm petri dishes at 37°C with 5% CO2 95% air atmosphere and >95% humidity.
The cell monolayers were grown in DMEM (Mediatech) supplemented with 10% fetal bovine serum, 100 kiu/L penicillin, 100 mg/L streptomycin, l-glutamine (2 mmol/L), and nonessential amino acids. All experiments were performed in the same medium without 10% fetal bovine serum, i.e. in the serum-free medium.
Calcium switch assay.
Fully confluent Caco-2 cell monolayers (usually within 72 h after confluence) were washed with calcium-free GIBCO Minimum Essential Medium (S-MEM) and treated with S-MEM for 16 h.
S-MEM was removed and cell monolayers were washed 3 times with serum-free DMEM. The cell monolayers were then incubated in the regular serum-free DMEM for the indicated time periods under standard cell culture conditions. Reassembly of tight junctions and restoration of barrier function were determined at various time points by measuring the TER.
Measurement of TER.
We used 24-well trans-well system plates with collagen-coated membrane inserts (Corning) for this assay as described in detail previously (13). Briefly, 1.5 × 106 Caco-2 cells in 0.5 mL medium were seeded in the apical chamber that bathed in the basal chamber with 1.5 mL of medium and the changes of TER were measured with an epithelial volt-ohmmeter (WPI). Once grown to confluence when TER reached ∼140Ω/insert, the cells were rinsed with PBS and incubated in serum-free medium alone or with other reagents as indicated. Values were corrected for background resistance due to the membrane insert and the collagen layer and calculated as Ω·cm2.
Paracellular flux assay.
The procedure was performed as described in detail previously with some modification (13). Briefly, Caco-2 cells were plated into 24-well trans-well filter inserts for the assay. At 72 h following incubation with different reagents, 50 μL of fluorescein isothiocyanate-conjugated inulin (inulin-FITC) (1 g/L; Sigma) was added into the apical chamber and 3 parallel aliquots were taken from the basal chamber after 1 h of incubation at 37°C. The fluorescence emission at 535 nm was measured after excitation at 480 nm by using a microplate fluorescence reader (SPECTRAmax GEMINI XS, Molecular Devices).
Immunofluorescence microscopy.
Caco-2 cells were seeded on glass slide covers placed in 6-well plates and grown in DMEM. After rinsing with PBS, the treated monolayers were fixed in cold methanol (−20°C) and blocked in 10% normal goat serum for 1 h at room temperature. The cells were then labeled with mouse anti-occludin or mouse anti-ZO-1 (Zymed Laboratory) for 1 h, followed by incubation for 1 h with fluorescein-conjugated goat anti-mouse IgG (Santa Cruz Biotechnology). The distribution of tight junction proteins was visualized and analyzed by a Zeiss LSM510 inverted confocal laser-scanning microscopy. Digital images were processed using LSM software and Adobe Photoshop.
Immunoblot analysis.
Proteins in different samples were separated with 8% SDS-polyacrylamide gel or 10–20% precast Tris-HCl gel electrophoresis and transferred into polyvinylidene difluoride membranes. Membranes were probed for ZO-1, occludin, claudin-1, claudin-4, phosphorylated AMPK (pAMPK), total AMPK, phosphorylated acetyl-CoA carboxylase (pACC), and actin with corresponding primary antibodies (Claudin antibodies from Zymed Laboratory and others from Cell Signaling), respectively, in combination with horseradish peroxidase-conjugated second antibodies. The density of blotting bands was then analyzed by using ImageJ software (NIH). Quantification of AMPK activation status was determined as the ratio of the phosphorylated form of the protein to the total AMPK protein content for each sample. Furthermore, because acetyl-CoA carboxylase (ACC) is a direct substrate of AMPK (20,21), the degree of ACC phosphorylation was also used to reflect the in situ level of AMPK activity.
Statistics.
All of the experiments were repeated at least 3 times. Group data from all experiments are presented as means ± SE except where noted and the numbers in each group used for statistical analysis are mentioned, respectively, in the figure legends. One-way ANOVA was used for all of the statistical analyses among multiple groups other than 1 set of data in Figure 1A, in which 2-way ANOVA was used for statistical analysis. Groups were compared at each time point by post hoc Tukey tests as appropriate. A P-value < 0.05 was considered significant in all cases. All statistical analyses were performed using Microcal Origin 6.0 for Windows.
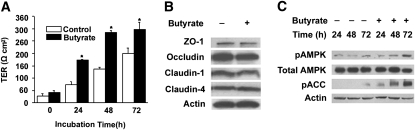
FIGURE 1 .
Effects of butyrate on barrier function, expressions of tight junction proteins, and AMPK phosphorylation in Caco-2 cell monolayers. (A) Cells were grown in the trans-well inserts until confluence and then incubated with or without 2 mmol/L of butyrate for 72 h. Values are means + SE, n = 6. *Different from control at that time, P < 0.05. (B) After 72 h treatment with medium alone or with 2 mmol/L of butyrate, cell lysates were immunoblotted with the specific antibodies indicated. (C) Total cell lysates from the indicated time points after treatment with medium alone or with 2 mmol/L of butyrate were subjected to immunoblotting for pAMPK, total AMPK, pACC, and actin with specific antibodies.
