Abstract
Current therapies for meniscal injury seek to preserve and repair damaged tissue since loss of meniscal tissue is associated with degenerative changes in the joint, ultimately leading to osteoarthritis (OA). After a meniscal tear, the difficulty of integrating juxtaposed meniscal surfaces continues to be an obstacle. In order to determine the local factors that are necessary for successful tissue repair, previous studies have developed in vitro model systems that allow both biological and quantitative biomechanical measures of meniscus repair. Many studies have shown the importance of individual factors in meniscus metabolism, but there is a complex interplay among a variety of factors that influence meniscal healing, including inflammatory cytokines, growth factors, mechanical loading, and zonal differences in cell and tissue properties. In particular, the upregulation of inflammatory cytokines following joint injury appears to have significant catabolic influences on meniscal cell metabolic activity that must be overcome in order to promote repair. In the presence of inflammatory cytokines, such as interleukin-1 (IL-1) or tumor necrosis factor alpha (TNF-α), intrinsic meniscal repair in vitro is significantly inhibited. While anabolic growth factors, such as transforming growth factor-β1 (TGF-β1), enhance meniscal repair, they cannot completely overcome the IL-1-mediated inhibition of repair. The mechanisms by which these mediators influence meniscal repair, and their interactions with other factors in the microenvironment, such as mechanical loading, remain to be determined. Future studies must address these complex interactions during meniscal healing to ultimately enhance meniscal repair.
Keywords: articular cartilage, matrix metalloproteinases, fibrochondrocyte, cell migration, meniscectomy, mechanical compression
1. Introduction
The menisci are C-shaped fibrocartilaginous tissues situated between the femoral condyles and the tibial plateau in the knee joint. These tissues are required for the normal biomechanical function of the knee, in particular for load bearing, shock absorption, joint congruity, and joint stability [1–3]. The meniscus is composed of 60–70% water and approximately 70% collagen by dry weight [4] and smaller amounts of proteoglycans, non-collagenous proteins, lipids, and cells [5]. Meniscal cells can exhibit phenotypic characteristics of both fibroblasts and chondrocytes and are responsible for maintaining the extracellular matrix [6]. The meniscus is an inhomogeneous tissue that possesses significant differences in composition, cellularity, and vascularity, depending on the distance from the peripheral edge. The outer one-third of the meniscus is composed of type I collagen [4, 7, 8], the cells in this region are fibroblast-like [6], and the tissue is vascularized [9]. On the other hand, the inner region of the meniscus contains fibrochondrocyte-like cells [6], contains both types I and II collagen [7, 8], and a higher aggrecan content than the outer region [9]. Importantly, the inner zone of the meniscus lacks a vascular supply [10].
Current clinical therapies for meniscal injury seek to preserve and repair the damaged tissue, since damage or loss of meniscal tissue is associated with degenerative changes in the joint that ultimately lead to osteoarthritis (OA) [11–13]. In this respect, surgical repair of meniscal lesions in the outer, vascularized zone is often successful, but in the inner, avascular zone little or no repair is observed [10]. This difference in repair potential between the inner and outer regions of the meniscus is generally attributed to differences in the transport of vascular-derived factors, including progenitor cells and/or soluble mediators, such as growth factors and inflammatory mediators. Therefore, a variety of techniques have been developed in an attempt to increase the vascular supply to meniscal lesions in the inner zone [10, 14–21]. However, the clinical success of these techniques is still unclear.
After a meniscal tear, the difficulty of integrating juxtaposed meniscal surfaces continues to be an obstacle with regard to the long-term efficacy of meniscus repair strategies. Previous work on integrative repair of cartilaginous tissues has been performed using a model of cartilage repair developed by Sah and co-workers [22–26]. Using this model, the strength of cartilage integrative repair was determined by a modified single lap test [22] or ‘T’ peel test [27]. These important studies have shown that cartilage exhibits intrinsic integrative repair in vitro that can be modified by the biochemical environment. On the other hand, few studies have developed meniscus organ culture systems to investigate the in vitro repair of meniscal defects or tears. Here we review a number of studies that have used in vitro model systems of meniscal tissue or organ culture to examine the role of various factors, such as cell viability, exogenous biomaterials, soluble mediators, or site in the meniscus, on meniscal repair.
