Abstract
NMR spectroscopy is used to investigate the heterotrimeric nature of a collagen model peptide. Two distinct peptide chains (A and B) were synthesized to model a site in heterotrimeric basement membrane type IV collagen. For NMR studies, four amino acids in the B chain were labeled with 15N/13C. CD spectroscopy and DSC thermal stability results on a solution with both A and B peptides (molar ratio 2A:1B) are consistent with the presence of one heterotrimeric triple-helical molecular species. HSQC experiments on homotrimers of the B peptide show trimer peaks which disappear at temperatures higher than 10°C, while the 2A:1B mixture has trimer peaks with increased stability and altered chemical shifts. The reduction in the number of Leu trimer peaks from three to one and the increased stability of trimer resonances confirm the participation of B chains in an AAB heterotrimer molecule.
Triple-helical peptides serve as valuable models for collagen, a critical protein in normal tissue structure and in disease. Close packing of 3 polyproline-like chains in the collagen triple-helix structure generates the requirement for glycine as every third residue, (Gly-X-Y)n.1 Peptides with Gly as every third residue and a high imino acid content will spontaneously self-assemble into homotrimers with a triple-helical structure. Such stable homotrimer peptides have been well characterized in terms of stability, folding, and dynamics, and molecular structures have been obtained by NMR and X-ray crystallography.2 These homotrimer peptides serve as good models for collagen molecules with three identical chains, such as type II collagen in cartilage. It has proved more difficult to obtain and characterize hybrid triple-helical peptides composed of chains with different amino acid sequences (heterotrimers), which can serve as models for heterotrimeric collagens such as Type I collagen in bone and type IV collagen in basement membranes.
Heterotrimer peptide design strategies have included covalent linkage to force the selection of three chains and their alignment,3 while recent studies used electrostatic interactions within the (Gly-X-Y)n sequences to direct desired self-assembly.4 Thus far, techniques for biophysical characterization of heterotrimers have probed average properties of the triple-helix. For instance, Gauba and Hartgerink used circular dichroism (CD) spectroscopy to follow the thermal stabilities of single peptide vs. mixed peptide solutions, and differences in stability are interpreted in terms of homotrimer and heterotrimer molecules.4 In contrast, NMR has the capacity to follow the properties of individual residues and individual chains within trimers.2a Complexes where one subunit is labeled and others are not have been studied by NMR to define the features of the labeled complex and its interaction.5 Here, a similar strategy is applied to the triple-helix, using NMR to monitor labeled residues within one peptide chain to follow specific structural and chemical properties within a heterotrimer versus a homotrimer context.
The design strategy presented here is based on mixing an 15N/13C labeled peptide chain which has a low propensity to self-associate into a triple-helix together with an unlabeled peptide chain of different sequence and high triple-helix propensity. The peptides studied model a site of the heterotrimeric type IV collagen molecule composed of two α1(IV) and one α2(IV) chains.6 The site modeled contains a natural interruption in the Gly-X-Y repeating sequence of both the α1(IV) and α2(IV) chains. Peptide A, the unlabeled peptide, includes residues 289-302 (Table 1) from the sequence of the α1(IV) chain of type IV collagen with an interruption. Peptide B includes residues 288-295 from the corresponding sequence in the α2(IV) chain at the same site with a corresponding interruption (Table 1). Both collagen sequences are flanked by stabilizing triplets and a terminal Tyr for concentration determination. Four residues in peptide B were 15N/13C-labeled, including three residues Ile, Ser and Leu in the interruption and an Ala in the stabilizing C-terminal sequence.
Table 1.
