Abstract
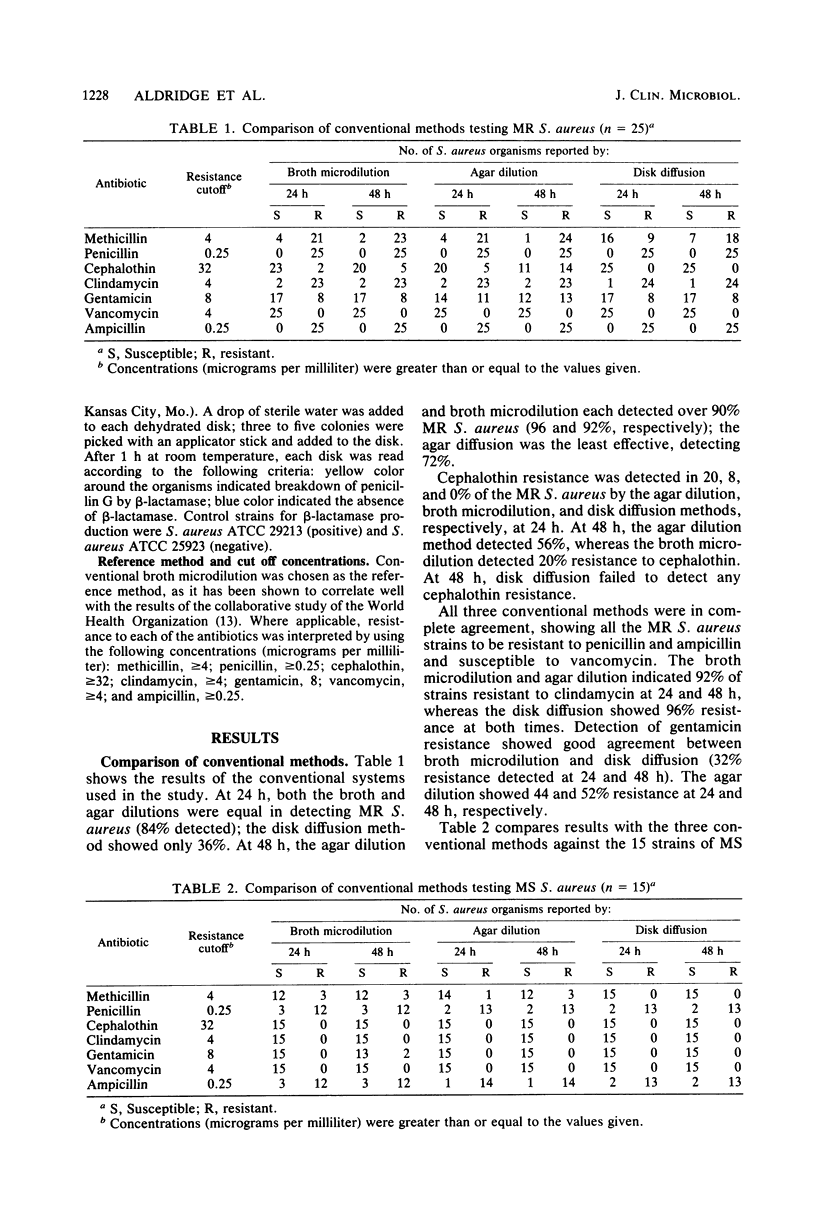
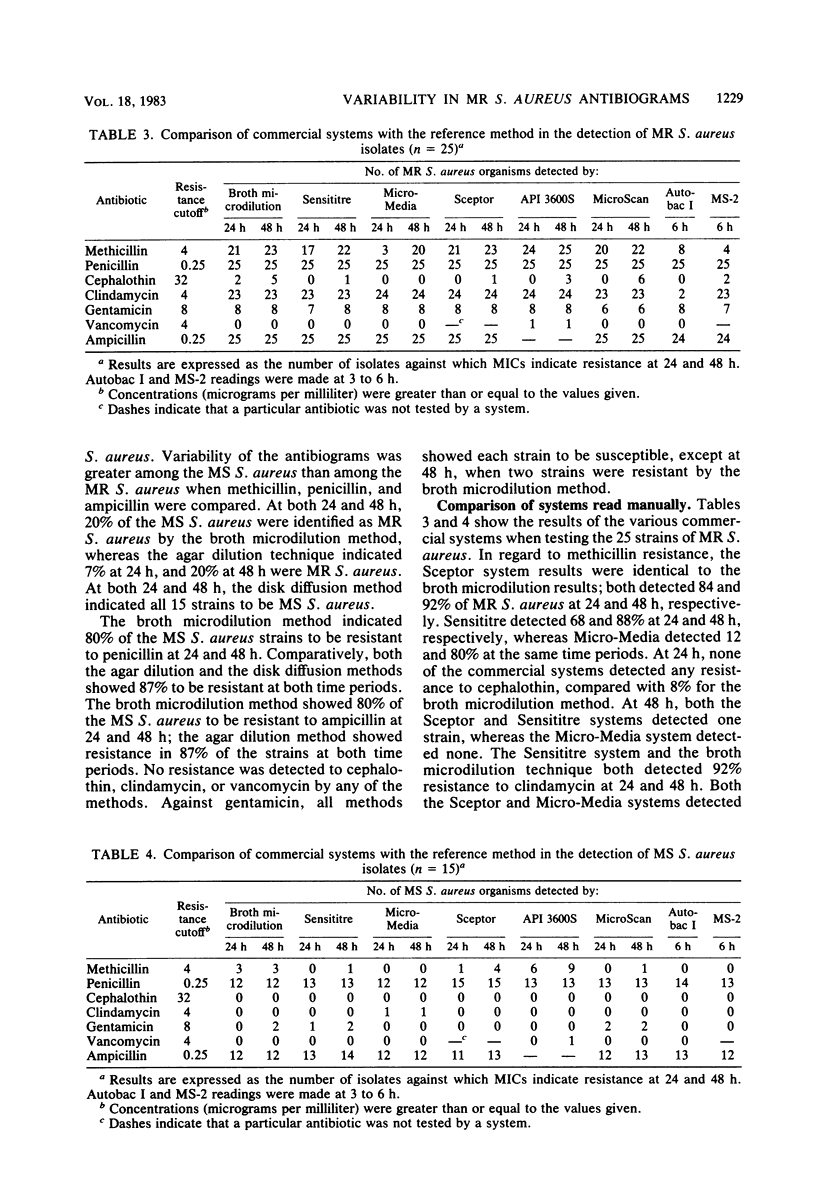
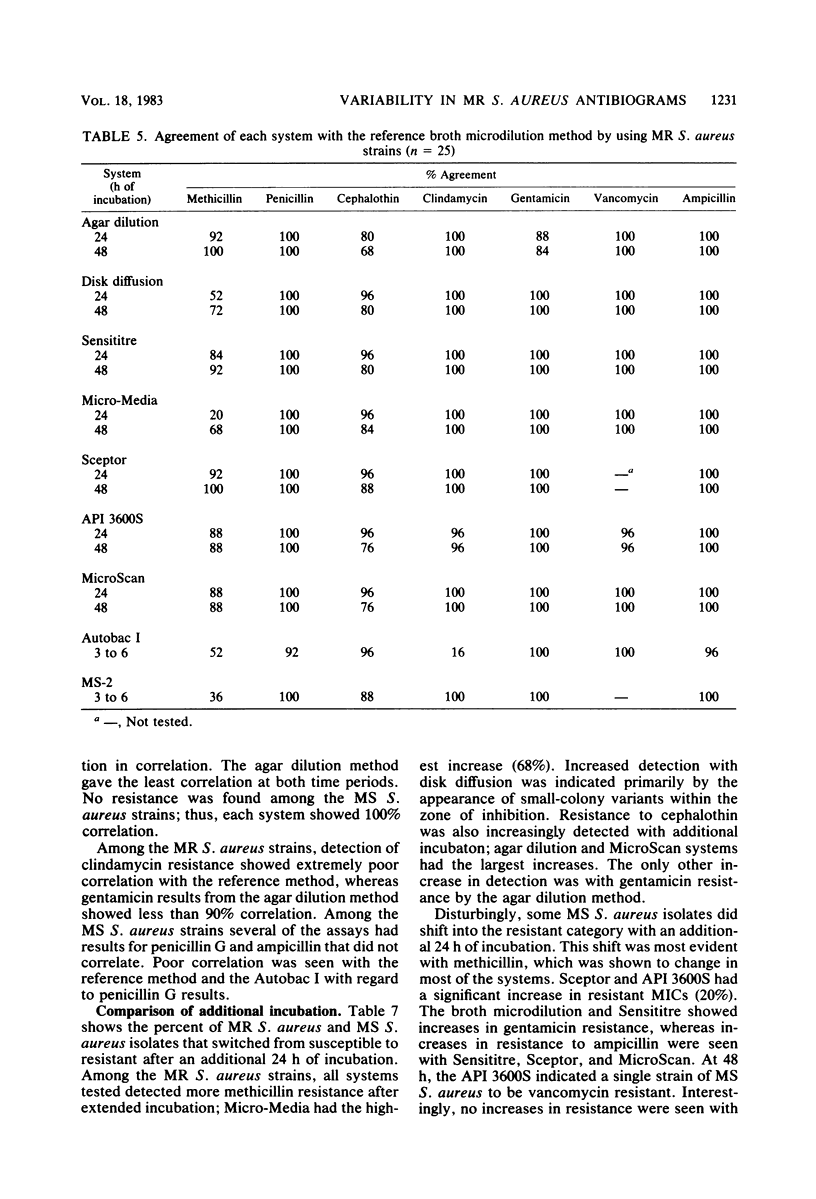
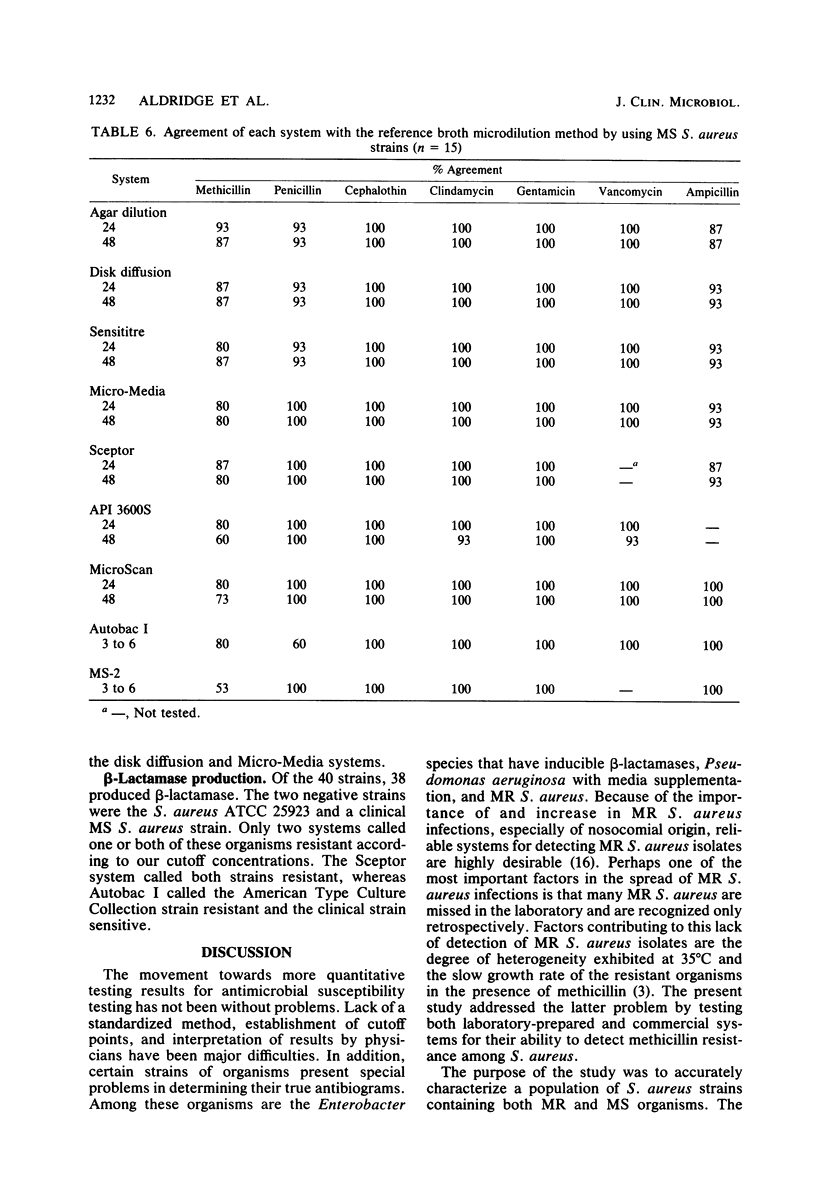
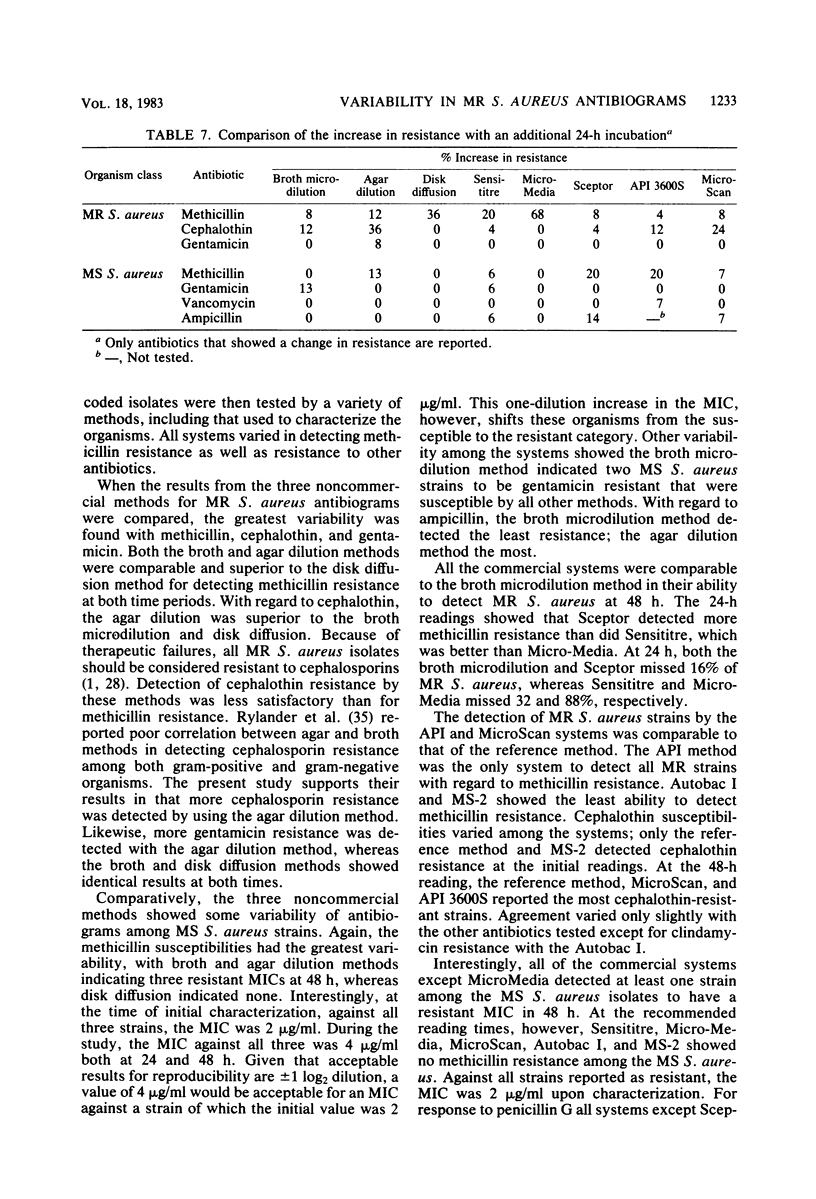
Laboratory-prepared (conventional) and commercial susceptibility testing systems were compared by using a group of methicillin-resistant (MR) and methicillin-susceptible (MS) strains of Staphylococcus aureus. A group of 25 MR and 15 MS S. aureus strains were coded and tested blindly by disk diffusion, agar dilution, broth microdilution, Sensititre, Micro-Media, Sceptor, API 3600S, MicroScan, Autobac