Abstract
Nonglucose carbohydrates such as mannose and inositol are important in early growth and development, although little is known about their metabolism. Our aim in this study was to determine the plasma appearance rates (Ra) for mannose and inositol in newborns as an index of utilization and as an improved guide to supplementation practices. We studied late-preterm (n = 9) and term (n = 5) infants (median 34 wk gestation, range 33–41 wk) using a multiple isotope infusion start time protocol to determine Ra for each carbohydrate. The plasma mannose concentration [median (range)] was 69.83 (48.60–111.75) μmol/L and the Ra was 0.59 (0.42–0.98) μmol·kg−1·min−1 (854 μmol·kg−1·d−1). The plasma inositol concentration was 175.74 (59.71–300.60) μmol/L and Ra was 1.06 (0.33–1.75) μmol·kg−1·min−1 (1521 μmol·kg−1·d−1). The Ra for mannose and inositol are >10-fold higher than the amounts a breast-fed infant typically ingests, which are ∼6 μmol·kg−1·d−1 mannose and 150 μmol·kg−1·d−1 inositol. Thus, for both mannose and inositol, the newborn infant must produce these compounds from glucose at rates sufficient to meet nutritional requirements.
See commentary: Kalhan, J. Nutr. 1611-2, 2009
Introduction
Nonglucose carbohydrates such as inositol (present in biological systems primarily as myo-inositol) and mannose (biologically active as d-mannose) have specific functions in fetal and neonatal nutrition and development. Inositol plays a role in many important biological functions, including the regulation of cell osmolality (1), phosphoinositide-mediated processes of cell signaling (2,3), formation of the neural system (4), pulmonary surfactant phospholipid production (5,6), and host defense (7). Several studies have proposed an important nutritional role for inositol in early human development. As early as the first trimester of pregnancy, inositol is concentrated in the intervillus, coelomic, and amniotic fluids compared with maternal serum, consistent with production of inositol by the conceptus (8). At term, inositol concentrations are highest in the umbilical artery compared with the umbilical vein and maternal serum (9). An uptake of inositol into the placenta from the fetal circulation indicates that there is high fetal inositol production to meet fetal requirements. Furthermore, inositol is present at relatively high concentrations in human breast milk (∼1200 μmol/L), 3rd only to lactose and glucose, suggesting exogenous inositol requirements postnatally (10).
Mannose is a biologically important molecule for N- and O-glycosylation, mannosylation, and glycosylphosphatidylinositol anchor synthesis (11). Mannose may be essential to the developing fetus, because there is a considerable uptake of mannose into the fetal circulation despite the relatively low plasma concentrations in the maternal circulation (9). Free mannose also is present in breast milk (∼40 μmol/L) (10), although there may be even more mannose available in milk in the form of oligosaccharides, which contribute to the establishment of nonpathogenic colonic flora and inhibit binding of pathogens to the intestinal epithelial cell (12,13).
Although there have been many studies of glucose metabolic rates due to the relatively high frequency of plasma glucose abnormalities in neonates (14–17), there have been no metabolic studies in infants or children, to our knowledge, that aimed to define the utilization rates of inositol and mannose. Given the high concentration of inositol in breast milk, inositol has been widely supplemented in term and preterm formulas without basic information about utilization rates in the newborn. Furthermore, most parenteral nutrition (PN)3 solutions contain little or no inositol (18), with no known consequences attributable to inositol deficiency. Thus, in this study, we aimed to determine the plasma appearance rate (Ra) for inositol and mannose in late-preterm and term infants as an index of utilization and as an improved guide to more accurate supplementation practices.
Methods
Participants.
This study was approved by the Institutional Review Board at the University of Colorado Denver, Aurora, CO, and was supported by the NIH-University of Colorado Denver Pediatric Clinical Translational Research Center. Informed consent was obtained from the parent or guardian of the infants (both parents when possible) prior to the study by Clinical Translational Research Center nurses. Infants were recruited from the neonatal intensive care unit at the University of Colorado Hospital and were considered eligible for the study if they were ≥32 wk gestation, clinically stable, and had an i.v. in place for other clinical indications. Infants with respiratory distress syndrome who required a brief period of minimally supportive mechanical ventilation, nasal continuous positive airway pressure, or supplemental oxygen were included. The nutritional intake of the infants varied from PN only, milk (breast milk or standard formula) feedings only, or both modes of nutritional support. PN at our institution contains no mannose or inositol. Per our previous study, we estimated that breast milk, term formula, and preterm formula inositol concentration ranges from 400 to 2200 μmol/L and mannose content ranges from 40 to 140 μmol/L (10). If the infant was receiving enteral feeds, the study was performed during the 3-h time period between feedings to best approximate endogenous carbohydrate production.