Triple-helix content (MRE225), thermal stability (Tm) and calorimetric enthalpy (ΔHcal) for peptides A, B and a mixture with a 2A:1B molar ratio. Underlined residues are sequences from α1(IV) and α2(IV) chains; bold residues in peptide B are 15N/13C labeled.
| Peptide | Sequence | MRE225 deg.cm2. dmol−1 |
Tm °C |
ΔHcal KJ/mol |
|---|---|---|---|---|
| A | (GPO)3GMOGVGEKG EOGKO(GPO)2GYa |
2800 | 16.5 | 190 |
| B | POGDO(GPO)2GISLKG EEGPOGPAGPOGYOGa |
1150 | 3.5b | 60 |
| 2A:1B | 2500 | 14.5 | 140 |
O stands for hydroxyproline
Estimated value since the thermal transition is incomplete
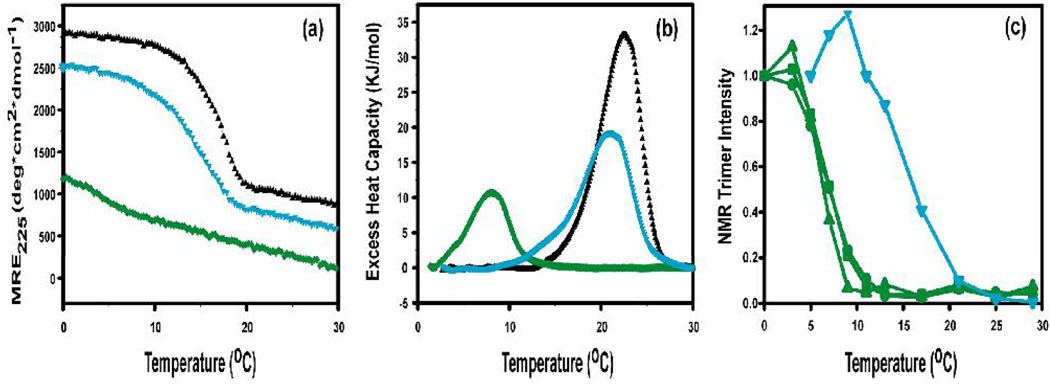
First, the properties of each peptide as a homotrimer were characterized. Homotrimeric peptides models with interruptions have been shown to form triple-helical molecules with a low thermal stability and a lower than normal CD maximum at 225nm.7 Dissolving peptide A in solution at low temperature (c=0.33 mM) leads to formation of stable homotrimeric triple-helical molecules with a characteristic CD triple-helix peak at 225nm (MRE225=2800 deg.cm2.dmol−1) and a melting temperature of 16.5°C. Dissolving peptide B in solution leads to the formation of some homotrimers with a low MRE225 value (1150 deg.cm2.dmol−1) and a partial thermal transition (Figure 1a). Differential scanning calorimetry (DSC) scans confirm that B homotrimers are less stable than A homotrimers (Fig. 1b).
Figure 1.
Stability of peptide A(black), B(green) and a mixture with molar ratio 2A:1B (cyan) (a) CD thermal transitions; (b) DSC profiles. The DSC thermal transitions are at higher temperatures than seen for CD melts, as expected for faster heating rates under non-equilibrium conditions.8 (c) HSQC residue-specific melting for the 3 Leu peaks in peptide B as a homotrimer (green) and of the single Leu peak seen for the mixture of 2A:1B (cyan). Peptide A contains no labeled residues for HSQC studies. Peak intensities are normalized to values observed at lowest temperature.