I, and MS-2 systems. All systems were incubated at 35 degrees C and read with either a manual or automated reader at the recommended times. Where applicable, systems were also read at 48 h. Among the conventional assays, the broth and agar dilution methods were comparable, both detecting 88% of the MR strains at 24 h and detecting 92 and 96%, respectively, at 48 h. The disk diffusion method was less efficient, detecting only 36 and 72% at 24 and 48 h, respectively. Detection of cephalothin resistance was low for all systems at both time periods, with agar dilution and disk diffusion being the most and least efficient, respectively. Some variability was also seen with detection of resistance to clindamycin and gentamicin. Among the MS strains, variability among the conventional systems occurred with methicillin, gentamicin, ampicillin, and penicillin. Comparison of the commercial systems with manual readers with the broth microdilution method (reference method) showed that for MR strains, the Sceptor system gave identical results at 24 and 48 h. Sensititre detected 68 and 88% of the MR strains, whereas Micro-Media was least effective detecting 12 and 80% at 24 and 48 h, respectively. None of the commercial systems detected cephalothin resistance well, with only one strain being indicated by the Sceptor and Sensititre systems at 48 h. Slight differences were also seen among the systems with clindamycin and gentamicin. With regard to the MS strains, variability among the systems was seen with methicillin, penicillin, ampicillin, clindamycin, and gentamicin. Among commercial systems with automated readers, the API system detected a greater number of MR strains than did the reference method at 24 and 48 h, 96 and 100%, respectively. The MicroScan method was comparable to the reference method detecting 80 and 88% of the MR strains at both time periods, respectively. Both Autobac I and MS-2 were much less effective in detecting MR strains, noting only 32 and 16%, respectively, at the 3- to 6-h readings. Poor detection of cephalothin resistance among MR strains was evident in all systems. Variability also occurred among the systems with clindamycin, gentamicin, and ampicillin. A single strain of the MR group was reported to be vancomycin resistant by the API system. Among the MS group, the greatest variability was seen with methicillin. Less variability occurred with penicillin, ampicillin, gentamicin, and vancomycin.