For each infant studied, the following information was collected: gestational age, postnatal age, birthweight, infant weight at the time of the study, sex, milk type, intake volume/kg body weight (for breast-fed infants, intake volume was determined by weighing the infant before and after the feeding), composition of PN, and clinical indication for i.v. placement.
Study protocol.
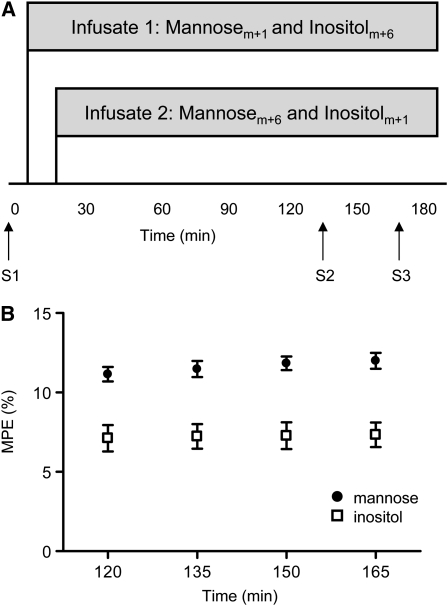
A multiple infusion start time protocol was used in this study, which staggers the start time of stable isotope infusions. Each infusate contained a different isotopomer of mannose and inositol and therefore minimized the number of blood samples required to measure the steady-state Ra of each carbohydrate in an infant (19). Isotopes were reconstituted and diluted in dextrose 5% in water at 0.3 g/L. After sterilization by filtration (0.22-μm filter), samples were tested for pyrogen content and sterility cultures were performed before the isotopes were utilized in infants. Baseline blood samples were obtained by heel stick for molar percent enrichment (MPE) and plasma concentration measurements. Infusate 1 contained [1-13C] mannose (mannosem+1) and [2H6] myo-inositol (inositolm+6) (both from Cambridge Isotope Laboratories) and was started at time 0 at an infusion rate of 2 mL·kg−1·h−1 (Fig. 1A). Infusate 2 contained 2 different isotopomers of mannose and inositol, [13C6] mannose (mannosem+6, Isotec) and [2-2H] myo-inositol (inositolm+1, Cambridge Isotope Laboratories) and was started 15 min later at the same rate of infusion. Two more blood samples were obtained 30 min apart at 135 and 165 min after starting infusate 1. Approximately 0.5 mL of blood was obtained per sample, resulting in a total of 1.5 mL of blood withdrawn from each patient during the study. Because different isotopomers of each carbohydrate were started 15 min apart, these 2 blood samples provided MPE measurements at 4 time points (120, 135, 150, and 165 min).
FIGURE 1 .
Experimental study design (A) in which term and late-preterm infants were infused with mannose and inositol and samples obtained at baseline (S1) and under steady-state conditions (S2, S3). MPE for mannose and inositol were determined at 4 time points after infusion began (B). MPE were corrected for the minor differences in tracer infusate concentrations between infusate 1 and infusate 2. Values are means ± SEM, n = 14.
Plasma carbohydrate concentration and enrichment analysis.
Allose and chiro-inositol, which are stereoisomers of mannose and inositol, respectively, were used as internal standards to account for the loss of carbohydrate during the following sample preparation and derivatization steps. Human plasma glucose concentration is ∼40-fold higher than mannose concentration. Chromatographically, the high-glucose concentration interferes with the determination of mannose concentration. Therefore, glucose was removed from each sample by the action of glucose oxidase (20). Plasma (0.05 mL) was added to 0.2 mL water and 0.05 mL of internal standards (10 mg/L of chiro-inositol and 10 mg/L of allose) followed by 280 U of catalase and 12 U of glucose oxidase. After incubating at ambient temperatures for 90 min, the reaction was stopped by the addition of 0.15 mL of 0.3 mol/L ZnSO4 and 0.15 mL of 0.3 mol/L Ba(OH)2. The protein precipitate was removed by centrifugation at 16,000 × g for 4 min at 40°C. The supernatant was transferred to a test tube and dried under a vacuum.