Results
Butyrate effects on the barrier function and AMPK activity in Caco-2 cell monolayers.
Consistent with what we showed previously, incubation with butyrate at 2 mmol/L led to significant increases in TER in the Caco-2 cell monolayers. The increases in TER occurred after 24 h of butyrate treatment and reached maximal levels within 48–72 h. The TER levels of control cell monolayers also increased spontaneously with time but were at significantly lower levels than those of butyrate-treated monolayers (Fig. 1A).
The effect of butyrate on the epithelial barrier could be mediated by regulating the expressions of tight junction proteins. We determined whether the expressions of 4 major tight junction proteins (occludin, claudin-1, claudin-4, and ZO-1) in Caco-2 cells were regulated by butyrate by western blots. The treatment with 2 mmol/L butyrate for 72 h did not significantly alter the expressions of these tight junction proteins (Fig. 1B). No significant expression changes of these proteins occurred after 24 or 48 h of butyrate treatment (data not shown). This indicates that the effect of butyrate on the Caco-2 cell model of intestinal barrier is unlikely mediated by regulating the levels of these 4 major tight junction proteins.
To determine whether AMPK is involved in the pathway through which butyrate regulates the function of tight junctions, we examined the phosphorylation status of AMPK Thr-172 after stimulation with 2 mmol/L of butyrate in Caco-2 cells. The amount of pAMPK increased after the treatment with butyrate in a time-dependent manner (Fig. 1C). An antibody against pACC at Ser-79 was utilized to determine the ACC phosphorylation in the same cell extracts. The levels of ACC phosphorylation were also increased after butyrate treatment (Fig. 1C), which was in good agreement with the corresponding changes in the levels of AMPK phosphorylation, reflecting an increase in AMPK activity.
AMPK activation is involved in the butyrate regulation of the barrier function.
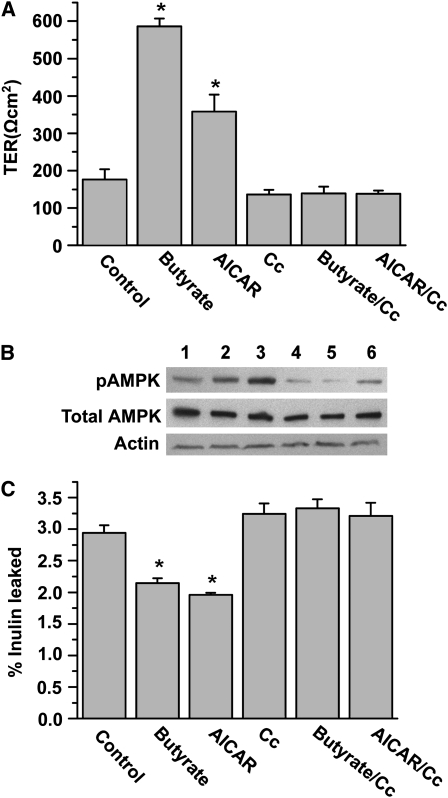
To further determine whether the facilitating effect of butyrate on barrier function involves the AMPK pathway, we compared the effects of butyrate on TER and AMPK phosphorylation in Caco-2 cell monolayers with that of 5-aminoimidazole-4-carboxamide 1-[β]-D-ribonucleoside (AICAR), an AMP analogue, in the presence or absence of compound C, a specific inhibitor of AMPK. As expected, incubation with butyrate at 2 mmol/L for 72 h led to significant increases in TER in the Caco-2 cell monolayers. AICAR (Cell Signaling) at 0.5 mmol/L had a similar effect compared with that of butyrate. However, these effects were abolished by the presence of 10 μmol/L of compound C (Sigma) (Fig. 2A). Corresponding to the changes of TER, both butyrate and AICAR induced an increased level of AMPK phosphorylation and these effects were inhibited by compound C (Fig. 2B). We also examined permeability of the tight junctions by measuring paracellular flux of inulin-FITC in Caco-2 cell monolayers in the presence of 2 mmol/L of butyrate or 0.5 mmol/L of AICAR, with or without 10 μmol/L of compound C, respectively. Incubation of butyrate or AICAR led to a significant decrease in the flux rate across the Caco-2 cell monolayers at the 72-h time point. These effects were also abolished by the treatment of compound C (Fig. 2C).
FIGURE 2 .
AMPK is involved in butyrate regulation of the barrier function in Caco-2 cell monolayers. (A) Caco-2 cells were incubated for 72 h in trans-well inserts with 2 mmol/L butyrate, 0.5 mmol/L AICAR, 10 μmol/L compound C (Cc), or butyrate with Cc and AICAR with Cc, respectively. (B) Extracts from Caco-2 cells at 72 h were immunoblotted for pAMPK, total AMPK, and actin. Lanes 1–6 are medium control, butyrate at 2 mmol/L, AICAR at 0.5 mmol/L, Cc at 10 μmol/L, butyrate+Cc, and AICAR+Cc, respectively. (C) Paracellular flux was determined using FITC-inulin probes in Caco-2 cell monolayers treated similarly for 72 h. In A and C, values are means + SE, n = 6. *Different from control, P < 0.05.
