Abstract
Technological advances have led to greater use of both structural and functional brain imaging to assist with the diagnosis of dementia for the increasing numbers of people with cognitive decline as they age. In current clinical practice, structural imaging (CT or MRI) is used to identify space-occupying lesions and stroke. Functional methods, such as PET scanning of glucose metabolism, could be used to differentiate Alzheimer’s disease from frontotemporal dementia, which helps to guide clinicians in symptomatic treatment strategies. New neuroimaging methods that are currently being developed can measure specific neurotransmitter systems, amyloid plaque and tau tangle concentrations, and neuronal integrity and connectivity. Successful co-development of neuroimaging surrogate markers and preventive treatments might eventually lead to so-called brain-check scans for determining risk of cognitive decline, so that physicians can administer disease-modifying medications, vaccines, or other interventions to avoid future cognitive losses and to delay onset of disease.
Introduction
Advances in medical technology have helped to increase life expectancy, but age-associated cognitive impairment often diminishes the quality of life for the increasing numbers of older adults. Developments in diagnosis and treatment of age-related cognitive decline have led to better detection of and symptomatic treatment for Alzheimer’s disease (AD), and neuroimaging is often used to assist physicians in making an accurate diagnosis. The prospect of more effective treatments for AD and other dementias, and the potential for improving memory and other cognitive functions in people with mild, age-related decline, as well as delaying their progression, has led to new brain imaging technologies that might not only further improve diagnostic accuracy but also accelerate treatment discovery. In this Review, we describe current clinical uses of common neuroimaging techniques, as well as scanning methods and strategies in development that could eventually be used to detect neurodegeneration before clinical symptoms are obvious, so that physicians can identify treatment candidates for future preventive therapies.
Normal ageing, mild cognitive impairment, and dementia
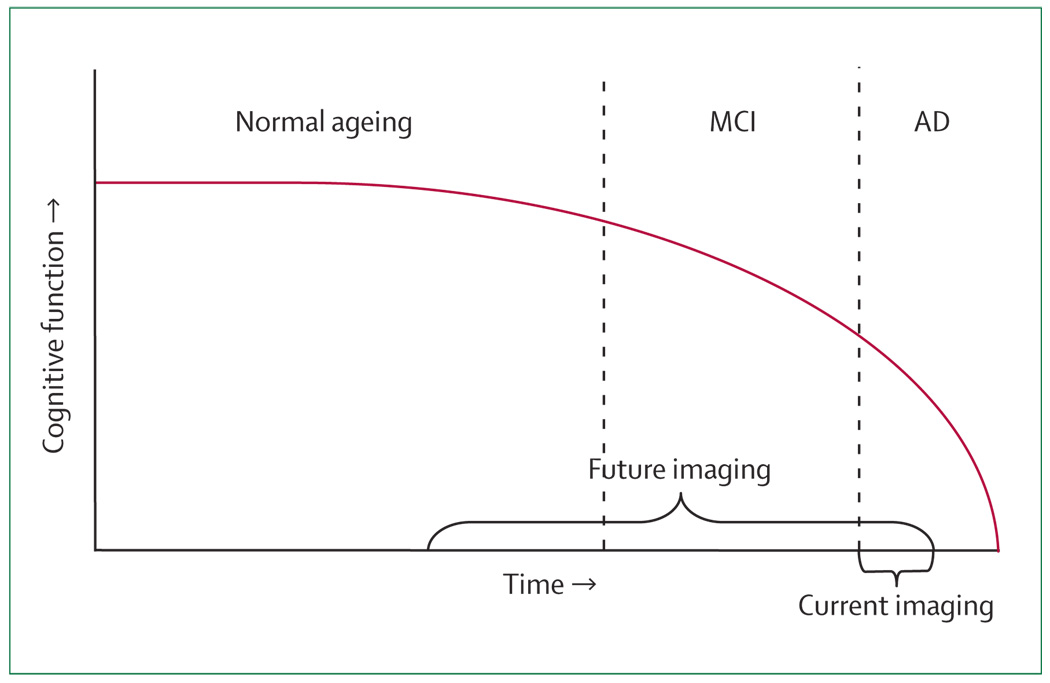
Cognitive impairment associated with ageing develops along a continuum (figure 1, panel). In normal ageing, people are aware of mild age-related memory changes, but objective cognitive deficits are minimal. By contrast, mild cognitive impairment (MCI) often represents a transitional state between normal ageing and dementia.1 Patients with MCI are able to live independently, are aware of their memory changes, and typically show problems with delayed recall, although non-memory cognitive domains might also be impaired. The prevalence of MCI could be as high as 19% in people over the age of 65 years and 29% in those over the age of 85 years.2 The term amnestic MCI describes cognitive complaints that are primarily in the area of memory. Patients with the amnestic subtype are likely to progress to AD.3
Figure 1. Hypothetical course of the continuum of brain ageing.
Neuroimaging is currently used once dementia develops. Future use is likely to involve detection of neurodegeneration before symptoms are obvious in preclinical stages such as normal ageing and MCI.
When cognitive decline interferes with daily functioning and impairs not only memory but also other mental abilities, dementia is often diagnosed.4 AD accounts for most common cases and is insidious in its onset and progressive in its course.5,6 By contrast, vascular dementia is often diagnosed when onset is abrupt, cognitive deficits progress in a stepwise manner, and features of cerebrovascular disease are present.7,8 Other causes include dementia associated with Lewy bodies, characterised by visual hallucinations, Parkinsonian signs, and alterations of alertness or attention,9 and frontotemporal dementia, which often presents with executive dysfunction and personality changes.10 Depression, medical illnesses, delirium, drug toxic effects, and many other medical conditions can present as a dementia syndrome.11 Often, dementia results from several causes: for example, cerebrovascular disease is often seen in patients with AD pathology.
Pharmacological treatments currently available for AD include the cholinesterase inhibitors donepezil, rivastigmine, and galantamine, and the glutamate NMDA receptor antagonist memantine.11,12 A randomised placebo-controlled clinical trial in patients with MCI showed that donepezil delayed progression to AD compared with placebo after 1 year of treatment, but no difference between drug and placebo was observed after 3 years of treatment.13 The prescribing of cholinesterase inhibitors for patients with MCI would be considered off-label use in current practice.
Recently, revised research criteria for the diagnosis of AD have been proposed in light of the growing scientific knowledge about neuroimaging and other biomarkers for AD.14 These criteria include the presence of early episodic memory impairment with at least one or more abnormal biomarkers that could be detected using techniques such as structural neuroimaging with MRI, molecular neuroimaging with PET, and CSF analysis of amyloid β or tau proteins.
Panel: Terms for the continuum of brain ageing
Age-associated memory impairment
Often referred to as normal ageing because it is so common. It is characterised by self-perception of memory loss and a standardised memory test score that shows a decline in objective memory performance compared with younger adults.
MCI
A transitional state between normal ageing and dementia. MCI is associated with an increased risk for dementia. Such patients are able to live independently, are aware of their memory changes, and typically show problems with delayed recall, although non-memory cognitive domains can also be impaired.
Amnestic MCI
A subtype of MCI with memory impairment, but without deficits in other cognitive domains. Patients with this condition are at increased risk of developing AD.
Dementia
Cognitive decline that interferes with daily functioning and impairs not just memory but other mental abilities.
AD
This most common form of dementia has an insidious onset and progressive course and is definitively diagnosed at autopsy by the presence of high cerebral concentrations of amyloid senile plaques and tau neurofibrillary tangles.
Brain alterations in age-related cognitive decline
Many of the brain changes that occur along this neurodegenerative continuum can be quantified by various neuroimaging technologies designed to measure a range of biological processes (table). The most obvious age-related brain change is atrophy from neuronal synaptic degeneration and loss, which occurs throughout the brain, but can vary regionally according to the form of dementia.15 For example, patients with AD show medial temporal atrophy early in the disease, whereas those with frontotemporal dementia have greater frontal and temporal atrophy.10
Table.
Imaging techniques and the biological processes they measure
| Biological process | |
|---|---|
| CT | Atrophy, space-occupying lesions |
| MRI | Atrophy, space-occupying lesions, white-matter hyperintensity |
| [18F]FDG-PET | Glucose metabolism |
| [99mTc]Tc-HMPAO SPECT | Blood flow |
| [18F]FDDNP-PET | Amyloid plaques and tau tangles |
| [11C]PIB-PET | Amyloid plaques |
| [11C]SB-13 PET | Amyloid plaques |
| [18F]-BAY94-9172-PET | Amyloid plaques |
| [18F]MPPF-PET | Hippocampal neuronal integrity |
| Functional MRI | Blood flow, functional connectivity |
| DTI | Neuronal connectivity, white-matter integrity |
| MRS | Metabolite concentrations |
| [11C]-nicotine PET | Nicotinic binding sites |
| [11C](R)-PK11195 PET | Microglial activation (inflammation) |
| [11C]β-CFT PET | Dopamine reuptake |
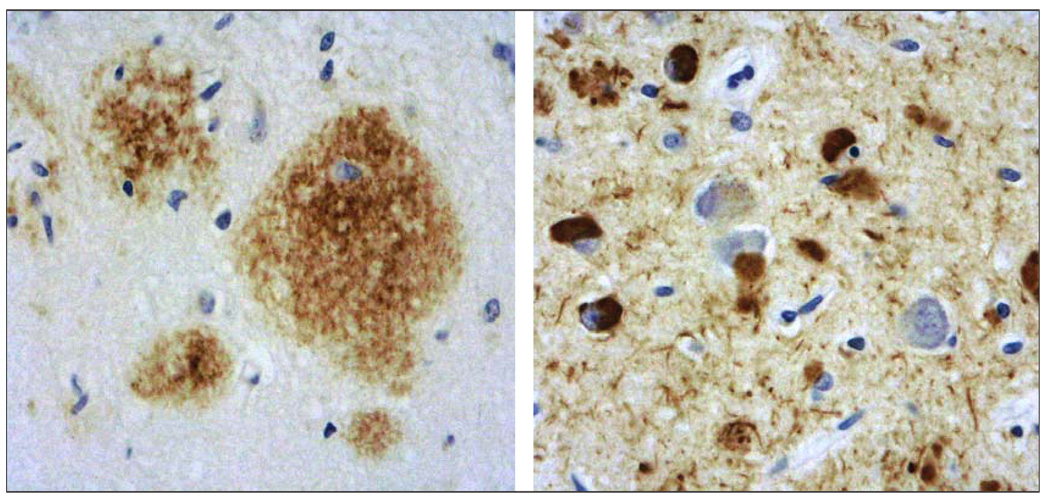
Amyloids are insoluble protein aggregates consisting of beta-pleated sheet structures with high affinity binding to Congo red.16 Two proteins that share such amyloid properties, amyloid β (in plaques) and tau (in neurofibrillary tangles; figure 2), have been observed in autopsy studies in young and middle-aged adults, and they accumulate in a predictable spatial pattern in normal ageing, MCI, and AD.3,17,18 Neurofibrillary tangles have been observed in the hippocampus and other medial temporal regions in MCI, and spread to parietal and frontal neocortical areas as MCI progresses to AD. Although rarer in normal ageing, neuritic plaques begin to accumulate in MCI in the hippocampus and neocortex, where they become more prevalent in AD. A definite diagnosis of AD requires the presence at autopsy of high numbers of neocortical amyloid senile plaques and tau neurofibrillary tangles.5
Figure 2. Neuropathological microscopic examination of the hippocampus after brain autopsy of a patient with AD, by use of immunohistochemical staining.
