Abstract
Background
Dietary intake of genistein by patients with prostate cancer has been associated with decreased metastasis and mortality. Genistein blocks activation of p38 mitogen-activated protein kinase and thus inhibits matrix metalloproteinase-2 (MMP-2) expression and cell invasion in cultured cells and inhibits metastasis of human prostate cancer cells in mice. We investigated the target for genistein in prostate cancer cells.
Methods
Prostate cell lines PC3-M, PC3, 1532NPTX, 1542NPTX, 1532CPTX, and 1542CPTX were used. All cell lines were transiently transfected with a constitutively active mitogen-activated protein kinase kinase 4 (MEK4) expression vector (to increase MEK4 expression), small interfering RNA against MEK4 (to decrease MEK4 expression), or corresponding control constructs. Cell invasion was assessed by a Boyden chamber assay. Gene expression was assessed by a quantitative reverse transcription–polymerase chain reaction. Protein expression was assessed by Western blot analysis. Modeller and AutoDock programs were used for modeling of the structure of MEK4 protein and ligand docking, respectively. MMP-2 transcript levels were assessed in normal prostate epithelial cells from 24 patients with prostate cancer from a phase II randomized trial comparing genistein treatment with no treatment. Statistical significance required a P value of .050 or less. All statistical tests were two-sided.
Results
Overexpression of MEK4 increased MMP-2 expression and cell invasion in all six cell lines. Decreased MEK4 expression had the opposite effects. Modeling showed that genistein bound to the active site of MEK4. Genistein inhibited MEK4 kinase activity with a half maximal inhibitory concentration of 0.40 μM (95% confidence interval [CI] = 0.36 to 0.45 μM). The MMP-2 transcript level in normal prostate epithelial cells was statistically significantly higher in the untreated group (100%) than in the genistein-treated group (24%; difference = 76%, 95% CI = 38% to 115%; P = .045).
Conclusions
We identified MEK4 as a proinvasion protein in six human prostate cancer cell lines and the target for genistein. We showed, to our knowledge for the first time, that genistein treatment, compared with no treatment, was associated with decreased levels of MMP-2 transcripts in normal prostate cells from prostate cancer–containing tissue.
CONTEXT AND CAVEATS
Prior knowledge
Consumption of foods with high levels of genistein has been associated with decreased metastasis and mortality among patients with prostate cancer. Genistein has been shown to inhibit matrix metalloproteinase-2 (MMP-2) expression and cell invasion.
Study design
Six prostate cell lines were used to study the effects of genistein treatment on the expression of mitogen-activated protein kinase kinase 4 (MEK4), a protein kinase, and various activities, including cell invasion. The structure of MEK4 protein was modeled, and genistein docking was studied. MMP-2 expression was assessed in normal prostate epithelial cells from 24 patients with prostate cancer from a phase II randomized trial comparing genistein treatment with no treatment.
Contribution
MEK4 expression was associated with MMP-2 expression and cell invasion in all six cell lines. Genistein appeared able to bind to the active site of MEK4 by computer modeling. Genistein inhibited MEK4 kinase activity. MMP-2 expression was statistically significantly higher in normal prostate epithelial cells from untreated patients than in those from genistein-treated patients.
Implications
MEK4 was identified as a proinvasion protein and a target for genistein. These results may indicate a mechanism to link high dietary consumption of genistein-containing foods with lower rates of prostate cancer metastasis and mortality.
Limitations
The possibility that genistein has at least one more target cannot be ruled out. MEK3 function was not investigated in all six cell lines. The target for genistein action has not yet been localized to a specific component in a signaling pathway in human tissue specimens.
From the Editors
Prostate cancer is the second most common cause of cancer-related death in men in the United States (1), with essentially all such deaths being caused by metastatic disease (2). Targeted therapy to prevent prostate cancer metastasis could potentially reduce the morbidity and mortality of this disease. Unfortunately, no therapy has been developed that successfully targets metastasis-associated processes of any human cancer type (3).
Before cells can metastasize, they must progress through the steps of the metastatic cascade (4). Inhibiting early steps of this cascade precludes development of later steps. Cell invasion is an initial step in metastasis and a defining feature that is required for a diagnosis of invasive prostate cancer (5). Elevated extracellular protease activity increases the invasive activity of many cancer cell types, including prostate cancer cells (6). Matrix metalloproteinase-2 (MMP-2) is elevated in invasive prostate cancer tissue (7). In human prostate cancer cells, p38 mitogen-activated protein kinase (MAPK) increases MMP-2 expression and cell invasion (8). p38 MAPK is involved in many cellular processes, in a cell type–specific manner (9). In human prostate cancer cells, transforming growth factor β activates p38 MAPK, p38 MAPK then phosphorylates MAPK-activated protein kinase 2 (MAPKAPK2), MAPKAPK2 phosphorylates heat shock protein 27, and heat shock protein 27 stimulates the expression of MMP-2 and increases cell invasion (8,10). The level of heat shock protein 27 is higher in prostate cancer cells than in normal prostate cells (11).
Epidemiological studies indicate that an increased level of dietary genistein is associated with reduced rates of metastatic prostate cancer, whereas studies in prostate cancer cells show that genistein treatment blocks activation of the p38 MAPK proinvasion pathway (8,12). Consumption of dietary genistein has been linked to lower rates of prostate cancer metastasis and mortality (13,14). Individuals who consume a diet that is high in soy products have blood concentrations of genistein that are approximately two orders of magnitude higher than those who consume a Western-style red meat–based diet (15). In human prostate cancer cells, genistein blocks phosphorylation of p38 MAPK on threonine-180 and tyrosine-182, which are located in the activation motif, and thus prevents activation of the p38 MAPK kinase activity (8). Genistein has also been shown to inhibit phosphorylation of MAPKAPK2 and heat shock protein 27 (which are downstream components of the p38 MAPK proinvasion pathway), induction of MMP-2 expression, and invasion in cell lines and metastasis of human prostate cancer cells in mice (12,16). Importantly, genistein is active in cultured cells at low-to-mid nanomolar concentrations, which corresponds to levels of genistein in blood that are typically attained through dietary consumption of soy products (15).
Although inhibiting p38 MAPK activation is therapeutically important, genistein's pharmacological target for inhibiting cell invasion is not known. We investigated whether upstream activators of p38 MAPK, including mitogen-activated protein kinase kinase 3 (MEK3), MEK4, and MEK6 (17,18), regulated MMP-2 expression and prostate cancer cell invasion and were pharmacological targets for genistein.
Patients, Materials, and Methods
Antibodies, Plasmids, and Drugs
Polyclonal antibodies against the following proteins were used: phosphorylated MEK4 (clone 9156), MEK4 (clone 9152), MEK3 (clone 9232), MEK6 (clone 9264), phosphorylated p38 MAPK (clone 9211S; all from Cell Signaling Technology, Beverly, MA), and p38 MAPK (clone C-20; Santa Cruz Biotechnology, Santa Cruz, CA). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) monoclonal antibody was from Stressgen (Victoria, CA; product CSA-335E), and anti-mouse immunoglobulin and anti-rabbit immunoglobulin, each coupled to horseradish peroxidase, were from GE Life Science (Piscataway, NJ). The following constructs were purchased: the β-galactosidase expression vector pCMV-β-gal (Stratagene, La Jolla, CA), constitutive active MEK4EE (plasmid 14813; Addgene, Cambridge, MA), small interfering RNA (siRNA) against MEK4 (SMARTPool siMEK4; product L-003574-00; Dharmacon, Lafayette, CO), and nontargeting control siRNA (siCO; product D-001810-10-20; Dharmacon). Because p38 MAPK can be activated by several pathways, we used the constitutively active MEK4 construct, MEK4EE (19), to specifically evaluate MEK4 function. Genistein (Sigma Chemical Co, St Louis, MO) was stored in 10-μL aliquots of a 50 mM stock in dimethyl sulfoxide, was thawed just before use, and, unless otherwise stated, was used at a final concentration of 50 μM. Recombinant human transforming growth factor β1 (R&D Systems, Minneapolis, MN) was reconstituted and stored according to manufacturer's instructions and was used at a final concentration of 2 ng/mL.
Cell Culture
The origin and culture conditions of human PC3 and PC3-M metastatic prostate cancer cell lines, 1532NPTX and 1542NPTX immortalized normal human prostate epithelial cell lines, and 1532CPTX and 1542CPTX human prostate cancer cell lines have been described previously (20–22). PC3 cells originated from a human metastatic bone lesion and are metastatic in mice. PC3-M cells were derived from parental PC3 cells and represent a more metastatic derivative cell line. The other four cell lines originated from prostate tissue from patients with localized prostate cancer, have been transformed with human papillomavirus, and thus represent early-stage prostate cancer cells. Normal prostate epithelial cells and cancer cells from one patient gave rise to 1532NPTX and 1532CPTX cell lines, and those from another patient gave rise to 1542NPTX and 1542CPTX cell lines. Compared with PC3 and PC3-M metastatic cells, early-stage cells have a smaller percentage of proliferating cells, slower growth rates, normal DNA ploidy, more organized actin networks, and uniform cell adhesion profiles (22). PC3 and PC3-M cells were cultured in RPMI 1640 medium (Gibco, Grand Island, NY) supplemented with 10% heat-inactivated fetal bovine serum (product SH30071-03; Fisher/Hyclone, Waltham, MA), 2 mM glutamine, and 10 mM Hepes (pH 7.2). Early-stage 1532NPTX, 1542NPTX, 1532CPTX, and 1542CPTX cells were maintained in keratinocyte serum-free medium with epidermal growth factor (5 ng/mL) and bovine pituitary extract (25 μg/mL) (all from Gibco) supplemented with 2 mM glutamine, 10 mM Hepes (pH 7.2), and 5% heat-inactivated fetal bovine serum. All cells were maintained at 37°C in a humidified atmosphere of 5% carbon dioxide and 95% air under subconfluent exponential growth conditions with biweekly changes of medium, were routinely monitored for mycoplasma infection, and were replaced with fixed-passage cells from frozen stock every 3 months. Unless otherwise stated, cells were treated with 50 μM genistein (1 μL of stock added to 1 mL of culture medium) for 24 hours before being used in an assay. Control cells were treated with an equal amount of dimethyl sulfoxide. For experiments with transforming growth factor β, cells were treated with transforming growth factor β at 2 ng/mL for 45 minutes and then were harvested immediately. Cell viability was routinely monitored by trypan blue exclusion and was not adversely altered in any experimental condition used compared with untreated control cells. All six cell lines were evaluated in all in vitro experiments.