2. In vitro models of meniscal defect repair
An early organ culture model of meniscus repair demonstrated the ability of meniscal fibrochondrocytes to invade 2 mm defects in rabbit menisci filled with a fibrin clot [28]. Organ culture model systems have also been established using full thickness defects in the avascular region of either human [29] or dog menisci [30]. For example, defects filled with autogenous synovial grafts demonstrated histologic repair that bridged the defect gap, as compared to unfilled defects where cells only migrated to the edges of the gap [29]. On the other hand, collagen gels loaded with platelet-derived growth factor (PDGF) and hepatocyte growth factor (HGF) in a meniscal defect revealed increases in proteoglycan staining, collagen formation, and cell number [30]. In order to assess regional differences in meniscus healing, Kobayashi et al. established an organ culture model in which 1.5 mm defects in the inner zone of the meniscus were filled with tissue from either the inner or the outer zone [31]. While, these model systems have provided important histological indications of tissue repair, the complex geometric structure of a whole, intact meniscus can complicate quantitative measures of meniscal repair.
3. Models of integrative repair of the meniscus
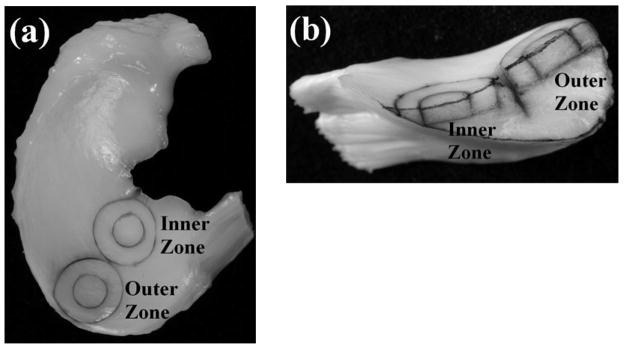
In order to determine the local factors that are necessary for successful tissue repair, our laboratory has developed an in vitro model system that allows both biological and quantitative biomechanical measures of meniscus repair [32–35]. This model of meniscal repair allows the analysis of biochemical interventions on the time course, strength, and histology of the repair process. The model uses cylindrical explants (8mm diameter, 2mm thick) from the femoral surface of either the outer one-third and/or the inner two-thirds of medial menisci from 2–3 year-old skeletally mature female pigs (Figure 1). To simulate a full-thickness tear, a central core (4 mm in diameter) is removed with a biopsy punch and reinserted immediately. Explants are cultured for up to 6 weeks, at which time a push-out test is used to determine the shear strength of repair between the inner core and outer ring. Additional measures of integrative repair include a fluorescence viability assay with confocal microscopy to visualize cell death and cell accumulation at the interface and histological staining to detect tissue repair in the interface. Using this model system, we show that the meniscus exhibits an intrinsic repair capacity in vitro and identify a variety of factors that influence meniscal healing [32–35].
Fig. 1.
Images of the site of harvest and cross section of meniscal constructs. A. Medial meniscus showing location of explants harvested from the inner and outer zones. B. Cross section of the meniscus within the center of the explants. From each meniscus, concentric cylindrical explants (8 mm outer ring and 4 mm inner core diameter) were obtained from the outer and inner zone. C. Interfacial shear strength of specimens from the inner two-thirds and outer one-third of the meniscus at the day of harvest and 2, 4, and 6 weeks of culture. Significant changes in repair strength were observed with time in culture, but no differences between inner and outer zone were found. Data are presented as mean + standard error of the mean. * p < 0.05. ** p < 0.01. *** p < 0.001 (n = 12–18 per zone and time point). Reprinted from [33] with permission from SAGE Publications, Inc.
4. Inner versus outer zone of the meniscus and repair potential
Previous studies have shown that the outer zone of the meniscus exhibits significantly higher repair potential in vivo than the inner zone [10]. In this respect, an in vitro explant model system can be used to investigate the intrinsic healing abilities of tissue from the inner versus the outer zones of the meniscus, in the absence of a vascular supply [31, 33]. For example, meniscal plugs transplanted from the outer zone to the inner zone show improved gross and histological healing (Figure 2A and 2C), as compared to plugs from the inner zone (Figure 2B and 2D), suggesting a superior intrinsic healing potential of outer zone meniscal tissue, even without the influence of the vasculature [31]. On the other hand, meniscal repair model explants from both the inner and outer zones of the meniscus have similar intrinsic repair capacities when analyzed by the shear strength of repair (Figure 1C), cell migration into the repair site, and tissue formation in the interface [33]. This quantitative model of meniscal repair suggests that healing in the inner zone could be improved under the appropriate intra-articular conditions.
