Abstract
Background
The 5-year survival rate for individuals with neuroblastoma is approaching 70%. Few data exist, however, on the long-term outcomes of these patients, who are often treated at a very young age.
Methods
Outcome data were obtained for 954 5-year neuroblastoma survivors who were diagnosed in 1970–1986 and enrolled in the Childhood Cancer Survivor Study (CCSS). Late mortality, second malignant neoplasms, and chronic health conditions were analyzed in relation to treatment factors using Poisson regression models and their modification with generalized estimating equations. Neuroblastoma survivors were compared with a cohort of 3899 siblings of CCSS participants for risk of chronic health conditions and selected sociodemographic outcomes. All statistical tests were two-sided.
Results
Six percent of patients died more than 5 years after their diagnosis (standardized mortality ratio = 5.6; 95% confidence interval [CI] = 4.4 to 6.9). The most common causes of death were disease recurrence (n = 43) and second malignant neoplasms (n = 13). The cumulative incidence of second malignant neoplasms was 3.5% at 25 years and 7.0% at 30 years after diagnosis. Compared with the sibling cohort, survivors had an increased risk of selected chronic health conditions (risk ratio [RR] = 8.3; 95% CI = 7.1 to 9.7) with a 20-year cumulative incidence of 41.1%. The most prevalent outcomes involved the neurological, sensory, endocrine, and musculoskeletal systems, with 20-year cumulative incidences of 29.8%, 8.6%, 8.3%, and 7.8%, respectively. Neuroblastoma survivors who were treated with multimodality therapy were more likely to develop a chronic health condition than survivors treated with surgery alone (RR = 2.2; 95% CI = 1.6 to 3.0). Neuroblastoma survivors were less likely than siblings to have ever been employed (P = .04) or to be married (P < .001) and had a lower personal income (P = .009).
Conclusions
Neuroblastoma survivors have an increased rate of mortality and second malignant neoplasms, relative to the age- and sex-comparable US population, and of chronic health conditions, relative to their siblings, which underscores the need for long-term medical surveillance.
CONTEXT AND CAVEATS
Prior knowledge
Very limited information has been available about long-term outcomes among survivors of childhood cancers including neuroblastoma. The Childhood Cancer Survivor Study (CCSS) was initiated to study long-term outcomes among those diagnosed in the United States and Canada in 1970–1986.
Study design
Late mortality was assessed among 1358 5-year survivors of neuroblastoma who were CCSS participants. Of these, 954 participating survivors self-reported demographic information, second malignant neoplasms, and chronic health conditions, from which cumulative incidences could be calculated. Data from 3899 nondiseased siblings were used as a control.
Contribution
Nearly 6% of patients died more than 5 years after diagnosis, usually from disease recurrence or second malignant neoplasms. Neuroblastoma survivors were eight times more likely than the sibling cohort to have chronic health conditions, and by 20 years, nearly a third would develop neurological complications and about 8% each would develop endocrine, sensory, and musculoskeletal complications. Those who received multimodality therapy were more than twice as likely to develop a chronic health condition as those treated by surgery alone.
Implications
Emerging therapies should be evaluated for their potential long-term effects.
Limitations
The data gathered were for participants only, demographic and medical outcomes among survivors were self-reported, and data concerning neuroblastoma stage at diagnosis were unavailable.
From the Editors
Neuroblastoma is the most common extracranial solid tumor in childhood, and it affects children at a very young age, with 41% of cancers in infants diagnosed within the first 3 months of life (1). The 5-year relative survival rate has increased from 54% for patients diagnosed in 1975–1984 to 68.5% for those diagnosed in 1996–2003 (1). Substantial variation in rates of 5-year relative survival exists among subpopulations of neuroblastoma patients based on age at diagnosis, extent of disease, and tumor biology. Such prognostic classifications have resulted in substantial changes in therapy during the past 40 years, which include treatment with myeloablative therapy and autologous stem cell transplantation (2,3).
Ongoing research is providing important insights into the long-term health-related and psychosocial problems faced by some childhood cancer survivors. For example, in a large retrospective cohort study of 10 397 adult survivors of childhood cancer (4), 62.3% of survivors of neuroblastoma had at least one chronic health condition and 27.5% had a severe or life-threatening condition, an average of 17.5 years (range = 6.0–31.0 years) after cancer diagnosis, and the likelihood of a chronic condition increased substantially over time. Although most childhood cancer survivors remain psychologically healthy, certain subgroups of survivors, including women, survivors of solid tumors, and those with lower income and lower educational attainment, are at higher risk of psychological distress and poor health-related quality of life (5,6).
Currently available data regarding long-term treatment-related outcomes among neuroblastoma survivors are limited to a few case series and to small cohort studies of survivors with a combination of low- and intermediate-risk (mostly localized tumors) and high-risk (mostly metastatic) disease. These studies have reported musculoskeletal, neurological, and endocrine problems (7–13), as well as severe sensorineural hearing loss (14–16) and increased risk of second malignant neoplasms (17).
The study of long-term outcomes among neuroblastoma survivors reflects an excellent opportunity to assess the impact of a wide variety of treatment approaches, both in terms of modality and intensity, on cancer survivors who were diagnosed at very young ages (2). Here, we analyzed data from 954 participants in the Childhood Cancer Survivor Study (CCSS) to investigate the occurrence of, and risk factors for, selected long-term outcomes, including sociodemographic outcomes (marital status, employment, personal income), mortality, second malignant neoplasms, and chronic health conditions (musculoskeletal, endocrine, sensory, neurological). To estimate the relative risk of these outcomes, the cohort of childhood cancer survivors was compared with a cohort of siblings.
Methods
Childhood Cancer Survivor Study
CCSS is a multicenter retrospectively ascertained cohort of 20 346 childhood cancer survivors diagnosed before age 21 years between 1970 and 1986 and of approximately 4000 siblings of survivors, who serve as a comparison group (18,19). The cohort was assembled through the efforts of 26 participating clinical research centers in the United States and one in Canada (see Supplementary Materials available online for a complete list of participating centers). Recruitment began in August 1994 for cancer survivors and in 1996 for siblings, and participants remain in active follow-up. The detailed methods that were used to establish the CCSS cohort of 5-year survivors of childhood cancer have been described previously (18–20). The CCSS was initiated to establish a resource for assessing a broad spectrum of late adverse effects following treatment for childhood and adolescent cancer. The study and baseline questionnaires were approved by the Institutional Review Board at all participating centers at the initiation of the study. Participating survivors and siblings provided written informed consent for study participation and medical record release when they were recruited in the cohort (20).