Cell Transfection
Cells from all six lines were transfected with constitutive active MEK4EE plasmid or with empty vector, as indicated. The MEK4EE plasmid carries a MEK4 that contains the Ser220Glu and Thr224Glu mutations, which render it constitutively active (19). The empty vector plasmid lacks the MEK4EE insert and serves as a transfection control. Briefly, 1 day after plating 3 × 106 cells into each well of a six-well plate, cells were transfected with 4 μg of the indicated plasmid by use of the TransIT-LT1 Transfection Reagent (Mirus, Madison, WI), as described previously (23). For cell invasion assays, cells were transfected with 3 μg of an expression plasmid (MEK4EE or empty vector, as indicated), along with 1 μg of the reporter plasmid, pCMV-β-gal. For RNA interference studies, cells were transfected with siRNAs against MEK4 or with nontargeting control siRNAs, as indicated, with DharmaFECT (Dharmacon) 5 hours after transfection with the reporter pCMV-β-gal plasmid, as described previously (24).
Cell Invasion Assays
Cell invasion was measured by a Boyden chamber invasion assay, as described previously (23). Briefly, the upper and lower chambers of the 48-well Boyden chamber (product AP48; Neuro Probe, Gaithersburg, MD) was separated by a Nuclepore Track-Etch Membrane with 8-μm pores (product NC 983-1643; Whatman, Clifton, NJ) that had been precoated with denatured collagen (product 214340; Difco-Becton Dickinson, Sparks, MD). A total of 1 × 104 cells were suspended in 52 μL of serum-free culture medium containing 0.1% bovine serum albumin, added to the upper well in the Boyden chamber apparatus, and allowed to migrate for 15 hours through the membrane toward serum-free NIH-3T3–conditioned medium in the lower chamber. The percentage of invading cells was counted under light microscopy. A total of two experiments were performed, with four replicate samples for each experimental condition. Some Boyden chamber experiments used cells that had been transfected with MEK4EE plasmid or vector control plasmid, along with pCMV-β-gal, so that the cells could be identified in a three-dimensional collagen matrix. Transfected cells were visualized with a β-galactosidase staining kit (product 200384; Stratagene) by following the manufacturer's instructions.
Quantitative Reverse Transcription–Polymerase Chain Reaction for Cell Lines and Normal Human Prostate Epithelial Cells
The quantitative reverse transcription–polymerase chain reaction (qRT-PCR) was performed, and data were analyzed as described previously (25,26) by using the TaqMan universal RT-PCR kit (product N8080234; Applied Biosystems, Foster City, CA), an Applied Biosystems 7500 Real Time PCR Workstation, and exon-spanning gene-specific sets of two primer and one probe TaqMan Gene Expression Assays for GAPDH, MEK4, MEK3, and MMP-2 (assays Hs99999905_m1, Hs00387426_m1, Hs00177127_m1, and Hs00234422_m1, respectively). Reverse transcription was performed with 40 ng of RNA from normal prostate epithelial cells or 100 ng of RNA from cell lines and TaqMan reverse transcriptase and random hexamers in a total reaction volume of 10 or 50 μL for RNA from normal prostate epithelial cells or from cell lines, respectively, with the following incubation conditions: 25°C for 10 minutes, 48°C for 30 minutes, and 95°C for 5 minutes. For the quantitative PCR, the total reaction volume was 20 μL, and the reaction conditions were 10 minutes at 50°C and 10 minutes at 95°C, followed by 40 cycles of 15 seconds at 95°C and 1 minute at 60°C. The threshold cycle for individual reactions was identified through Applied Biosystems 7500 Real Time PCR System software. Relative gene expression was normalized to that of GAPDH, by using the 2−ΔΔCt method (27). Negative controls were reaction mixtures that lacked reverse transcriptase. Each reaction point was in replicates of two; each experiment was repeated two times.
Western Blot Analysis
Cell lysis and Western blot analysis of all six cell lines were performed as described previously (12). Briefly, cells were lysed at 4°C in a solution of 0.5% Triton X-100, 20 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA, 2.5 mM sodium pyrophosphate, and 1 mM β-glycerol phosphate containing a mixture of protease inhibitors (leupeptin at 1 μg/mL, aprotinin at 1 μg/mL, and 1 mM phenylmethylsulfonyl fluoride; all from Sigma) and a mixture of phosphatase inhibitors (10 mM NaF, 1 mM orthovanadate, and phosphatase inhibitor mixtures I and II [both at a 1:100 dilution]; all from Sigma). Cell lysates were centrifuged at 14 000g for 30 minutes at 4°C. Equal amounts of clarified lysate protein, as determined by Bradford dye-binding assay (Bio-Rad, Hercules, CA), were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and transferred onto 0.45-μm (pore size) nitrocellulose (Whatman Schleicher and Schuell, Keene, NH). After blocking with 5% nonfat dry milk for 1 hour at room temperature, membranes were probed with polyclonal anti-phosphorylated MEK4 (diluted 1:1000), anti-phosphorylated p38 MAPK (diluted 1:1000), anti-MEK4 (diluted 1:1000), anti-MEK3 (diluted 1:1000), anti-MEK6 (diluted 1:1000), or anti-p38 MAPK (diluted 1:1000), for 16 hours at 4°C, and then with secondary antibody. Bands were visualized with the Enhanced Chemiluminescence System (GE Life Science) by following the manufacturer's instructions, and band density was read with the FUJI film LAS-3000 system and quantified with AlphaEaseFC software (Alpha Innotech Corporation, San Leandro, CA). After probing for phosphorylated proteins, membranes were stripped of bound antibodies by treating with stripping buffer (100 mM β-mercaptoethanol, 2% sodium dodecyl sulfate, and 62.5 mM Tris–HCl at pH 6.7) at 50°C for 30 minutes. After washing, membranes were reexposed (after readdition of the substrate for horseradish peroxidase) to confirm removal of previously bound antibody, before being reblocked and reprobed with an antibody against the corresponding total protein. All data were within the linear range of detection for each antibody used, as determined by assessing signal intensity after various lengths of film exposure time. GAPDH was used as the loading control. All experiments were performed twice, at separate times; all results were similar. Unless otherwise stated, Western blot analysis was performed 24 hours after transfection.
In Vitro Kinase Assay with Recombinant MEK4
The MEK4 kinase assay system from Millipore Upstate Biotechnology (Billerica, MA) was used by following the manufacturer's instructions, with modifications. The system contained recombinant active MEK4 (product 14-377), recombinant inactive mutant JNK3 (K55R) as the substrate (product 13-126), buffer (product 20-108), ATP (product 20-113), and anti-phosphorylated JNK antibody (product 07-175). The following compounds were added in the order listed to kinase buffer at 4°C for a final reaction volume of 20 μL: 0.06 U of recombinant MEK4; genistein at 0.01, 0.1, 1.0, 10, or 100 μM or a no genistein control; 375 μM MgCl2; 2.5 μM ATP; and 1 μg of JNK3 (K55R). All reactions were incubated simultaneously. The K55R mutation of JNK3 renders it inactive (28), and thus, it is a suitable substrate for MEK4. After a 5-minute incubation at 30°C, denaturing and reducing gel loading buffer (final concentrations: 125 mM Tris–HCl at pH 6.8, 1% sodium dodecyl sulfate, 5% [vol/vol] glycerol, 0.8% dithiolthreitol, and 0.05% bromophenol blue) was added and incubated at 95°C for 5 minutes and then at 4°C for 5 minutes. The resultant reaction product was detected by Western blot analysis, by following the manufacturer's instructions. For each experiment, all six different reaction conditions were assayed at the same time but with only a single sample for each condition. Two separate experiments were performed, at separate times.
Homology Model of MEK4 Structure and Docking of Genistein
Models of the three-dimensional structure of MEK4 were constructed by use of the ModWeb implementation of the Modeller program (29). This program aligned the MEK1 and MEK4 amino acid sequences, substituted the MEK1 amino acid residues in the three-dimensional structure with the corresponding amino acid residues of MEK4, and eliminated steric conflicts by energy minimization while spatially restraining the model to the template structure. The genetic algorithm implemented in the AutoDock program (30) was used to calculate an ensemble of possible poses of genistein bound to the MEK4 model. The pose yielding the highest score was selected.
Phase II Clinical Trial
Patients with localized, biopsy-proven adenocarcinoma of the prostate who had been scheduled for radical prostatectomy, had a Gleason's score of 6 or 7, a prostate-specific antigen level of less than 20 ng/mL, clinical stage T1 or T2 cancer, and intact end organ function, and were not on other therapy were accrued onto a Northwestern University Institutional Review Board–approved protocol, after they provided verbal and written informed consent. The trial was registered with the National Cancer Institute (PDQ Registration No. NCT00058266). Patients were randomly assigned to genistein treatment or no treatment in a 1:1 ratio, before radical prostatectomy. Randomization used the block method with a block size of 4, as designed by the study statistician (B. Jovanovic). Individual patients were randomly assigned through a process in which the study coordinator called a central number in the central coordinating Clinical Research Office to reach a dedicated full-time quality control officer, who then made the treatment assignment.
The genistein that was used in this trial was manufactured by Protein Technology International (PTI, St Louis, MO) and supplied by the US National Cancer Institute as PTI G-4660 in 150-mg gel cap pills. Genistein (150 mg/d) was administered, as described previously (31). This dose and formulation have been shown to give blood concentrations of genistein that are comparable with those measured in men consuming a soy-based diet (15,31). Estimates of daily genistein consumption by those on a soy-based diet range from 0.25 to 1.0 mg/kg (32–36). Thus, genistein was administered to patients in this trial at two to eight times the estimated dietary doses. During the trial, patients were asked to refrain from consuming foods and supplements that were high in soy and/or genistein. All data from all subjects were collected and stored in a central and secure location by the Clinical Research Office of the Robert H. Lurie Cancer Center of Northwestern University.