Effect of butyrate on the assembly of tight junctions.
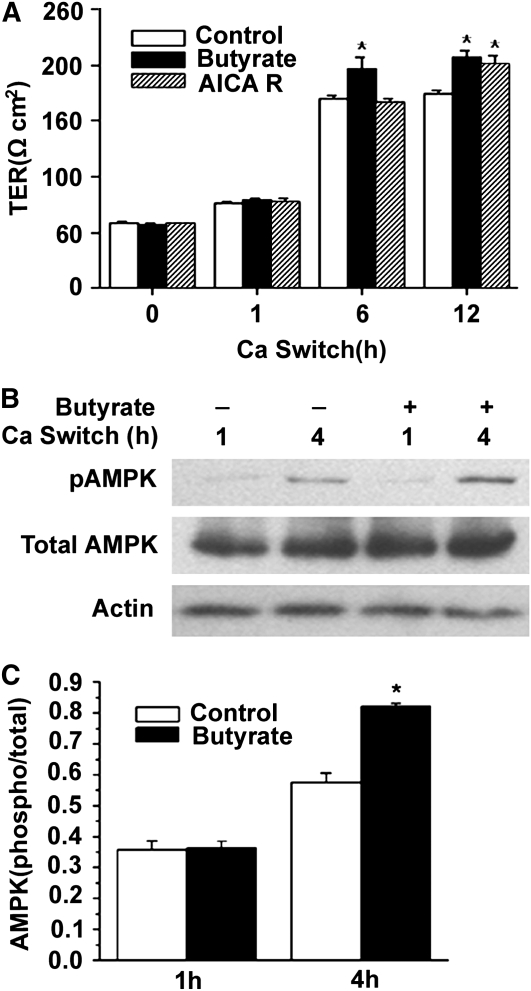
We next investigated the effect of butyrate on the process of tight junction assembly by a calcium switch assay, which has been widely used to study the assembly of epithelial tight junctions in Madin-Darby canine kidney epithelial cell monolayer. Using the same principle, we performed the calcium switch assay in the Caco-2 cell monolayers. We used AICAR as a positive control in the experiment. Both butyrate at 2 mmol/L and AICAR at 0.5 mmol/L enhanced the assembly of the tight junctions after a calcium switch in Caco-2 cell monolayers as measured by the changes in TER. Butyrate caused a more rapid effect than AICAR in enhancing the establishment of TER (Fig. 3A).
FIGURE 3 .
Effects of butyrate on the tight junction assembly and AMPK activation in Caco-2 cell monolayers after calcium switch. (A) After incubation in a low-calcium medium for 16 h, cell monolayers were switched to a regular calcium medium alone or with 2 mmol/L of butyrate, or with 0.5 mmol/L of AICAR. Values are means + SE, n = 5. *Different from control at that time, P ≤ 0.05. B. Total cell lysates from untreated cells or those treated with 2 mmol/L of butyrate at indicated calcium-switch time points were subjected to immunoblotting for pAMPK, total AMPK, and actin with specific antibodies. (C) AMPK activity was expressed as the ratio of the phosphorylated form of the protein to total protein. Values are means + SE, n = 8. *Different from control at that time, P ≤ 0.05.
To determine the effect of butyrate on the activation of AMPK during calcium switch-induced assembly of tight junctions in Caco-2 cell monolayers, we performed a time-dependent assay to measure the levels of AMPK phosphorylation with immunoblotting. At the 4-h time point, however, the level of AMPK Thr-172 phosphorylation increased significantly with 2 mmol/L butyrate compared with that of medium control (Fig. 3B,C). The increases in AMPK Thr-172 phosphorylation happened before the corresponding changes in TER (Fig. 3A), indicating that the biochemical events may happen before the function changes. These data demonstrate that the promoting effect of butyrate on the activation of AMPK also occurs in intestinal epithelial cells during calcium-induced assembly of tight junctions.
Butyrate promotes redistribution of tight junction protein ZO-1 and occludin.
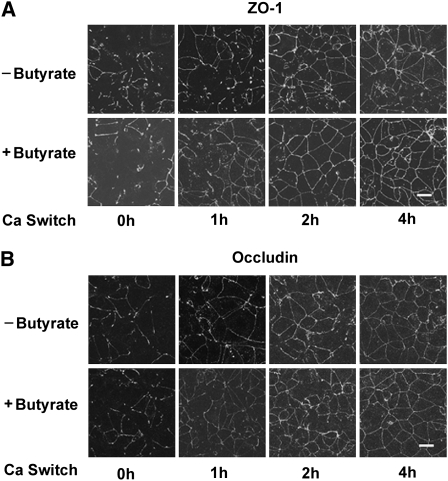
At the molecular level, depletion of calcium in the medium causes translocation of the tight junction proteins ZO-1 and occludin from the cell periphery to the cytoplasm. Upon readdition of calcium, tight junction proteins ZO-1 and occludin move back to the tight junction as they assemble. Thus, we used immunofluorescence staining and confocal microscopy to examine the effect of butyrate on the time courses of the relocation of ZO-1 and occludin after the calcium switch. The depletion of calcium in the medium resulted in the disruption of the junctional organization of ZO-1 and occludin. One hour after readdition of calcium, only partial and sparse reorganization of ZO-1 at the intercellular junctions was achieved in the control cells. However, in the presence of 2 mmol/L of butyrate, the majority of ZO-1 was relocalized to the cell periphery (Fig. 4A). Similarly, at the 1-h time point, immunofluorescence for occludin displayed a weak but continuous pericellular band in the cells incubated in medium with butyrate, but the staining band in the control cells still remained in a relatively interrupted state (Fig. 4B). By 2 h after calcium switch, both ZO-1 and occludin relocalization to the tight junctions were almost completed in the cells treated with butyrate. In contrast, some discontinuous fluorescence bands for ZO-1 were still present in the control cells (Fig. 4A). The relocation of occludin appeared to be completed by 4 h in both the control and butyrate-treated cell monolayers (Fig. 4B). These results morphologically demonstrate that butyrate accelerates relocation of ZO-1 and occludin to the tight junctions during calcium switch-induced tight junction assembly, which enhances the barrier function as determined by the increases in TER in the Caco-2 cell monolayer model.