When recruited in the study between 1994 and 1996, CCSS participants completed a comprehensive baseline questionnaire, which was answered by the survivor if aged 18 years or older or by a parent proxy if younger than 18 years or deceased, with follow-up surveys distributed in 2000 and 2002. These questionnaires collected data on sociodemographic outcomes (marital status, employment, personal income), the occurrence of second malignant neoplasms, and chronic health conditions, which were self-reported. At the time of study entry, detailed information was abstracted from the medical record of each participant including all treatments related to diagnosis and any relapse(s). Information was collected on 42 chemotherapeutic agents, including cumulative dose for 22 specific drugs (distribution of cumulative doses for all drugs are provided at www.stjude.org/ccss under supplementary information for reference 18). Alkylating agent exposure was calculated according to the method described by Tucker et al. (21). Radiation therapy records were photocopied and sent to the CCSS Radiation Physics Center to abstract relevant data and assess patient exposures in terms of field size, site, and dose. Surgical procedures were classified according to International Classification of Diseases, Ninth Revision, procedure codes. Copies of medical record abstraction forms and study questionnaires are available at www.stjude.org/ccss
Neuroblastoma Study Population
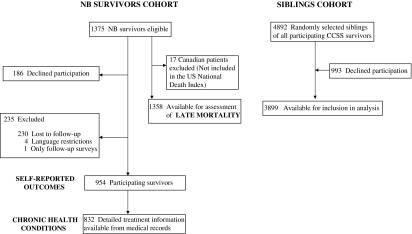
Of the 1375 neuroblastoma survivors eligible for the CCSS cohort, 954 completed the baseline questionnaire (participating survivors for self-reported outcomes) and 421 were excluded for the following reasons: 186 (8.9%) declined participation, 230 (16.7%) were considered to be lost to follow-up after extensive attempts to trace them, four (<1%) were unable to participate because they spoke neither English nor Spanish fluently, and one participated only in the follow-up surveys (Figure 1). The CCSS previously compared demographic and cancer-related characteristics for the entire cohort among participants, nonparticipants, and those who were lost to follow-up and found that these three groups were similar (18,22). For the neuroblastoma survivors cohort, comparison of demographic and cancer-treatment characteristics of the 954 participating survivors with the 421 nonparticipating survivors revealed that both groups were similar overall, except that the participating survivors were more likely to be female (52% of the participating group was female vs 40% of the nonparticipating group; P < .001) and to have been diagnosed during a later time period (41% of the participating group vs 48% of the nonparticipating group was diagnosed between 1970 and 1978 and 59% of the participating group vs 52% of the nonparticipating group was diagnosed between 1979 and 1986).
Figure 1.
Flow diagram of participants in study cohorts. CCSS = Childhood Cancer Survivor Study; NB = neuroblastoma.
A comparison group of nearest-age siblings was identified from randomly selected participating CCSS survivors across all cancer diagnoses. Of the 4892 randomly selected siblings, 3899 (80%) were available for inclusion in the analysis (Figure 1).
Assessment of late mortality included 1358 patients of the 1375 eligible 5-year neuroblastoma survivors and excluded 17 Canadians who were not included in the US National Death Index (NDI). Analysis of the self-reported outcomes (second malignant neoplasms, chronic health conditions, and sociodemographic factors) was restricted to the 954 subjects who completed the baseline questionnaire. Analysis of risk factors for chronic health conditions was restricted to the 832 survivors who completed the baseline questionnaire and for whom we had abstracted medical records. For analyses of sociodemographic outcomes (eg, highest level of schooling, employment history, total household and personal income, health insurance, and marital history), only survivors and siblings aged 25 years and older at last follow-up were included because this was an age at which independence from parents might be anticipated.
Statistical Analyses
Descriptive data were summarized for 954 neuroblastoma survivors and compared with those of the 3899 siblings. Age-adjusted comparisons were also made between the two groups for demographics and health status using bootstrap techniques to account for potential within-family correlations (23).
Overall and cause-specific mortality among the 1358 eligible survivors were assessed according to our previously described methods (24). In brief, participants whose vital status as of December 31, 2002, was unknown and those who were reported to have died after cohort entry were included in a NDI search for deaths that occurred between January 1, 1979, and December 31, 2002. For those who died in the United States, cause of death information was provided by the NDI. For deaths that occurred in 1975 through 1978 (ie, the years not covered by the NDI), copies of the death certificates were requested from all states where such deaths occurred. Canadian participants were censored on the last survey date (because the survey response was the only indicator for their survival status) or on December 31, 2002, whichever came earlier. Death certificates were not available for Canadian participants, so they were excluded from the cause-specific mortality analysis but were included in all other analyses. Standardized mortality ratios (SMRs) were computed by dividing the observed number of deaths by the expected number of deaths, which was calculated based on age-, sex-, and calendar year–specific US mortality rates. A 95% confidence interval (CI) for each SMR was calculated based on Poisson probability models (25).
Cumulative incidence of second malignant neoplasms was estimated by considering death as a competing risk (26). Standardized incidence ratios (SIRs) of overall and specific types of second malignant neoplasms that occurred following the cohort entry were calculated using the US Surveillance, Epidemiology, and End Results cancer incidence rates (1).
Health status (27) and chronic health conditions were assessed from the study questionnaires; the criteria for defining specific chronic health conditions are provided in the footnotes of Table 3. Cumulative incidence of chronic health conditions was also estimated by considering death as a competing risk. A subset of female neuroblastoma survivors were analyzed for the incidence of acute ovarian failure using the methods previously reported (28). Poisson regression models modified with generalized estimating equations (25) were used to compare age-adjusted risk of chronic health conditions between survivors and siblings. Similarly, Poisson regression was used for case-to-case comparisons among survivors, adjusting for attained age (natural cubic spline with knots at 5, 15, 25, and 35 years), age at diagnosis (categorical, <1, 1–4, 5+ years), year of diagnosis (categorical, 1970–1975, 1976–1980, 1981–1986), and sex. The constancy of the multiplicative effect of each covariate was assessed by testing an interaction of the covariate by time.
Table 3.