Fig. 2.
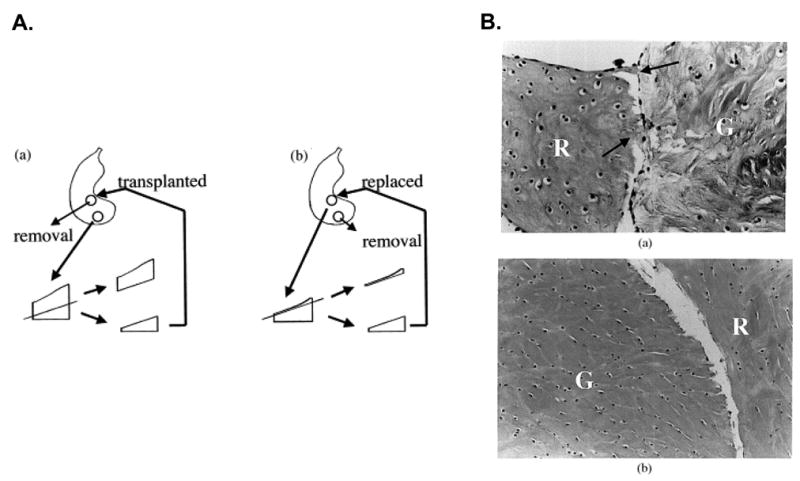
A. Illustration of the Kobayashi meniscal defect model system and histological healing of meniscal defects after 6 weeks in culture. A 1.5 mm diameter full-thickness circular defect was created normal to the inner zone. A. Grafts of the same diameter were obtained from the outer zone in the same meniscus. The femoral side of the graft was cut to match the depth of the defect, and transplanted into the defect in the inner zone. B. Alternatively, a defect of the inner zone was created and after making a thin cut in the femoral side, the punch was replaced into the same defect. A column of tissue was removed from the outer zone to produce the same defect generated in A. C. In explants transplanted from the outer zone to the inner zone (A), fibrous continuity (arrow) was observed between the recipient (R) tissue and the graft (G) (H&E, original magnification ×45). D. In explants from the inner zone that were replaced in the inner zone (B), cells and little extracellular matrices were observed in the interface between the two components and no fibrous continuity was detected (H&E, original magnification×30). Reprinted from [31] with permission from Elsevier.
There are a variety of other zonal specific characteristics that could attribute to the differences in the intrinsic healing potential. For example, the inner zone of the meniscus constitutively expresses higher mRNA levels for nitric oxide synthase 2 (NOS2), an enzyme necessary for the synthesis of the pro-inflammatory mediator nitric oxide (NO) [36]. Additionally, NO production is increased in the central region of the meniscus following partial medial meniscectomy [37]. Cells of the inner zone are also more sensitive to dynamic compression, and exhibit greater increases in the production of the pro-inflammatory mediators prostaglandin E2 (PGE2) and NO than cells of the outer zone in response to loading [38]. These differences may be intrinsic to the cells or may depend on variations in the local biomechanical environment during compressive loading. Modeling predictions also propose that inner and outer zone cells experience different micromechanical forces during joint loading, which could translate into regionally specific changes in gene and protein regulation [39]. Each of these zonal specific characteristics of the meniscus may contribute to the poor healing capacity of the inner zone in vivo.
5. Inflammatory cytokines and meniscal repair
Inflammatory cytokines, such as interleukin-1 (IL-1) and tumor necrosis factor-α (TNF-α), are upregulated in injured and diseased joints. The synovial fluid concentrations in patients undergoing arthroscopy following a meniscal tear are between 25 – 175 pg/mL IL-1α and 10–150 pg/mL TNF-α [40]. Many degradative and pro-inflammatory pathways are activated by IL-1 and TNF-α in the joint [41–44]. IL-1 increases the production of NO [45–49] and PGE2 [45, 48, 50], upregulates matrix metalloproteinases (MMPs) [47, 48, 51], increases proteoglycan release [46, 48], suppresses collagen synthesis [48], and upregulates TNF-α expression [47] in meniscal cells and explants. Similarly, TNF-α upregulates the production of NO [48] and inhibits proteoglycan synthesis in meniscal cells and explants [45, 52].