FIGURE 4 .
Butyrate facilitates redistribution of ZO-1 and occludin during calcium switch-induced tight junction assembly Caco-2 cells were grown in normal DMEM for 2–3 d to confluence and subjected to incubation with a low-calcium medium for 16 h. When calcium was switched back into the medium in the absence or presence of butyrate at 2 mmol/L for indicated times, the cell monolayers were fixed and the distribution of ZO-1 (A) and occludin (B) in intercellular junctions was visualized by probing the target proteins with specific antibodies. The fluorescence images were obtained with a confocal laser scanning microscope (scale bars = 20 μm).
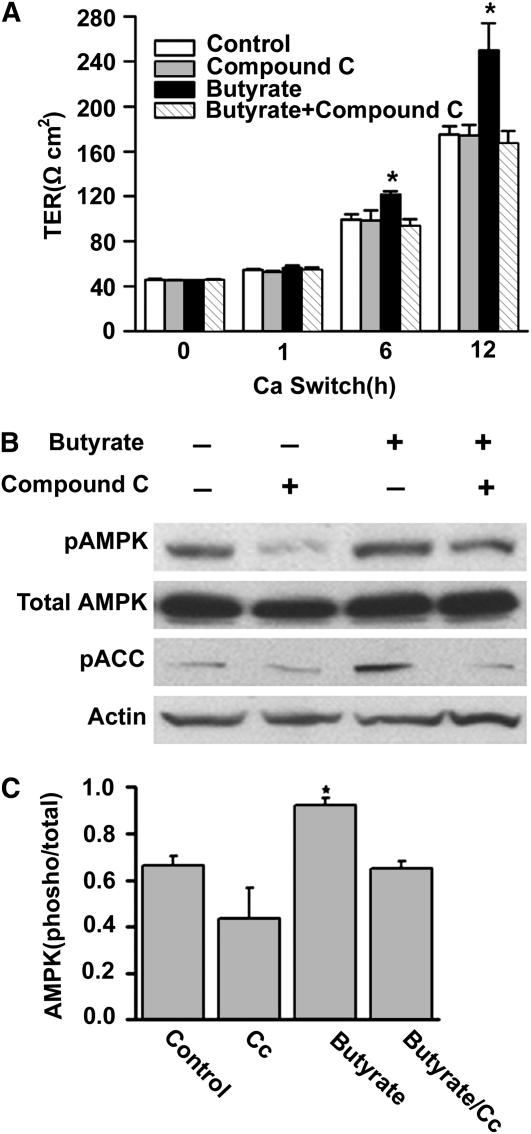
Effect of AMPK inhibitor on the butyrate regulation of tight junction assembly.
To further determine the role of AMPK activation in the facilitating effect of butyrate on the tight junction assembly, we further performed a calcium switch assay in Caco-2 cell monolayers in the presence of compound C. After a 16-h incubation with low calcium medium, Caco-2 cells were treated with medium with vehicle alone, medium with 2 mmol/L butyrate, medium with 10 μmol/L compound C, and medium with 2 mmol/L butyrate plus 10 μmol/L compound C. As expected, the normal assembly of tight junctions as reflected by the establishment of TER after the calcium switch was facilitated by the treatment with butyrate (Fig. 5A). This effect was abolished in the cell monolayers by the co-treatment with compound C. Furthermore, the normal establishment of TER in the cell monolayers after the calcium switch was not altered by the presence of 10 μmol/L compound C. However, at a higher concentration (40 μmol/L), compound C also inhibited the normal establishment of TER in the cell monolayers after the calcium switch (data not shown). Immunoblotting results showed that the butyrate-induced activation of AMPK was totally inhibited by the treatment with compound C, as evidenced by the obvious inhibition of butyrate effect on the levels of both pAMPK and pACC at the 4-h time point after calcium switch (Fig. 5B,C). These data further support our hypothesis that the facilitating effect of butyrate on the tight junction assembly is mediated by the activation of AMPK.
FIGURE 5 .