Age-adjusted rate ratios of selected chronic health conditions first occurring 5 or more years following diagnosis in neuroblastoma survivors compared with siblings*
| Condition | Cumulative incidence at 20 y since diagnosis of survivors (%) | Survivors | Siblings | RR (95% CI) | P |
| Any condition | 41.1 | 252.2 | 30.4 | 8.3 (7.1 to 9.7) | <.001 |
| Musculoskeletal conditions | 7.8 | 53.2 | 2.6 | 20.1 (12.1 to 35.3) | <.001 |
| Severe scoliosis† | 5.8 | 42.9 | 1.6 | 27.0 (13.6 to 53.4) | <.001 |
| Osteoporosis | 2.3 | 12.5 | 0.9 | 13.2 (5.5 to 32.1) | <.001 |
| Neurological conditions | 29.8 | 152.9 | 20.0 | 7.6 (6.4 to 9.1) | <.001 |
| Weakness in arms or legs | 13.7 | 35.3 | 1.6 | 22.7 (16.4 to 31.6) | <.001 |
| Decreased sense of touch and feeling in hands, fingers, arms, or legs | 10.7 | 45.5 | 4.4 | 10.2 (7.6 to 13.7) | <.001 |
| Prolonged pain or abnormal sensations in arms, legs, or back | 13.7 | 82.4 | 11.8 | 7.0 (5.4 to 9.1) | <.001 |
| Problems with balance | 8.1 | 36.4 | 3.8 | 9.6 (6.8 to 13.5) | <.001 |
| Seizures | 6.6 | 24.4 | 4.1 | 6.0 (4.2 to 8.7) | <.001 |
| Tremor or movement problems | 5.4 | 13.7 | 1.2 | 11.2 (7.0 to 18.0) | <.001 |
| Paralysis, unspecified | 5.5 | 14.5 | 1.3 | 11.3 (7.3 to 17.5) | <.001 |
| Endocrine conditions | 8.3 | 67.9 | 5.21 | 13.0 (8.9 to 19.1) | <.001 |
| Medication needed to initiate puberty | 3.1 | 41.7 | 0.8 | 55.4 (16.1 to 191.1) | <.001 |
| Growth hormone deficiency | 4.2 | 24.6 | 0.6 | 38.5 (15.2 to 97.3) | <.001 |
| Growth hormone injections | 2.8 | 14.3 | 0.4 | 37.0 (12.7 to 107.6) | <.001 |
| Hypothyroidism | 4.1 | 33.7 | 4.0 | 8.4 (5.1 to 13.6) | <.001 |
| Sensory conditions | 8.6 | 22.6 | 2.8 | 8.0 (5.6 to 11.3) | <.001 |
| Cataract | 3.1 | 10.8 | 0.7 | 16.2 (7.3 to 35.8) | <.001 |
| Hearing loss/deafness‡ | 3.8 | 6.8 | 0.8 | 8.7 (5.2 to 14.6) | <.001 |
| Blindness§ | 3.1 | 11.0 | 1.6 | 6.9 (4.0 to 11.8) | .004 |
Rates are listed per 10 000 person-years. Two-sided statistical inference (Wald confidence intervals and tests) from Poisson regression was used with the modification by generalized estimating equations to account for potential within-family correlation. Incidence rates per 10 000 person-years were age-adjusted rates at age 15 years. Statistical tests were two-sided. CI = confidence interval; RR = rate ratio.
Severe scoliosis was further analyzed within survivors in relation to receiving laminectomy, thoracotomy, and radiotherapy to the spine: The results are described in the text.
Hearing loss requiring a hearing aid or deafness not corrected by a hearing aid.
Legally blind or loss of an eye.
Second malignant neoplasms and chronic health conditions that first occurred during the first 5 years after diagnosis of neuroblastoma were included as prevalent cases at the cohort entry, except for the standardized incidence ratio calculation of second malignant neoplasms, following the method of Oeffinger et al. (4). All statistical tests were two-sided.
Results
Characteristics of the 954 neuroblastoma survivors and 3899 siblings are provided (Table 1). The median age of the survivors was 0.9 years (range = 0–20.7 years) at diagnosis, 17.2 years (range = 5.7–44.2 years) at baseline survey, and 23.3 years (range = 5.7–45.2 years) at completion of the latest questionnaire. Neuroblastoma survivors were less likely to be married than their nonafflicted siblings (54.9% vs 67.9%; P < .001) and more likely to have been never employed (1.3 % vs 0.2%; P = .04). Both personal and household incomes were also lower among the neuroblastoma survivors than the siblings (Table 2). The disparity between neuroblastoma survivors and their siblings in terms of marital rate, employment rate, and income levels showed no statistical association with age (data not shown).
Table 1.
Characteristics of neuroblastoma survivors and siblings cohorts
| Characteristic | Survivors (n = 954), No. (%) | Siblings (n = 3899), No. (%) | P* |
| Sex | |||
| Male | 455 (48) | 1875 (48) | .83 |
| Female | 499 (52) | 2024 (52) | |
| Vital status at baseline questionnaire | |||
| Alive | 919 (96) | ||
| Dead | 35 (4) | ||
| Age at interview at baseline, y | |||
| <10 | 33 (3) | 76 (2) | <.001 |
| 10–19 | 616 (65) | 1010 (26) | |
| 20–29 | 280 (29) | 1381 (35) | |
| 30–39 | 23 (2) | 1116 (29) | |
| 40–49 | 2 (0) | 316 (8) | |
| Age at interview at latest follow-up, y | |||
| <10 | 10 (1) | 12 (<1) | <.001 |
| 10–19 | 263 (28) | 379 (10) | |
| 20–29 | 519 (54) | 1353 (35) | |
| 30–39 | 155 (16) | 1332 (34) | |
| 40–49 | 7 (1) | 823 (21) | |
| Age at diagnosis, y | |||
| <1 | 525 (55) | NA | |
| 1–4 | 340 (36) | NA | |
| 5–9 | 66 (7) | NA | |
| 10–14 | 15 (2) | NA | |
| 15–21 | 8 (1) | NA | |
| Year of diagnosis | |||
| 1970–1975 | 228 (24) | NA | |
| 1976–1980 | 291 (31) | NA | |
| 1981–1986 | 435 (46) | NA | |
| Survival time at baseline, y | |||
| 5–9 | 103 (11) | NA | |
| 10–14 | 344 (36) | NA | |
| 15–19 | 283 (30) | NA | |
| 20–24 | 187 (20) | NA | |
| >25 | 37 (4) | NA | |
| Survival time at latest follow-up, y | |||
| 5–9 | 32 (3) | NA | |
| 10–14 | 62 (6) | NA | |
| 15–19 | 272 (29) | NA | |
| 20–24 | 298 (31) | NA | |
| >25 | 290 (30) | NA | |
| Treatment group† | |||
| Surgery only | 200 (24) | NA | |
| Surgery + chemotherapy | 216 (26) | NA | |
| Surgery + radiotherapy | 132 (16) | NA | |
| Surgery + chemotherapy + radiotherapy | 268 (32) | NA | |
| Other‡ | 16 (2) | NA | |
Survivors and siblings were compared using χ2 tests with bootstrap to account for potential within-family correlations. All statistical tests were two-sided.