Isotopic enrichments were determined using GC-MS (Model 5975, Agilent Technologies) equipped with a HP5-MS column (30 m × 0.25 mm × 0.25 μm). Helium was used as the carrier gas at a flow rate of 1 mL/min. The injector inlet and the transfer line were both maintained at 280°C. The initial column temperature was 120°C and held for 1 min. The column temperature was then ramped at a rate of 12°C/min to 179°C and held for 8 min. Finally, column temperature was ramped at a rate of 50°C/min to 320°C and held for 1 min to clean the column. Mannose was converted to the aldononitrile peracetate and inositol was converted to the peracetate (21). A total of 0.1 mL hydroxylamine hydrochloride (20 g/L in anhydrous pyridine) was added to the dried residue. The pyridine solution was incubated at 90°C for 30 min. After cooling, 0.075 mL of acetic anhydride was added and the solution was incubated for another 30 min at 90°C. After cooling, 1 mL of 1 mol/L HCl was added, followed by 2 mL chloroform. The mixture was vortexed and after the separation of the phases, the aqueous layer was removed and discarded. The chloroform solution was washed with another 1 mL of 1 mol/L HCl, then washed sequentially 3 times using 1 mL of water and then dried. The residue was dissolved in 0.05 mL acetonitrile. Mannose was monitored at a mass/charge ratio of 314, 315, 316, 319, 320, and 321. Inositol was monitored at a mass:charge ratio of 373, 374, 375, 379, 380, and 381. For mannose, the ratio of the 315:314 peaks was used to monitor the m+1 MPE and the ratio of the 319:314 peaks was used to monitor the m+6 MPE. For inositol, the ratio of the 374:373 peaks was used to monitor the m+1 MPE and the ratio of the 379:373 peaks was used to monitor the m+6 MPE. The plasma concentrations of mannose and inositol were calculated using the total ion counts of the corresponding ions. The total ion counts were compared with standard curves constructed using unlabeled mannose and inositol.
The Ra of mannose and of inositol were calculated using the equation Ra = IR/TTR, where IR is the tracer infusion rate (μmol·kg−1·min−1) and TTR is the tracer:tracee ratio (22). The plasma clearance rates for mannose and inositol were calculated by dividing IR by the plasma tracer concentration.
Statistical methods.
All analyses assumed a 2-sided test of the hypothesis with an overall significance level of 0.05 and were conducted in SAS v 9.1 (SAS Institute). Values in the text are presented as median and range, unless otherwise specified. We used Wilcoxon's rank-sum test to compare MPE and Ra between mannose and inositol isotopomers. Linear regression was used to model inositol and mannose Ra as a function of plasma concentration, birthweight, and gestational age.
Results
Fourteen infants were evaluated (n = 9 late-preterm and n = 5 term) (Table 1). This distribution of infants reflected the selection criteria, which required that the infants had an i.v. line present for other clinical indications. All patients were appropriate for gestational age based on percentile standards for growth (23) and by fetal growth standards, with the exception of 1 term infant who measured large for gestational age with no clinical evidence of maternal gestational diabetes.
TABLE 1.