Specific AMPK inhibitor abolishes the facilitating effect of butyrate on tight junction assembly. After 16 h of incubation with low-calcium medium, Caco-2 cells were treated with normal medium/vehicle (medium), 10 μmol/L of compound C, 2 mmol/L of butyrate, and 2 mmol/L of butyrate plus 10 μmol/L of compound C, respectively. (A) The facilitating effect of butyrate on the increases in TER after calcium switch was abolished by the co-treatment of compound C. Values are means + SE, n = 4. *Different from other groups at that time, P < 0.05. (B) Total lysates were isolated from the cells after 4 h of treatment and subjected to immunoblotting for pAMPK, total AMPK, pACC, and actin with specific antibodies. Compound C significantly inhibited the butyrate-induced activation of AMPK. (C) Active AMPK is defined as the band intensity of pAMPK Thr-172 to that of total AMPK. Values are means + SE, n = 8. *Different from control, P < 0.05.
Discussion
Enteral feeding and normal bacterial colonization play important roles in the postnatal development and maintenance of the intestinal barrier. The absence of luminal nutrition is usually associated with deleterious consequences to the intestinal barrier (22). As the important end products of bacterial fermentation in the intestinal lumen, SCFA have been proposed to be the key molecules that mediate the regulatory effect of normal intestinal flora on mucosal immune system (23). The presence of luminal SCFA may also be very important for the establishment and maintenance of the intestinal mucosal barrier based on the previous in vitro studies, which demonstrate that SCFA in physiological concentrations enhance the intestinal barrier function (13,14). Our current study further demonstrates that butyrate enhances the intestinal barrier function by facilitating the assembly of tight junctions via activating AMPK.
The Caco-2 cell monolayer has been widely used as an in vitro model of the intestinal barrier (24,25). By using this model, we have previously shown that butyrate in physiological concentration promotes intestinal barrier function as measured by increases in TER and decreases in inulin permeability (13). It has been speculated that the effect of SCFA on the epithelial barrier may be mediated by regulating the expression of tight junction-associated proteins (14). Among the tight junction proteins, occludin and claudins are the main transmembrane proteins that contribute to the paracellular seal. ZO-1 is a cytoplasmic plaque protein that interacts with both transmembrane proteins and cytoskeletal proteins (26,27). In the present study in Caco-2 cells, we found that the treatment with butyrate did not significantly alter the expression of some main tight junction proteins, including occludin, claudin-1, claudin-4, and ZO-1. The regulation of barrier function by butyrate in the Caco-2 cell model is therefore unlikely to be explained by the changes in the expression of these proteins. Rather, we demonstrate that the butyrate treatment increases AMPK activity and accelerates the assembly of tight junctions as demonstrated by the reorganization of tight junction proteins as well as the development of TER. AMPK activity is also upregulated by butyrate during calcium switch-induced tight junction assembly. These data suggest that butyrate facilitation of the assembly and organization of the tight junction proteins involves activation of AMPK.
The assembly of the tight junction proteins is dynamically regulated and very important for the formation and maintenance of the barrier function (28). Tight junction assembly involves translocation of tight junction protein ZO-1 and occludin from cytoplasm to the tight junction (17,18). AICAR, the AMP analogue, has been demonstrated to facilitate tight junction assembly in Madin-Darby canine kidney cells by activating AMPK (17). AICAR rapidly enters into cells and is phosphorylated to form the AMP mimetic S-aminoimidazole-4-carboxamide ribonucleoside monophosphate, which activates AMPK without changing the intracellular levels of AMP or ATP (29). We show here that butyrate not only increases the AMPK activity but also has a similar effect as AICAR in facilitating the assembly of tight junctions in Caco-2 cells. Furthermore, compound C, a cell-permeable competitive specific AMPK inhibitor (30,31), inhibits the butyrate-induced activation of AMPK. The facilitating effect of butyrate on the increases in TER in regular culture condition as well as after calcium switch is abolished by compound C. These data further indicate that butyrate enhances intestinal barrier function by facilitating tight junction assembly and the regulatory effect of butyrate on the assembly of the tight junction proteins depends on the activation of AMPK. Our data are consistent with the recent findings by others in which tight junction assembly is impaired when AMPK activity is downregulated by a genetic manipulation strategy (17,18) and consistent with the hypothesis that the activation of AMPK is involved in butyrate regulation of tight junction assembly in intestinal epithelial cells. Of note, the effect of butyrate on AMPK activation and the barrier functions is not exactly the same compared with that of AICAR, as demonstrated in Figures 2 and 3. This difference suggests that the mechanism by which AMPK is activated by butyrate may be different from AICAR. Furthermore, in addition to AMPK activation, other mechanisms may also be involved in butyrate regulation of barrier function.