Based on the 832 patients with medical records abstracted. Of these patients, 20 had bone marrow transplantation in addition to treatment described, including 16 who received total body irradiation.
Among 16 patients who did not have surgery, four had chemotherapy only, three had radiotherapy only, six had both chemotherapy and radiotherapy, and three had no treatment.
Table 2.
Demographics and health status of study subjects (age adjusted)
| Characteristic | Survivors, % (n = 954) | Siblings, % (n = 3899) | P* |
| Highest level of schooling† | |||
| Not high school graduate | 3.2 | 2.7 | .68 |
| High school graduate | 48.3 | 45.4 | |
| College graduate | 48.5 | 51.9 | |
| Ever employed† | |||
| No | 1.3 | 0.2 | .04 |
| Total household income, $† | |||
| <19 999 | 14.7 | 10.4 | .003 |
| 20 000–39 999 | 30.9 | 24.2 | |
| 40 000–59 999 | 22.9 | 23.3 | |
| More than 60 000 | 31.5 | 42.1 | |
| Total personal income, $† | |||
| <19 999 | 40.7 | 31.9 | .009 |
| 20 000–39 999 | 35.6 | 36.9 | |
| 40 000–59 999 | 16.8 | 18.3 | |
| More than 60 000 | 6.9 | 12.9 | |
| Health insurance† | |||
| Yes | 80.0 | 79.5 | .51 |
| No | 13.1 | 11.7 | |
| Canadian | 6.9 | 8.8 | |
| Ever married† | |||
| Yes | 54.9 | 67.9 | <.001 |
| General health status‡ | |||
| Not adverse | 91.5 | 95.4 | .006 |
| Adverse | 8.5 | 4.6 | |
| Mental health status‡ | |||
| Not adverse | 93.9 | 95.6 | .33 |
| Adverse | 6.1 | 4.4 | |
Survivors and siblings were compared using χ2 tests with bootstrap to account for potential within-family correlations. All statistical tests were two-sided.
Demographic data shown are for neuroblastoma survivors and siblings aged 25 years and older at last follow-up (to allow for independence from parents).
Health status data are shown only for those who were aged 18 years and older at baseline.
Late Mortality
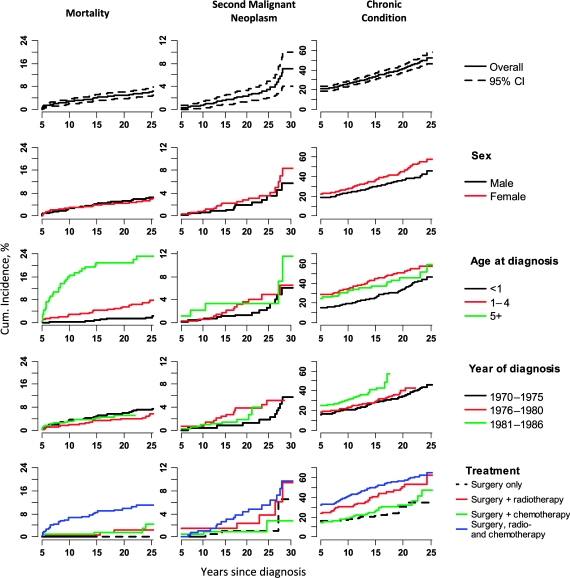
Among the 1358 neuroblastoma survivors for whom mortality data were available, 84 deaths occurred more than 5 years after diagnosis, resulting in a 25-year cumulative mortality of 6% (Figure 2). The SMR for all causes of death was 5.6 (95% CI = 4.4 to 6.9). The most common cause of late mortality was disease recurrence (43 patients; 51% of all deaths). Other causes of death included second malignant neoplasms (n = 13; SMR = 10.9, 95% CI = 5.8 to 18.7), pulmonary complications (n = 4; SMR = 11.4, 95% CI = 3.1 to 29.3), cardiac complications (n = 3; SMR = 5.0, 95% CI = 1.0 to 14.6), external causes (n = 11; SMR = 1.1, 95% CI = 0.5 to 1.9), other causes (n = 6), and unknown causes (n = 7). Cumulative mortality was associated with older age at diagnosis, such that patients who were diagnosed at 5 years of age or older demonstrated an absolute 23 percentage point increase in 25-year cumulative mortality (Figure 2).
Figure 2.
Cumulative incidence of mortality, second malignant neoplasms, and selected chronic health conditions among 5-year survivors of neuroblastoma. The mortality curves are statistically significantly different across the categories of sex (P < .001) and treatment (P < .001). The chronic condition curves are statistically significantly significant across the categories of sex (P = .01), age at diagnosis (P < .001), year of diagnosis (P < .001), and treatment (P < .001). Cumulative incidence curves were compared using Gray's method (29). All statistical tests were two-sided. CI = confidence interval.
Chronic Health Conditions
The 20-year cumulative incidence of a chronic health condition was 41.1% for neuroblastoma survivors. Compared with siblings, neuroblastoma survivors were at increased risk for at least one of the following chronic health conditions: musculoskeletal and neurological complications, endocrine complications, and sensory complications (RR = 8.3; 95% CI = 7.1 to 9.7; Table 3) as further described below. Survivors who were treated with multimodality therapy were more than twice as likely to develop any chronic health condition as survivors who were treated with surgery alone (RR = 2.2; 95% CI = 1.6 to 3.0) (Figure 2).
Musculoskeletal and Neurological Complications
The 20-year cumulative incidence of musculoskeletal complications was 7.8% for neuroblastoma survivors, who were 20.1 times (95% CI = 12.1 to 35.3 times) more likely than siblings to report any musculoskeletal conditions. For example, survivors had a much higher risk of severe scoliosis that required surgery as compared with siblings (RR = 27.0; 95% CI = 13.6 to 53.4). Laminectomy (RR = 11.0; 95% CI = 5.8 to 21.1), thoracotomy (RR = 3.1; 95% CI = 1.6 to 6.1), and radiotherapy to the spine (RR = 5.6; 95% CI = 1.7 to 18.4) were statistically significantly associated with the development of severe scoliosis.