Left: amyloid-β protein (length 1–42 amino acids; brown staining). Right: phosphorylated tau protein (brown staining).
As neurodegeneration progresses in the ageing brain, other alterations have been observed. Post-mortem and CSF studies have shown deficits in cholinergic, serotonergic, dopaminergic, somatostatinergic, noradrenergic, and glutaminergic neurotransmitters.19 Inflammatory and oxidative alterations are also thought to contribute to neural dysfunction and neural death.20,21 These and various other pathogenic mechanisms, such as hyper tension and insulin resistance, further contribute to compromised cerebral blood flow, regional decreases in glucose metabolism, white-matter hyperintensities, and small strokes.22
Currently available neuroimaging methods
Traditionally, neuroimaging techniques have been categorised as either structural or functional, according to the primary information they provide. However, methods generally used to look at structure can also be altered to observe function (eg, functional MRI). Similarly, traditional functional methods, such as PET, can also be used to view structure (eg, amyloid plaque imaging).
Commonly used structural methods include CT and MRI. Studies of brain function are often done using single photon emission computed tomography (SPECT), with [99mTc]technetium-labelled D,L-hexamethylpropylene amine oxime ([99mTc]technetium-HMPAO) to measure blood flow, or PET, with the 2-deoxy-2-[18F]fluoro-D-glucose (FDG) tracer to measure glucose metabolism. In clinical practice, imaging studies are usually used to increase diagnostic accuracy to assist with treatment planning. However, diagnostic information offers benefits to patients and families beyond drug treatment decisions. For example, accurate knowledge of diagnosis often reduces anxiety and helps families clarify caregiving tasks, even though the learning of a diagnosis of a progressive dementia can be initially upsetting to patients and their family members.23
Structural imaging is often done to identify possible treatable causes of dementia, such as normal pressure hydrocephalus or a space-occupying lesion, or to clarify the diagnosis by detecting strokes or atrophy. The most recent American Academy of Neurology Practice Parameter guidelines recommended structural neuroimaging with either a non-contrast CT or MRI scan in the initial assessment of patients with dementia.24
Functional imaging technologies provide information about brain structure, but their spatial resolution is lower than with CT or MRI. The added value of functional scanning is the information provided on cerebral blood flow or glucose metabolism, even when structural deficits are not present, which can assist the physician in the diagnosis and differential diagnosis of dementia.25,26 For example, patients with AD typically show parietal, temporal, posterior cingulate, and frontal deficits, whereas those with frontotemporal dementia show frontal and temporal deficits.25 Dementia with Lewy bodies has a pattern similar to that of AD, but with additional occipital deficits.27 Both PET and SPECT can be used to measure regional cerebral blood flow, which is physiologically coupled to brain metabolism in many patients with dementia. The radiotracer is assumed to accumulate in areas of the brain in proportion to the rate of delivery of nutrients to that volume of brain tissue. PET also provides measures of regional cerebral glucose metabolism.
CT
CT scanning provides high-resolution (~1 mm) information on brain structure, and can be used to identify stroke, hydocephalus, atrophy, or space-occupying lesions (eg, tumour, haemorrhage). The use of intravenous contrast material will enhance imaging of bleeding, neoplasms, infection, and inflammation. Radiation exposure is approximately 1 · 3 mSv, which is about a third to a half the natural background exposure per year.28 Despite this radiation exposure, CT scanning is often used instead of structural MRI in clinical settings because of its low cost and wide availability.
MRI
Structural MRI
MRI provides high-resolution (~1 mm) information on brain structure, including differentiation of grey matter and white matter (T1-weighted images) and delineation of white-matter hyperintensity (T2-weighted images).29 Gadolinium contrast agents are sometimes used to enhance visualisation of brain lesions. Although patients can have multiple scans because the method does not involve ionising radiation, some patients do not tolerate MRI because the long, narrow tube makes them feel claustrophobic, or their body metal from hip replacements or pacemakers, for example, cannot be subjected to the high magnetic fields of the scanners.
In clinical practice, structural MRI scanning is widely used, and radiologists interpret results on the basis of visual readings. However, image analysis programs that quantify regional volumes in MRI (and CT) have shown that medial temporal or hippocampal atrophy measures can distinguish patients with a clinical diagnosis of AD from controls.30 Although hippocampal atrophy can predict memory progression,31 these changes might not be specific to AD and might occur in other dementia disorders. Other studies have charted the progression of cortical grey-matter atrophy as AD progresses,32 and show early involvement of temporal and limbic cortices, with atrophy sweeping forward into the frontal brain regions and sparing the primary sensory and motor cortices until late in the disease. Structural MRI measures of atrophy in the entorhinal cortex and hippocampus are associated with increased risk of developing AD,33,34 and predict future memory decline in healthy adults.35 Related studies have shown a more generalised pattern of cortical atrophy in early versus late-onset dementia,36 and specific cortical changes have been associated with a specific decline in language and an increase in apathy.37 Boundary shift interval measures derived from serial MRI scans have shown reduced measurement variability, which offers the possibility of reducing the sample sizes required to test new treatments.38 Such measures have indicated different rates of cerebral atrophy for some but not all forms of neurodegeneration.39
Subcortical white-matter hyperintensity has been observed on MRI scans both in AD and normal ageing and is not strongly associated with disease severity.40 Older people with severe white-matter changes are at greater risk for rapid global functional decline than those with mild changes.41 The extent and spatial location of these lesions seem to determine their influence on cognition.42 Large volumes of white-matter hyperintensity, which are associated with cerebrovascular disease, are found in non-demented people with lower cognitive performance scores,43 particularly in executive functioning.44 Although the presence of vascular risk factors might contribute to the initial expression of AD, not all studies find that they predict the rate of future cognitive decline.45 Severity of baseline white-matter hyperintensity, rather than diagnosis or severity of dementia, is a significant predictor of lesion progression.46,47 In patients with MCI, medial temporal atrophy seems to be a better predictor of cognitive function than does small-vessel disease.48 Although MRI evidence of infarcts that indicate that cerebrovascular disease and medial temporal atrophy independently contribute to the risk of AD, the presence of white-matter hyperintensity has not been useful in clinical practice for the diagnosis of dementia or for predicting future cognitive decline.49 Cognitive decline associated with white-matter changes seems less extensive than that from other causes, such as AD.50 As automated quantitative analytic programs become more user friendly and widely available, clinicians might wish to use visual rating scales.
Functional MRI
Functional MRI is used to measure signal intensities that are associated with relative cerebral blood flow during memory or other cognitive tasks.51 The MRI signal intensity associated with a particular task in comparison to the control condition corresponds with blood flow and neural activity, but only indirectly. Functional MRI studies of patients with AD show lowered brain activity in parietal and hippocampal regions and relatively higher activity in primary cortices unaffected by the disease.52
A relatively new application of functional MRI involves the measurement of the extent to which different areas of the brain are functionally connected. Compared with controls, patients with AD show, during mental rest, less coordinated activity in a default brain network (posterior cingulate, hippocampus, and inferior parietal lobes).53 Resting and activation functional MRI studies have reported similar disruptions in functional connectivity in AD,54,55 which might account in part for the decreased resting glucose metabolism found with [18F]FDG-PET scanning.
Functional MRI has also shown greater brain activation during tasks requiring memory in cognitively intact APOE ε4 carriers than in non-carriers, and the degree of activation predicts subsequent memory decline.56 Thus, combining genetic risk measures and functional MRI during memory task performance has future potential as a presymptomatic predictor of cognitive decline. Whereas patients with MCI undergoing functional MRI can perform similar memory tasks that activate the hippocampus during successful memory encoding,57 patients with dementia are usually too impaired to perform such memory tasks. However, use of less demanding memory encoding paradigms during functional MRI has shown significant correlations between regional activation and cognitive performance scores.58 Functional MRI activation responses to a face-matching paradigm have been found to differentiate patients with MCI from healthy controls.59 Although functional MRI might eventually be used to assist in diagnosis and treatment monitoring, the identification of cognitive paradigms appropriate for varying levels of cognitive impairment poses a practical challenge.
Diffusion tensor imaging
Diffusion tensor imaging (DTI) provides images of neuronal connectivity in the form of quantitative data on the directionality (anisotropy) of water diffusion, which can indicate local fibre orientation and integrity of white-matter tracts. A DTI study of hippocampal water diffusion changes and temporal white matter found that patients with AD had decreased fibre density in the temporal white matter, probably related to secondary degeneration of medial temporal grey matter.60 DTI has also been reported to differentiate patients with AD from those with dementia with Lewy bodies,61 and to show decreases in white-matter fractional anisotropy in presymptomatic carriers of familial AD mutations.62 APOE status of healthy middle-aged and older adults was found to affect the trajectory of age-related myelin breakdown in late-myelinating brain regions.63 Fractional anisotropy of the cingulum fibres has been reported to be significantly reduced in patients with MCI, and even more so in those with AD.64 When DTI results were added to hippocampal volume measures, significantly better differentiation among MCI, AD, and control groups was found.64 Thus, DTI might eventually be useful as a diagnostic tool with measures of regional brain volume. However, the meaning of DTI findings in ageing remains unclear since various factors could influence results, including mental ability during childhood, as well as early developmental changes.65
Magnetic resonance spectroscopy
Magnetic resonance spectroscopy (MRS) provides information on tissue substrate or metabolite concentrations. This technique has been used to show that the neuronal marker N-acetyl aspartate is reduced in the hippocampus in patients with AD and MCI compared with controls.66 MRS has also been used to monitor metabolite changes in clinical trials and to differentiate patients with MCI from those with AD.67,68 In a 3-month trial of donepezil in patients with AD, baseline MRS parietal lobe patterns predicted positive treatment outcome.69 Further study with this technique is necessary to determine its potential clinical use in diagnosis.
SPECT
SPECT provides measures of cerebral blood flow by detecting a single-photon emitting tracer after its intravenous injection and brain uptake. Spatial resolution is about 10 mm, and radiation exposure is approximately 9 mSv (three times the annual natural background). Although comparison studies suggest that SPECT has lower diagnostic accuracy than PET, recent advances in voxel-based statistical analysis have improved the accuracy of brain perfusion SPECT in diagnosing early AD.70 In clinical practice, SPECT imaging can be helpful in diagnosing dementia and differentiating AD from frontotemporal dementia. In the past, SPECT has been used more extensively because of its wider availability and lower cost. However, with increased use of PET for oncology studies, it is now widely available in many geographical areas.