AMPK functions as a cellular fuel gauge that regulates metabolic pathways in glucose and fatty acid metabolism and protein synthesis (19). The increases of pAMPK and pACC in Caco-2 cells by butyrate imply that butyrate can activate AMPK in intestinal epithelial cells either directly or indirectly. There are at least 3 possible mechanisms involved in the activation of AMPK: 1) allosteric activation of the phosphorylated enzyme; 2) promotion of phosphorylation of Thr-172 by the upstream kinase; and 3) inhibition of dephosphorylation of Thr-172 by protein phosphatases (32,33). AMPK activity is exquisitely regulated by the changes in the cellular AMP:ATP ratio. AMPK is activated by the elevation in cellular 5′-AMP that accompanies a decrease in the ATP:ADP ratio due to the reaction catalyzed by adenylate kinase (34). In the activation of fatty acids to fatty acyl-CoA, AMP is generated in the cytoplasm. Indeed, it has been shown that acetate, one of the SCFA, can increase the AMP:ATP ratios (35) and activates AMPK activity in hepatocytes (35,36). Other pathways through which AMPK is activated may involve the upstream kinase(s). So far, the upstream kinases identified to be involved in the activation of AMPK include LKB1 and calcium/calmodulin-dependent protein kinase. Some studies have strongly suggested that AMPK activity is regulated by changes in intracellular calcium concentrations through changes in calcium/calmodulin-dependent protein kinase activity (37–39). Whether an increased AMP:ATP ratio in Caco-2 cells is the main mechanism underlying the butyrate activation of AMPK or the upstream kinases are involved in the butyrate activation of AMPK needs to be determined. Furthermore, how AMPK activation promotes the assembly of tight junction proteins remains to be elucidated. Occludin phosphorylation at threonine residues (T403 and T404) has been found to play a crucial role in the assembly of tight junctions in Caco-2 cell monolayers (40). However, occludin phosphorylation at Tyr-398 and Tyr-402 seems to prevent its interaction with ZO-1 (41). The phosphorylation status of tight junction components is clearly important in the assembly of tight junctions but more complex than we expected.
In humans, the proximal-to-distal passage of undigested carbohydrates across the ileo-cecal valve occurs normally once enteral feeding is introduced and normal bacterial colonization is established. In the anaerobic environment of the colon, bacteria rapidly ferment undigested carbohydrates such as dietary fiber to SCFA (8). The absorption of SCFA in the colon is coupled with sodium absorption (42). Once the SCFA are absorbed by the colonocyte, they are either locally used as fuel for the colonic mucosal epithelial cells or enter the portal bloodstream (43,44). It has been shown previously that increasing luminal SCFA concentration by dietary fiber in normal rats improves mucosal barrier function in the colon (45). This was further supported by a recent ex vivo study in which it was found that physiological concentrations of SCFA promote epithelial barrier functions in rat cecal walls mounted on Ussing-type chambers (46). However, it remains to be determined whether the activation of AMPK is also involved as a mechanism underlying the in vivo effect of SCFA on the intestinal barrier. If the pathway of AMPK activation is indeed involved in the SCFA regulation of the intestinal barrier in vivo, this may form a basis for the design of therapeutic or preventive strategies for the diseases related to an impaired intestinal barrier.
In summary, the results of this study demonstrate that the butyrate regulation of barrier function in the Caco-2 cell monolayer model is unlikely to be explained by the changes in the levels of expression of some major tight junction proteins. Rather, butyrate enhances the intestinal barrier function, at least partly, by facilitating the assembly of tight junctions. This dynamic process is mediated by the activation of AMPK. These results suggest an intriguing link between SCFA and intracellular energy sensor for the maintenance of intestinal barrier function. How AMPK is activated by butyrate remains to be elucidated. Furthermore, it remains to be determined if the in vivo effect of SCFA on intestinal barrier and other metabolic pathways also involves activation of AMPK.
Supported by NIH R03-HD060753.
Author disclosures: L. Peng, Z.-R. Li, R. S. Green, I. R. Holzman, and J. Lin, no conflicts of interest.
Abbreviations used: ACC, acetyl-CoA carboxylase; AICAR, 5-aminoimidazole-4-carboxamide 1-[β]-d-ribonucleoside; AMPK, AMP-activated protein kinase; inulin-FITC, fluorescein isothiocyanate-conjugated inulin; pACC, phosphorylated acetyl-CoA carboxylase; pAMPK, phosphorylated AMP-activated protein kinase; TER, transepithelial electrical resistance.