Survivors have a 20-year cumulative incidence of neurological complications of 29.8% and were more likely to report neurological conditions than siblings (RR = 7.6; 95% CI = 6.4 to 9.1). Statistically significant risk factors for the development of a neurological complication included diagnosis at less than 1 year of age (RR = 2.6; 95% CI = 1.9 to 3.5) and laminectomy (RR = 4.0; 95% CI = 2.7 to 5.9). There was no difference in the risk of developing any neurological complication between survivors who received surgery alone and survivors who received other therapies in addition to surgery (P = .08). When risk of developing neurological complications was analyzed with respect to chemotherapy exposure alone, there was no statistically significant difference in risk between survivors who were and were not so exposed (P = .54).
Endocrine Complications
Neuroblastoma survivors were statistically significantly more likely to report an endocrine condition, as compared with siblings (RR = 13.0; 95% CI = 8.9 to 19.1). The cumulative 20-year incidence of an endocrine complication was 8.3%. Hypothyroidism was reported by 3.5% of the survivors, an 8.4-fold increase when compared with the siblings (95% CI = 5.1 to 13.6). Exposure to radiotherapy to the neck was associated with an increased risk of hypothyroidism. Neuroblastoma survivors who did have neck radiotherapy were much more likely to be hypothyroid than survivors who did not (RR = 17.9; 95% CI = 8.0 to 40.0).
Growth hormone (GH) deficiency was reported by 3.3% of neuroblastoma survivors (RR = 38.5; 95% CI = 15.2 to 97.3), and 75% of those who were GH deficient reported receiving GH replacement therapy. Exposure to cranial radiotherapy was statistically significantly associated with risk of developing GH deficiency (RR = 33.0; 95% CI = 14.7 to 73.9).
Thirteen of 204 evaluable women developed ovarian failure. Univariate analysis showed that radiotherapy to the ovaries (odds ratio [OR] = 8.4, 95% CI = 1.1 to 67.7), exposure to alkylating agents with a score of 3 (OR = 12, 95% CI = 2.0 to 71.0) or 2 (OR = 2.0, 95% CI = 1.0 to 33.2), and exposure to cumulative doses of cyclophosphamide above 5 g (OR = 7.1, 95% CI = 1.5 to 34.0) were statistically significantly associated with the development of ovarian failure. In a multivariable logistic regression model that considered age at diagnosis, cyclophosphamide exposure, radiotherapy, surgery, and alkylating agent score as potential covariates to enter the model in a stepwise variable selection, only radiotherapy to the ovaries emerged as a statistically significant risk factor for ovarian failure (P < .05).
Sensory Complications
Neuroblastoma survivors were 8.0 times (95% CI = 5.6 to 11.3) more likely than siblings to report any sensory complication, with a cumulative 20-year incidence of 8.6%. Survivors reported hearing loss more often than siblings (RR = 8.7; 95% CI = 5.2 to 14.6). Hearing loss was statistically significantly associated with cumulative exposure to cisplatin and cranial radiotherapy (Table 4). Survivors were also 6.9 times more likely than siblings to be blind (95% CI = 4.0 to 11.8) and 16.2 times more likely to have cataract (95% CI = 7.3 to 35.8). Cranial radiotherapy was a statistically significant risk factor for the development of adverse visual outcome (RR = 20.3; 95% CI = 9.7 to 42.7) and blindness (RR = 13.6; 95% CI = 7.7 to 24.0). Survivors who did not receive cranial radiation therapy also had a mildly increased risk of blindness (RR = 2.9; 95% CI = 1.2 to 7.2) compared with the sibling group.
Table 4.
Specific risk factors for the occurrence of hearing loss and deafness in survivors*
| Treatment | RR (95% CI) | P |
| Cisplatin | ||
| None | 1.0 | |
| 0.1–299 mg/m2 | 0.0 (0.0 to 999) | |
| 300–600 mg/m2 | 13.1 (4.7 to 36.9) | <.001 |
| >600 mg/m2 | 69.0 (25.8 to 184) | <.001 |
| Cranial radiation therapy | ||
| Yes | 3.2 (1.0 to 10.3) | .046 |
| No | 1.00 | |
Age, age at diagnosis, year at diagnosis, and sex adjusted. Two-sided statistical inference (Wald confidence intervals and tests) from Poisson regression was used. CI = confidence interval; RR = rate ratio.
Second Malignant Neoplasms
Thirty neuroblastoma survivors developed a second malignant neoplasm (SIR = 8.0; 95% CI = 5.4 to 11.4) 5 or more years following the diagnosis. In addition, three second malignant neoplasms (acute lymphoblastic leukemia, thyroid cancer, and soft tissue sarcoma) occurred before entry into the cohort (ie, within 5 years of the diagnosis of neuroblastoma). The cumulative incidence of second malignant neoplasms after 5 years from diagnosis was 3.5% (95% CI = 2.1% to 4.9%) at 25 years after diagnosis (Figure 2). The second malignant neoplasms that occurred 5 or more years after the original cancer diagnosis were thyroid cancer (n = 8; SIR = 23.6, 95% CI = 10.2 to 46.5), renal cell carcinoma (n = 5; SIR = 89.4, 95% CI = 28.8 to 208.7), soft tissue sarcomas (n = 3; SIR = 20.6, 95% CI = 4.1 to 60.1), acute myeloid leukemia (n = 2; SIR = 186.9, 95% CI = 21.0 to 674.9), breast cancer (n = 2; SIR [among female survivors] = 12.8, 95% CI = 1.4 to 46.2), brain tumor (n = 1; SIR = 5.2, 95% CI = 0.6 to 18.9), acute lymphoblastic leukemia (n = 1), Hodgkin lymphoma (n = 1), and others (n = 7). The other secondary cancers included two case patients with squamous cell carcinoma (one on the border of the tongue and the other at the cervix uteri), two serous cystadenomas of the ovary, one mucoepidermoid carcinoma (in the submandibular gland), one bladder cancer, and one teratoma (of the pineal gland). Two of the eight survivors who developed thyroid cancer were known to have received radiotherapy to the neck, one had received total body irradiation, and one additional patient had received radiotherapy but the exact site was unknown. Two of the five survivors who developed renal cell carcinoma had received radiotherapy to the abdomen. One of the two patients who developed acute myeloid leukemia received etoposide, and the patient who developed breast cancer received chest radiotherapy. In the multivariable Poisson regression analysis, exposure to any radiotherapy (P = .05) and to etoposide (P = .01) were statistically significant risk factors for the development of a second malignant neoplasm.