The catabolic and anti-anabolic effects of IL-1 and TNF-α have been shown to inhibit the repair of meniscal lesions in vitro [32, 34]. At high concentrations (10 ng/mL), IL-1 or TNF-α completely inhibit the intrinsic repair process, as measured by repair strength, histology, and cell accumulation [32]. Furthermore, these suppressive effects are dose-dependent over a wide range of cytokine concentrations, and even low, physiologically relevant concentrations (10 – 1000 pg/mL) of IL-1 or TNF-α significantly decrease the shear strength of repair, cell accumulation, and tissue formation in the interface over 14, 28, or 42 days of culture [34]. Furthermore, acute exposure to IL-1 (1 or 3 days at 100 pg/mL) is sufficient to suppress meniscal repair at 14 and 28 days, and this suppression of repair correlates with an upregulation of MMP activity in the culture media [35].
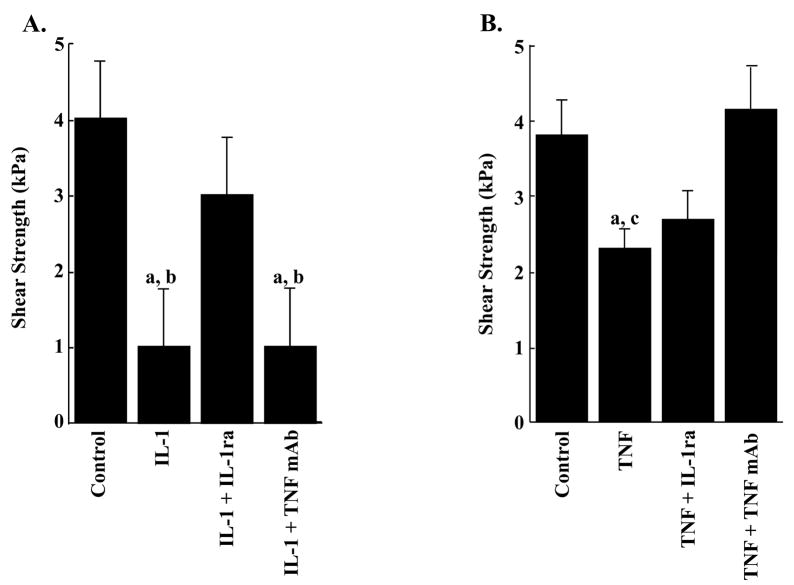
The potent inhibitory effects of chronic IL-1 and TNF-α on integrative meniscal repair can be blocked by the administration of IL-1 receptor antagonist (IL-1ra, Figure 3A) or anti-TNF monoclonal antibody (TNF mAb, Figure 3B), respectively [34]. These findings suggest that meniscus repair in vivo could also be inhibited by joint inflammation following injury or due to arthritis, and thus therapeutic interventions, such as IL-1ra and/or TNF mAb, targeted at improving the early stages of repair may have significant benefit on clinical outcomes of meniscal injury.
Fig. 3.
IL-1ra and TNF mAb prevent the IL-1 and TNF-α mediated decrease in the interfacial shear strength of repair. A. The interfacial shear strength of repair was measured after treatment of meniscal repair model explants with the following: no treatment (control); 100 pg/mL IL-1; 100 pg/mL IL-1 and 150 ng/mL IL-1ra; and 100 pg/mL IL-1 and 2.4 μg/mL TNF mAb for 14 days (n = 8 – 14 explants per treatment group). a: p < 0.05 as compared to control. b: p < 0.05 as compared to the IL-1 + IL-1ra treated samples. B. The interfacial shear strength of repair was measured after treatment of meniscal repair model explants with the following: no treatment (control); 1000 pg/mL TNF-α; 1000 pg/mL TNF-α and 150 ng/mL IL-1ra; and 1000 pg/mL TNF-α and 2.4 μg/mL TNF mAb for 14 days (n = 8–12 explants per treatment group). c: p < 0.05 as compared to the TNF-α + TNF mAb treated samples. The bars indicate the mean shear strength in kPa + standard error. Reprinted from [34].