Patient clinical data
| Patient | GA1 | PNA1 | BW1 | Sex | Nutrition1 | Milk intake | GIR from PN1 | Indications for i.v. placement |
|---|---|---|---|---|---|---|---|---|
| wk | d | kg | mL/kg | mg·kg−1·min−1 | ||||
| 1 | 34 | 1 | 2.08 | M | PN | 5.5 | RDS, i.v. fluids, antibiotics | |
| 2 | 33 | 0 | 1.86 | M | PN | 5.4 | i.v. fluids, antibiotics | |
| 3 | 33 | 1 | 2.12 | F | PN | 4.7 | RDS, i.v. fluids | |
| 4 | 33 | 1 | 1.80 | F | PN | 5.6 | i.v. fluids | |
| 5 | 34 | 1 | 1.89 | F | PN+M | 6 | 5.6 | i.v. fluids |
| 6 | 33 | 2 | 2.25 | F | PN+M | 2 | 6.9 | RDS, i.v. fluids, antibiotics |
| 7 | 33 | 1 | 2.05 | F | PN+M | 12 | 5.5 | i.v. fluids, antibiotics |
| 8 | 36 | 3 | 2.22 | F | M | 12 | Poor feeding, RDS, antibiotics | |
| 9 | 34 | 2 | 2.30 | M | M | 17 | i.v. fluids | |
| 10 | 40 | 1 | 3.03 | F | PN | 5.2 | Respiratory distress, i.v. fluids | |
| 11 | 38 | 1 | 3.98 | F | PN | 5.2 | Respiratory distress, i.v. fluids | |
| 12 | 41 | 1 | 2.97 | M | PN+M | 5 | 6.9 | Respiratory distress, i.v. fluids |
| 13 | 37 | 1 | 2.78 | F | PN+M | 5 | 0.9 | Respiratory distress, i.v. fluids |
| 14 | 41 | 1 | 4.11 | M | M | 10 | Respiratory distress, antibiotics | |
| Median | 34.0 | 1.0 | 2.2 | 8 | 5.5 | |||
| Mean | 35.7 | 1.2 | 2.5 | 8.6 | 5.2 | |||
| SD | 3.1 | 0.7 | 0.75 | 5.0 | 1.6 |
GA, Gestational age; PNA, postnatal age; BW, birthweight; GIR, glucose infusion rate; RDS, respiratory distress syndrome; PN+M, PN and milk; M, milk.
The MPE of mannose and inositol at the 4 time points analyzed were relatively constant in all infants for both carbohydrates (Fig. 1B). Ra and MPE obtained from mannosem+1 in infusate 1 did not differ from that of mannosem+6 in infusate 2. This was also true for the comparison of Ra in inositolm+1 and inositolm+6.
The median plasma concentrations, Ra, and plasma clearance rates for mannose and inositol are shown in Table 2. Previously published glucose concentrations and Ra in term infants are included in the table for reference (15). Ra (μmol·kg−1·min−1) was modeled as a function of plasma concentration (μmol/L), birthweight, and gestational age. Plasma concentration was the only significant predictor of both mannose and inositol Ra, with r2 estimates of 0.73 and 0.74, respectively. In both cases, this was a positive relationship with a mannose slope estimate of 0.0074 (95% CI 0.005–0.010; P < 0.0001) and an inositol slope estimate of 0.005 (95% CI 0.003–0.006; P < 0.0001).
TABLE 2.
Plasma concentrations and Ra of mannose and inositol in term and late-preterm infants1
| Plasma concentration | Ra | Plasma clearance | |
|---|---|---|---|
| μmol/L | μmol·kg−1·min−1 | mL·kg−1·min−1 | |
| Mannose | 69.83 (48.6–111.75) | 0.59 (0.42–0.98) | 11.52 (8.52–14.38) |
| Inositol | 175.74 (59.71–300.60) | 1.06 (0.33–1.75) | 7.67 (3.97–13.88) |
| Glucose2 | 3780.01 (3047.36–5001.22) | 30.30 (27.56–32.50)† |
Values are median (range), n = 14.
From (15), n = 7.
Discussion
The present study is the first, to our knowledge, to establish Ra for d-mannose and myo-inositol under baseline conditions in the late-preterm and term neonate. We used the multiple infusion start time protocol, which staggers the start time of isotope infusions containing different isotopomers of a substrate that are metabolized in the same way as the naturally occurring forms. This protocol minimizes the number of blood samples required from each infant to accurately measure the steady-state Ra of a substrate. Therefore, we were able to measure the Ra of both mannose and inositol with only 2 blood samples from each patient instead of 4. We also found that plasma concentrations of mannose and inositol predicted their respective Ra. Our results contribute to a growing body of literature that supports the biological and nutritional importance of nonglucose carbohydrates such as mannose and inositol in early growth and development.