The phase II study was designed to detect the effects of genistein on nuclear morphometry, as measured by quantitative image analysis. The primary endpoint was nuclear morphology. Nuclear stretching, as determined by an increase in nuclear length or area, provides a measure of increased cell attachment (16). The study was designed to detect a statistically significant (P < .050) increase in nuclear flattening in formalin-fixed prostate tissue with 80% power.
In this article, the primary goal was to measure MMP-2 expression by qRT-PCR in normal prostate epithelial cells that were dissected from fresh-frozen prostate tissue by laser capture microdissection. From the larger pool of 38 subjects on the phase II trial, fresh-frozen prostate tissue was available from 12 genistein-treated patients and 12 untreated control patients, who were the subjects of study described in this article. These patients were accrued onto the study between September 1, 2004, and February 28, 2006. All clinical parameters reported correspond to these 24 patients.
Laser Capture Microdissection of Normal Prostate Epithelial Cells and RNA Isolation
Prostate tissue from radical prostatectomy specimens, from 12 genistein-treated patients and 12 untreated control patients, was harvested, annotated, and processed according to standard operating procedures of the Northwestern Prostate SPORE Tissue Bank (25). Briefly, tissue for laser capture microdissection was snap frozen within 30 minutes of surgical removal and stored at −80°C. Because, in our experience, there was rapid and unpredictable RNA degradation with a prolongation of time until freezing, tissue that could not be frozen within 30 minutes of removal was not used. Laser capture microdissection and RNA isolation were performed as previously described (25). Briefly, 6-μm serial cryosections were cut, end sections were stained with hematoxylin–eosin, and areas of cancer and normal tissue were identified by a single genitourinary pathologist (X. J. Yang) who was blinded to treatment status. Normal prostate epithelial cells that were separated from malignant tissue by more than one field, as observed under a ×40 objective lens (ie, 500 μm), and that were not associated with areas of benign prostatic hypertrophy were dissected on a PixCell II laser capture microdissection workstation (Arcturus, Mountain View, CA). Dissections were conservative (ie, epithelial–stromal boundaries were not approached), and images before and after laser capture microdissection were examined as a quality control measure to ensure that stromal tissue was not dissected. RNase-free conditions were maintained during all steps of tissue cutting, transport, microdissection, and transport of dissected specimens, including the use of RNase-free reagents and supplies, gloves, and barrier methods to block exposure to exhaled air. RNA was extracted from approximately 50 000 microdissected cells with a PicoPure RNA extraction kit (Arcturus) and treated with 30 Kunitz units of RNase-free DNase I (Qiagen, Valencia, CA) for 20 minutes at room temperature. A 1-μL aliquot of purified RNA was loaded on to an Agilent RNA LabChip and separated on an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA). Separation on the LabChip is continuous and is based on molecular weight, in a manner that is analogous to the separation of RNA by agarose gel electrophoresis. From the resultant chromatogram of eluted RNA, peaks corresponding to 28s and 18s RNAs were readily identified. RNA was considered nondegraded and of sufficient quality for use when 28s and 18s RNA peaks were evident and when the area of the 28s RNA peak was greater than that of the 18s RNA peak. Degraded RNA was not processed further. By using known amounts of stock RNA on the same LabChip that was used for the patient samples, a standard curve was generated in which the amount of known stock RNA was plotted against the total combined area of 28s and 18s RNA peaks. This standard curve was used to determine the amount of RNA in each patient sample from the total combined area of their 28s and 18s RNA peaks.
Measurement of Plasma Genistein Concentration
Plasma was collected (31) from the 24 patients above, and total genistein concentration was measured in a blinded fashion by a single person (J. Killmer), as described previously (16), with modifications. Briefly, plasma was treated overnight at 37°C with Helix pomatia β-glucuronidase and sulfatase (product G7017; Sigma) to remove glucuronidate and sulfate that are conjugated to genistein by metabolism in the liver; total genistein was extracted with methyl tert-butyl ether, resuspended in a solution of 20% methanol and 80% aqueous ammonium formate and then loaded on to a Waters Alliance high-pressure liquid chromatography system (Waters, Milford, MA), a 717 plus autosampler with Empower software (Waters), a Luna Phenyl-Hexyl reverse-phase analytical column (150 × 4.6 mm, 5 μm; Phenomenex, Torrance, CA), a Zorbax Eclipse XDB-phenyl guard column (Agilent Technologies, Santa Clara, CA), a step gradient mobile phase; and genistein was detected with a 2487 dual-wavelength absorbance detector with Millenium software (Waters) at 259 nm. Mobile phases were A (50 mM aqueous ammonium formate, pH 4.00) and B (50% acetonitrile and 50% methanol). The gradient program was 0% solution B for 1 minute, ramped to 40% solution B in 0.5 minute, held at 40% solution B for 11 minutes, ramped to 80% solution B in 1 minute, held at 80% solution B for 3 minutes, ramped down to 0% solution B in 1.5 minutes, and reequilibrated at 0% solution B for 8.5 minutes. The genistein calibration curve, which was constructed with genistein standards, was linear over genistein concentrations of 15.6–5000 ng/mL. Assay-to-assay and day-to-day variability was less than 10%. Any sample in which the genistein concentration was less than the lower limit of quantification was assigned the value of the lower limit of quantification.
Histology
Fresh-frozen prostate tissue from the 24 patients above was cut on a cryostat in 5-μm sections for staining with hematoxylin–eosin or in 6-μm sections for laser capture microdissection. Tissue was stained for hematoxylin–eosin as indicated and was visualized and photographed on a standard light microscope. Tissue for laser capture microdissection was immediately processed by incubation in 70%, 95%, and then 100% ethanol (each for 30 seconds); was then washed for two 2-minute periods in xylene; and was finally air-dried. After overlaying tissue with a CapMacro laser capture microdissection cap (Arcturus) on a PixCell II laser capture microdissection Workstation (Arcturus), images of tissue were photographed. All photomicrographs were taken with a ×40 objective.
Statistical Analysis
Differences between genistein-treated and untreated groups were compared by use of a two-sided Student t test. Data were considered statistically significantly different for P values of .050 or less. All statistical tests were two-sided.
Results
Genistein Treatment and Early-Stage Human Prostate Cancer Cells
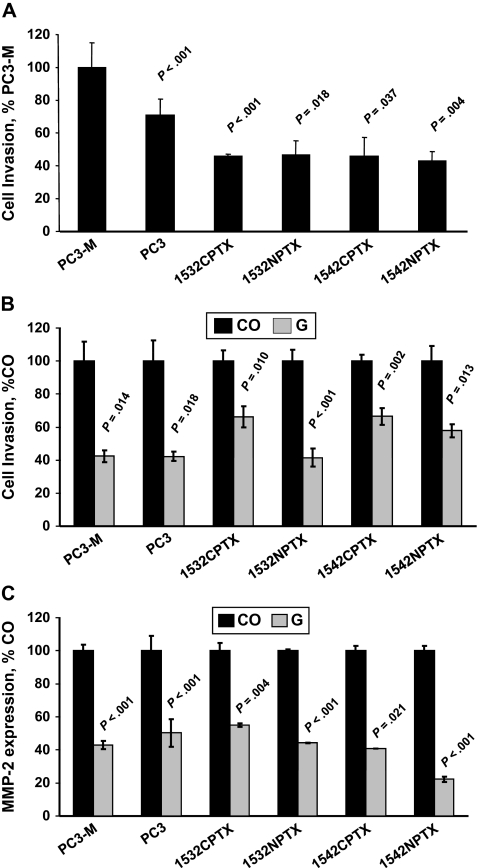
Boyden chamber assays were used to investigate the invasion activity of the metastatic prostate cancer cells (PC3 and PC3-M); early-stage, immortalized, localized prostate cancer cells (1532CPTX and 1542CPTX); and immortalized, normal prostate epithelial cells (1532NPTX and 1542NPTX). We found that early-stage cells were statistically significantly less invasive than metastatic cells (eg, the invasion of 1542NPTX cells was only 43% of that of PC3-M cells [set at 100%], difference = 57%, 95% confidence interval [CI] of difference = 44% to 70%, P = .004) (Figure 1, A). We then investigated whether a 24-hour treatment with 50 μM genistein altered the invasive activity of these six cell lines and found that the invasive activity of all six lines, including the early-stage cell lines, was statistically significantly lower than that of untreated cells (eg, for 1532NPTX cells, genistein decreased invasion to 41% of that of untreated cells [set at 100%], difference = 59%, 95% CI of difference = 42% to 75%, P < .001) (Figure 1, B).
Figure 1.
Genistein treatment and invasion in PC3-M, PC3, 1532CPTX, 1532NPTX, 1542CPTX, and 1542NPTX human prostate cell lines. A) Cell invasion. A Boyden chamber assay was used to assess the invasive activity of each cell line. Data are expressed as the percentage of invasion of PC3-M cells. B) Cell invasion and genistein treatment. Cell invasion was measured with a Boyden chamber assay in control (CO) and genistein-treated (G) cells and expressed as a percentage of the expression in the corresponding untreated control cells. C) Matrix metalloproteinase-2 (MMP-2) expression and genistein treatment. The expression of MMP-2 was by quantitative reverse transcription–polymerase chain reaction in control and genistein-treated cells. Data are expressed as a percentage of the expression in the corresponding untreated control cells. For invasion, each data point is the mean of three replicates (A) or four replicates (B) from one experiment. For MMP-2 expression, each data point is the mean of two replicates from one experiment. All experiments were conducted twice, at separate times and with similar findings. All error bars = 95% confidence intervals. P values were for comparisons with invasion of PC3-M cells in (A) and with untreated control cells in (B) and (C). All statistical tests were two-sided.
Genistein has previously been shown (8) to decrease the expression of the immature pro-MMP-2 zymogen in human prostate cells. To determine whether genistein was acting at the level of MMP-2 RNA or protein, we first investigated whether a 24-hour treatment with 50 μM genistein altered the level of MMP-2 transcripts by use of qRT-PCR. In all six cell lines, genistein-treated cells had statistically significantly lower levels of MMP-2 transcripts than the corresponding untreated cells (Figure 1, C).