Discussion
In this study of 5-year neuroblastoma survivors, we observed that the cumulative 20-year incidence of a chronic health condition is 41.1%, with an 8.3-fold increased risk when compared with their siblings. The most prevalent outcomes involved the neurological, sensory, endocrine, and musculoskeletal systems, with 20-year cumulative incidences of 29.8%, 8.6%, 8.3%, and 7.8%, respectively. Survivors treated with multimodality therapy were at increased risk of developing a chronic health condition compared with those treated with surgery alone. Six percent of patients died more than 5 years after their diagnosis, with the most common causes of death being disease recurrence and second malignant neoplasms.
The published literature on late outcomes for neuroblastoma survivors, which is based on case series and small cohorts of neuroblastoma survivors, reports treatment-related outcomes including musculoskeletal and neurological problems (7–10), severe sensorineural hearing loss (14–16), endocrine deficits (8,9,11–13), and increased risk of second malignant neoplasms (17). The current study of more than 900 neuroblastoma survivors who were followed up to 30 years from diagnosis, to our knowledge, represents the largest comprehensive investigation of long-term outcomes in this specific population. Our data confirm the results of many of the previous smaller studies and provide additional information regarding the incidence or prevalence and risk factors for specific outcomes. We demonstrate that the risks of mortality and of developing a chronic health condition are statistically significantly higher for neuroblastoma survivors than would be expected in the general population or observed among siblings. In expanding on our previous report about chronic health conditions 30 years after a diagnosis of neuroblastoma (4), we now demonstrate that risks were particularly high for development of a second malignant neoplasm and musculoskeletal or neurological complications. It is also noteworthy that neuroblastoma survivors who were diagnosed and treated more recently (1981–1986) had the highest cumulative rate of chronic health conditions. The latter finding may reflect the introduction of more intensive therapies for patients who were judged by risk stratification to have a poor prognosis and the treatment of low- or intermediate-risk patients with relapsed disease. Increased therapeutic exposure in this population could result from greater dose intensity, stem cell transplantation, and regimens using multimodality approaches (2,3).
The 25-year cumulative risk of late mortality in this study was 6%. Main causes for late mortality were disease recurrence, second malignant neoplasms, and cardiac complications. These findings are similar to those reported for survivors of all types of childhood cancer (30). Also, the increased risk of late mortality for patients who were older than 5 years at diagnosis is consistent with the findings of previous studies of childhood cancer survivors (30,31).
Similar to observations among all survivors of childhood cancer combined (32), the cumulative incidence of second malignant neoplasm among neuroblastoma survivors was 3.5% at 25 years and increased to 7.0% at 30 years, with risk being statistically significantly associated with exposure to radiation therapy. As in previous smaller series, thyroid cancer, breast cancer, and acute myeloid leukemia were among the most common second malignant neoplasms observed in neuroblastoma survivors (13,17,32,33). The occurrence of five patients with renal cell carcinoma in our cohort is particularly noteworthy. The occurrence of renal cell carcinoma after neuroblastoma has been described previously (34). Although some cases of renal cell carcinoma have occurred following exposure to chemotherapy, particularly with cyclophosphamide, the fact that others have occurred in neuroblastoma patients who were not exposed to any chemotherapy suggests the possibility of an underlying genetic predisposition (35). Recently, Altinok et al. (36) described an Xp11.2/TFE3 translocation in a neuroblastoma patient who developed renal cell carcinoma after exposure to chemotherapy. The same translocation has been described in patients with renal cell carcinoma after chemotherapy in individuals with diagnoses other than neuroblastoma, suggesting that cytotoxic chemotherapy may predispose to the development of this specific form of renal cell carcinoma (35).
Musculoskeletal and neurological late effects have been reported in small series of low- or intermediate-risk neuroblastoma survivors (7). The most common of these is the development of scoliosis that is associated with moderate to high doses of radiation therapy (1500–5000 cGy, frequently administered in an asymmetric spinal field). Neurological complications, including mild to severe paresis, paraplegia, and neurogenic bladder, have also been described. These conditions, which occurred at 3- to 20-fold higher rates in neuroblastoma survivors than siblings, could be disease related (eg, intraspinal tumors) and/or could be complications of surgery. In addition, the data indicated that age at diagnosis was a statistically significant risk factor for the development of a neurological complication, with infants who were diagnosed at less than 1 year of age being at greatest risk. This increased risk may be a reflection of the difficulties inherent in operating on small infants, in cases of epidural neuroblastoma.
We also found that the risk of blindness was increased in neuroblastoma survivors compared with siblings. Cranial radiation therapy was associated with increased risk of blindness. Blindness was most likely to be due to disease that involved either the base of the skull or the orbits and that was treated with radiation therapy.
Not unexpectedly, we found neuroblastoma survivors to be at a statistically significantly increased risk of hearing loss. Exposure to cisplatin was the major risk factor for hearing loss. Hearing loss at a young age has a substantial impact on speech acquisition and academic achievement and may contribute to an increased need for special education services (37). Nonetheless, neuroblastoma survivors reported educational attainment similar to siblings. However, the neuroblastoma survivors differed substantially from the sibling cohort in psychosocial outcomes, including having never been employed, having lower individual and household income, and being less likely to have ever married. These outcomes are suggestive of a diminished level of social integration among neuroblastoma survivors. Few studies have specifically investigated the quality of life of neuroblastoma survivors (16,38,39). A recent Children's Oncology Group study (16) found that the 137 neuroblastoma survivors did not score substantially different from population norms on validated quality-of-life measures but those with hearing loss had substantially increased risk of academic problems and indicators of psychosocial distress. Barrera et al. (38) also reported neuroblastoma survivors to be at risk for decreased emotional well-being and adverse social outcomes.
Several issues need to be considered when interpreting our findings. First, the medical conditions are self-reported without external verification, with the exception of mortality and second malignant neoplasms, and self-reporting has the potential to result in misclassification of outcome status and imprecision in risk estimates. Second, it was necessary to restrict the current report to selected outcomes because it is not possible to adequately present results on the full range of potential late effects. Many additional possible outcomes (eg, chronic pain, hepatitis C, posttraumatic stress, etc) will be the subject of future CCSS publications and will present findings stratified on initial diagnosis.
Third, the CCSS cohort is hospital based as opposed to population based, and a proportion of eligible subjects did not participate in the baseline survey and thus are not included in analyses of risk factors for chronic health conditions. These features must be considered within the context of the generalizability of the results and the potential validity of the rates obtained. Although the similarity of demographic- and treatment-specific characteristics of participants and nonparticipants is reassuring, it is still possible that nonparticipants systematically differ with respect to prevalence of chronic health conditions, thus resulting in biased estimates. Nonetheless, previously published results from the CCSS relative to late mortality and morbidity are similar to those in smaller population-based studies from Europe (40–43). With this in mind, it is still important to note that the population of neuroblastoma survivors in the current project represents one of the largest series reported to date.