PET
For PET, the patient receives an intravenous injection of tracer that emits a positron that annihilates and releases two gamma photons travelling in opposite directions. The scanner records the photons at detectors 180° apart and determines the line along which the annihilation occurs to construct the PET images. Spatial resolution is 3–5 mm, and radiation exposure is similar to that of a SPECT brain study.
[18F]FDG-PET
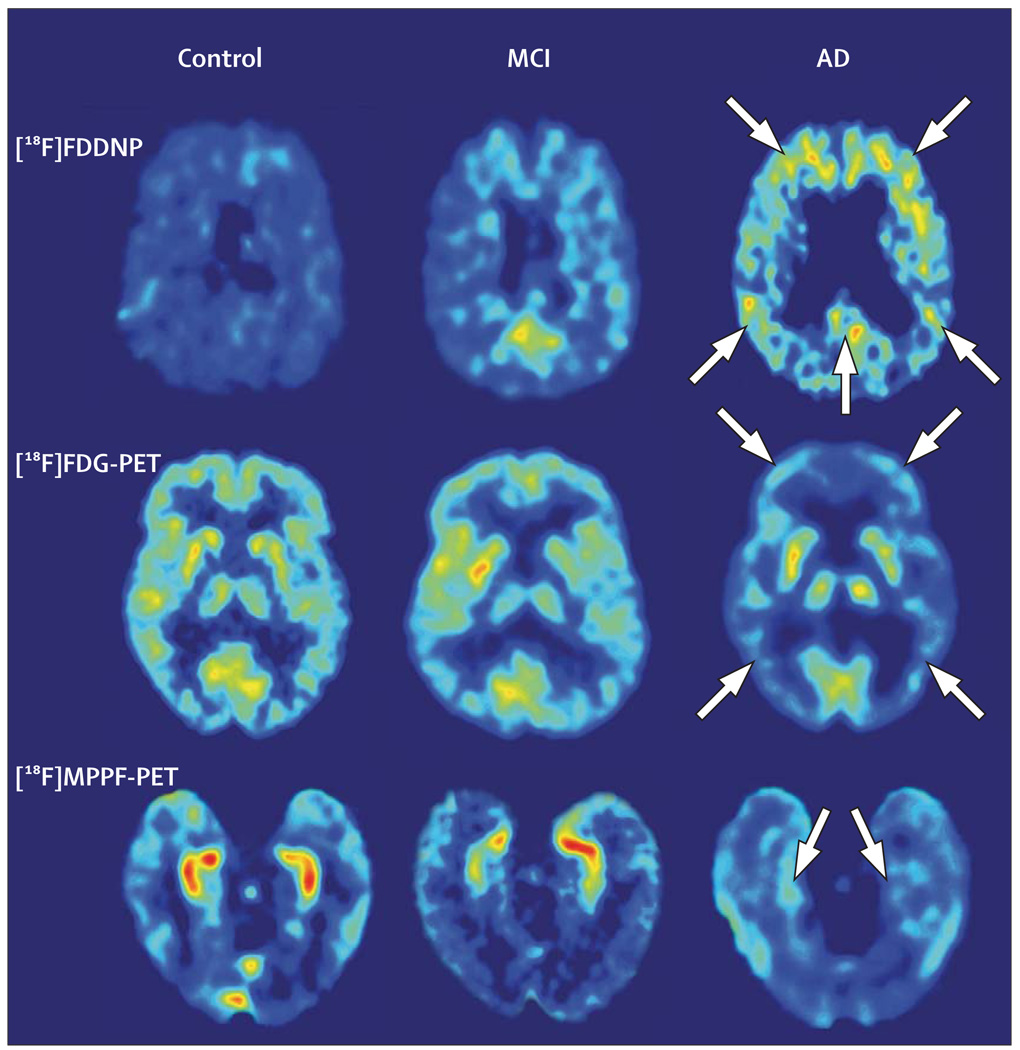
Studies of AD often use the radiolabelled glucose analogue FDG to measure cerebral glucose metabolism, which indicates levels of neurosynaptic activity. Such studies have shown characteristic cortical brain alterations in AD that begin in the posterior cingulate and parietal regions, and spread to the temporal and prefrontal cortices. Such images can differentiate patients with AD from those with other dementias and from cognitively intact people.25 Regional cortical hypometabolism correlates with greater cognitive losses, and [18F]FDG-PET can also differentiate patients with MCI from those with AD and controls (figure 3).71,72 Diagnostic accuracy for AD seems better when [18F]FDG-PET is added to the standard clinical assessment of dementia, providing increased sensitivity and specificity of diagnosis that is similar to that observed on clinical assessment 4 years after baseline.73 In September 2004, the US Centers for Medicare and Medicaid Services approved Medicare reimbursement for [18F]FDG-PET scans to assist with the differential diagnosis of AD and frontotemporal dementia, which is useful clinical information since the latter dementia does not seem to respond well to currently available symptomatic treatments. Lower scanner costs and greater availability of PET tracers is expected to lead to wider use of PET for dementia diagnosis.
Figure 3. Representative examples of brain [18F]FDG-PET images showing cognitive impairment.
For each imaging method, the patient with MCI has intermediate values between the control and the patient with AD. Absolute scales are comparable within each patient with a specific probe, and warmer colours indicate higher values. In the patient with AD, the arrows point to frontal, parietal, and posterior cingulate (upper), frontal and temporal-parietal (middle), and medial temporal (lower) regions. Reproduced with permission from the National Academy of Sciences.72
Because MCI and AD are often difficult to differentiate, some clinicians will use [18F]FDG-PET in patients with MCI to help guide treatment. Longitudinal studies of patients with MCI have found that if the baseline [18F]FDG-PET scan suggests an AD-like pattern, the probability of clinical progression within several years is extremely high. 74,75
Hypometabolism in the posterior cingulate, parietal, prefrontal, entorhinal, and temporal regions has been found to predict future cognitive decline in normal ageing, 76,77 and middle-aged and older APOE ε4 carriers have greater rates of cerebral metabolic decline than non-carriers.78 Significantly lower metabolism in these regions has been reported in APOE ε4 carriers compared with non-carriers in cognitively intact young adults in their 20s and 30s.79 Several [18F]FDG-PET studies have shown that AD-like metabolic patterns in patients with MCI are highly predictive of conversion to AD within several years, particularly in patients who are APOE ε4 carriers.80,81 Confirmation of such results is likely to lead to greater clinical use of [18F]FDG-PET in patients with MCI.
Plaque and tangle PET
Before the development of neuroimaging technologies that could measure amyloid-β plaques and tau tangles, these protein deposits could only be observed at autopsy, or, rarely, at biopsy. Several small molecule probes for use with PET have been developed to provide measures of these deposits in vivo.82–86
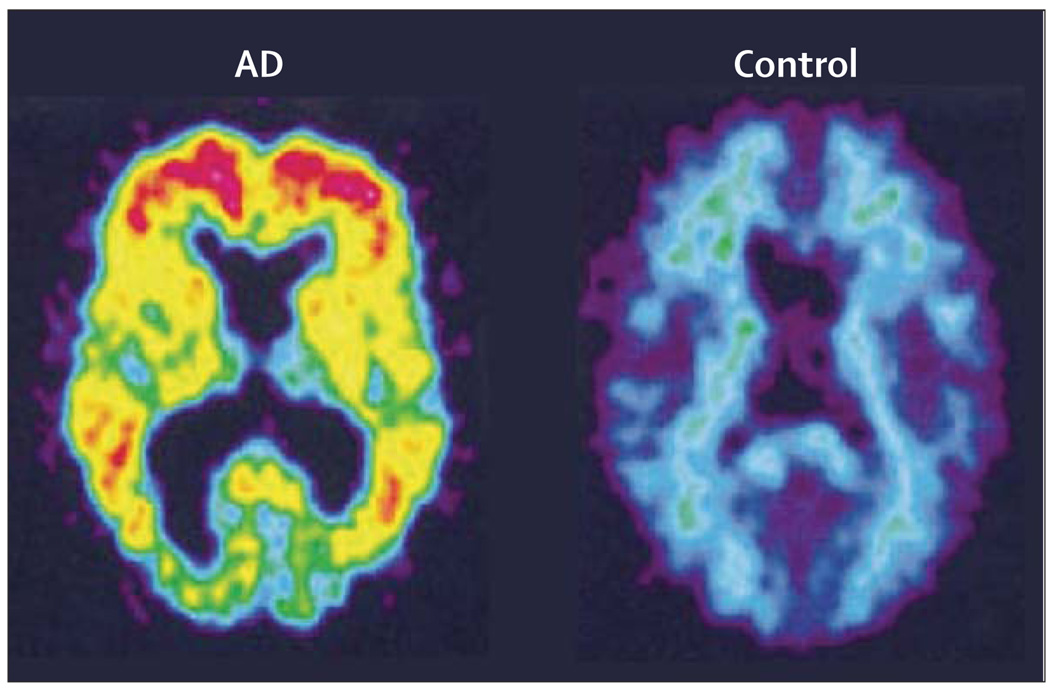
The most extensive experience has been with the amyloid-binding radiotracer [11C]-labelled Pittsburgh compound B (2-[4L’-(methylamino)phenyl]-6-hydrobenzothiazole; PIB), a derivative of thioflavin-T amyloid dye that binds to amyloid-β plaques but not tangles. PET studies using [11C]PIB show significantly greater cortical retention in patients with AD compared with controls (figure 4).82 Studies of MCI show a bimodal distribution with approximately 50% of MCI patients showing high [11C]PIB binding.87,88 Initial 2-year longitudinal follow-up has shown stable levels of [11C]PIB retention despite declines in glucose metabolism and cognitive function.89 [11C]PIB shows retention in patients with cerebral amyloid angiopathy, so it could potentially be used to detect cerebrovascular amyloid.90 Initial PET studies with [11C]PIB in patients with dementia with Lewy bodies indicate lower binding than in AD, and no cortical binding in patients with frontotemporal dementia.88
Figure 4. [11C]PIB-standardised uptake images showing higher [11C]PIB retention in a 79-year-old patient with AD than in a 67-year-old healthy control.