The Ra for mannose (854 μmol·kg−1·d−1) and inositol (1521 μmol·kg−1·d−1) were >10-fold higher than the amounts a breast-fed infant typically ingests, which are ∼6 μmol·kg−1·d−1 of mannose and 150 μmol·kg−1·d−1 of inositol (based on 150 mL·kg−1·d−1 of ingested breast milk) (10,24). Thus, the newborn infant must produce both mannose and inositol from glucose at rates sufficient to meet biological requirements. Inositol can either be transported across the intestinal wall by a sodium-dependent transport system (25) or synthesized from d-glucose through the cyclization of glucose-6-phosphate and dephosphorylation of inositol-1-phosphate (26). Mannose also can be effectively absorbed from the gastrointestinal tract and transported into the cell by a specific mannose transporter or synthesized from glucose by isomerization via fructose-6-phosphate (11).
Due to the high de novo synthesis rates of mannose and inositol from glucose, the healthy, full-term, breast-fed infant should not be at risk for developing a deficiency of either carbohydrate. However, this may not be true for infants who are critically ill, such as infants born prematurely and infants of diabetic mothers (IDM). Fetuses and infants born prematurely have higher plasma concentrations of inositol than term infants, which decline to adult levels by ∼8 wk of postnatal age (24,27–29). Extremely low-birthweight babies who require prolonged PN devoid of nonglucose carbohydrates may not be able to maintain endogenous production to meet requirements. Previous studies have demonstrated that prolonged PN in preterm infants is an important predictor of lower plasma inositol concentrations (24,27). Furthermore, supplementation of inositol to preterm infants increases plasma inositol concentrations, reduces neonatal mortality, increases survival without chronic lung disease, and decreases the incidence and severity of retinopathy of prematurity, all findings consistent with inositol deficiency and subsequent adverse effects in the preterm infant (6).
IDM also may be at increased risk for developing a deficiency in inositol and are at increased risk of neural tube defects, which may be in part the result of maternal hyperglycemia and subsequent deficiency of inositol in offspring (30). In rodent models of neural tube defects, a hyperglycemic embryonic environment results in decreased inositol uptake either by competitive inhibition with glucose or by compromised incorporation of inositol into lipid components (31). Inositol supplementation to cultured rat embryos exposed to hyperglycemia and as well as to a mouse model of gestational diabetes reduces the incidence of neural tube defects (4,32,33). Further investigation is needed to determine whether a deficiency in inositol does occur in preterm, prolonged PN-dependent neonates or IDM and whether supplementation to such at-risk groups is therapeutic. Less is known about the potential for mannose deficiency in a preterm neonate or IDM, although an external supply of mannose may be required in some tissues for an optimal rate of mannosylation (11,34).
Plasma concentration was the only significant predictor of both mannose and inositol Ra when Ra was modeled as a function of plasma concentration, birthweight, and gestational age. This finding suggests that supplementation of the diet with either carbohydrate might increase utilization rates, which would be consistent with the metabolism of other major substrates in the fetus and neonate, such as glucose and certain amino acids (35–38). We studied a relatively small number of patients receiving each of the various dietary intakes; thus, we were unable to test the effect of dietary intake on Ra in our study. Previous studies have found positive correlations between inositol intake and plasma inositol concentrations after 2–3 wk of age (24,27,39) and supplementation of inositol in premature infants has been shown to increase plasma concentrations (6). However, supplementing infants with inositol and mannose above what is present in milk might simply suppress endogenous mannose and inositol production from glucose, especially in the healthy, full-term infant.
The Ra of mannose and inositol under fasting steady-state plasma concentrations using a single-pool model equals the sum of endogenous production and exogenous infusion (22). Our study was performed under relatively short-term, fasting conditions (2 h after a milk feeding). We have previously reported in term neonates that a single milk feeding does not change postprandial plasma concentrations of either mannose or inositol (40). Neither carbohydrate was present in PN administered to the infants. We did not specifically measure urinary losses of either substrate, although for inositol, urinary losses are only a small fraction of the total disposal (41). Statistically, Ra and MPE did not differ among the samples at the 4 time points measured for both mannose and inositol, indicating steady-state conditions. Therefore, we assumed that the Ra measured in our study approximates the utilization rate in the infant. However, one must use caution when assuming equilibrium among all compartments that utilize these carbohydrates, which is a limitation to the methodology used in this study. Stable isotope methodology using isotopes labeled with deuterium may also overestimate utilization due to unintended measurements of futile cycling of deuterium in exchange for hydrogen. This scenario is not likely in our study, because m+1 and m+6 measurements did not differ and there was no measurable enrichment of m+2, m+3, m+4, and m+5 for inositol above the natural abundance for each isotopomer.