6. Growth factors and meniscus repair
Previous studies have shown strong anabolic and mitogenic effects of certain growth factors on meniscal cells. Transforming growth factor-β1 (TGF-β1) increases proteoglycan production and cell division in monolayer cultures [53]. Additionally, transduction of meniscal cells with TGF-β1 also results in increased production of collagens and proteoglycans [54]. On the other hand, PDGF increases cell proliferation in meniscal explants [55], along with proteoglycan synthesis [56, 57] and increased DNA synthesis in monolayer meniscal cells [58]. Other studies have shown that the production of collagen and proteoglycans is increased by TGF-β1 but not PDGF, insulin-like growth factor 1 (IGF1), or basic fibroblast growth factor (bFGF) in meniscal cells [59]. Treatment of meniscal fibrochondrocyte seeded poly-glycolic acid (PGA) scaffolds with TGF-β1 increased collagen and proteoglycan synthesis in a dose-dependent manner [60]. Furthermore, in meniscal explants TGF-β1 was found to be the strongest stimulator of proteoglycan and protein synthesis, while bFGF was the least potent stimulator, with PDGF and IGF demonstrating intermediate stimulation [61]. However, IGF1 increased cell proliferation and extracellular matrix synthesis in isolated meniscal cells, with the greatest response being observed in cells from the inner zone of the meniscus [62]. These studies demonstrate the potential benefits of anabolic factors for promoting the healing of meniscal lesions.
The effects of TGF-β1 on the repair of meniscal lesions in vitro have recently been investigated. Either meniscal cells or mesenchymal stem cells transduced with adenoviral vectors expressing TGF-β1 cDNA and seeded in type I collagen-glycosaminoglycan (GAG) matrices were transplanted into lesions in the avascular zone of bovine menisci and cultured ex vivo for 3 weeks [63]. The constructs containing TGF-β1 cDNA demonstrate increased cellularity, increased proteoglycan and collagen synthesis, and filling of the lesions with repair tissue. However, the biomechanical strength of these constructs was not assessed.
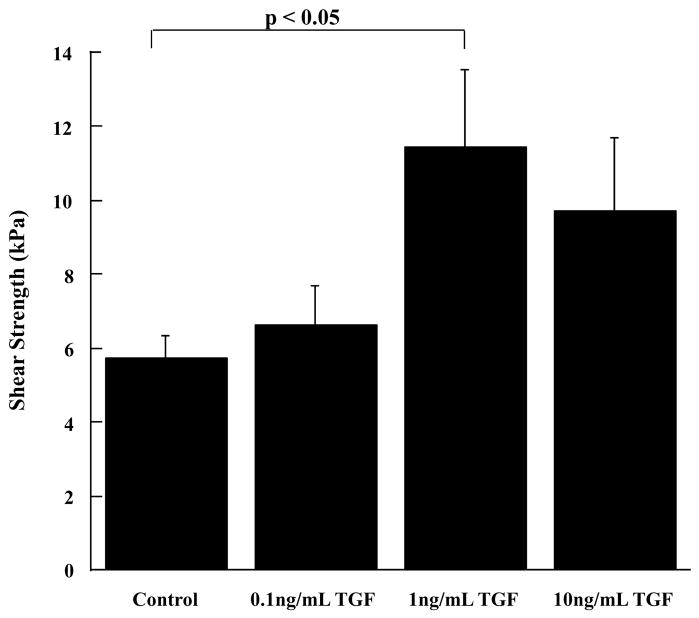
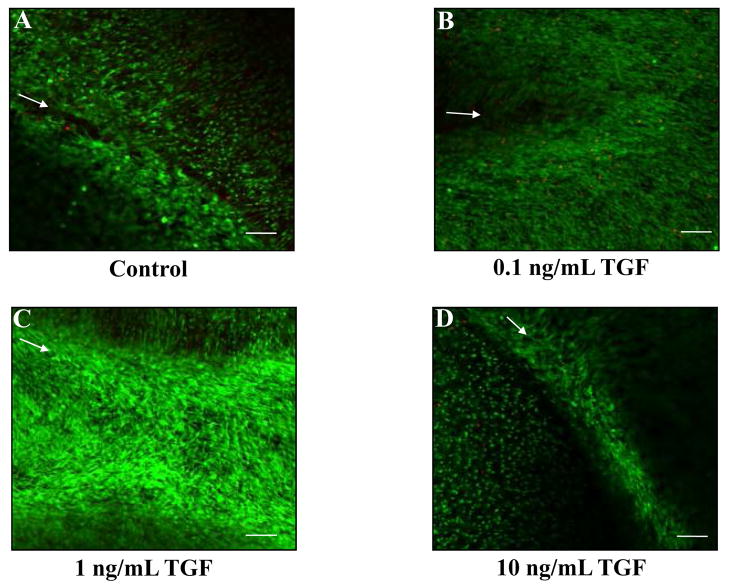
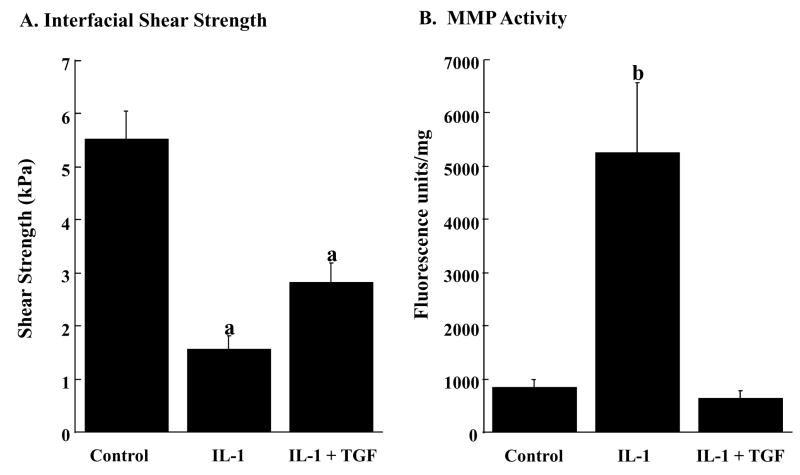