Reproduced with permission from John Wiley and Sons, Inc.82
Two newly developed amyloid PET ligands use fluorine-18, which has a half-life long enough to make these compounds potentially suitable for clinical use. [18F]-BAY94-9172-PET or trans-4-(N-methyl-amino)-4L’-{2-[2-(2-[18F]fluoro-ethoxy)-ethoxy]-ethoxy}-stilbene, an amyloid-β ligand, has been used with PET to discriminate patients with AD from those with frontotemporal dementia and healthy controls.91
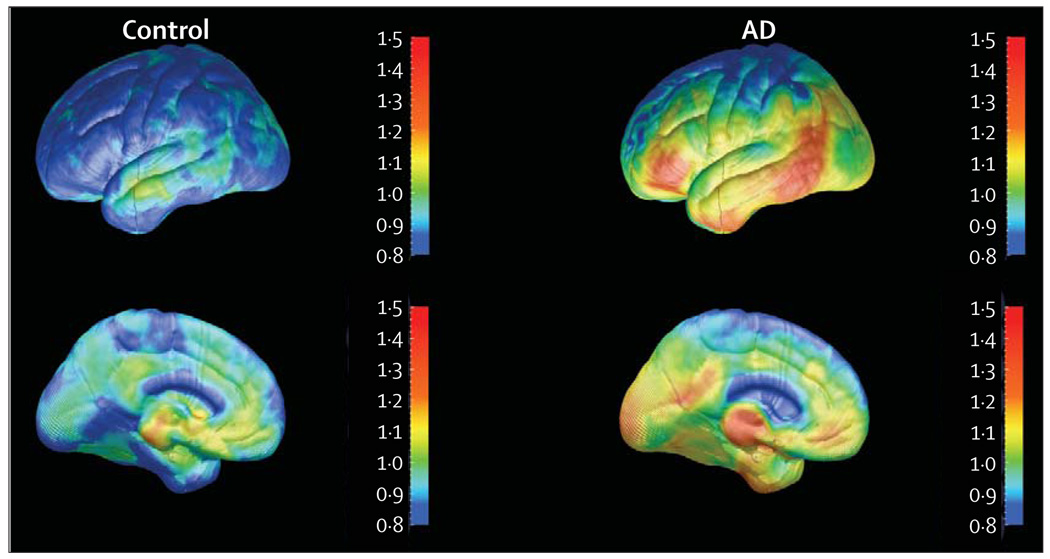
Our group has developed a fluorine-18 tau and amyloid PET probe, [18F]FDDNP (2-(1-[6-[(2-[18F]fluoroethyl] (methyl)amino]-2-naphthyl]ethylidene)malononitrile). Both [18F]FDDNP and its non-fluorinated analogue, DDNP, are fluorescent and provide clear images in vitro of plaques and tangles in AD brain specimens examined with confocal fluorescent microscopy.92 Neuropathological studies done at autopsy of patients with AD who previously received [18F]FDDNP-PET scans show close matching of in-vitro brain sections concentrated with plaques and tangles, and brain regions that show increased [18F]FDDNP-PET signals in vivo. [18F]FDDNP-PET scanning differentiates patients with AD from those with MCI and cognitively intact controls, and initial longitudinal studies show that [18F]FDDNP binding values increase as cognitive symptoms progress (figure 3).71 Three-dimensional cortical surface projection images of [18F]FDDNP-PET show patterns remarkably similar to those expected from autopsy studies that show regional brain accumulation patterns of plaques and tangles (figure 5).
Figure 5. Three-dimensional cortical surface projection images of [18F]FDDNP-PET scans from a control and a patient with AD.
Lateral (upper) and medial (lower) brain surfaces are shown. Warmer colours indicate higher numbers of plaques and tangles.
Other PET ligands that measure neurotransmitter systems
Hippocampal pyramidal neurons have high concentrations of serotonin 5-HT1A receptors, and loss of these pyramidal neurons has been correlated with lower 5-HT1A receptor densities.72 Recent studies have found that a selective molecular imaging probe for 5-HT1A receptors, 4-[18F]fluoro-N-{2-[1-(2-methoxyphenyl)-piperazinyl]ethyl}-N-(2-pyridinyl)benzamide (MPPF), provides excellent images of the hippocampus in cognitively intact individuals. Patients with AD show diminished hippocampal signals, whereas patients with MCI show binding values intermediate between controls and patients with AD (figure 3).72 The differential binding potential of MPPF in the hippocampus is indicative of neuronal losses and might eventually provide an early diagnostic measure even before symptoms are present.
Investigators have also used PET radioligands to visualise cholinergic nicotinic receptors in patients with AD, which have been found to correlate with cognitive measures of attention.93 PET measures of the cholinergic system with 11C-nicotine have been used to assess nicotine binding sites in the brain before and after treatment with cholinesterase inhibitors.94 Studies of the brain dopaminergic system have shown decreased striatal uptake of the dopamine reuptake ligand [11C]2β-carbomethoxy-3β-(4-fluorophenyl) tropane ([11C]β-CFT) in patients with AD.95 PET studies using [11C](R)-PK11195, a measure of microglial activation and the brain’s immune response to neuronal degeneration, have shown greater binding in patients with AD than in controls.96 However, most of these ligands have been used only in research settings and require further study to better understand their clinical usefulness.
Future uses of neuroimaging
As clinical use of available neuroimaging techniques expands, investigators are exploring novel applications of these techniques and developing others that measure various biological processes relevant to neuro degeneration. Combining neuroimaging studies with other procedures that provide data on genetic risk and biomarker measures from other tissues (eg, serum, CSF) might increase diagnostic sensitivity and specificity, as well as the predictive value of the information.
Biomarkers for diagnosis and treatment monitoring
The AD Neuroimaging Initiative is a large, multisite study that is doing serial structural MRI scans and [18F]FDG-PET scans, and acquiring other biomarkers from patients with MCI and AD and normal controls. Data from this study are likely to improve current knowledge of these techniques and how they can be used in combination to improve diagnostic accuracy and treatment monitoring.97 This initiative and other investigations will increase understanding of neuroimaging measures that might also be used to track the biological effects of treatments in clinical trials.
In this context, a biomarker has been defined as an indicator of disease activity, whereas a surrogate marker can substitute for a clinically meaningful endpoint in a clinical trial.98 A useful neuroimaging biomarker would not only be an indirect measure of neurodegeneration, but it would also be detected in most patients. Moreover, the biomarker would be used to monitor a relevant aspect of disease pathophysiology, correlate with treatment-induced changes, and assist in identifying responders to a specific treatment. Such biomarkers might also be used to adjust dosing of dementia treatments, or to determine neuroreceptor brain distributions in treatment candidates.99 Examples of drugs that have been approved on the basis of their effects on surrogate markers include anti-hypertensives, cholesterol-lowering agents, and glaucoma treatments.98 Several of the neuroimaging technologies in development show promise in providing measurements of potential biomarkers or surrogate markers, but further research is necessary to validate their use.
Although symptomatic drug treatments are available for AD, the UK’s National Institute for Health and Clinical Excellence recently issued guidance restricting the use of these drugs in the National Health Service.100 The search for new disease-modifying treatments is a high priority for many current investigative groups. When used to monitor treatment response in clinical trials, the particular neuroimaging modality chosen for the measurement of a biomarker will depend on the specific intervention and the biological processes it presumably targets. For example, [18F]FDG-PET and functional MRI might be used to monitor treatments that affect cerebral blood flow, metabolism, or neuronal dysfunction, whereas DTI might track the effects of treatments that strengthen or protect neuronal connectivity and white-matter integrity.
The relation between a particular neuroimaging measure, treatment, and neurodegeneration is not always straightforward. For example, regional atrophy predicts cognitive decline and effective treatments would be expected to reduce such atrophy. However, patients with AD who experienced cognitive benefits from an anti-amyloid immunotherapy trial had greater brain volume decreases rather than increases on MRI, perhaps because of amyloid-β removal and associated cerebral fluid shifts.101
Other potentially informative biomarkers
Neuroimaging measures have the potential of offering relatively direct biomarker windows into the brain, and such central measures might indicate neurodegenerative events more accurately than measures derived from peripheral tissues. By contrast, biomarkers of other tissues and organs might be more readily accessible and less expensive to obtain. Measures of genetic risk for dementia, such as APOE ε4, can be obtained from blood samples.102 Although APOE has been helpful in research studies, APOE ε4 is neither necessary nor sufficient for developing AD, and is thus not recommended as a predictive test. In families with autosomal dominant inheritance patterns, wherein half the relatives develop dementia when they reach the age at onset for the family (usually mid-life), blood tests could provide information on genetic mutations that cause the disease.103 Genome-wide surveys of single-nucleotide polymorphisms are being used to characterise and confirm additional susceptibility genes.104 Inherited variants in the SORL1 neuronal sorting receptor gene have been associated with late-onset AD.105 Although future research might determine a genetic profile that assists in diagnosis, the large number of potential genetic risks poses a major challenge. Signalling proteins in blood plasma were recently found to classify AD patients and controls with 90% accuracy.106
CSF concentrations of proteins associated with plaques and tangles (eg, increased phosphorylated tau and low Aβ1-42) have been found to distinguish patients with AD from normal controls.107 Various other biomarkers are currently being studied, including olfactory identification tests,108 serum markers of inflammation,20 a pilocarpine eye-drop test,109 and a skin test to determine endothelial alterations.110
Early detection and prevention of neurodegeneration
An important potential application of these emerging technologies is in the early detection and prevention of minimum cognitive decline, since the feasibility of protecting a healthy brain is always greater than trying to repair one that is already damaged. Of course, the true power of a novel neuroimaging surrogate marker is not only in its diagnostic specificity and sensitivity, but also in the effectiveness of the treatments it can monitor. Even if a particular biomarker were perfectly accurate in identifying a dementia decades before symptoms begin, its widespread use would be unlikely if effective preventive treatments were not available. Fortunately, scientists are actively studying various potential treatments that could delay or prevent neurodegeneration.
Monitoring treatments in development
Several therapeutic strategies for preventing or diminishing insoluble amyloid-β accumulation—such as secretase inhibitors or modulators, active or passive vaccines, aggregate (oligomer) and tau kinase inhibitors, cholesterol-lowering statins, anti-inflammatory drugs, omega-3 fatty acids, and the yellow curry pigment curcumin—are being evaluated as interventions for delaying onset or slowing progression of AD, in addition to non-pharmacological interventions, such as physical exercise, nutrition, and cognitive training.111–114 Some of these approaches might provide disease-modifying effects and slow progression of neurodegeneration. Although a neuroimaging biomarker for insoluble brain amyloid β might measure the biological effects of the intervention, a clinical trial design that would assess the progression of AD (eg, randomised withdrawal or randomised start design) would be necessary to prove disease-modifying effects.115,116 Combining neuroimaging with genetic risk measures or other biological markers might improve diagnostic accuracy, reduce the number of individuals needed for a clinical trial of a new treatment, and identify genetically defined subgroups of patients who are most likely to respond.117 However, successful disease-modifying trials in AD will require careful attention to methodological issues such as trial design, duration, choice of primary and secondary endpoints, and selection of participants.118
To the extent that these neuroimaging technologies provide measures of the neuropathological hallmarks of AD, plaque and tangle imaging has face validity even if the amyloid β or tau protein deposits do not cause the disease but rather result from it. Anti-plaque treatments would be expected to have a greater effect on plaque-dense lateral temporal regions, whereas anti-tangle treatments would have a greater impact on tangle-rich medial temporal regions, both of which might be assessed at the earliest stages of the disease.