Fourth, neuroblastoma staging was not collected for participants in the CCSS cohort. Although lack of staging data might appear to be a limitation, it actually had minimal consequences because there has been a marked shift in risk- and stage-based tailoring of therapy, which makes it more relevant to considering late effects among survivors relative to the actual treatment exposure of the patients rather than to their stage of disease.
In summary, young adult neuroblastoma survivors who were treated in the 1970s and 1980s are at risk for many adverse outcomes, including second malignant neoplasms, chronic health conditions, and unfavorable psychosocial outcomes. It will be important to continue to follow this relatively young adult cohort of survivors to determine whether the pattern of adverse outcomes will change as they age. For children who are currently undergoing intensive treatment for neuroblastoma, the results of the current study are quite relevant and underscore the need for close surveillance and lifelong follow-up to ameliorate potential medical and psychosocial late effects. Future research should build on these data and investigate risk factors for long-term complications of neuroblastoma treatment and second malignant neoplasms, including genetic studies for the risk of renal cell carcinoma.
Funding
CCSS is supported by a grant from the National Cancer Institute (U24 CA55727 to L.L.R.) and the American Lebanese Syrian Associated Charities (St. Jude Children's Research Hospital). National Cancer Institute of Canada with funds from the Terry Fox Run (C.L.).
Footnotes
The study sponsors were not involved in the design of the study; the collection, analysis, or interpretation of the data; the writing of the article; or the decision to submit the article for publication.
References
- 1.Surveillance, Epidemiology, and End Results (SEER) Program, National Cancer Institute, Division of Cancer Prevention and Control, Surveillance Program, Cancer Statistics Branch, registry data 1975–2000. www.seer.cancer.gov. Accessed September 15, 2008. [Google Scholar]
- 2.Maris JM, Hogarty MD, Bagatell R, Cohn SL. Neuroblastoma. Lancet. 2007;369(9579):2106–2120. doi: 10.1016/S0140-6736(07)60983-0. [DOI] [PubMed] [Google Scholar]
- 3.Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999;341(16):1165–1173. doi: 10.1056/NEJM199910143411601. [DOI] [PubMed] [Google Scholar]
- 4.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 5.Zebrack BJ, Zevon MA, Turk N, et al. Psychological distress in long-term survivors of solid tumors diagnosed in childhood: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2007;49(1):47–51. doi: 10.1002/pbc.20914. [DOI] [PubMed] [Google Scholar]
- 6.Zeltzer LK, Recklitis C, Buchbinder D, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study [published online ahead of print on March 2, 2009] J Clin Oncol. 2009;27(14):2396–2404. doi: 10.1200/JCO.2008.21.1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kajanti M. Neuroblastoma in 88 children: clinical features, prognostic factors, results and late effects of therapy. Ann Clin Res. 1983;15(suppl 39):1–68. [PubMed] [Google Scholar]
- 8.Paulino AC, Mayr NA, Simon JH, Buatti JM. Locoregional control in infants with neuroblastoma: role of radiation therapy and late toxicity. Int J Radiat Oncol Biol Phys. 2002;52(4):1025–1031. doi: 10.1016/s0360-3016(01)02713-4. [DOI] [PubMed] [Google Scholar]
- 9.Laverdiere C, Cheung NK, Kushner BH, et al. Long-term complications in survivors of advanced stage neuroblastoma. Pediatr Blood Cancer. 2005;45(3):324–332. doi: 10.1002/pbc.20331. [DOI] [PubMed] [Google Scholar]
- 10.Flandin I, Hartmann O, Michon J, et al. Impact of TBI on late effects in children treated by megatherapy for Stage IV neuroblastoma. A study of the French Society. Int J Radiat Oncol Biol Phys. 2006;64(5):1424–1431. doi: 10.1016/j.ijrobp.2005.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Hovi L, Saarinen-Pihkala UM, Vettenranta K, Lipsanen M, Tapaneinen P. Growth in children with poor-risk neuroblastoma after regimens with or without total body irradiation in preparation for autologous bone marrow transplantation. Bone Marrow Transplant. 1999;24(10):1131–1136. doi: 10.1038/sj.bmt.1702021. [DOI] [PubMed] [Google Scholar]
- 12.Van Santen, de Kraker J, Vulsma T. Endocrine late effects from multi-modality treatment of neuroblastoma. Eur J Cancer. 2005;41(12):1767–1774. doi: 10.1016/j.ejca.2005.02.036. [DOI] [PubMed] [Google Scholar]
- 13.Trahair TN, Vowels MR, Johnston K, et al. Long-term outcomes in children with high-risk neuroblastoma treated with autologous stem cell transplantation. Bone Marrow Transplant. 2007;40(8):741–746. doi: 10.1038/sj.bmt.1705809. [DOI] [PubMed] [Google Scholar]
- 14.Parsons SK, Neault MW, Lehmann LE, et al. Severe ototoxicity following carboplatin-containing conditioning regimen for autologous marrow transplantation for neuroblastoma. Bone Marrow Transplant. 1998;22(7):669–674. doi: 10.1038/sj.bmt.1701391. [DOI] [PubMed] [Google Scholar]
- 15.Kushner BH, Budnick A, Kramer K, Modak S, Cheung NK. Ototoxicity from high-dose use of platinum compounds in patients with neuroblastoma. Cancer. 2006;107(2):417–422. doi: 10.1002/cncr.22004. [DOI] [PubMed] [Google Scholar]
- 16.Gurney JG, Tersak JM, Ness KK, Landier W, Matthay KK, Schmidt ML. Hearing loss, quality of life, and academic problems in long-term neuroblastoma survivors: a report from the Children's Oncology Group. Pediatrics. 2007;120(5):1229–1236. doi: 10.1542/peds.2007-0178. [DOI] [PubMed] [Google Scholar]
- 17.Kushner BH, Cheung NK, Kramer K, Heller G, Jhanwar SC. Neuroblastoma and treatment-related myelodysplasia/leukemia: the Memorial Sloan-Kettering experience and a literature review. J Clin Oncol. 1998;16(12):3880–3889. doi: 10.1200/JCO.1998.16.12.3880. [DOI] [PubMed] [Google Scholar]