Genistein Treatment and MEK4 Kinase Activity in Cell Lines
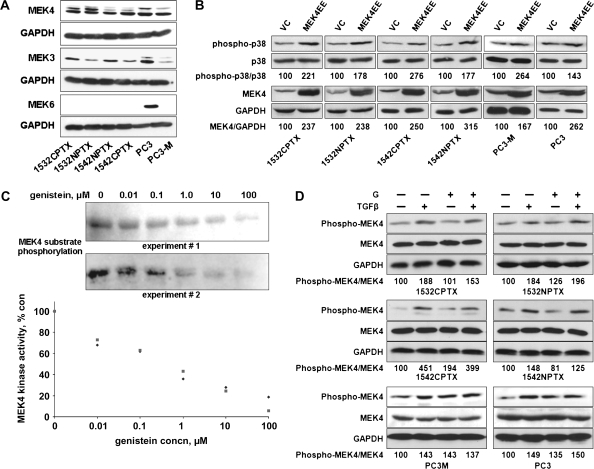
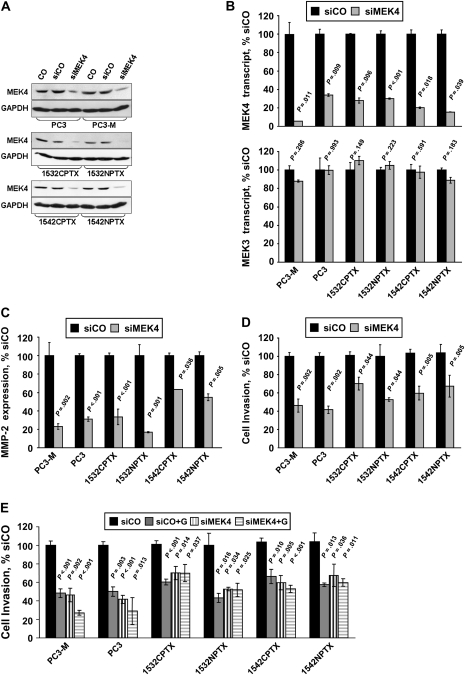
Because genistein blocks phosphorylation of p38 MAPK on its activation motif (8) and because MEK3, MEK4, and MEK6 activate p38 MAPK (17,18), we measured the expression of MEK3, MEK4, and MEK6 protein in lysates from all six prostate cell lines (PC3, PC3-M, 1532NPTX, 1532CPTX, 1542NPTX, and 1542CPTX cells). The expression of MEK3 and MEK4 protein was readily detected in all six cell lines, whereas only PC3 cells expressed readily detectable levels of MEK6 (Figure 2, A). Consequently, we focused on MEK4 because high expression was observed across all six cell lines and because the expression of MEK4 is elevated in invasive prostate cancer tissue (37).
Figure 2.
Genistein treatment and mitogen-activated protein kinase kinase 4 (MEK4) kinase activity in 1532CPTX, 1532NPTX, 1542NPTX, 1542CPTX, PC3, and PC3-M human prostate cell lines. A) Expression of MEK subtypes that activate p38 mitogen-activated protein kinase (MAPK). Lysates from each of the six cell lines were probed for the expression of MEK3, MEK4, or MEK6 protein by Western blot analysis. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was the loading control. The experiment was conducted two times, with similar findings in each. B) MEK4 overexpression and activation of p38 MAPK. Cells were transfected with a constitutively active form of MEK4, MEK4EE, or an empty vector control (VC), and then probed for phosphorylated (phospho-p38) and total (p38) p38 MAPK, MEK4, and GAPDH with specific antibodies by Western blot analysis. The density of the resultant bands was determined with AlphaEaseFc software, and mean ratios of the expression of the indicated proteins, from two separate experiments, are shown below the respective bands. Data are expressed as the percentage of the expression in VC-transfected cells. C) Genistein and the kinase activity of purified MEK4. MEK4 kinase activity in the presence of genistein (0–100 μM) was assessed with commercially available kits that used an inactive JNK3 as the substrate. Top) Western blots. The phosphorylated substrate was detected by Western blot analysis and the density of bands was determined with AlphaEaseFc software. Bottom) MEK4 kinase activity. Data are presented as the percentage of activity in the untreated control reaction. The experiment was conducted two separate times. All data from both experiments are shown. D) Genistein treatment and phosphorylation of MEK4. Cells were pretreated with 50 μM genistein (G) for 24 hours and then with transforming growth factor β (TGFβ) at 2 ng/mL for 45 minutes, as indicated. Lysates were prepared and probed for phosphorylated and total MEK4 and GAPDH with specific antibodies by Western blot analysis. The density of resultant bands was determined with AlphaEaseFc software, normalized to that of GAPDH, and mean values, from two separate experiments, are shown below the respective bands. Data are expressed as the percentage of the expression in untreated control cells. Data in (B) and (D) were from a representative experiment of two experiments, with similar findings. Each point was repeated two times. con = control; conc = concentration.
We investigated the function of MEK4 in human prostate cells by transfecting all six cell lines with MEK4EE, expressing a constitutively active form of MEK4, or with a control vector. Across all six cell lines, levels of phosphorylated p38 MAPK were higher in cells transfected with MEK4EE than in cells transfected with the control vector (eg, the ratio of phosphorylated to total p38 MAPK from MEK4EE-transfected 1532CPTX cells was 221% and that for control vector–transfected cells was 100%; difference = 121%, 95% CI of difference = 100% to 142%, P = .040) (Figure 2, B)
We investigated whether genistein treatment directly inhibits MEK4 kinase activity by using a commercially available MEK4 assay with an inactive recombinant JNK3 as the substrate. Phosphorylation of JNK3 decreased as the concentration of genistein increased (Figure 2, C). Specifically, genistein inhibited MEK4 kinase activity with a mean half maximal inhibitory concentration (IC50) of 0.40 μM (95% CI = 0.36 to 0.45 μM).
Because genistein treatment of human prostate cancer cells has been shown to block transforming growth factor β–stimulated phosphorylation of p38 MAPK (8), we measured phosphorylated and total MEK4 protein in all six cell lines. Cells were treated with genistein (50 μM for 24 hours) and/or with transforming growth factor β (2 ng/mL for 45 minutes); untreated cells were the control (Figure 2, D). Treatment with transforming growth factor β increased the ratio of phosphorylated to total MEK4 level by more than 20% across all six cell lines, and this increase was not blocked by genistein treatment. Similar results were obtained in two independent experiments. For example, after transforming growth factor β treatment of 1532NPTX cells in the absence of genistein, the ratio of phosphorylated to total MEK4 increased from a baseline of 100% in untreated cells to 184%. After transforming growth factor β treatment in the presence of genistein, the ratio increased to 196%. In 1532NPTX, 1542CPTX, PC3, and PC3-M cells, genistein treatment alone increased phosphorylated MEK4 levels; this result supports the existence of a feedback regulatory loop, as described previously in this pathway (10).
Structure of MEK4 and Genistein Docking
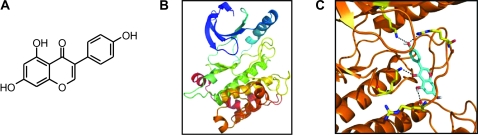
We used computer-assisted modeling to further investigate whether genistein inhibited MEK4 activity by directly binding to MEK4. The structure of genistein is shown in Figure 3, A. The structure of MEK1 (Protein Data Base [PDB] code 1S9J) had the highest sequence similarity to MEK4 (38% identity in the modeled region of residues 97–385) and a higher resolution structure than that of the next best template, MEK2 (PDB code 1S9I). So we used the structure of MEK1 to approximate the structure of MEK4. Because the structure of MEK1 used for modeling contained bound ATP and inhibitor (PD318088) (38), the resultant MEK4 model was in a closed, inactive conformation (Figure 3, B). We used AutoDock to examine all possible positions in three-dimensional space, or conformations, by which genistein could interact with the MEK4 active site and selected the conformation with the highest score, as assessed by the genetic algorithm implemented in the AutoDock program (Figure 3, C). In this predicted model for genistein binding to MEK4, genistein spanned the cleft between the domains by making hydrogen bonds with lysine-231 and arginine-267. These findings, as well as those involving the use of purified MEK4 in an in vitro kinase assay (Figure 2, C), support the hypothesis that genistein directly inhibits MEK4 kinase activity.
Figure 3.
Computer modeling of the mitogen-activated protein kinase kinase 4 (MEK4) active site and genistein binding. A) The structure of genistein (4′,5,7-trihydroxyisoflavone). B) Three-dimensional model of the MEK4 active site without genistein. C) Three-dimensional model of the MEK4 active site with bound genistein.
MEK4, MMP-2 Expression, and Invasion of Human Prostate Cancer Cells
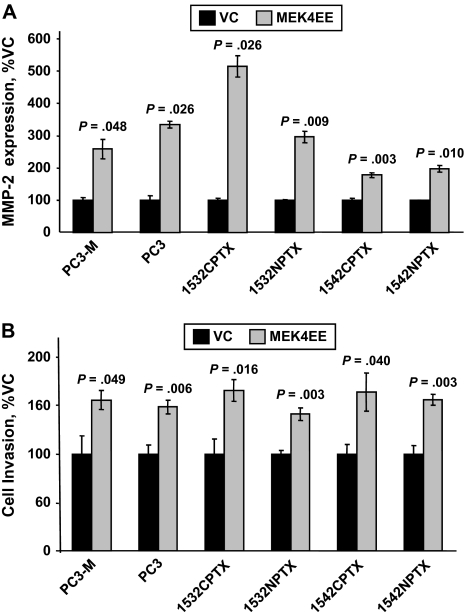
We next investigated whether MEK4 increased MMP-2 expression and cell invasion in all six human prostate cell lines. To increase MEK4 expression, cells were transfected with constitutively active MEK4EE vector or control vector, and 24 hours later, MMP-2 expression was assessed by qRT-PCR (Figure 4, A) and cell invasion was assessed by Boyden chamber assays (Figure 4, B). Across all six cell lines, overexpression of MEK4 statistically significantly increased MMP-2 expression (eg, in MEK4EE- and vector control–transfected PC3-M cells, MMP-2 expression was 267% and 100%, respectively; difference = 167%, 95% CI = 100% to 235%, P = .048) and cell invasion (eg, in the same cell combination, cell invasion was 157% and 100%, respectively; difference = 57%, 95% CI of the difference = 19% to 96%, P = .049).
Figure 4.