Full text
PDF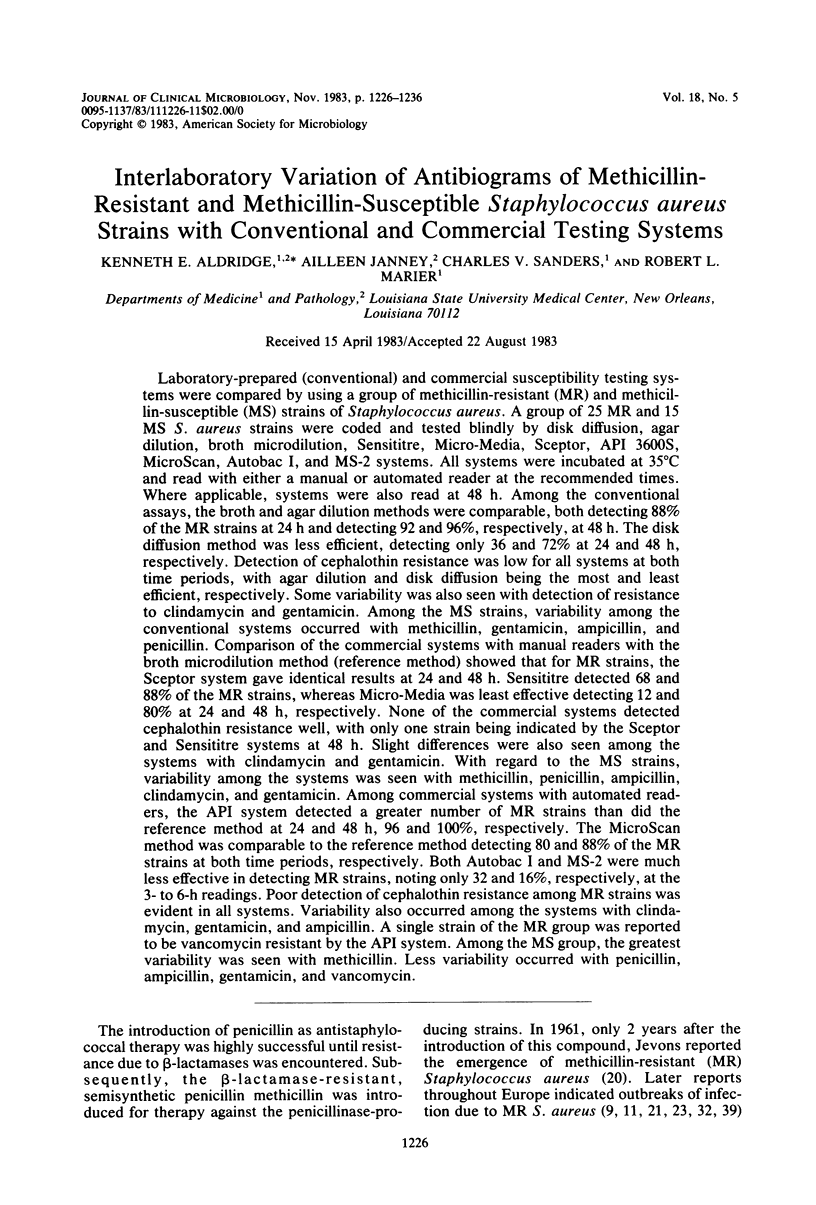





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrett F. F., McGehee R. F., Jr, Finland M. Methicillin-resistant Staphylococcus aureus at Boston City Hospital. Bacteriologic and epidemiologic observations. N Engl J Med. 1968 Aug 29;279(9):441–448. doi: 10.1056/NEJM196808292790901. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Badal R. E. Reliability of the microdilution technic for detection of methicillin-resistant strains of staphylococcus aureus. Am J Clin Pathol. 1977 May;67(5):489–495. doi: 10.1093/ajcp/67.5.489. [DOI] [PubMed] [Google Scholar]
- Barry A. L., Jones R. N., Gavan T. L. Evaluation of the micro-media system for quantitative antimicrobial drug susceptibility testing: a collaborative study. Antimicrob Agents Chemother. 1978 Jan;13(1):61–69. doi: 10.1128/aac.13.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Blair H. C., Cleary T. J. Susceptibility testing of multidrug-resistant Staphylococcus aureus with the Sceptor microdilution system. J Clin Microbiol. 1983 Jul;18(1):194–196. doi: 10.1128/jcm.18.1.194-196.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyce J. M., White R. L., Bonner M. C., Lockwood W. R. Reliability of the MS-2 system in detecting methicillin-resistant Staphylococcus aureus. J Clin Microbiol. 1982 Feb;15(2):220–225. doi: 10.1128/jcm.15.2.220-225.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHABBERT Y. A., BAUDENS J. G. [Staphylococcus strains naturally resistant to methicillin and 5-methyl-3-phenyl-4-iso-oxazolyl-penicillin]. Ann Inst Pasteur (Paris) 1962 Aug;103:222–230. [PubMed] [Google Scholar]
- COLLEY E. W., MCNICOL M. W., BRACKEN P. M. METHICILLIN-RESISTANT STAPHYLOCOCCI IN A GENERAL HOSPITAL. Lancet. 1965 Mar 13;1(7385):595–597. doi: 10.1016/s0140-6736(65)91165-7. [DOI] [PubMed] [Google Scholar]
- Carlson J. R., Conley F. E., Cahall D. L. Methicillin-resistant Staphylococcus aureus susceptibility testing with Abbott MS-2 system. Antimicrob Agents Chemother. 1982 Apr;21(4):676–677. doi: 10.1128/aac.21.4.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary T. J., Maurer D. Methicillin-resistant Staphylococcus aureus susceptibility testing by an automated system, Autobac I. Antimicrob Agents Chemother. 1978 May;13(5):837–841. doi: 10.1128/aac.13.5.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crossley K., Loesch D., Landesman B., Mead K., Chern M., Strate R. An outbreak of infections caused by strains of Staphylococcus aureus resistant to methicillin and aminoglycosides. I. Clinical studies. J Infect Dis. 1979 Mar;139(3):273–279. doi: 10.1093/infdis/139.3.273. [DOI] [PubMed] [Google Scholar]