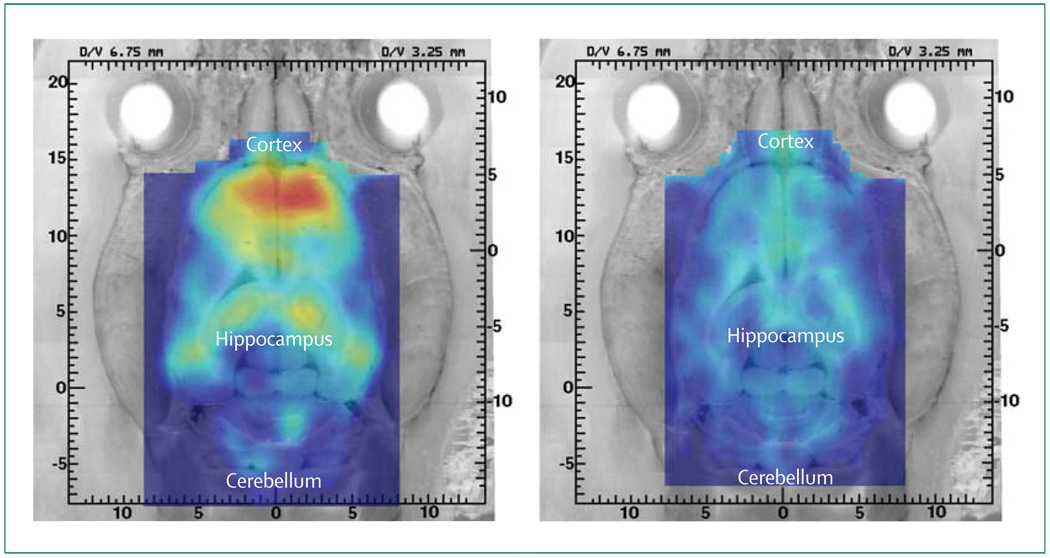
Animal models of AD could be useful in the co-development of neuroimaging measures and treatments. For example, microPET studies (ie, using high-resolution PET scanners for imaging small animals) have shown high hippocampal and cortical [18F]FDDNP binding in 15-month-old transgenic amyloid rat models of AD.119 These same transgenic animals showed minimum [18F]FDDNP binding after in-vivo blocking with the non-steroidal anti-inflammatory drug naproxen, a competitive inhibitor of [18F]FDDNP binding,90 suggesting specificity of [18F]FDDNP binding in vivo (figure 6). Such findings also indicate that commonly used medicines can interfere with neuroimaging results, and patients should be instructed to discontinue their use of certain anti-inflammatory drugs before [18F]FDDNP-PET scanning. In addition, with a suitable anti-aggregation treatment approach, initial testing of compounds in animals could be followed by clinical trials in small animals with microPET used to measure a biomarker for plaques or tangles or both, to guide investigators on further testing in human beings.
Figure 6. MicroPET imaging of amyloid-β deposits in vivo with [18F]FDDNP in a triple transgenic amyloid-β rat model of AD.
Transgenic rat model developed by Cephalon, Inc. (West Chester, PA) and Xenogen Biosciences (Cranbury, NJ). The 15-month-old animal showed the expected increase in binding in the cerebral cortex and hippocampus but not in white matter (left). Specificity of cortical [18F] binding was shown by blockage of the FDDNP signal (right) when the animal was rescanned after three 8 mg doses of naproxen (given over 2 days), which brought the [18F]FDDNP-binding signal to a value similar to that of a control animal.119
Monitoring available interventions
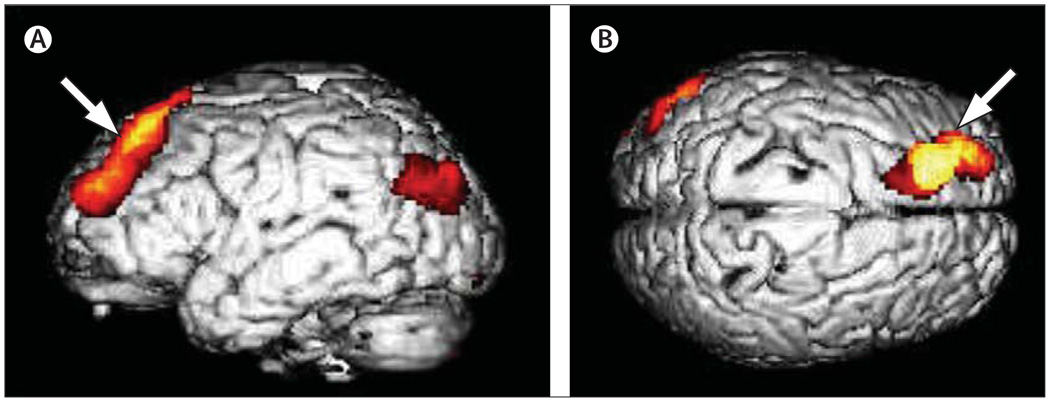
[18F]FDG-PET has been used to monitor cerebral metabolism during clinical trials in patients with AD, and has indicated that cholinesterase inhibitors increase or stabilise cerebral glucose metabolic rates.120–122 [18F]FDG-PET has also been used to track non-pharmacological interventions that might improve brain health and delay cognitive decline. Conventional acupuncture has been reported to increase glucose metabolism in temporal, parietal, and thalamic regions.123 A short-term healthy lifestyle programme that combines mental and physical exercise, stress reduction, and healthy diet was associated with significant effects on both cognitive function and brain metabolism (figure 7).124
Figure 7. Statistical parametric mapping of results of [18F]FDG-PET scanning.
Shows a 5% decline in metabolic activity in the left dorsolateral prefrontal cortex after a 2-week programme of memory training, physical conditioning, relaxation exercises, and healthy diet, whereas no such decline was observed in the control group.124 The colour scale indicates the location of all cortical voxels that show significantly greater decline (p<0·01) in the intervention group compared with the control group. Left: left lateral viewpoint; right: from the top of the brain. The arrows point to the voxels of peak significance (p<0·001). Reproduced with permission from the American Association for Geriatric Psychiatry.124
Conclusions
In current clinical practice, CT or MRI is routinely used and SPECT or [18F]FDG-PET is sometimes used to assist in the diagnosis of dementia. With the recent advances in neuroimaging technology, additional methods are emerging as potentially useful. Many of these methods require validation before they become widely used in clinical settings, and the relatively high costs and lack of wide availability is likely to limit their initial adoption. A cost analysis of CT scanning for identifying theoretically treatable causes of dementia concluded that CT would be cost-effective in patients with dementia aged under 65 years and should be undertaken selectively in older patients.125 A decision-analytic model that compared costs and quality-adjusted life-years associated with adding SPECT, dynamic susceptibility-weighted contrast material-enhanced MRI, or [18F]FDG-PET to the standard clinical examination found that despite the high diagnostic accuracy of PET, these benefits incurred high costs.126 Other analyses, however, have shown that for the same cost, [18F]FDG-PET provides more accurate diagnoses.127,128 Improved diagnostic accuracy would be expected to result in treatment of more patients with the correct diagnosis and fewer patients with a false-positive diagnosis. Thus, the proposed new diagnostic criteria for AD that incorporate biomarkers and that improve early diagnostic accuracy could lead to cost savings, as well as higher quality of care.14 With more extensive use of new technology, costs generally decline, and the assumptions of any of these analytic models will change as new and more effective treatments emerge.
Available structural and functional imaging techniques have improved diagnostic accuracy, helping in the identification of patients who might respond to current anti-dementia symptomatic treatments. However, current technologies and those under development might have the greatest future impact in presymptomatic detection and preventive treatment management of age-related cognitive decline and neurodegeneration. If co-development of neuroimaging surrogate markers and preventive treatments succeeds, future management of neurodegeneration could be similar to current management of blood cholesterol or blood pressure surrogate markers, which are used in the initiation and monitoring of medicines to prevent future disease. Healthy adults with risk factors for cognitive decline (eg, age, previous head trauma, family history) might undergo a scan or so-called brain check for measures of cognitive decline risk, and physicians would administer a vaccine, preventive medication, or other interventions to avoid future cognitive losses and delay or prevent onset of disease. The potential impact of such strategies on the future prevalence of dementia would be substantial.
Search strategy and selection criteria
For each relevant topic area for this Review, peer-reviewed articles published between 1980 and November, 2007, were obtained through PubMed searches by use of appropriate keyword search terms, including “age-related memory loss”, “Alzheimer’s disease”, “amyloid”, “biomarker”, “CT”, “dementia”, “dementia with Lewy bodies”, “diagnosis”, “DTI”, “frontotemporal dementia”, “functional and structural MRI”, “genetics”, “mild cognitive impairment”, “MRS”, “neurotransmitter”, “PET”, “prevention”, “SPECT”, “surrogate marker”, “treatment”, and “vascular dementia”. The most recent references that the authors judged to be representative of the topic area and most relevant were used. Only papers published in English were included.
Acknowledgments
We acknowledge support from NIH grants P01-AG024831, AG13308, P50-AG16570, MH/AG58156, MH52453, AG10123, M01-RR00865, the US Department of Energy (DOE contract DE-FC03-87-ER60615), the Fran and Ray Stark Foundation Fund for Alzheimer’s Disease Research, the Ahmanson Foundation, the Judith Olenick Elgart Fund for Research on Brain Aging, the Parlow-Solomon Professorship (GWS), and the Elizabeth and Thomas Plott endowed Chair in Gerontology (JRB). We thank Harry Vinters for preparing the immunostained images of plaques and tangles.
Footnotes
Conflicts of interest
The University of California, Los Angeles, owns a US patent (6,274,119) entitled “Methods for labeling β-amyloid plaques and neurofibrillary tangles”, which uses an approach outlined in this Review and has been licensed to Siemens. GWS, GMC, S-CH, and JRB are among the inventors, have received royalties, and will receive royalties on future sales. GWS has served as a consultant and/or received lecture fees from Abbott, Dakim, Eisai, Forest, Myriad Genetics, Novartis, Ortho-McNeil, Pfizer, Radica, Servier, Siemens, and VerusMed. GWS has also received stock options from Dakim. S-CH has received lecture fees from GlaxoSmithKline. JRB has served as a consultant and received lecture fees from Nihon Medi-Physics Co, Bristol-Meyer Squibb, PETNet Pharmaceuticals, and Siemens.
Contributor Information
Gary W Small, Department of Psychiatry and Biobehavioral Sciences and Semel Institute for Neuroscience and Human Behavior, University of California–Los Angeles, Los Angeles, California, USA; Alzheimer’s Disease Center, University of California–Los Angeles, Los Angeles, California, USA; Center on Aging, University of California–Los Angeles, Los Angeles, California, USA.
Susan Y Bookheimer, Department of Psychiatry and Biobehavioral Sciences and Semel Institute for Neuroscience and Human Behavior, University of California–Los Angeles, Los Angeles, California, USA; Center for Cognitive Neurosciences, University of California–Los Angeles, Los Angeles, California, USA.
Paul M Thompson, Department of Neurology, University of California–Los Angeles, Los Angeles, California, USA; Laboratory of Neuro Imaging, University of California–Los Angeles, Los Angeles, California, USA.