Overexpression of mitogen-activated protein kinase kinase 4 (MEK4); expression of matrix metalloproteinase-2 (MMP-2); and invasion in PC3-M, PC3, 1532CPTX, 1532NPTX, 1542CPTX, and 1542NPTX human prostate cell lines. Prostate cells were transfected with the constitutively active MEK4 vector, MEK4EE, or an empty control vector (VC) and evaluated 24 hours later for MMP-2 expression or cell invasion. A) MMP-2 expression. The expression of MMP-2 mRNA was measured by quantitative reverse transcription–polymerase chain reaction; values were normalized to the expression of glyceraldehyde 3-phosphate dehydrogenase. Data are the mean percentage of MMP-2 expression in control cells from a single experiment; each point was assayed in two replicates. The experiment was conducted two times, with similar results in each. B) Cell invasion. Cell invasion was measured by Boyden chamber cell invasion assay. Data are the mean percentage of invading cells, in control cells, from a single experiment; each point was assayed in four replicates. The experiment was conducted two times, with similar results in each. All error bars = 95% confidence intervals. P values for comparisons with the control were from Student t test. All statistical tests were two-sided.
To decrease the expression of MEK4, all six cell lines were transfected with MEK4-specific siRNA or a control siRNA. Across all six cell lines, cells transfected with MEK4-specific siRNA contained substantively lower levels of MEK4 protein (Figure 5, A) and transcripts (Figure 5, B) than cells transfected with control siRNA. Given that cells also express MEK3 protein (Figure 2, A), it should be noted that transfection with siMEK4 had little to no effect on the level of MEK3 transcripts (Figure 5, B). In addition, across all six cell lines, cells transfected with MEK4 siRNA had statistically significantly lower levels of MMP-2 expression (Figure 5, C) and cell invasion (Figure 5, D) than cells transfected with control siRNA. For example, in MEK4 siRNA– and control siRNA–transfected PC3-M cells, MMP-2 expression was 23% and 100% (difference = 77%, 95% CI of the difference = 63% to 91%, P = .002), respectively, and cell invasion was 46% and 100% (difference = 54%, 95% CI of the difference = 38% to 70%, P = .002).
Figure 5.
Decreased expression of mitogen-activated protein kinase kinase 4 (MEK4); expression of matrix metalloproteinase-2 (MMP-2); invasion; and genistein treatment in PC3-M, PC3, 1532CPTX, 1532NPTX, 1542CPTX, and 1542NPTX human prostate cell lines. Cells were transfected with small interfering RNA (siRNA) against MEK4 (siMEK4) or a control siRNA (siCO) and evaluated 24 hours later for cell invasion or mRNA expression. Cell invasion was assessed with a Boyden chamber assay. Data are expressed as the percentage of invasion in control cells transfected with siCO. Transcript levels for MEK4, MEK3, and MMP-2 were measured by quantitative reverse transcription–polymerase chain reaction and normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Data are expressed as the percentage of expression in control cells transfected with siCO. A) Expression of MEK4 protein after transfection with siRNAs. MEK4 expression was measured with specific antibodies by Western blot analysis; GAPDH was the loading control. Untransfected control cells = CO. Results from a representative experiment of two experiments are shown, both with similar results. B) Expression of MEK4 and MEK3 transcripts after transfection with siRNAs. Results are from a representative experiment, with each point assayed in two replicates; two experiments were conducted, both with similar results. C) Expression of MMP-2 transcripts after transfection with siRNAs. Results are from a representative experiment, with each point assayed in two replicates; two experiments were conducted, both with similar results. D) Cell invasion after transfection with siRNAs. Results are from a representative experiment, with each point assayed in four replicates; two experiments were conducted, both with similar results. E) Decreased expression of MEK4, genistein treatment, and cell invasion after transfection with siRNAs. Twenty-four hours after transfection with siCO or siMEK4, cells were treated with 50 μM genistein for 24 hours as indicated (+G), and cell invasion was measured. Data are from a representative experiment of two separate experiments, with each point assayed in four replicates; each experiment had similar results. All error bars = 95% confidence intervals. P values for comparisons with siCO-transfected cells or cells not treated with genistein were from Student t test. All statistical tests were two-sided.
MEK4 and Genistein Action
If MEK4 was the only site of action for genistein-mediated inhibition of cell invasion, then cells lacking MEK4 expression should be resistant to genistein. We investigated whether genistein treatment decreased invasion of cells transfected with MEK4 siRNA compared with control cells (Figure 5, E). Genistein treatment did not further inhibit the invasive activity of cells transfected with MEK4 siRNA. It should be noted that genistein treatment appeared to decrease the invasion of metastatic PC3 and PC3-M cells, although not statistically significantly so. Thus, in early-stage 1532NPTX, 1532CPTX, 1542NPTX, and 1542CPTX cells, MEK4 appears to be the only target for genistein-mediated inhibition of invasion, whereas in the metastatic cells, MEK4 may have additional targets.
Genistein and MMP-2 Expression in Normal Human Prostate Epithelial Cells
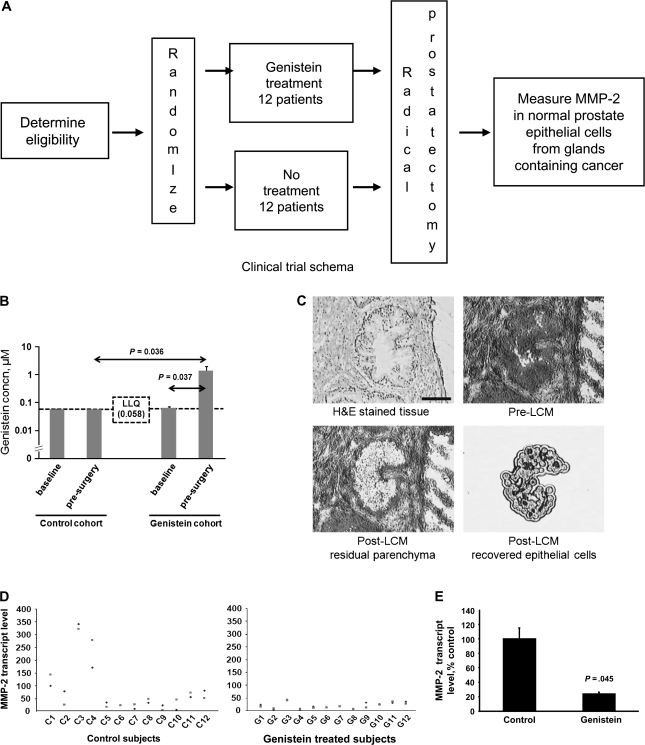
A drug designed to inhibit cell motility in human prostate tissue should be directed to cells that are at high risk of developing into invasive cancer but have not yet acquired a motile phenotype, such as normal prostate epithelial cells in a prostate gland containing prostate cancer. We thus focused on this cell population and used laser capture microdissection to isolate these cells from prostate tissue that was obtained from 24 patients with localized prostate cancer who participated in a randomized phase II clinical trial. This randomized trial examined genistein treatment (150 mg daily) or no treatment before radical prostatectomy (Figure 6, A). No statistically significant differences in the clinical characteristics were observed between genistein-treated and untreated groups (Table 1). The mean presurgical treatment time was 4.0 weeks (SD = 2.1 weeks), and no serious adverse events were observed during the trial. The concentration of genistein in the blood was measured before treatment (baseline) and before surgery (Figure 6, B). In the genistein cohort, the mean blood concentration of genistein had increased from a baseline level of 0.066 μM (95% CI = 0.060 to 0.070 μM) to 1.42 μM (95% CI = 0.990 to 1.852 μM) before surgery.
Figure 6.
Normal human prostate epithelial cells from prostate tissue containing prostate cancer, genistein treatment, and matrix metalloproteinase-2 (MMP-2) expression. A) Flow diagram of the randomized, phase II clinical trial. B) Blood concentration of genistein in genistein-treated and untreated control groups. The concentration of genistein in the blood was measured at the time of randomization (baseline) and just before surgery (presurgery). Any sample in which the genistein concentration was less than the lower limit of quantification (LLQ) was assigned the value of the LLQ, which in this experiment was 0.058 μM. C) Representative photomicrographs of normal tissue in a prostate gland containing cancer. Micrographs shown are of hematoxylin and eosin (H&E)–stained tissue and tissue before (pre-) and after (post-) laser capture microdissection (LCM). Length of bar = 50 μm. D) Genistein treatment and the level of MMP-2 transcripts in normal prostate epithelial cells. The transcript levels in normal prostate epithelial cells isolated by LCM was measured by quantitative reverse transcription–polymerase chain reaction (qRT-PCR) and normalized to that of GAPDH. Each data point is the mean value of two replicates from one qRT-PCR assay. For each subject, the qRT-PCR assay was performed twice at separate times. Individual control and genistein-treated subjects are denoted as C1–C12 and G1–G12, respectively. The MMP-2 transcript level for subject C1 was arbitrarily set to 100. E) Summary data for the MMP-2 transcript level by cohort. Data are the mean MMP-2 transcript level from 12 samples. Error bars = 95% confidence interval of control.
Table 1.
Characteristics of patients in the phase II randomized trial*
| Characteristic | Genistein treatment group | Untreated control group |
| Patients, No. | 12 | 12 |
| Mean age (range), y | 57 (44–67) | 58 (48–73) |
| Race, No. (%) | ||
| White | 9 (75) | 9 (75) |
| African American | 2 (17) | 2 (17) |
| Other | 1 (8) | 1 (8) |
| Clinical stage, No. (%) | ||
| T1 | 7 (58) | 6 (50) |
| T2 | 4 (33) | 4 (33) |
| Unknown | 1 (8) | 2 (17) |
| Mean PSA level (SD), ng/mL | 6 (2.0) | 6 (1.9) |
| Gleason score, No. (%) | ||
| 6 | 7 (58) | 7 (58) |
| 7 | 5 (42) | 5 (42) |
| Mean presurgery treatment time (SD), wk | 4 (2.1) | N/A |
Two-sided t tests were used to obtain P values. A P value of more than .05 was considered not statistically significant for differences between treatment and control groups. All P values were not statistically significant. No serious adverse events, defined as grade of 2 or greater for clinical toxicity according to the National Cancer Institute's Common Toxicity Criteria, version 2.0, were observed. PSA = prostate-specific antigen; N/A = not applicable.