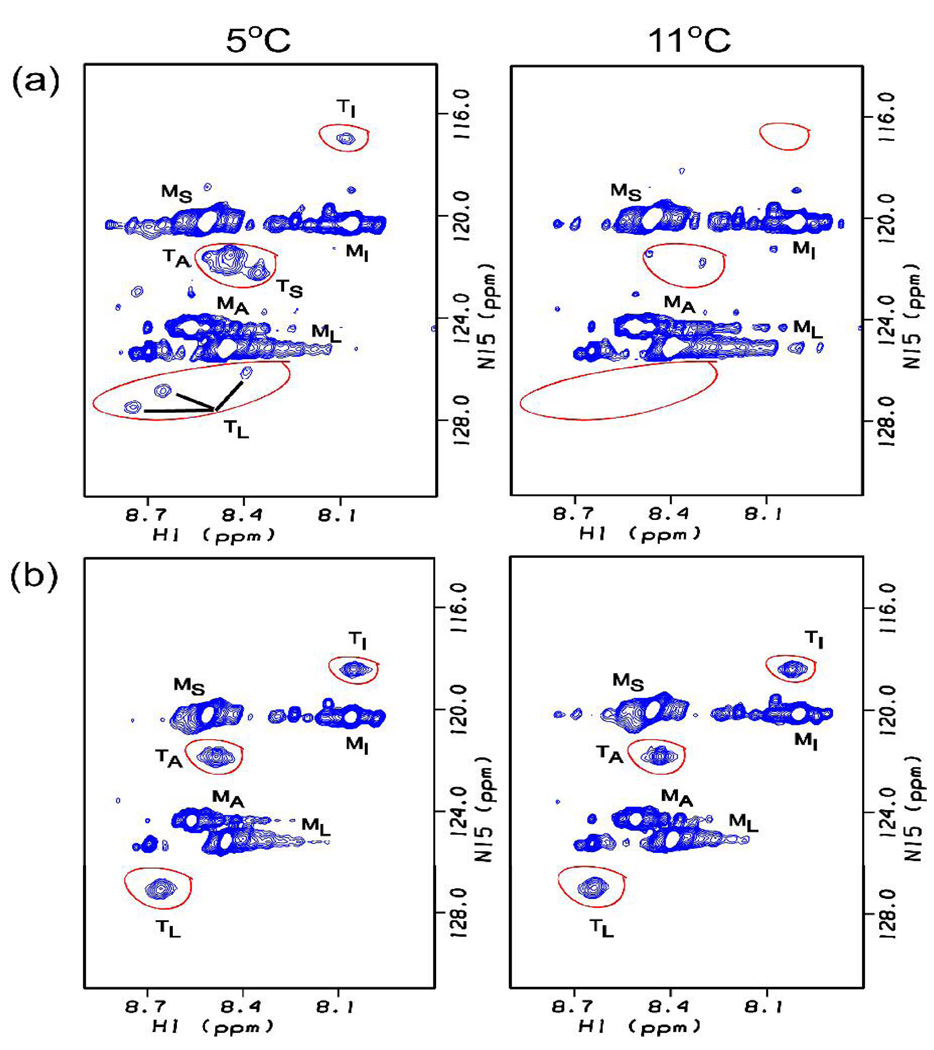
NMR studies were carried out on the labeled B homotrimer at high concentrations (6 mM). As seen in previous spectra of triple helical peptides, the HSQC spectrum at 5°C shows monomer and trimer peaks for each labeled residue.2a,e Three trimer peaks are seen for Leu (Fig. 2a), indicating different environments for the Leu residues located in the interruption region in each of the three chains of the homotrimer. All three Leu peaks show a thermal transition at the same temperature, Tm=7°C (Fig. 1c). The absence of three distinct trimer peaks for Ile, Ser and Ala residues, which show only one or two trimer resonances, is likely due to overlapping trimer resonances. Raising the temperature to 11°C leads to loss of almost all trimer peaks, indicating the complete melting of the B homotrimer triple helix by this temperature (Fig. 2(a) right). NMR melting curves are not obtained for the A peptide, which contains no 15N/13C labeled residues.
Figure 2.
Comparison of 1H-15N HSQC spectra of labeled Ile, Ser, Leu and Ala residues in peptide B for (a) a peptide B solution (homotrimer) and (b) a 2A:1B peptide mixture. Trimer peaks for all residues of peptide B in a homotrimer (a) are present at 5°C (left) and absent at 11°C (right), while trimer peaks are present at both temperatures when peptide B is present in a 2A:1B peptide mixture. The peaks corresponding to monomer and trimer state are denoted with superscript M or T. The large number of minor monomer peaks are due to cis/trans isomerization in the unfolded state in this proline rich chain.9 Other small peaks between the monomer and trimer peaks may be due to noise or impurities.