Using our in vitro meniscal repair model explants, we observed that TGF-β1 significantly increased the interfacial shear strength of repair (Figure 4, p < 0.05) and cellular accumulation in the repair interface (Figure 5), as compared to control samples. While all tested concentrations of TGF-β1 increased cell accumulation in the interface (Figure 5), the concentration of 1 ng/mL TGF-β1 demonstrated the most dramatic enhancement in cellular accumulation (Figure 5C), and this observation was consistent with the increase in the shear strength of repair (Figure 4). Based on these results, we assessed the ability of TGF-β1 to overcome the catabolic effects of IL-1 on integrative meniscal repair. In the presence of IL-1, TGF-β1 increased the interfacial shear strength of repair, as compared to IL-1 alone (Figure 6A, p < 0.05). Additionally, TGF-β1 suppressed the IL-1 mediated MMP activity to control levels (Figure 6B). These findings are consistent with previous results that TGF-β blocks MMP-13 and MMP-14 upregulation by IL-1 [64] and suppresses collagenase activity in the presence of TNF-α in cartilage explants [65]. As has been shown in cartilage repair model systems, the strength of repair is positively correlated with collagen deposition [25], as well as collagen crosslinking [23, 26]. Furthermore, new protein synthesis is required for interface integration in cartilage explants [25]. These results from cartilage and meniscus repair models suggest that anabolic factors that enhance the synthesis of extracellular matrix components and decrease the catabolic effects of inflammatory cytokines have potential to promote the healing of meniscal lesions.
Fig. 4.
TGF-β1 increases the interfacial shear strength of repair. The interfacial shear strength of repair was measured after treatment of meniscal repair model explants with 0 ng/mL, 0.1 ng/mL, 1 ng/mL, and 10 ng/mL TGF-β1 for 14 days (n = 9 – 11 explants per treatment group). The bars indicate the mean shear strength in kPa + standard error.
Fig. 5.
TGF-β1 increases cell accumulation at the interface of meniscal repair explants. Fluorescence confocal microscopy was utilized to visualize the cells in the meniscal repair interface. The green signal indicates live cells, while the red signal marks dead cells. Arrows mark the interface of the inner core and outer ring on all images. Representative images for each treatment are shown: control samples (A), 0.1 ng/mL TGF-β1 (B), 1 ng/mL TGF-β1 (C), and 10 ng/mL TGF-β1 (D). Scale bar = 100 μm.
Fig. 6.