Given that overexpression of MEK4 increased the level of MMP-2 transcript and that genistein inhibited MEK4 kinase activity and decreased the level of MMP-2 transcripts and protein in all six prostate cell lines, we investigated whether genistein treatment of patients with prostate cancer was associated with decreased levels of MMP-2 transcripts in normal prostate epithelial cells isolated from fresh-frozen prostate tissue by laser capture microdissection (Figure 6, C). Briefly, RNA was isolated from the dissected cells and treated with DNase, and then, the levels of MMP-2 and GAPDH transcripts were measured in each subject by qRT-PCR (Figure 6, D). The mean level of MMP-2 transcripts in normal epithelial cells from untreated control subjects was taken as baseline and expressed as 100% (Figure 6, E). In genistein-treated patients at surgery, the mean level of MMP-2 expression had decreased statistically significantly to 24% of that in control patients (100%; difference = 76%, 95% CI of the difference = 38% to 115%; P = .045).
Discussion
Our findings demonstrate for the first time, to our knowledge, that MEK4 was a regulator and stimulator of MMP-2 expression and cell invasion in human prostate cells. MEK4 was also identified as a therapeutic target for genistein-mediated inhibition of MMP-2 expression and cell invasion. Furthermore, genistein was found to directly inhibit MEK4 kinase activity at nanomolar concentrations. The clinical relevance of genistein treatment to inhibit signaling pathways that are involved prostate cell motility was shown by using normal human prostate epithelial cells that were isolated from prostate tissue of patients participating in a prospective, randomized, phase II trial examining genistein treatment. Cells from patients treated with genistein had lower levels of MMP-2 transcripts than cells from untreated patients. This result demonstrates that cancer motility processes in patients with prostate cancer can be targeted by genistein. We also observed no adverse side effects during the trial.
The exact role of MEK4 in human cancer biology is not well understood (39). Our findings support those of others (17,37,40–43), however. MEK4 has been shown to activate p38 MAPK in fibroblast and kidney cells (17,40) and to increase the invasive activity of human breast and pancreatic cells (41). Decreasing the expression of MEK4 by knockdown approaches inhibits metastasis of human pancreatic cancer cells in mice (42). Increased MEK4 expression (37) and increased MMP-2 expression (43) were found in invading human prostate cancer cells. Increased MMP-2 expression has been associated with a higher Gleason score, a more advanced disease stage (44), and decreased disease-free survival (45). We have shown that MEK4 induces MMP-2 and cell invasion in early-stage 1532NPTX, 1532CPTX, 1542NPTX, and 1542CPTX cells. Thus, these studies support the hypothesis that MEK4-mediated MMP-2 induction is an event in prostate cancer progression that precedes the development of metastasis.
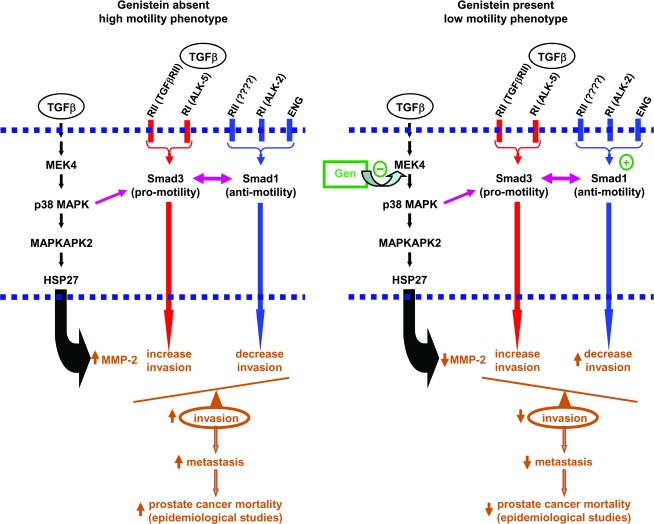
Because cell invasion is a basic cell function that is critical for the development of invasive cancer, and a central determinant of metastatic potential, it is a tightly regulated process that involves cooperation across many signaling pathways. If MEK4 is an important regulator of cell invasion, it should, and in fact does, interact with other regulatory pathways. On the basis of this study and earlier studies (8,10,16,22,23,46,47), we propose a model by which human prostate cancer cells regulate invasion and metastasis (Figure 7). Briefly, transforming growth factor β, which is produced by prostate cells and other cell types in prostate tissue, activates MEK4; MEK4 phosphorylates p38 MAPK and thus activates downstream signaling proteins (such as MAPKAPK2 and heat shock protein 27), thereby increasing MMP-2 expression, cell invasion, and metastasis (8,10,16,22). Through signaling pathway cross talk; p38 MAPK can phosphorylate the proinvasive protein Smad3 (46). Smad3 is also activated via canonical transforming growth factor β signaling in the human prostate (23); this signaling pathway involves the binding of transforming growth factor β to transforming growth factor β superfamily receptors type I and II, termed activin-like kinase receptor (ALK)-5 and type II TGFβ receptor (TGFβRII), respectively, after which ALK-5 phosphorylates Smad3. Endoglin (47) and ALK-2 (23), which is also a transforming growth factor β type I receptor, inhibit invasion and thereby counterbalance proinvasion signaling by activating Smad1, which acts to inhibit invasion (23). An important determinant of whether cells ultimately invade or not appears to be the ratio of proinvasive Smad3 to anti-invasive Smad1 (23).
Figure 7.
Proposed model for the mitogen-activated protein kinase kinase 4 (MEK4) regulation of human prostate cell motility and for its inhibition by genistein. Left) High motility phenotype. Transforming growth factor β (TGFβ) activates MEK4. Activated MEK4 in turn activates downstream effector proteins, including p38 mitogen-activated protein kinase (MAPK) (8), MAPK-activated protein kinase 2 (MAPKAPK2) (10), and heat shock protein 27 (HSP27) (10), all of which act to increase expression of matrix metalloproteinase-2 (MMP-2) and cell invasion. Smad3 can be activated by p38 MAPK (46) or by the TGFβ superfamily receptors, activin-like kinase receptor (ALK)-5 and type II TGFβ receptor (TGFβRII) (23). The proinvasive action of Smad3 is mitigated by the anti-invasion action of Smad1 (23). Smad1 is constitutively activated by endoglin (ENG) in an ALK-2–dependent fashion (23). Under the combined influence of proinvasion MEK4 and Smad3 signaling and of anti-invasion Smad1 signaling, cells have a high-motility phenotype characterized by high invasion and metastatic potential (16), which in turn causes mortality from prostate cancer. Right) Genistein treatment and low-motility phenotype. When genistein is present, it binds to and inhibits MEK4 kinase activity. Genistein-mediated decreases in MEK4 kinase activity in turn blocks activation of p38 MAPK (8), MAPKAPK2 (12), and HSP27 (12), all of which of block TGFβ-mediated increases in MMP-2 and cell invasion. In addition, genistein activates the antimotility action of Smad1, in a manner that is dependent on ALK-2 kinase activity (24). Genistein's action serves to inhibit proinvasion MEK4 and Smad3 signaling and to enhance anti-invasion Smad1 signaling, resulting in cells with a low-motility phenotype characterized by low invasion and metastatic potential (16), which in turn decreases mortality from prostate cancer (13,14). − = inhibitory activity of genistein on a molecular pathway; + = stimulatory activity of genistein on a molecular pathway; ↓ = inhibition of individual cellular and systemic processes by genistein; ↑ = stimulation of individual cellular and systemic processes by genistein; TGFβRI = type I TGFβ receptor.
Genistein has many disparate effects, such as inhibition of breast cancer cell growth (48), induction of apoptosis in prostate cancer cells (49), inhibition of angiogenesis in human umbilical vein endothelial cells (50), inhibition of topoisomerase in K562 cells (51), and estrogenic activity in breast cancer cells (52). Consequently, it should be emphasized that all of these effects are concentration dependent and require at least micromolar concentrations, so that as the concentration of genistein increases, its specificity decreases. The concentrations of free genistein in the blood of men consuming a soy-based diet (15), as well as in men who have been treated with genistein supplements (31,53), are in the nanomolar range. Epidemiological studies of patients with prostate cancer have found associations between these blood concentrations of genistein and decreased metastasis and mortality (13,14). Because of these findings, we had focused on mechanisms operative at nanomolar concentrations. Our current finding that the genistein has an IC50 value in the nanomolar range for MEK4 is consistent with our previous reports that genistein at mid-to-low nanomolar concentrations inhibits prostate cell invasion in vitro (8) and inhibits metastasis of human prostate cancer cells in mice (16). Related mechanistic studies by us corroborate these findings by demonstrating that genistein inhibits activation of p38 MAPK (8), MAPKAPK2 (12), and heat shock protein 27 (12), thereby inhibiting MMP-2 expression (8,12), cell invasion (8,12), and metastasis of human prostate cancer cells in a murine model (16). We have shown previously that genistein activates Smad1, thereby decreasing human prostate cancer cell invasion (24), and that endoglin and ALK-2 cooperated to activate Smad1 and activated Smad1-suppressed human prostate cancer cell invasion (23). It is unclear whether genistein-induced activation of Smad1 is due to genistein-mediated MEK4 inhibition or whether it involves other components. It is clear, however, that activation of Smad1 by genistein requires the function of intact ALK-2 kinase (24).
It has been proposed that MEK4 is able to suppress prostate cancer metastasis (39), but the supporting results were from experiments in rat prostate cancer cells (54). The actions of MEK4 appear to be species specific because in a study of rat prostate cancer metastasis, p38 MAPK has been shown not to be involved in MEK4-mediated suppression of metastasis, but activation of JNK was required (55). Thus, caution should be exercised when extrapolating functions of signaling pathways across species.
The finding that the invasive activity of early-stage 1532NPTX, 1532CPTX, 1542NPTX, and 1542CPTX cells transfected with MEK4 siRNA, which lack MEK4 expression, was essentially resistant to genistein treatment indicates that MEK4 is the only target for genistein's anti-invasion activity. However, the findings with metastatic PC3 and PC3-M cells transfected with MEK4 siRNA indicate that genistein may be acting on at least one more target during prostate cancer progression. The identity of these targets for genistein should be the subject of future investigations.