In conclusion, we report the Ra of 2 nonglucose carbohydrates, mannose and inositol, which indicate a relatively high requirement for de novo production from glucose or other carbon source despite their high content in breast milk and formula. Whereas healthy infants should not require supplemental mannose and inositol due to endogenous production, the need for and possible benefit of supplementation to those infants at risk for developing mannose or inositol deficiency requires further study.
Acknowledgments
We thank the Perinatal Clinical Translational Research Center nurses (Kathryn Hale, Donna Rodden, Lucy Fashaw, Christine Reed, Barbara Pruckler) for all of their hard work and contributions to this study. We also thank Ken Easterday for his preparation of the isotopes used in this study.
Supported by the NIH grant 5 RO1 HD34837; Colorado Clinical and Translational Science Award grant 1 UL1 RR025780 from National Center for Research Resources/NIH; the NIH Building Interdisciplinary Careers in Women's Health Award, K12 HD057022; and the Research Institute of The Children's Hospital of Denver.
Author disclosures: L. D. Brown, A. Cheung, J. E. F. Harwood, and F. C. Battaglia, no conflicts of interest.
Abbreviations used: IDM, infant of a diabetic mother; IR, tracer infusion rate; MPE, molar percent enrichment; PN, parenteral nutrition; Ra, plasma appearance rate.
References
- 1.Wiese TJ, Dunlap JA, Conner CE, Grzybowski JA, Lowe WL Jr, Yorek MA. Osmotic regulation of Na-myo-inositol cotransporter mRNA level and activity in endothelial and neural cells. Am J Physiol. 1996;270:C990–7. [DOI] [PubMed] [Google Scholar]
- 2.Holub BJ. Metabolism and function of myo-inositol and inositol phospholipids. Annu Rev Nutr. 1986;6:563–97. [DOI] [PubMed] [Google Scholar]
- 3.Majerus PW, Ross TS, Cunningham TW, Caldwell KK, Jefferson AB, Bansal VS. Recent insights in phosphatidylinositol signaling. Cell. 1990;63:459–65. [DOI] [PubMed] [Google Scholar]
- 4.Greene ND, Copp AJ. Mouse models of neural tube defects: investigating preventive mechanisms. Am J Med Genet C Semin Med Genet. 2005;135C:31–41. [DOI] [PubMed] [Google Scholar]
- 5.Hallman M, Saugstad OD, Porreco RP, Epstein BL, Gluck L. Role of myoinositol in regulation of surfactant phospholipids in the newborn. Early Hum Dev. 1985;10:245–54. [DOI] [PubMed] [Google Scholar]
- 6.Hallman M, Bry K, Hoppu K, Lappi M, Pohjavuori M. Inositol supplementation in premature infants with respiratory distress syndrome. N Engl J Med. 1992;326:1233–9. [DOI] [PubMed] [Google Scholar]
- 7.Schanler RJ, Atkinson SA. Human milk. In: Tsang RC, Uauy R, Koletzko B, Zlotkin SH, editors. Nutrition of the preterm infant: scientific basis and practical guidelines. Cincinnati (OH): Digital Educational Publishing, Inc.; 2005. p. 333–56.