References
- 1.Walker WA. Development of the intestinal mucosal barrier. J Pediatr Gastroenterol Nutr. 2002;34:S33–9. [DOI] [PubMed] [Google Scholar]
- 2.de Santa Barbara P, van den Brink GR, Roberts DJ. Development and differentiation of the intestinal epithelium. CMLS. Cell Mol Life Sci. 2003;60:1322–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rouwet EV, Heineman E, Buurman WA, Riet GT, Ramsay G, Blanco CE. Intestinal permeability and carrier-mediated monosaccharide absorption in preterm neonates during the early postnatal period. Pediatr Res. 2002;51:64–70. [DOI] [PubMed] [Google Scholar]
- 4.Udall JN, Pang K, Fritze L, Kleinman R, Walker WA. Development of gastrointestinal mucosal barrier. I. The effect of age on intestinal permeability to macromolecules. Pediatr Res. 1981;15:241–4. [DOI] [PubMed] [Google Scholar]
- 5.Udall JN, Colony P, Fritze L, Pang K, Trier JS, Walker WA. Development of gastrointestinal mucosal barrier. II. The effect of natural versus artificial feeding on intestinal permeability to macromolecules. Pediatr Res. 1981;15:245–9. [DOI] [PubMed] [Google Scholar]
- 6.Schumann A, Nutten S, Donnicola D, Comelli EM, Mansourian R, Cherbut C, Corthesy-Theulaz I, Garcia-Rodenas C. Neonatal antibiotic treatment alters gastrointestinal tract developmental gene expression and intestinal barrier transcriptome. Physiol Genomics. 2005;23:235–45. [DOI] [PubMed] [Google Scholar]
- 7.Kansagra K, Stoll B, Rognerud C, Niinikoski H, Ou CN, Harvey R, Burrin D. Total parenteral nutrition adversely affects gut barrier function in neonatal piglets. Am J Physiol Gastrointest Liver Physiol. 2003;285:G1162–70. [DOI] [PubMed] [Google Scholar]
- 8.Bond JH, Levitt MD. Fate of soluble carbohydrate in the colon of rats and man. J Clin Invest. 1976;57:1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakata T. Stimulatory effect of short-chain fatty acids on epithelial cell proliferation in the rat intestine: a possible explanation for trophic effects of fermentable fiber, gut microbes and luminal trophic factors. Br J Nutr. 1987;58:95–103. [DOI] [PubMed] [Google Scholar]
- 10.Roediger WEW. Utilization of nutrients by isolated epithelial cells of rat colon. Gastroenterology. 1982;83:424–9. [PubMed] [Google Scholar]
- 11.Koruda MJ, Rolendalli RH, Bliss DZ, Hastings J, Rombeau JL, Settle RG. Parenteral nutrition supplemented with short-chain fatty acids: effect on the small-bowel mucosa in normal rats. Am J Clin Nutr. 1990;51:685–9. [DOI] [PubMed] [Google Scholar]
- 12.Nankova BB, Chua J, Mishra R, Kobasiuk CD, La Gamma EF. Nicotinic induction of preproenkephalin and tyrosine hydroxylase gene expression in butyrate-differentiated rat PC12 cells: a model for adaptation to gut-derived environmental signals. Pediatr Res. 2003;53:113–9. [DOI] [PubMed] [Google Scholar]
- 13.Peng L, He ZJ, Chen W, Holzman IR, Lin J. Effects of short chain fatty acid on intestinal barrier function in a Caco-2 cell monolayer model. Pediatr Res. 2007;61:37–41. [DOI] [PubMed] [Google Scholar]
- 14.Mariadason JM, Barkla DH, Gibson PR. Effect of short-chain fatty acids on paracellular permeability in Caco-2 intestinal epithelium model. Am J Physiol. 1997;272:G705–12. [DOI] [PubMed] [Google Scholar]
- 15.Anderson JM, Van Itallie CM. Tight junctions and the molecular basis for regulation of paracellular permeability. Am J Physiol. 1995;269:G467–75. [DOI] [PubMed] [Google Scholar]
- 16.Mitic LL, Van Itallie CM, Anderson JM. Molecular physiology and pathophysiology of tight junctions. I. Tight junction structure and function: lessons from mutant animals and proteins. Am J Physiol Gastrointest Liver Physiol. 2000;279:G250–4. [DOI] [PubMed] [Google Scholar]
- 17.Zhang L, Li J, Young LH, Caplan M. AMP-activated protein kinase regulates the assembly of epithelial tight junctions. Proc Natl Acad Sci USA. 2006;103:17272–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zheng B, Cantley LC. Regulation of epithelial tight junction assembly and disassembly by AMP-activated protein kinase. Proc Natl Acad Sci USA. 2007;104:819–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hardie DG, Hawley SA, Scott JW. AMP-activated protein kinase-development of the energy sensor concept. J Physiol. 2006;574:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardie DG. The AMP-activated protein kinase pathway: new players upstream and downstream. J Cell Sci. 2004;117:5479–87. [DOI] [PubMed] [Google Scholar]
- 21.Hardie DG, Pan DA. Regulation of fatty acid synthesis and oxidation by the AMP-activated protein kinase. Biochem Soc Trans. 2002;30:1064–70. [DOI] [PubMed] [Google Scholar]
- 22.MacFie J. Enteral versus parenteral nutrition: the significance of bacterial translocation and gut-barrier function. Nutrition. 2000;16:606–11. [DOI] [PubMed] [Google Scholar]
- 23.Sanderson IR. Short chain fatty acid regulation of signaling genes expressed by the intestinal epithelium. J Nutr. 2004;134:2450S–4S. [DOI] [PubMed] [Google Scholar]