This study has several limitations. It has not been definitively proven that genistein inhibits MEK4 by binding to its active site, although it is clear that genistein directly inhibited MEK4 kinase activity and that our computer-simulated structural model for MEK4 predicted that genistein binds to the active site of MEK4. However, such models may not be reliable predictors of actual structure (56). The most accurate way to determine the three-dimensional structure of a protein and its ligand-binding site is through x-ray crystallographic analysis. A second limitation is that because MEK4 siRNA transfection did not affect the MEK3 expression in all six cell lines, we did not evaluate the function of MEK3 in these cells. So we cannot rule out the possibility that MEK3 may be involved in regulating cell invasion in human metastatic prostate cancer cells. A third limitation relates to the pharmacodynamics of genistein in human subjects. Although our results on the mechanism of action of genistein is consistent with inhibition of MEK4 kinase, we have not localized the action of genistein to a specific component of a signaling pathway in human tissue specimens because the high levels of prostatic acid phosphatase in prostate tissue preclude the accurate measurement of phosphorylated protein levels in clinical specimens. A fourth limitation relates to the fact that we cannot exclude the possibility that the antimotility action of genistein may be directed, at least in part, to at least one more target, in addition to MEK4. A fifth limitation relates to the strength of the association between the antimotility activity of genistein acquired through dietary consumption and decreased prostate cancer mortality. Additional studies on this association should include the analysis of MMP-2 expression in subjects who were consuming diets that contain either high or low levels of genistein.
Genistein appears to have selectivity toward MMP-2, compared with other MMP subtypes, in human prostate epithelial cells (8). MMP-2 is a secreted protein that can be produced by prostate stromal cells, which are adjacent to epithelial cells in human prostate tissue (44). We therefore specifically isolated prostate epithelial cells by laser capture microdissection and showed that genistein specifically decreased MMP-2 transcript levels in these cells. Previous clinical studies (3) that used MMP active site inhibitors found that adverse clinical toxic effects were a limiting factor. These toxic effects appeared to be related to the non-MMP subtype-specific nature of the inhibitors and the fact that there are more than 20 MMP subtypes that play vital roles in normal human biology. Given that MMPs are extracellular proteases, they have key roles in defining how cancer cells physically interact with their surrounding microenvironment. Other important regulators of how cancer cells physically interact with their microenvironment include other classes of proteases (57) and cell adhesion molecules (58,59). Cell–microenvironment interactions, including those mediated by proteases (60) and cell adhesion molecules (61), represent currently popular therapeutic targets. However, because interactions between cells and their microenvironment are fundamental to human life and because differences in such interactions between normal and cancer tissue are largely caused by differences in the level of protein expression, such therapeutic approaches should be examined cautiously. Although our current finding that MMP-2 can be targeted with genistein therapy is only one example, it supports the feasibility of targeting regulatory pathways rather than physical interactions. By using normal prostate epithelial cells from prostate cancer–containing tissue, which were presumably at risk of progression to prostate cancer cells, we show that it is possible to decrease MMP-2 expression. This finding supports the possibility that destabilization of a premalignant field can potentially be prevented by therapeutically targeting cell motility processes that are linked to cancer, as has been proposed previously (62,63).
In summary, the expression of MEK4 was shown to be associated with the expression of MMP-2 and with invasive activity of human prostate PC3, PC3-M, 1532NPTX, 1532CPTX, 1542NPTX, and 1542CPTX cells. We found evidence that MEK4 was the target for the anti-invasive activity of genistein in human prostate cancer cell lines. By use of a randomized phase II trial of genistein treatment vs no treatment of patients with prostate cancer, we found lower levels of MMP-2 transcripts in normal prostate epithelial cells from the treated group than from the untreated group. MMP-2 is an important stimulator of cell invasion and metastasis. Thus, we have shown that it is possible to target motility-associated processes with genistein in patients with prostate cancer, have identified MEK4 as the therapeutic target for genistein in all six prostate cell lines examined, and have provided a possible mechanism to link high dietary consumption of genistein-containing foods with lower rates of prostate cancer metastasis and mortality.
Funding
Veterans Administration (merit review to R.C.B.); National Institutes of Health (CA099263, CA122985, CA37403, Prostate SPORE CA90386, RR00048 to R.C.B.).
Footnotes
The authors had full responsibility for the design of the study, the collection of the data, the analysis and interpretation of the data, the decision to submit the manuscript for publication, and the writing of the manuscript.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001;58(6):843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 3.Overall CM, Lopez-Otin C. Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer. 2002;2(9):657–672. doi: 10.1038/nrc884. [DOI] [PubMed] [Google Scholar]
- 4.Ruoslahti E. How cancer spreads. Sci Am. 1996;275(3):72–77. doi: 10.1038/scientificamerican0996-72. [DOI] [PubMed] [Google Scholar]
- 5.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111(1):58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 6.Stetler-Stevenson WG, Yu AE. Proteases in invasion: matrix metalloproteinases. Semin Cancer Biol. 2001;11(2):143–152. doi: 10.1006/scbi.2000.0365. [DOI] [PubMed] [Google Scholar]
- 7.Stearns ME, Stearns M. Immunohistochemical studies of activated matrix metalloproteinase-2 (MMP-2a) expression in human prostate cancer. Oncol Res. 1996;8(2):63–67. [PubMed] [Google Scholar]
- 8.Huang X, Chen S, Xu L, et al. Genistein inhibits p38 map kinase activation, matrix metalloproteinase type 2, and cell invasion in human prostate epithelial cells. Cancer Res. 2005;65(8):3470–3478. doi: 10.1158/0008-5472.CAN-04-2807. [DOI] [PubMed] [Google Scholar]
- 9.Cuenda A, Rousseau S. p38 MAP-kinases pathway regulation, function and role in human diseases. Biochim Biophys Acta. 2007;1773(8):1358–1375. doi: 10.1016/j.bbamcr.2007.03.010. [DOI] [PubMed] [Google Scholar]
- 10.Xu L, Chen S, Bergan RC. MAPKAPK2 and HSP27 are downstream effectors of p38 MAP kinase-mediated matrix metalloproteinase type 2 activation and cell invasion in human prostate cancer. Oncogene. 2006;25(21):2987–2998. doi: 10.1038/sj.onc.1209337. [DOI] [PubMed] [Google Scholar]
- 11.Rocchi P, Beraldi E, Ettinger S, et al. Increased Hsp27 after androgen ablation facilitates androgen-independent progression in prostate cancer via signal transducers and activators of transcription 3-mediated suppression of apoptosis. Cancer Res. 2005;65(23):11083–11093. doi: 10.1158/0008-5472.CAN-05-1840. [DOI] [PubMed] [Google Scholar]
- 12.Xu L, Bergan RC. Genistein inhibits matrix metalloproteinase type 2 activation and prostate cancer cell invasion by blocking the transforming growth factor beta-mediated activation of mitogen-activated protein kinase-activated protein kinase 2-27-kDa heat shock protein pathway. Mol Pharmacol. 2006;70(3):869–877. doi: 10.1124/mol.106.023861. [DOI] [PubMed] [Google Scholar]
- 13.Severson RK, Nomura AM, Grove JS, Stemmermann GN. A prospective study of demographics, diet, and prostate cancer among men of Japanese ancestry in Hawaii. Cancer Res. 1989;49(7):1857–1860. [PubMed] [Google Scholar]
- 14.Adlercreutz H. Western diet and Western diseases: some hormonal and biochemical mechanisms and associations. Scand J Clin Lab Invest. 1990;50(suppl 201):3–23. [PubMed] [Google Scholar]
- 15.Adlercreutz H, Markkanen H, Watanabe S. Plasma concentrations of phyto-oestrogens in Japanese men. Lancet. 1993;342(8881):1209–1210. doi: 10.1016/0140-6736(93)92188-y. [DOI] [PubMed] [Google Scholar]
- 16.Lakshman M, Xu L, Ananthanarayanan V, et al. Dietary genistein suppresses metastasis of human prostate cancer in mice. Cancer Res. 2008;68:2024–2032. doi: 10.1158/0008-5472.CAN-07-1246. [DOI] [PubMed] [Google Scholar]
- 17.Derijard B, Raingeaud J, Barrett T, et al. Independent human MAP-kinase signal transduction pathways defined by MEK and MKK isoforms. Science. 1995;267(5198):682–685. doi: 10.1126/science.7839144. [DOI] [PubMed] [Google Scholar]
- 18.Raingeaud J, Whitmarsh AJ, Barrett T, Derijard B, Davis RJ. MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol Cell Biol. 1996;16(3):1247–1255. doi: 10.1128/mcb.16.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Whitmarsh AJ, Shore P, Sharrocks AD, Davis RJ. Integration of MAP kinase signal transduction pathways at the serum response element. Science. 1995;269(5222):403–407. doi: 10.1126/science.7618106. [DOI] [PubMed] [Google Scholar]
- 20.Kozlowski JM, Fidler IJ, Campbell D, Xu ZL, Kaighn ME, Hart IR. Metastatic behavior of human tumor cell lines grown in the nude mouse. Cancer Res. 1984;44(8):3522–3529. [PubMed] [Google Scholar]
- 21.Bright RK, Vocke CD, Emmert-Buck MR, et al. Generation and genetic characterization of immortal human prostate epithelial cell lines derived from primary cancer specimens. Cancer Res. 1997;57(5):995–1002. [PubMed] [Google Scholar]