- 18.Robison LL, Mertens AC, Boice JD, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Med Pediatr Oncol. 2002;38(4):229–239. doi: 10.1002/mpo.1316. [DOI] [PubMed] [Google Scholar]
- 19.Robison LL, Armstrong GT, Boice JD, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research [published online ahead of print April 13, 2009] J Cin Oncol. 2009;27(14):2308–2318. doi: 10.1200/JCO.2009.22.3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leisenring WM, Mertens AC, Armstrong GT, et al. Pediatric cancer survivorship research: experience of the Childhood Cancer Survivor Study [published online ahead of print April 13, 2009] J Clin Oncol. 2009;27(14):2319–2327. doi: 10.1200/JCO.2008.21.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tucker MA, D'Angio GJ, Boice JD, Jr, et al. Bone sarcomas linked to radiotherapy and chemotherapy in children. N Engl J Med. 1987;317(10):588–593. doi: 10.1056/NEJM198709033171002. [DOI] [PubMed] [Google Scholar]
- 22.Mertens AC, Walls RS, Taylor L, et al. Characteristics of childhood cancer survivors predicted their successful tracing. J Clin Epidemiol. 2004;57(9):933–944. doi: 10.1016/j.jclinepi.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 23.Efron B, Tibshirani RJ. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 24.Mertens AC, Liu Q, Neglia JP, et al. Cause-specific late mortality among 5-year survivors of childhood cancer. J Natl Cancer Inst. 2008;100:1–12. doi: 10.1093/jnci/djn310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Breslow NE, Day NE. Statistical Methods in Cancer Research. Vol. 2. The Design and Analysis of Cohort Studies (IARC Scientific Publications No. 82) Lyon, France: International Agency for Research on Cancer; 1987. [PubMed] [Google Scholar]
- 26.Gooley TA, Leisenring W, Crowley J, Storer JE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 27.Hudson MM, Mertens AC, Yasui Y, et al. Health status of adult long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. JAMA. 2003;290(12):1583–1592. doi: 10.1001/jama.290.12.1583. [DOI] [PubMed] [Google Scholar]
- 28.Chemaitilly W, Merterns AC, Mitby P, Whitton J, Stovall M, Yasui Y. Acute ovarian failure in the Childhood Cancer Survivor Study. J Clin Endocrinol Metab. 2006;91(5):1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 29.Gray R. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 30.Mertens AC, Yasui Y, Neglia J, et al. Late mortality experience in five-year survivors of childhood and adolescent cancer: the Childhood Cancer Survivor Study. J Clin Oncol. 2001;19(13):3163–3172. doi: 10.1200/JCO.2001.19.13.3163. [DOI] [PubMed] [Google Scholar]
- 31.Möller TR, Garwicz S, Perfekt R, et al. Late mortality among five-year survivors of cancer in childhood and adolescence. Acta Oncol. 2004;43(8):711–718. doi: 10.1080/02841860410002860. [DOI] [PubMed] [Google Scholar]
- 32.Neglia JP, Friedman DL, Yasui Y, et al. Second malignant neoplasms in five-year survivors of childhood cancer: Childhood Cancer Survivor Study. J Natl Cancer Inst. 2001;93(8):618–629. doi: 10.1093/jnci/93.8.618. [DOI] [PubMed] [Google Scholar]
- 33.Rubino C, Adjadj E, Guerin S, et al. Long-term risk of second malignant neoplasms after neuroblastoma in childhood: role of treatment. Int J Cancer. 2003;107(5):791–796. doi: 10.1002/ijc.11455. [DOI] [PubMed] [Google Scholar]
- 34.Fleitz JM, Wootton-Gorges SL, Wyatt-Ashmead J, et al. Renal cell carcinoma in long-term survivors of advanced stage neuroblastoma in early childhood. Pediatr Radiol. 2003;33(8):540–545. doi: 10.1007/s00247-003-0913-x. [DOI] [PubMed] [Google Scholar]
- 35.Argani P, Lae M, Ballard ET, et al. Translocation carcinomas of kidney after chemotherapy in childhood. J Clin Oncol. 2006;24(10):1529–1534. doi: 10.1200/JCO.2005.04.4693. [DOI] [PubMed] [Google Scholar]
- 36.Altinok G, Kattar MM, Mohamed A, Poulik J, Grignon D, Rabah R. Pediatric renal cell carcinoma associated with Xp 11.2 translocations/TFE3 gene fusions and clinicopathologic associations. Pediatr Dev Pathol. 2005;8(2):168–180. doi: 10.1007/s10024-004-9106-3. [DOI] [PubMed] [Google Scholar]
- 37.Knight KR, Kraemer DF, Neuwelt EA. Ototoxicity in children receiving platinum chemotherapy: underestimating a commonly occurring toxicity that may influence academic and social development. J Clin Oncol. 2005;23(34):8588–8596. doi: 10.1200/JCO.2004.00.5355. [DOI] [PubMed] [Google Scholar]
- 38.Barrera M, Shaw AK, Speechley KN, Maunsell E, Pogany L. Educational and social late effects of childhood cancer and related clinical, personal and familial characteristics. Cancer. 2005;104(8):1751–1760. doi: 10.1002/cncr.21390. [DOI] [PubMed] [Google Scholar]
- 39.Nathan PC, Ness KK, Greenberg M, et al. Health-related quality of life in adult survivors of childhood Wilms tumor or Neuroblastoma: a report from the Childhood Cancer Survivor Study. Pediatr Blood Cancer. 2007;49(5):704–715. doi: 10.1002/pbc.20949. [DOI] [PubMed] [Google Scholar]
- 40.Geenen MM, Cardous-Ubbink MC, Kremer LC, et al. Medical assessment of adverse health outcomes in long-term survivors of childhood cancer. JAMA. 2007;297:2705–2715. doi: 10.1001/jama.297.24.2705. [DOI] [PubMed] [Google Scholar]
- 41.Möller TR, Garwicz S, Barlow L, et al. Decreasing late mortality among five-year survivors of cancer in childhood and adolescence. J Clin Oncol. 2001;19:3173–3181. doi: 10.1200/JCO.2001.19.13.3173. [DOI] [PubMed] [Google Scholar]
- 42.Oeffinger KC, Robison LL. Childhood cancer survivors, late effects, and a new model for understanding survivorship. JAMA. 2007;297:2762–2764. doi: 10.1001/jama.297.24.2762. [DOI] [PubMed] [Google Scholar]
- 43.Simone JV. Late mortality in childhood cancer: two excellent studies bring good news tempered by room for improvement. J Clin Oncol. 2001;19:3161–3162. doi: 10.1200/JCO.2001.19.13.3161. [DOI] [PubMed] [Google Scholar]