Greg M Cole, Department of Neurology, University of California–Los Angeles, Los Angeles, California, USA; Alzheimer’s Disease Center, University of California–Los Angeles, Los Angeles, California, USA.
S-C Huang, Department of Molecular and Medical Pharmacology, University of California–Los Angeles, Los Angeles, California, USA.
Vladimir Kepe, Department of Molecular and Medical Pharmacology, University of California–Los Angeles, Los Angeles, California, USA.
Jorge R Barrio, Department of Molecular and Medical Pharmacology, University of California–Los Angeles, Los Angeles, California, USA.
References
- 1.Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- 2.Lopez OL, Jagust WJ, DeKosky ST, et al. Prevalence and classification of mild cognitive impairment in the Cardiovascular Health Study Cognition Study. Arch Neurol. 2003;60:1385–1389. doi: 10.1001/archneur.60.10.1385. [DOI] [PubMed] [Google Scholar]
- 3.Petersen RC, Parisi JE, Dickson DW, et al. Neuropathology of amnestic mild cognitive impairment. Arch Neurol. 2006;63:665–672. doi: 10.1001/archneur.63.5.665. [DOI] [PubMed] [Google Scholar]
- 4.American Psychiatric Association. Diagnostic and statistical manual of mental disorders (IV-Tr) 4th edn—text revised. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 5.McKhann G, Drachman D, Folstein M, et al. Clinical diagnosis of Alzheimer’s disease—report of the NINCDS-ADRDA work group under the auspices of the Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 6.Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA. Alzheimer disease in the US population—prevalence estimates using the 2000 census. Arch Neurol. 2003;60:1119–1122. doi: 10.1001/archneur.60.8.1119. [DOI] [PubMed] [Google Scholar]
- 7.Meguro K, Ishii H, Kasuya M, et al. Incidence of dementia and associated risk factors in Japan: the Osaki-Tajiri Project. J Neurol Sci. 2007;260:175–182. doi: 10.1016/j.jns.2007.04.051. [DOI] [PubMed] [Google Scholar]
- 8.Gascón-Bayarri J, Reñé R, Del Barrio JL, et al. Prevalence of dementia subtypes in El Prat de Llobregat, Catalonia, Spain: the PRATICON Study. Neuroepidemiology. 2007;28:224–234. doi: 10.1159/000108597. [DOI] [PubMed] [Google Scholar]
- 9.McKeith IG, Galasko D, Kosaka K, et al. Consensus guidelines for the clinical and pathological diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47:250–260. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 10.Neary D, Snowden J, Mann D. Frontotemporal dementia. Lancet Neurol. 2005;4:771–780. doi: 10.1016/S1474-4422(05)70223-4. [DOI] [PubMed] [Google Scholar]
- 11.Small GW, Rabins PV, Barry PP, et al. Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA. 1997;278:1363–1371. [PubMed] [Google Scholar]
- 12.Feldman HH, Gauthier S, Chertkow H, et al. Progress in clinical neurosciences: Canadian guidelines for the development of antidementia therapies: a conceptual summary. Can J Neurol Sci. 2006;33:6–26. doi: 10.1017/s0317167100004649. [DOI] [PubMed] [Google Scholar]
- 13.Petersen RC, Thomas RG, Grundman M, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–2388. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- 14.Dubois B, Feldman HH, Jacova C, et al. Research criteria for the diagnosis of Alzheimer’s disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6:734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 15.Josephs KA, Whitwell JL, Ahmed Z, et al. Beta-amyloid burden is not associated with rates of brain atrophy. Ann Neurol. 2007 doi: 10.1002/ana.21223. published online Sept 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glenner GG. Amyloid deposits and amyloidosis. The β-fibrilloses (first of two parts) N Engl J Med. 1980;302:1283–1292. doi: 10.1056/NEJM198006053022305. [DOI] [PubMed] [Google Scholar]
- 17.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 18.Price JL, Morris JC. Tangles and plaques in nondemented aging and “preclinical” Alzheimer’s disease. Ann Neurol. 1999;45:358–368. doi: 10.1002/1531-8249(199903)45:3<358::aid-ana12>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Reinikainen KJ, Soininen H, Riekkinen PJ. Neurotransmitter changes in Alzheimer’s disease: implications to diagnostics and therapy. J Neurosci Res. 1990;27:576–586. doi: 10.1002/jnr.490270419. [DOI] [PubMed] [Google Scholar]
- 20.Tan ZS, Beiser AS, Vasan RS, et al. Inflammatory markers and the risk of Alzheimer disease: the Framingham Study. Neurology. 2007;68:1902–1908. doi: 10.1212/01.wnl.0000263217.36439.da. [DOI] [PubMed] [Google Scholar]
- 21.Atamna H, Boyle K. Amyloid-beta peptide binds with heme to form a peroxidase: relationship to the cytopathologies of Alzheimer’s disease. Proc Natl Acad Sci USA. 2006;103:3381–3386. doi: 10.1073/pnas.0600134103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Craft S. Insulin resistance syndrome and Alzheimer disease: pathophysiologic mechanisms and therapeutic implications. Alzheim Dis Assoc Disord. 2006;20:298–301. doi: 10.1097/01.wad.0000213866.86934.7e. [DOI] [PubMed] [Google Scholar]
- 23.Wadley VG, Haley WE. Diagnostic attributions versus labeling: impact of Alzheimer’s disease and major depression diagnoses on emotions, beliefs, and helping intentions of family members. J Gerontol B Psychol Sci Soc Sci. 2001;56:P244–P252. doi: 10.1093/geronb/56.4.p244. [DOI] [PubMed] [Google Scholar]
- 24.Knopman DS, DeKosky ST, Cummings JL, et al. Practice parameter: diagnosis of dementia (an evidence-based review): report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2001;56:1143–1153. doi: 10.1212/wnl.56.9.1143. [DOI] [PubMed] [Google Scholar]
- 25.Silverman DHS, Small GW, Chang CY, et al. Positron emission tomography in evaluation of dementia: regional brain metabolism and long-term clinical outcome. JAMA. 2001;286:2120–2127. doi: 10.1001/jama.286.17.2120. [DOI] [PubMed] [Google Scholar]
- 26.Jagust W, Thisted R, Devous MD, Sr, et al. SPECT perfusion imaging in the diagnosis of Alzheimer’s disease: a clinical-pathological study. Neurology. 2001;56:950–956. doi: 10.1212/wnl.56.7.950. [DOI] [PubMed] [Google Scholar]
- 27.Minoshima S, Foster NL, Sima AA, Frey KA, Albin RL, Kuhl DE. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann Neurol. 2001;50:358–365. doi: 10.1002/ana.1133. [DOI] [PubMed] [Google Scholar]
- 28.Blodgett TM, Meltzer CC, Townsend DW. PET/CT: form and function. Radiology. 2007;242:360–385. doi: 10.1148/radiol.2422051113. [DOI] [PubMed] [Google Scholar]
- 29.Shellock FG, Morisoli S, Kanal E. MR procedures and biomedical implants, materials, and devices: 1993 update. Radiology. 1993;189:587–599. doi: 10.1148/radiology.189.2.8210394. [DOI] [PubMed] [Google Scholar]
- 30.Jack CR, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer’s disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mungas D, Harvey D, Reed BR, et al. Longitudinal volumetric MRI change and rate of cognitive decline. Neurology. 2005;65:565–571. doi: 10.1212/01.wnl.0000172913.88973.0d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson PM, Hayashi KM, de Zubicaray G, et al. Dynamics of gray matter loss in Alzheimer’s disease. J Neurosci. 2003;23:994–1005. doi: 10.1523/JNEUROSCI.23-03-00994.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stoub TR, Bulgakova M, Leurgans S, et al. MRI predictors of risk of incident Alzheimer disease: a longitudinal study. Neurology. 2005;64:1520–1524. doi: 10.1212/01.WNL.0000160089.43264.1A. [DOI] [PubMed] [Google Scholar]
- 34.Apostolova LG, Dutton RA, Dinov ID, et al. Conversion of mild cognitive impairment to Alzheimer disease predicted by hippocampal atrophy maps. Arch Neurol. 2006;63:693–699. doi: 10.1001/archneur.63.5.693. [DOI] [PubMed] [Google Scholar]
- 35.Rodrigue KM, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. J Neurosci. 2004;24:956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frisoni GB, Pievani M, Testa C, et al. The topography of gray matter involvement in early and late-onset Alzheimer’s disease. Brain. 2007;130:720–730. doi: 10.1093/brain/awl377. [DOI] [PubMed] [Google Scholar]
- 37.Apostolova LG, Akopyan GG, Partiali N, et al. Structural correlates of apathy in Alzheimer’s disease. Dement Geriatr Cogn Disord. 2007;24:91–97. doi: 10.1159/000103914. [DOI] [PubMed] [Google Scholar]
- 38.Schott JM, Price SL, Frost C, Whitwell JL, Rossor MN, Fox NC. Measuring atrophy in Alzhiemer disease: a serial MRI study over 6 and 12 months. Neurology. 2005;65:119–124. doi: 10.1212/01.wnl.0000167542.89697.0f. [DOI] [PubMed] [Google Scholar]
- 39.Whitwell JL, Jack CR, Jr, Parisi JE, et al. Rates of cerebral atrophy differ in different degenerative pathologies. Brain. 2007;130:1148–1158. doi: 10.1093/brain/awm021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen YF, Wang H, Chu Y, Huang YC, Su MY. Regional quantification of white matter hyperintensity in normal aging, mild cognitive impairment, and Alzheimer’s disease. Dement Geriatr Cogn Disord. 2006;22:177–184. doi: 10.1159/000094785. [DOI] [PubMed] [Google Scholar]
- 41.Inzitari D, Simoni M, Pracucci G, et al. Risk of rapid global functional decline in elderly patients with severe cerebral age-related white matter changes. Arch Intern Med. 2007;167:81–88. doi: 10.1001/archinte.167.1.81. [DOI] [PubMed] [Google Scholar]
- 42.Yoshita M, Fletcher E, Harvery D, et al. Extent and distribution of white matter hyperintensities in normal aging, MCI, and AD. Neurology. 2006;67:2192–2198. doi: 10.1212/01.wnl.0000249119.95747.1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 44.Kramer JH, Mungas D, Reed BR, et al. Longitudinal MRI and cognitive change in healthy elderly. Neuropsychology. 2007;21:412–418. doi: 10.1037/0894-4105.21.4.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Regan C, Katona C, Walker Z, et al. Relationship of vascular risk to the progression of Alzheimer disease. Neurology. 2006;67:1357–1362. doi: 10.1212/01.wnl.0000240129.46080.53. [DOI] [PubMed] [Google Scholar]