- 8.Jauniaux E, Hempstock J, Teng C, Battaglia FC, Burton GJ. Polyol concentrations in the fluid compartments of the human conceptus during the first trimester of pregnancy: maintenance of redox potential in a low oxygen environment. J Clin Endocrinol Metab. 2005;90:1171–5. [DOI] [PubMed] [Google Scholar]
- 9.Brusati V, Jozwik M, Jozwik M, Teng C, Paolini CL, Marconi AM, Battaglia FC. Fetal and maternal non-glucose carbohydrates and polyols concentrations in normal human pregnancies at term. Pediatr Res. 2005;58:700–4. [DOI] [PubMed] [Google Scholar]
- 10.Cavalli C, Teng C, Battaglia FC, Bevilacqua G. Free sugar and sugar alcohol concentrations in human breast milk. J Pediatr Gastroenterol Nutr. 2006;42:215–21. [DOI] [PubMed] [Google Scholar]
- 11.Davis JA, Freeze HH. Studies of mannose metabolism and effects of long-term mannose ingestion in the mouse. Biochim Biophys Acta. 2001;1528:116–26. [DOI] [PubMed] [Google Scholar]
- 12.Coppa GV, Pierani P, Zampini L, Bruni S, Carloni I, Gabrielli O. Characterization of oligosaccharides in milk and feces of breast-fed infants by high-performance anion-exchange chromatography. Adv Exp Med Biol. 2001;501:307–14. [DOI] [PubMed] [Google Scholar]
- 13.Engfer MB, Stahl B, Finke B, Sawatzki G, Daniel H. Human milk oligosaccharides are resistant to enzymatic hydrolysis in the upper gastrointestinal tract. Am J Clin Nutr. 2000;71:1589–96. [DOI] [PubMed] [Google Scholar]
- 14.Kalhan SC, Oliven A, King KC, Lucero C. Role of glucose in the regulation of endogenous glucose production in the human newborn. Pediatr Res. 1986;20:49–52. [DOI] [PubMed] [Google Scholar]
- 15.Kalhan SC, Parimi P, Van Beek R, Gilfillan C, Saker F, Gruca L, Sauer PJ. Estimation of gluconeogenesis in newborn infants. Am J Physiol Endocrinol Metab. 2001;281:E991–7. [DOI] [PubMed] [Google Scholar]
- 16.Sunehag AL, Haymond MW, Schanler RJ, Reeds PJ, Bier DM. Gluconeogenesis in very low birth weight infants receiving total parenteral nutrition. Diabetes. 1999;48:791–800. [DOI] [PubMed] [Google Scholar]
- 17.Zarlengo KM, Battaglia FC, Fennessey P, Hay WW Jr. Relationship between glucose utilization rate and glucose concentration in preterm infants. Biol Neonate. 1986;49:181–9. [DOI] [PubMed] [Google Scholar]
- 18.Parimi P, Kalhan SC. Carbohydrates including oligosaccharides and inositol. In: Tsang RC, Uauy R, Koletzko B, Zlotkin SH, editors. Nutrition of the preterm infant: scientific basis and practical guidelines. Cincinnati (OH): Digital Educational Publishing, Inc.; 2005. p. 81–95.
- 19.Paolini CL, Marconi AM, Pike AW, Fennessey PV, Pardi G, Battaglia FC. A multiple infusion start time (MIST) protocol for stable isotope studies of fetal blood. Placenta. 2001;22:171–6. [DOI] [PubMed] [Google Scholar]
- 20.Schadewaldt P, Hammen HW, Loganathan K, Bodner-Leidecker A, Wendel U. Analysis of concentration and (13)C enrichment of D-galactose in human plasma. Clin Chem. 2000;46:612–9. [PubMed] [Google Scholar]
- 21.Guerrant GO, Moss WC. Determination of monosaccharides as aldononitrile, O-methyloxime, alditol, and cyclitol acetate derivatives by gas chromatography. Anal Chem. 1984;56:633–8. [Google Scholar]
- 22.Wolfe RR, Chinkes DL. Isotope tracers in metabolic research: principles and practice of kinetic analysis. Hoboken (NJ): Wiley-Liss; 2005.