Peptides A and B were mixed in solution at a molar ratio of 2A:1B (4mM:2mM) to look for formation of heterotrimeric molecules. The mixture was heated to 50°C, cooled slowly to 0°C and incubated for 30hrs. The CD spectrum had a maximum at 225nm with MRE225 =2500 deg.cm2.dmol−1, which is somewhat higher than expected on the basis of simple addition of 2A and 1B (expected MRE225=2250 deg.cm2.dmol−1). The melting curve shows a single transition at 14.6°C, which is slightly less than the Tm value of A homotrimers and much higher than the Tm of B homotrimers (Figure 1a). DSC profiles (Figure 1b) confirm that the 2A:1B mixture has a single thermal transition with a stability slightly less than the A homotrimer. No DSC transition is seen at the B homotrimer position, confirming that no significant amount of B homotrimers are formed. These observations support the presence of a single heterotrimeric species containing chains of A and B.
NMR experiments were performed to probe chain specific resonances related to hybrid chain association. 1H-15N HSQC experiments were performed on the labeled B chain in a 2A:1B mixture after heating and cooling. In the HSQC at 5°C, there is only 1 Leu trimer resonance, compared with 3 distinct resonances for the B homotrimer. This is consistent with an AAB composition where there is only 1 B chain in a heterotrimer. A single peak is seen for Ile, but its position is shifted compared with the B homotrimer, suggesting a different environment in the heterotrimer compared with the homotrimer. A single resonance is seen for the Ala trimer and no Ser resonance is seen for the mixed peptides, which is likely due to overlap with monomer resonance.
The temperature dependence of the trimer resonances arising from the B chain in the mixed 2A:1B system was determined. The thermal stabilities of trimer resonances in the mixture differ markedly from those seen for the B homotrimer. All resonances of homotrimer B have disappeared by 11°C, while the resonances of B in the mixed system do not disappear until the temperature is higher than 20°C (Fig. 2). For example, the stability of the single Leu trimer resonance in the mixture (Tm~17°C) is shown to be markedly higher than the 3 Leu trimer resonances in the homotrimer (Tm~7°C) in Fig. 1c. The high stability of the residues in peptide B must arise as a result of interactions involved in heterotrimer association with A.
The formation and characterization of hybrid triple-helical peptide systems is important for modeling heterotrimeric collagens and for modeling diseases where there are one or two mutations in a collagen triple-helix.4c,10 The NMR data monitoring the chemical shifts and stability of defined residues within one chain in a homotrimer compared with a heterotrimer setting, complement the information on average properties obtained from CD and DSC studies. The NMR results show that in a 2:1 ratio mixture of A and B chains, peptide chain B is present in mixed trimers containing both types of chains and supports the presence of a single AAB molecular species. If both AAB and BBA species were present, multiple resonances with a more heterogeneous spectrum would be expected. It is not possible to eliminate the possibility of some A homotrimers, since these chains are not labeled and their stability is close to the heterotrimers. The simple approach presented here can be done on any hybrid system and provides evidence of the incorporation of a chain into a heterotrimer triple-helix as well as residue specific information about that chain.
Acknowledgments
This work was supported by NIH grants GM45302 (to J.B.) and GM60048 (to B.B.), and a BOYSCAST fellowship (DST, India) to B. M.