- 46.de Leeuw F-E, Barkhof F, Scheltens P. Progression of cerebral white matter lesions in Alzheimer’s disease: a new window for therapy? J Neurol Neurosurg Psychiatry. 2005;76:1286–1288. doi: 10.1136/jnnp.2004.053686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burton EJ, McKeith IG, Burn DJ, Firbank MJ, O’Brien JT. Progression of white matter hyperintensities in Alzheimer disease, dementia with Lewy bodies, and Parkinson disease dementia: a comparison with normal aging. Am J Geriatr Psychiatry. 2006;14:842–849. doi: 10.1097/01.JGP.0000236596.56982.1c. [DOI] [PubMed] [Google Scholar]
- 48.van de Pol LA, Korf ES, van der Flier WM, et al. Magnetic resonance imaging predictors of cognition in mild cognitive impairment. Arch Neurol. 2007;64:1023–1028. doi: 10.1001/archneur.64.7.1023. [DOI] [PubMed] [Google Scholar]
- 49.Rosano C, Aizenstein HJ, Wu M, et al. Focal atrophy and cerebrovascular disease increase dementia risk among cognitively normal older adults. J Neuroimaging. 17:148–155. doi: 10.1111/j.1552-6569.2007.00093.x. 207. [DOI] [PubMed] [Google Scholar]
- 50.Frisoni GB, Galluzzi S, Pantoni L, Filippi M. The effect of white matter lesions on cognition in the elderly—small but detectable. Nat Clin Pract Neurol. 2007;3:620–627. doi: 10.1038/ncpneuro0638. [DOI] [PubMed] [Google Scholar]
- 51.Wagner AD, Schacter DL, Rotte M, et al. Building memories: remembering and forgetting of verbal experiences as predicted by brain activity. Science. 1998;281:1188–1191. doi: 10.1126/science.281.5380.1188. [DOI] [PubMed] [Google Scholar]
- 52.Backman L, Andersson JLR, Nyberg L, Winblad B, Nordberg A, Almkvist O. Brain regions associated with episodic retrieval in normal aging and Alzheimer’s disease. Neurology. 1999;52:1861–1870. doi: 10.1212/wnl.52.9.1861. [DOI] [PubMed] [Google Scholar]
- 53.Greicius MD, Srivastava G, Reiss AL, Menon V. Default mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci USA. 2005;101:4637–4642. doi: 10.1073/pnas.0308627101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rombouts S, Scheltens P. Functional connectivity in elderly controls and AD patients using resting state fMRI: a pilot study. Curr Alzheim Res. 2005;2:114–115. doi: 10.2174/1567205053585783. [DOI] [PubMed] [Google Scholar]
- 55.Bokde AL, Lopez-Bayo P, Meindl T, et al. Functional connectivity of the fusiform gyrus during a face-matching task in subjects with mild cognitive impairment. Brain. 2006;129(pt 5):1113–1124. doi: 10.1093/brain/awl051. [DOI] [PubMed] [Google Scholar]
- 56.Bookheimer SY, Strojwas MH, Cohen MS, et al. Patterns of brain activation in people at risk for Alzheimer’s disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kircher TT, Weis S, Freymann K, et al. Hippocampal activation in patients with mild cognitive impairment is necessary for successful memory encoding. J Neurol Neurosurg Psychiatry. 2007;78:812–818. doi: 10.1136/jnnp.2006.104877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Diamond EL, Miller S, Dickerson BC, et al. Relationship of fMRI activation to clinical trial memory measures in Alzheimer disease. Neurology. 2007;69:1331–1341. doi: 10.1212/01.wnl.0000277292.37292.69. [DOI] [PubMed] [Google Scholar]
- 59.Teipel SJ, Bokde AL, Born C, et al. Morphological substrate of face matching in healthy ageing and mild cognitive impairment: a combined MRI-fMRI study. Brain. 2007;130:1745–1758. doi: 10.1093/brain/awm117. [DOI] [PubMed] [Google Scholar]
- 60.Hanyu H, Sakurai H, Iwamoto T, Takasaki M, Shindo H, Abe K. Diffusion-weighted MR imaging of the hippocampus and temporal white matter in Alzheimer’s disease. J Neurol Sci. 1998;156:195–200. doi: 10.1016/s0022-510x(98)00043-4. [DOI] [PubMed] [Google Scholar]
- 61.Firbank MJ, Blamire AM, Krishnan MS, et al. Diffusion tensor imaging in dementia with Lewy bodies and Alzheimer’s disease. Psychiatry Res. 2007;155:135–145. doi: 10.1016/j.pscychresns.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 62.Ringman JM, O’Neill J, Geschwind D, et al. Diffusion tensor imaging in preclinical and presymptomatic carriers of familial Alzheimer’s disease mutations. Brain. 2007;130:1767–1776. doi: 10.1093/brain/awm102. [DOI] [PubMed] [Google Scholar]
- 63.Bartzokis G, Lu PH, Geschwind DH, et al. Apolipoprotein E genotype and age-related myelin breakdown in healthy individuals: implications for cognitive decline and dementia. Arch Gen Psychiatry. 2006;63:63–72. doi: 10.1001/archpsyc.63.1.63. [DOI] [PubMed] [Google Scholar]
- 64.Zhang Y, Schuff N, Jahng GH, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Deary IJ, Bastin ME, Pattie A, et al. White matter integrity and cognition in childhood and old age. Neurology. 2006;66:505–512. doi: 10.1212/01.wnl.0000199954.81900.e2. [DOI] [PubMed] [Google Scholar]
- 66.Ackl N, Ising M, Schreiber YA, Atiya M, Sonntag A, Auer DP. Hippocampal metabolic abnormalities in mild cognitive impairment and Alzheimer’s disease. Neurosci Lett. 2005;384:23–28. doi: 10.1016/j.neulet.2005.04.035. [DOI] [PubMed] [Google Scholar]
- 67.Modrego PJ, Pina MA, Fayed N, Díaz M. Changes in metabolite ratios after treatment with rivastigmine in Alzheimer’s disease: a nonrandomised controlled trial with magnetic resonance spectroscopy. CNS Drugs. 2006;20:867–877. doi: 10.2165/00023210-200620100-00006. [DOI] [PubMed] [Google Scholar]
- 68.Falini A, Bozzali M, Magnani G, et al. A whole brain MR spectroscopy study from patients with Alzheimer’s disease and mild cognitive impairment. Neuroimage. 2005;26:1159–1163. doi: 10.1016/j.neuroimage.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 69.Jessen F, Traeber F, Freymann K, Maier W, Schild HH, Block W. Treatment monitoring and response prediction with proton MR spectroscopy in AD. Neurology. 2006;67:528–530. doi: 10.1212/01.wnl.0000228218.68451.31. [DOI] [PubMed] [Google Scholar]
- 70.Matsuda H. Role of neuroimaging in Alzheimer’s disease, with emphasis on brain perfusion SPECT. J Nucl Med. 2007;48:1289–1300. doi: 10.2967/jnumed.106.037218. [DOI] [PubMed] [Google Scholar]
- 71.Small GW, Kepe V, Ercoli L, et al. PET of brain amyloid and tau in mild cognitive impairment. N Engl J Med. 2006;355:2652–2663. doi: 10.1056/NEJMoa054625. [DOI] [PubMed] [Google Scholar]
- 72.Kepe V, Barrio JR, Huang S-C, et al. Serotonin 1A receptors in the living brain of Alzheimer’s disease patients. Proc Natl Acad Sci USA. 2006;103:702–707. doi: 10.1073/pnas.0510237103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jagust W, Reed B, Mungas D, Ellis W, DeCarli C. What does fluorodeoxyglucose PET imaging add to a clinical diagnosis of dementia? Neurology. 2007;69:871–877. doi: 10.1212/01.wnl.0000269790.05105.16. [DOI] [PubMed] [Google Scholar]
- 74.Drzezga A, Grimmer T, Riemenschneider M, et al. Prediction of individual clinical outcome in MCI by means of genetic assessment and (18)F-FDG PET. J Nucl Med. 2005;46:1625–1632. [PubMed] [Google Scholar]
- 75.Chételat G, Desgranges B, de la Sayette V, et al. Mild cognitive impairment: can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 76.Small GW, La Rue A, Komo S, Kaplan A, Mandelkern MA. Predictors of cognitive change in middle-aged and older adults with memory loss. Am J Psychiatry. 1995;152:1757–1764. doi: 10.1176/ajp.152.12.1757. [DOI] [PubMed] [Google Scholar]
- 77.de Leon MJ, Convit A, Wolf OT, et al. Prediction of cognitive decline in normal elderly subjects with 2-[(18)F]fluoro-2-deoxy-D-glucose/positron-emission tomography (FDG/PET) Proc Natl Acad Sci USA. 2001;98:10966–10971. doi: 10.1073/pnas.191044198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Small GW, Ercoli LM, Silverman DHS, et al. Cerebral metabolic and cognitive decline in persons at genetic risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 2000;97:6037–6042. doi: 10.1073/pnas.090106797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer’s dementia. Proc Natl Acad Sci USA. 2004;101:284–289. doi: 10.1073/pnas.2635903100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mosconi L, Perani D, Sorbi S, et al. MCI conversion to dementia and the APOE genotype: a prediction study with FDG-PET. Neurology. 2004;63:2332–2340. doi: 10.1212/01.wnl.0000147469.18313.3b. [DOI] [PubMed] [Google Scholar]
- 81.Chételat G, Eustache F, Viader F, et al. FDG-PET measurement is more accurate than neuropsychological assessments to predict global cognitive deterioration in patients with mild cognitive impairment. Neurocase. 2005;11:14–25. doi: 10.1080/13554790490896938. [DOI] [PubMed] [Google Scholar]
- 82.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 83.Shoghi-Jadid K, Small GW, Agdeppa ED, et al. Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer’s disease. Am J Geriatr Psychiatry. 2002;10:24–35. [PubMed] [Google Scholar]
- 84.Kung HF, Kung MP, Zhuang ZP, et al. Iodinated tracers for imaging amyloid plaques in the brain. Mol Imaging Biol. 2003;5:418–426. doi: 10.1016/j.mibio.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 85.Verhoeff NPLG, Wilson AA, Takeshita S, et al. In-vivo imaging of Alzheimer disease β-amyloid with [11C]SB-13 PET. Am J Geriatr Psychiatry. 2004;12:584–595. doi: 10.1176/appi.ajgp.12.6.584. [DOI] [PubMed] [Google Scholar]
- 86.Kudo Y, Okamura N, Furumoto S, et al. 2-(2-[2-Dimethylaminothiazol-5-yl]ethenyl)-6-(2-[fluoro]ethoxy)benzoxazole: a novel PET agent for in vivo detection of dense amyloid plaques in Alzheimer’s disease patients. J Nucl Med. 2007;48:553–561. doi: 10.2967/jnumed.106.037556. [DOI] [PubMed] [Google Scholar]
- 87.Kemppainen NM, Aalto S, Wilson IA, et al. PET amyloid ligand [11C]PIB uptake is increased in mild cognitive impairment. Neurology. 2007;68:1603–1606. doi: 10.1212/01.wnl.0000260969.94695.56. [DOI] [PubMed] [Google Scholar]