- Gavan T. L., Jones R. N., Barry A. L. Evaluation of the Sensititre system for quantitative antimicrobial drug susceptibility testing: a collaborative study. Antimicrob Agents Chemother. 1980 Mar;17(3):464–469. doi: 10.1128/aac.17.3.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill V. J., Manning C. B., Ingalls C. M. Correlation of penicillin minimum inhibitory concentrations and penicillin zone edge appearance with staphylococcal beta-lactamase production. J Clin Microbiol. 1981 Oct;14(4):437–440. doi: 10.1128/jcm.14.4.437-440.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley R. W., Hightower A. W., Khabbaz R. F., Thornsberry C., Martone W. J., Allen J. R., Hughes J. M. The emergence of methicillin-resistant Staphylococcus aureus infections in United States hospitals. Possible role of the house staff-patient transfer circuit. Ann Intern Med. 1982 Sep;97(3):297–308. doi: 10.7326/0003-4819-97-3-297. [DOI] [PubMed] [Google Scholar]
- Hone R., Keane C. T. Characteristics of methicillin resistant Staphylococcus aureus. Ir J Med Sci. 1974 May;143(3):145–154. doi: 10.1007/BF03004756. [DOI] [PubMed] [Google Scholar]
- JACKSON G. G., FINLAND M. Comparison of methods for determining sensitivity of bacteria to antibiotics in vitro. AMA Arch Intern Med. 1951 Oct;88(4):446–460. doi: 10.1001/archinte.1951.03810100030003. [DOI] [PubMed] [Google Scholar]
- JEVONS M. P., COE A. W., PARKER M. T. Methicillin resistance in staphylococci. Lancet. 1963 Apr 27;1(7287):904–907. doi: 10.1016/s0140-6736(63)91687-8. [DOI] [PubMed] [Google Scholar]
- Jessen O., Rosendal K., Bülow P., Faber V., Eriksen K. R. Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med. 1969 Sep 18;281(12):627–635. doi: 10.1056/NEJM196909182811201. [DOI] [PubMed] [Google Scholar]
- Jones R. N., Thornsberry C., Barry A. L., Gavan T. L. Evaluation of the Sceptor microdilution antibiotic susceptibility testing system: a collaborative investigation. J Clin Microbiol. 1981 Jan;13(1):184–194. doi: 10.1128/jcm.13.1.184-194.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KALLINGS L. O. The susceptibility of staphylococcal hospital strains to methyl-phenyl-isoxazolyl penicillin. Acta Pathol Microbiol Scand. 1962;54:127–128. [PubMed] [Google Scholar]
- Kayser F. H., Mak T. M. Methicillin-resistant staphylococci. Am J Med Sci. 1972 Sep;264(3):197–205. [PubMed] [Google Scholar]