References
- 1.(a) Ramachandran GN, Kartha G. Nature. 1955;176:593–595. doi: 10.1038/176593a0. [DOI] [PubMed] [Google Scholar]; (b) Rich A, Crick FHC. J. Mol. Biol. 1961;3:483–506. doi: 10.1016/s0022-2836(61)80016-8. [DOI] [PubMed] [Google Scholar]
- 2.(a) Baum J, Brodsky B. Curr. Opin. Struct. Biol. 1999;9:122–128. doi: 10.1016/s0959-440x(99)80016-5. [DOI] [PubMed] [Google Scholar]; (b) Brodsky B, Persikov AV. Adv. Prot. Chem. 2005;70:301–339. doi: 10.1016/S0065-3233(05)70009-7. [DOI] [PubMed] [Google Scholar]; (c) Jenkins CL, Raines RT. Nat. Prod. Rep. 2002;19:49–59. doi: 10.1039/a903001h. [DOI] [PubMed] [Google Scholar]; (d) Bella J, Eaton M, Brodsky B, Berman HM. Science. 1994;266:75–81. doi: 10.1126/science.7695699. [DOI] [PubMed] [Google Scholar]; (e) Li Y, Brodsky B, Baum J. J. Biol. Chem. 2007;282:22699–22706. doi: 10.1074/jbc.M702910200. [DOI] [PubMed] [Google Scholar]
- 3.(a) Slatter DA, Foley LA, Peachey AR, Nietlispach D, Farndale RW. J. Mol. Biol. 2006;359:289–298. doi: 10.1016/j.jmb.2006.02.071. [DOI] [PubMed] [Google Scholar]; (b) Fiori S, Sacca B, Moroder L. J. Mol. Biol. 2002;319:1235–1242. doi: 10.1016/S0022-2836(02)00365-0. [DOI] [PubMed] [Google Scholar]; (c) Koide T, Nishikawa Y, Takahara Y. Bioorg. Med. Chem. Lett. 2004;14:125–128. doi: 10.1016/j.bmcl.2003.10.005. [DOI] [PubMed] [Google Scholar]; (d) Fields GB, Prockop DJ. Biopolymers. 1996;40:345–357. doi: 10.1002/(SICI)1097-0282(1996)40:4%3C345::AID-BIP1%3E3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 4.(a) Gauba V, Hartgerink JD. J. Am. Chem. Soc. 2007;129:2683–2690. doi: 10.1021/ja0683640. [DOI] [PubMed] [Google Scholar]; (b) Gauba V, Hartgerink JD. J. Am. Chem. Soc. 2007;129:15034–15041. doi: 10.1021/ja075854z. [DOI] [PubMed] [Google Scholar]; (c) Gauba V, Hartgerink JD. J. Am. Chem. Soc. 2008;130:7509–7515. doi: 10.1021/ja801670v. [DOI] [PubMed] [Google Scholar]
- 5.Clore GM, Gronenborn AM. Curr. Opin. Chem. Biol. 1998;2:564–570. doi: 10.1016/s1367-5931(98)80084-7. [DOI] [PubMed] [Google Scholar]
- 6.(a) Kuhn K. Matrix Biol. 1995;14:439–445. doi: 10.1016/0945-053x(95)90001-2. [DOI] [PubMed] [Google Scholar]; (b) Khoshnoodi J, Pedchenko V, Hudson BG. Microsc Res Tech. 2008;71:357–370. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiagarajan G, Li Y, Mohs A, Strafaci C, Popiel M, Baum J, Brodsky B. J. Mol. Biol. 2008;376:736–748. doi: 10.1016/j.jmb.2007.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Persikov AV, Xu Y, Brodsky B. Protein Sci. 2004;13:893–902. doi: 10.1110/ps.03501704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buevich AV, Dai QH, Liu X, Brodsky B, Baum J. Biochemistry. 2000;39(15):4299–4308. doi: 10.1021/bi992584r. [DOI] [PubMed] [Google Scholar]
- 10.Makareeva E, Mertz EL, Kuznetsova NV, Sutter MB, Deridder AM, Cabral WA, Barnes AM, Mcbride DJ, Marini JC, Leikin S. J. Biol. Chem. 2008;283:4787–4798. doi: 10.1074/jbc.M705773200. [DOI] [PubMed] [Google Scholar]