- 88.Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology. 2007;68:1718–1725. doi: 10.1212/01.wnl.0000261919.22630.ea. [DOI] [PubMed] [Google Scholar]
- 89.Engler H, Forsberg A, Almkvist O, et al. Two-year follow-up of amyloid deposition in patients with Alzheimer’s disease. Brain. 2006;129:2856–2866. doi: 10.1093/brain/awl178. [DOI] [PubMed] [Google Scholar]
- 90.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 91.Rowe CC, Ackerman U, Browne W, et al. Imaging β-amyloid in Alzheimer’s disease with 18F-BAY94-9172, a novel fluorine-18 labeled positron emission tomography tracer. Lancet Neurol. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 92.Agdeppa ED, Kepe V, Petric A, et al. In vitro detection of (S)-naproxen and ibuprofen binding to plaques in the Alzheimer’s brain using the positron emission tomography molecular imaging probe 2-(1-{6-[(2-[18F]fluoroethyl)(methyl)amino]-2-naphthyl}ethylid ene)malononitrile. Neuroscience. 2003;117:723–730. doi: 10.1016/s0306-4522(02)00907-7. [DOI] [PubMed] [Google Scholar]
- 93.Kadir A, Almkvist O, Wall A, Långström B, Nordberg A. PET imaging of cortical 11C-nicotine binding correlates with the cognitive function of attention in Alzheimer’s disease. Psychopharmacol. 2006;188:509–520. doi: 10.1007/s00213-006-0447-7. [DOI] [PubMed] [Google Scholar]
- 94.Kadir A, Darreh-Shori T, Almkvist O, Wall A, Långström B, Nordberg A. Changes in brain 11C-nicotine binding sites in patients with mild Alzheimer’s disease following rivastigmine treatment as assessed by PET. Psychopharmacol. 2007;191:1005–1014. doi: 10.1007/s00213-007-0725-z. [DOI] [PubMed] [Google Scholar]
- 95.Rinne JO, Sahlberg N, Ruottinen H, Nagren K, Lehikoinen P. Striatal uptake of the dopamine reuptake ligand [11C]β-CFT is reduced in Alzheimer’s disease assessed by positron emission tomography. Neurology. 1998;50:152–156. doi: 10.1212/wnl.50.1.152. [DOI] [PubMed] [Google Scholar]
- 96.Cagnin A, Brooks DJ, Kennedy AM, et al. In-vivo measurement of activated microglia in dementia. Lancet. 2001;358:461–467. doi: 10.1016/S0140-6736(01)05625-2. [DOI] [PubMed] [Google Scholar]
- 97.Mueller SG, Weiner MW, Thal LJ, et al. Ways toward an early diagnosis in Alzheimer’s disease: the Alzheimer’s Disease Neuroimaging Initiative (ADNI) Alzheim Dement. 2005;1:55–66. doi: 10.1016/j.jalz.2005.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Katz R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx. 2004;1:189–195. doi: 10.1602/neurorx.1.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cohen RM, Podruchny TA, Bokde AL, et al. Higher in vivo muscarinic-2 receptor distribution volumes in aging subjects with an apolipoprotein E-epsilon4 allele. Synapse. 2003;49:150–156. doi: 10.1002/syn.10225. [DOI] [PubMed] [Google Scholar]
- 100.UK National Institute for Health and Clinical Excellence. [accessed Dec 12, 2007];Dementia: supporting people with dementia and their carers in health and social care. 2006/024 update on NICE guidance on the use of drugs to treat Alzheimer’s disease. http://guidance.nice.org.uk/cg42.
- 101.Fox NC, Black RS, Gilman S, et al. Effects of Abeta immunization (AN1792) on MRI measures of cerebral volume in Alzheimer disease. Neurology. 2005;64:1563–1572. doi: 10.1212/01.WNL.0000159743.08996.99. [DOI] [PubMed] [Google Scholar]
- 102.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science. 1993;261:921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 103.Rogaev EI, Sherrington R, Rogaeva EA, et al. Familial Alzheimer’s disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer’s disease type 3 gene. Nature. 1995;376:775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- 104.Reiman EM, Webster JA, Myers AJ, et al. GAB2 alleles modify Alzheimer’s risk in APOE epsilon4 carriers. Neuron. 2007;54:713–720. doi: 10.1016/j.neuron.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rogaeva E, Meng Y, Lee JH, et al. The neuronal sortilin-related receptor SORL1 is genetically associated with Alzheimer disease. Nat Genet. 2007;39:168–177. doi: 10.1038/ng1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ray S, Britschgi M, Herbert C, et al. Classification and prediction of clinical Alzheimer’s diagnosis based on plasma signaling proteins. Nat Med. 2007;13:1359–1362. doi: 10.1038/nm1653. [DOI] [PubMed] [Google Scholar]
- 107.Fagan AM, Roe CM, Xiong C, Mintun MA, Morris JC, Holtzman DM. Cerebrospinal fluid tau/beta-amyloid(42) ratio as a prediction of cognitive decline in nondemented older adults. Arch Neurol. 2007;64:343–349. doi: 10.1001/archneur.64.3.noc60123. [DOI] [PubMed] [Google Scholar]
- 108.Wilson RS, Schneider JA, Arnold SE, Tang Y, Boyle PA, Bennett DA. Olfactory identification and incidence of mild cognitive impairment in older age. Arch Gen Psychiatry. 2007;64:802–808. doi: 10.1001/archpsyc.64.7.802. [DOI] [PubMed] [Google Scholar]
- 109.Hanyu H, Hirao K, Shimizu S, Kanetaka H, Sakurai H, Iwamoto T. Phenylephrine and pilocarpine eye drop test for dementia with Lewy bodies and Alzheimer’s disease. Neurosci Lett. 2007;414:174–177. doi: 10.1016/j.neulet.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 110.Khalil Z, LoGiudice D, Khodr B, Maruff P, Masters C. Impaired peripheral endothelial microvascular responsiveness in Alzheimer’s disease. J Alzheim Dis. 2007;11:25–32. doi: 10.3233/jad-2007-11106. [DOI] [PubMed] [Google Scholar]
- 111.Jedrziewski MK, Lee Vm-Y, Trojanowski JQ. Lowering the risk of Alzheimer’s disease: evidence-based practices emerge from new research. Alzheim Dement. 2005;1:152–160. doi: 10.1016/j.jalz.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 112.Small GW. What we need to know about age related memory loss. BMJ. 2002;324:1502–1505. doi: 10.1136/bmj.324.7352.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cole GM, Lim GP, Yang F, et al. Prevention of Alzheimer’s disease: omega-3 fatty acid and phenolic anti-oxidant interventions. Neurobiol Aging. 2005;26 suppl 1:133–136. doi: 10.1016/j.neurobiolaging.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 114.Cummings JL, Doody R, Clark C. Disease-modifying therapies for Alzheimer disease. Neurology. 2007;69:1622–1634. doi: 10.1212/01.wnl.0000295996.54210.69. [DOI] [PubMed] [Google Scholar]
- 115.Cummings JL. Challenges to demonstrating disease-modifying effects in Alzheimer’s disease clinical trials. Alzheim Dement. 2006;2:263–271. doi: 10.1016/j.jalz.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mohs RC, Kawas C, Carrillo MC. Optimal design of clinical trials for drugs designed to show the course of Alzheimer’s disease. Alzheim Dement. 2006;2:131–139. doi: 10.1016/j.jalz.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 117.Reiman EM, Caselli RJ, Chen K, Alexander GE, Bandy D, Frost J. Declining brain activity in cognitively normal apolipoprotein E epsilon 4 heterozygotes: a foundation for using positron emission tomography to efficiently test treatments to prevent Alzheimer’s disease. Proc Natl Acad Sci USA. 2001;98:3334–3339. doi: 10.1073/pnas.061509598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Vellas B, Andrieu S, Sampaio C, et al. Disease-modifying trials in Alzhiemer’s disease: a European task force consensus. Lancet Neurol. 2007;6:56–62. doi: 10.1016/S1474-4422(06)70677-9. [DOI] [PubMed] [Google Scholar]
- 119.Kepe V, Cole GM, Liu J, et al. In vivo [F-18]FDDNP microPET imaging of brain β-amyloid in a transgenic rat model of Alzheimer’s disease. Alzheim Dement. 2005;1 suppl 1:S45. [Google Scholar]
- 120.Stefanova E, Wall A, Almkvist O, et al. Longitudinal PET evaluation of cerebral glucose metabolism in rivastigmine treated patients with mild Alzheimer’s disease. J Neural Transm. 2006;113:205–218. doi: 10.1007/s00702-005-0312-6. [DOI] [PubMed] [Google Scholar]
- 121.Mega MS, Cummings JL, O’Connor SM, et al. Cognitive and metabolic responses to metrifonate therapy in Alzheimer disease. Neuropsychiatry Neuropsychol Behav Neurol. 2001;14:63–68. [PubMed] [Google Scholar]
- 122.Tune L, Tiseo PJ, Ieni J, et al. Donepezil HCl (E2020) maintains functional brain activity in patients with Alzheimer disease: results of a 24-week, double-blind, placebo-controlled study. Am J Geriatr Psychiatry. 2003;11:169–177. [PubMed] [Google Scholar]
- 123.Huang Y, Chen J, Htut WM, Lai X, Wik G. Acupuncture increases cerebral glucose metabolism in human vascular dementia. Int J Neurosci. 2007;117:1029–1037. doi: 10.1080/00207450600936825. [DOI] [PubMed] [Google Scholar]
- 124.Small GW, Silverman DHS, Siddarth P, et al. Effects of a 14-day healthy longevity lifestyle program on cognition and brain function. Am J Geriatr Psychiatry. 2006;14:538–545. doi: 10.1097/01.JGP.0000219279.72210.ca. [DOI] [PubMed] [Google Scholar]
- 125.Foster GR, Scott DA, Payne S. The use of CT scanning in dementia. A systematic review. Int J Technol Assess Health Care. 1999;15:406–423. [PubMed] [Google Scholar]
- 126.McMahon PM, Araki SS, Sandberg EA, Neumann PJ, Gazelle GS. Cost-effectiveness of PET in the diagnosis of Alzheimer disease. Radiology. 2003;228:515–522. doi: 10.1148/radiol.2282020915. [DOI] [PubMed] [Google Scholar]
- 127.Silverman DH, Gambhir SS, Huang HW, et al. Evaluating early dementia with and without assessment of regional cerebral metabolism by PET: a comparison of predicted costs and benefits. J Nucl Med. 2002;43:253–266. [PubMed] [Google Scholar]
- 128.Moulin-Romsee G, Maes A, Silverman D, Mortelmans L, Van Laere K. Cost-effectiveness of 18F-fluorodeoxyglucose positron emission tomography in the assessment of early dementia from a Belgian and European perspective. Eur J Neurol. 2005;12:254–263. doi: 10.1111/j.1468-1331.2004.00940.x. [DOI] [PubMed] [Google Scholar]