- Klimek J. J., Marsik F. J., Bartlett R. C., Weir B., Shea P., Quintiliani R. Clinical, epidemiologic and bacteriologic observations of an outbreak of methicillin-resistant Staphylococcus aureus at a large community hospital. Am J Med. 1976 Sep;61(3):340–345. doi: 10.1016/0002-9343(76)90370-3. [DOI] [PubMed] [Google Scholar]
- Kluge R. M. Comparative in vitro activity of methicillin, oxacillin and cefamandole against clinical isolates of Staphylococcus aureus. J Antimicrob Chemother. 1980 May;6(3):412–415. doi: 10.1093/jac/6.3.412. [DOI] [PubMed] [Google Scholar]
- Lindsey N. J., Barnes W. G. Examination of major disagreements in susceptibility test results by Autobac-1 and MS-2. Antimicrob Agents Chemother. 1981 Jul;20(1):115–119. doi: 10.1128/aac.20.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. P., Linnemann C. C., Jr Bacteremia due to methicillin-resistant Staphylococcus aureus. J Infect Dis. 1982 Apr;145(4):532–536. doi: 10.1093/infdis/145.4.532. [DOI] [PubMed] [Google Scholar]
- PARKER M. T., JEVONS M. P. A SURVEY OF METHICILLIN RESISTANCE IN STAPHYLOCOCCUS AUREUS. Postgrad Med J. 1964 Dec;40:SUPPL–SUPPL:178. doi: 10.1136/pgmj.40.suppl.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. T., Hewitt J. H. Methicillin resistance in Staphylococcus aureus. Lancet. 1970 Apr 18;1(7651):800–804. doi: 10.1016/s0140-6736(70)92408-6. [DOI] [PubMed] [Google Scholar]
- Peacock J. E., Jr, Marsik F. J., Wenzel R. P. Methicillin-resistant Staphylococcus aureus: introduction and spread within a hospital. Ann Intern Med. 1980 Oct;93(4):526–532. doi: 10.7326/0003-4819-93-4-526. [DOI] [PubMed] [Google Scholar]
- Richmond A. S., Simberkoff M. S., Schaefler S., Rahal J. J., Jr Resistance of Staphylococcus aureus to semisynthetic penicillins and cephalothin. J Infect Dis. 1977 Jan;135(1):108–112. doi: 10.1093/infdis/135.1.108. [DOI] [PubMed] [Google Scholar]
- Rylander M., Brorson J. E., Johnsson J., Norrby R. Comparison between agar and broth minimum inhibitory concentrations of cefamandole, Cefoxitin, and cefuroxime. Antimicrob Agents Chemother. 1979 Apr;15(4):572–579. doi: 10.1128/aac.15.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEWART G. T., HOLT R. J. Evolutio of natural resistance to the newer penicillins. Br Med J. 1963 Feb 2;1(5326):308–311. doi: 10.1136/bmj.1.5326.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D. Chemical and physical factors influencing methicillin resistance of Staphylococcus aureus and Staphylococcus epidermidis. J Antimicrob Chemother. 1977 Nov;3 (Suppl 100):47–51. doi: 10.1093/jac/3.suppl_c.47. [DOI] [PubMed] [Google Scholar]