- 23.Ducan B, Lubchenco LO, Hansman C. Growth charts for children 0 to 18 years of age. Pediatrics. 1974;54:497–502. [PubMed] [Google Scholar]
- 24.Pereira GR, Baker L, Egler J, Corcoran L, Chiavacci R. Serum myoinositol concentrations in premature infants fed human milk, formula for infants, and parenteral nutrition. Am J Clin Nutr. 1990;51:589–93. [DOI] [PubMed] [Google Scholar]
- 25.Aouameur R, Da Cal S, Bissonnette P, Coady MJ, Lapointe JY. SMIT2 mediates all myo-inositol uptake in apical membranes of rat small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G1300–7. [DOI] [PubMed] [Google Scholar]
- 26.Holub BJ. The nutritional importance of inositol and the phosphoinositides. N Engl J Med. 1992;326:1285–7. [DOI] [PubMed] [Google Scholar]
- 27.Carver JD, Stromquist CI, Benford VJ, Minervini G, Benford SA, Barness LA. Postnatal inositol levels in preterm infants. J Perinatol. 1997;17:389–92. [PubMed] [Google Scholar]
- 28.Lewin LM, Melmed S, Passwell JH, Yannai Y, Brish M, Orda S, Boichis H, Bank H. Myoinositol in human neonates: serum concentrations and renal handling. Pediatr Res. 1978;12:3–6. [DOI] [PubMed] [Google Scholar]
- 29.Quirk JG Jr, Bleasdale JE. myo-Inositol homeostasis in the human fetus. Obstet Gynecol. 1983;62:41–4. [PubMed] [Google Scholar]
- 30.Groenen PM, Peer PG, Wevers RA, Swinkels DW, Franke B, Mariman EC, Steegers-Theunissen RP. Maternal myo-inositol, glucose, and zinc status is associated with the risk of offspring with spina bifida. Am J Obstet Gynecol. 2003;189:1713–9. [DOI] [PubMed] [Google Scholar]
- 31.Weigensberg MJ, Garcia-Palmer FJ, Freinkel N. Uptake of myo-inositol by early-somite rat conceptus. Transport kinetics and effects of hyperglycemia. Diabetes. 1990;39:575–82. [DOI] [PubMed] [Google Scholar]
- 32.Hashimoto M, Akazawa S, Akazawa M, Akashi M, Yamamoto H, Maeda Y, Yamaguchi Y, Yamasaki H, Tahara D, et al. Effects of hyperglycaemia on sorbitol and myo-inositol contents of cultured embryos: treatment with aldose reductase inhibitor and myo-inositol supplementation. Diabetologia. 1990;33:597–602. [DOI] [PubMed] [Google Scholar]
- 33.Khandelwal M, Reece EA, Wu YK, Borenstein M. Dietary myo-inositol therapy in hyperglycemia-induced embryopathy. Teratology. 1998;57:79–84. [DOI] [PubMed] [Google Scholar]
- 34.Eklund EA, Freeze HH. The congenital disorders of glycosylation: a multifaceted group of syndromes. NeuroRx. 2006;3:254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hay WW Jr, Meznarich HK, DiGiacomo JE, Hirst K, Zerbe G. Effects of insulin and glucose concentrations on glucose utilization in fetal sheep. Pediatr Res. 1988;23:381–7. [DOI] [PubMed] [Google Scholar]
- 36.Liechty EA, Boyle DW, Moorehead H, Auble L, Denne SC. Aromatic amino acids are utilized and protein synthesis is stimulated during amino acid infusion in the ovine fetus. J Nutr. 1999;129:1161–6. [DOI] [PubMed] [Google Scholar]
- 37.Thureen PJ, Baron KA, Fennessey PV, Hay WW Jr. Ovine placental and fetal arginine metabolism at normal and increased maternal plasma arginine concentrations. Pediatr Res. 2002;51:464–71. [DOI] [PubMed] [Google Scholar]
- 38.Thureen PJ, Melara D, Fennessey PV, Hay WW Jr. Effect of low versus high intravenous amino acid intake on very low birth weight infants in the early neonatal period. Pediatr Res. 2003;53:24–32. [DOI] [PubMed] [Google Scholar]
- 39.Bromberger P, Hallman M. Myoinositol in small preterm infants: relationship between intake and serum concentration. J Pediatr Gastroenterol Nutr. 1986;5:455–8. [DOI] [PubMed] [Google Scholar]
- 40.Brown LD, Cavalli C, Harwood JE, Casadei A, Teng CC, Traggiai C, Serra G, Bevilacqua G, Battaglia FC. Plasma concentrations of carbohydrates and sugar alcohols in term newborns after milk feeding. Pediatr Res. 2008;64:189–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clements RS Jr, Diethelm AG. The metabolism of myo-inositol by the human kidney. J Lab Clin Med. 1979;93:210–9. [PubMed] [Google Scholar]