- 22.Liu YQ, Kyle E, Patel S, et al. Prostate cancer chemoprevention agents exhibit selective activity against early stage prostate cancer cells. Prostate Cancer Prostatic Dis. 2001;4(2):81–91. doi: 10.1038/sj.pcan.4500506. [DOI] [PubMed] [Google Scholar]
- 23.Craft CS, Romero D, Vary CPH, Bergan RC. Endoglin inhibits prostate cancer motility via activation of the ALK2-Smad1 pathway. Oncogene. 2007;26(51):7240–7250. doi: 10.1038/sj.onc.1210533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Craft CS, Xu L, Romero D, Vary CP, Bergan RC. Genistein induces phenotypic reversion of endoglin deficiency in human prostate cancer cells. Mol Pharmacol. 2008;73(1):235–242. doi: 10.1124/mol.107.038935. [DOI] [PubMed] [Google Scholar]
- 25.Ding Y, Xu L, Chen S, et al. Characterization of a method for profiling gene expression in cells recovered from intact human prostate tissue using RNA linear amplification. Prostate Cancer Prostatic Dis. 2006;9(4):379–391. doi: 10.1038/sj.pcan.4500888. [DOI] [PubMed] [Google Scholar]
- 26.Ding Y, Xu L, Jovanovic BD, et al. The methodology used to measure differential gene expression affects the outcome. J Biomol Tech. 2007;18(5):321–330. [PMC free article] [PubMed] [Google Scholar]
- 27.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Derijard B, Hibi M, Wu IH, et al. JNK1: a protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76(6):1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 29.Eswar N, John B, Mirkovic N, et al. Tools for comparative protein structure modeling and analysis. Nucleic Acids Res. 2003;31(13):3375–3380. doi: 10.1093/nar/gkg543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forster MJ. Molecular modelling in structural biology. Micron. 2002;33(4):365–384. doi: 10.1016/s0968-4328(01)00035-x. [DOI] [PubMed] [Google Scholar]
- 31.Takimoto CH, Glover K, Huang X, et al. Phase I pharmacokinetic and pharmacodynamic analysis of unconjugated soy isoflavones administered to individuals with cancer. Cancer Epidemiol Biomarkers Prev. 2003;12(11, pt 1):1213–1221. [PubMed] [Google Scholar]
- 32.Adlercreutz H, Mazur W. Phyto-oestrogens and Western diseases. Ann Med. 1997;29(2):95–120. doi: 10.3109/07853899709113696. [DOI] [PubMed] [Google Scholar]
- 33.Kimira M, Arai Y, Shimoi K, Watanabe S. Japanese intake of flavonoids and isoflavonoids from foods. J Epidemiol. 1998;8(3):168–175. doi: 10.2188/jea.8.168. [DOI] [PubMed] [Google Scholar]
- 34.Wakai K, Egami I, Kato K, et al. Dietary intake and sources of isoflavones among Japanese. Nutr Cancer. 1999;33(2):139–145. doi: 10.1207/S15327914NC330204. [DOI] [PubMed] [Google Scholar]
- 35.Arai Y, Uehara M, Sato Y, et al. Comparison of isoflavones among dietary intake, plasma concentration and urinary excretion for accurate estimation of phytoestrogen intake. J Epidemiol. 2000;10(2):127–135. doi: 10.2188/jea.10.127. [DOI] [PubMed] [Google Scholar]
- 36.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21(2):113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 37.Lotan TL, Lyon M, Huo D, et al. Up-regulation of MKK4, MKK6 and MKK7 during prostate cancer progression: an important role for SAPK signalling in prostatic neoplasia. J Pathol. 2007;212(4):386–394. doi: 10.1002/path.2194. [DOI] [PubMed] [Google Scholar]
- 38.Ohren JF, Chen H, Pavlovsky A, et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat Struct Mol Biol. 2004;11(12):1192–1197. doi: 10.1038/nsmb859. [DOI] [PubMed] [Google Scholar]
- 39.Whitmarsh AJ, Davis RJ. Role of mitogen-activated protein kinase kinase 4 in cancer. Oncogene. 2007;26(22):3172–3184. doi: 10.1038/sj.onc.1210410. [DOI] [PubMed] [Google Scholar]
- 40.Guan Z, Buckman SY, Pentland AP, Templeton DJ, Morrison AR. Induction of cyclooxygenase-2 by the activated MEKK1 –> SEK1/MKK4 –> p38 mitogen-activated protein kinase pathway. J Biol Chem. 1998;273(21):12901–12908. doi: 10.1074/jbc.273.21.12901. [DOI] [PubMed] [Google Scholar]
- 41.Wang L, Pan Y, Dai JL. Evidence of MKK4 pro-oncogenic activity in breast and pancreatic tumors. Oncogene. 2004;23(35):5978–5985. doi: 10.1038/sj.onc.1207802. [DOI] [PubMed] [Google Scholar]
- 42.Cunningham SC, Gallmeier E, Hucl T, et al. Targeted deletion of MKK4 in cancer cells: a detrimental phenotype manifests as decreased experimental metastasis and suggests a counterweight to the evolution of tumor-suppressor loss. Cancer Res. 2006;66(11):5560–5564. doi: 10.1158/0008-5472.CAN-06-0555. [DOI] [PubMed] [Google Scholar]
- 43.Stearns ME, Wang M. Type IV collagenase (M(r) 72,000) expression in human prostate: benign and malignant tissue. Cancer Res. 1993;53(4):878–883. [PubMed] [Google Scholar]
- 44.Wood M, Fudge K, Mohler JL, et al. In situ hybridization studies of metalloproteinases 2 and 9 and TIMP-1 and TIMP-2 expression in human prostate cancer. Clin Exp Metastasis. 1997;15(3):246–258. doi: 10.1023/a:1018421431388. [DOI] [PubMed] [Google Scholar]
- 45.Trudel D, Fradet Y, Meyer F, Harel F, Tetu B. Significance of MMP-2 expression in prostate cancer: an immunohistochemical study. Cancer Res. 2003;63(23):8511–8515. [PubMed] [Google Scholar]
- 46.Hayes SA, Huang X, Kambhampati S, Platanias LC. Bergan RC. p38 MAP kinase modulates Smad-dependent changes in human prostate cell adhesion. Oncogene. 2003;22(31):4841–4850. doi: 10.1038/sj.onc.1206730. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y, Jovanovic B, Pins M, Lee C, Bergan RC. Over expression of endoglin in human prostate cancer suppresses cell detachment, migration and invasion. Oncogene. 2002;21(54):8272–8281. doi: 10.1038/sj.onc.1206117. [DOI] [PubMed] [Google Scholar]
- 48.Peterson G, Barnes S. Genistein inhibition of the growth of human breast cancer cells: independence from estrogen receptors and the multi-drug resistance gene. Biochem Biophys Res Commun. 1991;179(1):661–667. doi: 10.1016/0006-291x(91)91423-a. [DOI] [PubMed] [Google Scholar]
- 49.Kyle E, Neckers L, Takimoto C, Curt G, Bergan R. Genistein-induced apoptosis of prostate cancer cells is preceded by a specific decrease in focal adhesion kinase activity. Mol Pharmacol. 1997;51(2):193–200. doi: 10.1124/mol.51.2.193. [DOI] [PubMed] [Google Scholar]
- 50.Guo Y, Wang S, Hoot DR, Clinton SK. Suppression of VEGF-mediated autocrine and paracrine interactions between prostate cancer cells and vascular endothelial cells by soy isoflavones. J Nutr Biochem. 2007;18(6):408–417. doi: 10.1016/j.jnutbio.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 51.Lopez-Lazaro M, Willmore E, Austin CA. Cells lacking DNA topoisomerase II beta are resistant to genistein. J Nat Prod. 2007;70(5):763–767. doi: 10.1021/np060609z. [DOI] [PubMed] [Google Scholar]
- 52.Matsumura A, Ghosh A, Pope GS, Darbre PD. Comparative study of oestrogenic properties of eight phytoestrogens in MCF7 human breast cancer cells. J Steroid Biochem Mol Biol. 2005;94(5):431–443. doi: 10.1016/j.jsbmb.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 53.Fischer L, Mahoney C, Jeffcoat AR, et al. Clinical characteristics and pharmacokinetics of purified soy isoflavones: multiple-dose administration to men with prostate neoplasia. Nutr Cancer. 2004;48(2):160–170. doi: 10.1207/s15327914nc4802_5. [DOI] [PubMed] [Google Scholar]
- 54.Yoshida BA, Dubauskas Z, Chekmareva MA, Christiano TR, Stadler WM, Rinker-Schaeffer CW. Mitogen-activated protein kinase kinase 4/stress-activated protein/Erk kinase 1 (MKK4/SEK1), a prostate cancer metastasis suppressor gene encoded by human chromosome 17. Cancer Res. 1999;59(21):5483–5487. [PubMed] [Google Scholar]
- 55.Vander Griend DJ, Kocherginsky M, Hickson JA, Stadler WM, Lin A, Rinker-Schaeffer CW. Suppression of metastatic colonization by the context-dependent activation of the c-Jun NH2-terminal kinase kinases JNKK1/MKK4 and MKK7. Cancer Res. 2005;65(23):10984–10991. doi: 10.1158/0008-5472.CAN-05-2382. [DOI] [PubMed] [Google Scholar]
- 56.Schafferhans A, Klebe G. Docking ligands onto binding site representations derived from proteins built by homology modelling. J Mol Biol. 2001;307(1):407–427. doi: 10.1006/jmbi.2000.4453. [DOI] [PubMed] [Google Scholar]
- 57.Affara NI, Andreu P, Coussens LM. Delineating protease functions during cancer development. Methods Mol Biol. 2009;539:1–32. doi: 10.1007/978-1-60327-003-8_1. [DOI] [PubMed] [Google Scholar]
- 58.Fornaro M, Manes T, Languino LR. Integrins and prostate cancer metastases. Cancer Metastasis Rev. 2001;20(3–4):321–331. doi: 10.1023/a:1015547830323. [DOI] [PubMed] [Google Scholar]
- 59.Frame MC, Inman GJ. NCAM is at the heart of reciprocal regulation of E-cadherin- and integrin-mediated adhesions via signaling modulation. Dev Cell. 2008;15(4):494–496. doi: 10.1016/j.devcel.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 60.Blouse GE, Botkjaer KA, Deryugina E, et al. A novel mode of intervention with serine protease activity: targeting zymogen activation. J Biol Chem. 2009;284(7):4647–4657. doi: 10.1074/jbc.M804922200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ma WW, Adjei AA. Novel agents on the horizon for cancer therapy. CA Cancer J Clin. 2009;59(2):111–137. doi: 10.3322/caac.20003. [DOI] [PubMed] [Google Scholar]
- 62.Bergan R, Kyle E, Nguyen P, Trepel J, Ingui C, Neckers L. Genistein-stimulated adherence of prostate cancer cells is associated with the binding of focal adhesion kinase to beta-1-integrin. Clin Exp Metastasis. 1996;14(4):389–398. doi: 10.1007/BF00123398. [DOI] [PubMed] [Google Scholar]
- 63.Spencer VA, Xu R, Bissell MJ. Extracellular matrix, nuclear and chromatin structure, and gene expression in normal tissues and malignant tumors: a work in progress. Adv Cancer Res. 2007;97:275–294. doi: 10.1016/S0065-230X(06)97012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]