- 24.Li N, Lewis P, Don Samuelson D, Liboni K, Neu J. Glutamine regulates Caco-2 cell tight junction proteins. Am J Physiol Gastrointest Liver Physiol. 2004;287:G726–33. [DOI] [PubMed] [Google Scholar]
- 25.Basuroy S, Sheth P, Kuppuswamy D, Balasubramanian S, Ray RM, Rao RK. Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J Biol Chem. 2003;278:11916–24. [DOI] [PubMed] [Google Scholar]
- 26.Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM. The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem. 1998;273:29745–53. [DOI] [PubMed] [Google Scholar]
- 27.Yu D, Turner JR. Stimulus-induced reorganization of tight junction structure: the role of membrane traffic. Biochim Biophys Acta. 2008;1778:709–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson JM, Van Italie CM, Fanning AS. Setting up a selective barrier at the apical junction complex. Curr Opin Cell Biol. 2004;16:140–5. [DOI] [PubMed] [Google Scholar]
- 29.Corton JM, Gillespie JG, Hawley SA, Hardie DG. 5-Aminoimidazole-4-carboxamide ribonucleoside. A specific method for activating AMP-activated protein kinase in intact cells? Eur J Biochem. 1995;229:558–65. [DOI] [PubMed] [Google Scholar]
- 30.Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, et al. Role of AMP-activated protein kinase in mechanism of metformin action. J Clin Invest. 2001;108:1167–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gauthier MS, Miyoshi H, Souza SC, Cacicedo JM, Saha AK, Greenberg AS, Ruderman NB. AMP-activated protein kinase is activated as a consequence of lipolysis in the adipocyte. J Biol Chem. 2008;283:16514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crute BE, Seefeld K, Gamble J, Kemp BE, Witters LA. Functional domains of the alpha1 catalytic subunit of the AMP-activated protein kinase. J Biol Chem. 1998;273:35347–54. [DOI] [PubMed] [Google Scholar]
- 33.Kahn BB, Alquier T, Carling D, Hardie DG. AMP-activated protein kinase: Ancient energy gauge provides clues to modern understanding of metabolism. Cell Metab. 2005;1:15–25. [DOI] [PubMed] [Google Scholar]
- 34.Hardie DG, Hawley SA. AMP-activated protein kinase: the energy charge hypothesis revisited. Bioessays. 2001;23:1112–9. [DOI] [PubMed] [Google Scholar]
- 35.Kawaguchi T, Osatomi K, Yamashita H, Kabashima T, Uyeda K. Mechanism for fatty acid "sparing" effect on glucose-induced transcription: regulation of carbohydrate-responsive element-binding protein by AMP-activated protein kinase. J Biol Chem. 2002;277:3829–35. [DOI] [PubMed] [Google Scholar]
- 36.Sakakibara S, Yamauchi T, Oshima Y, Tsukamoto Y, Kadowaki T. Acetic acid activates hepatic AMPK and reduces hyperglycemia in diabetic KK-A (y) mice. Biochem Biophys Res Commun. 2006;344:597–604. [DOI] [PubMed] [Google Scholar]
- 37.Woods A, Dickerson K, Heath R, Hong SP, Momcilovic M, Johnstone SR, Carlson M, Carling D. Ca2+/calmodulin-dependent protein kinase kinase-beta acts upstream of AMP-activated protein kinase in mammalian cells. Cell Metab. 2005;2:21–33. [DOI] [PubMed] [Google Scholar]
- 38.Hawley SA, Pan DA, Mustard KJ, Ross L, Bain J, Edelman AM, Frenguelli BG, Hardie DG. Calmodulin-dependent protein kinase kinase-beta is an alternative upstream kinase for AMP-activated protein kinase. Cell Metab. 2005;2:9–19. [DOI] [PubMed] [Google Scholar]
- 39.Hurley RL, Anderson KA, Franzone JM, Kemp BE, Means AR, Witters LA. The Ca2+/Calmodulin-dependent protein kinase kinases are AMP-activated protein kinase kinases. J Biol Chem. 2005;280:29060–6. [DOI] [PubMed] [Google Scholar]
- 40.Suzuki T, Elias BC, Seth A, Shen L, Turner JR, Giorgianni F, Desiderio D, Guntaka R, Rao R. PKC eta regulates occludin phosphorylation and epithelial tight junction integrity. Proc Natl Acad Sci USA. 2009;106:61–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Elias BC, Suzuki T, Seth A, Giorgianni F, Kale G, Shen L, Turner JR, Naren A, Desiderio DM, et al. Phosphorylation of Tyr-398 and Tyr-402 in occludin prevents its interaction with ZO-1 and destabilizes its assembly at the tight junctions. J Biol Chem. 2009;284:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Krishnan S, Ramakrishna BS, Binder HJ. Stimulation of sodium chloride absorption from secreting rat colon by short-chain fatty acids. Dig Dis Sci. 1999;44:1924–30. [DOI] [PubMed] [Google Scholar]
- 43.Pouteau E, Nguyen P, Ballevre O, Krempf M. Production rates and metabolism of short-chain fatty acids in the colon and whole body using stable isotopes. Proc Nutr Soc. 2003;62:87–93. [DOI] [PubMed] [Google Scholar]
- 44.Kien CL, Chang JC, Cooper JR. Quantitation of colonic luminal synthesis of butyric acid in piglets. J Pediatr Gastroenterol Nutr. 2002;35:324–8. [DOI] [PubMed] [Google Scholar]
- 45.Mariadason JM, Catto-Smith A, Gibson PR. Modulation of distal colonic epithelial barrier function by dietary fiber in normal rats. Gut. 1999;44:394–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suzuki T, Yoshida S, Hara H. Physiological concentrations of short-chain fatty acids immediately suppress colonic epithelial permeability. Br J Nutr. 2008;100:297–305. [DOI] [PubMed] [Google Scholar]