TGF-β1 increases the interfacial shear strength of repair in the presence of IL-1 (A) and decreases the MMP activity in the media (B). A. The interfacial shear strength of repair was measured after treatment of meniscal repair model explants with control media, 100 pg/mL IL-1α, or 100 pg/mL IL-1α and 1 ng/mL TGF-β1 for 14 days (n = 13 – 16 explants per treatment group). The bars indicate the mean shear strength in kPa + standard error. a: p < 0.05 compared to all other treatments. B. Total specific MMP activity in the culture media at day 12 was measured using a quenched fluorescent substrate [35] (n = 6 – 8 explants per treatment group). The bars indicate the mean fluorescence units normalized by the wet weight of the meniscal tissue + standard error. b: p < 0.0005 compared to all other treatments.
7. Mechanical loading and repair of the meniscus
In addition to changes in the biochemical environment in the joint following meniscal injury or during arthritis, there are also changes in the biomechanical environment as well. In other cartilaginous tissues, such as articular cartilage, many studies have shown that the mechanical stress in the joint is an important factor that influences and presumably regulates chondrocyte activity [66]. The general consensus of these studies in cartilage is that static compression suppresses matrix biosynthesis, while cyclic or intermittent loading at specific frequencies can stimulate chondrocyte activity, as measured by gene expression and protein synthesis [67–70]. These responses have been reported over a wide range of loading magnitudes and frequencies, and are dependent on the magnitude of stress or strain ([66] for review).
Fewer studies have been performed on the response of meniscal cells to mechanical stress. Dynamic mechanical compression increases proteoglycan and total protein synthesis [46] and NO [71] and PGE2 production by meniscal explants [38]. On the other hand, in the presence of IL-1, NO production is decreased by mechanical compression. Imler et al. [61] show a decrease in biosynthetic activity, as measured by 35S-sulfate and 3H-proline incorporation, in explants of bovine meniscus subjected to static compression. Additionally, static compression of porcine meniscal explants results in an increase in MMP-1 mRNA and simultaneous decrease in type I collagen and decorin mRNA levels, suggesting an imbalance in biosynthesis under static loading [72]. Studies by Agarwal and co-workers have examined the effects of cyclic tensile stretch on isolated meniscal cells, showing that low-magnitude cyclic stretch inhibits the catabolic and pro-inflammatory effects of IL-1 [47, 73, 74], while high magnitudes of cyclic strain (10–15%) do not inhibit the effects of IL-1 on NO or MMP production. Cyclic tension also counteracts IL-1-dependent stimulation of NOS2, RANK, and RANKL in meniscal cells, potentially via inhibition of NF-κB [47, 73]. Static compression inhibits integrative cartilage repair [75], but further studies are needed to determine the effects of mechanical compression on the repair of the meniscus under both normal and inflammatory conditions.
8. Cellular effects on repair of the meniscus
Cell proliferation and migration appear to be important components of meniscal repair in vivo, and previous studies have shown complete repopulation of meniscal allografts [76] or a devitalized meniscal plug that was reinserted into the meniscus in an animal model [77]. The factors that influence meniscal cell migration are not well understood. For example, isolated outer zone bovine meniscal cells increase migration with 4 hours exposure to 1000 pg/mL of IL-1 [58]. However, porcine meniscal repair model explants treated with IL-1 show decreased cell accumulation, potentially due to suppression of cell proliferation and/or migration, in the repair interface [32, 34, 35]. On the other hand, anabolic growth factors, such as TGF-β1 [53], PDGF [55], and IGF [62], demonstrate enhanced proliferation of meniscal cells. The treatment of meniscal repair model explants with TGF-β1 also resulted in increased cell accumulation in the repair interface (Figure 5) and a coincident increase in the interfacial shear strength of repair (Figure 6). The 1 ng/mL concentration of TGF-β1 demonstrated the most striking increases in cell accumulation (Figure 5C), consistent with previous observations of meniscal fibrochondrocyte proliferation in PGA scaffolds in response to TGF-β1 [60]. While the effects of several cytokines and growth factors on the migration and proliferation of meniscal cells has been analyzed, thus far the effects of these factors on the migration and proliferation of meniscal cells in the context of the meniscal tissue and the resultant effects on meniscal repair have yet to be analyzed.
9. Challenges and future goals in meniscal repair
Meniscal injuries may occur due to overt trauma or meniscal pathology may reflect changes in knee joint function associated with aging, OA, rheumatoid arthritis, and/or gait disturbances [78, 79]. A healthy meniscus is critical for a normally functioning knee joint, and meniscal pathology frequently leads to overt OA and its associated morbidity. There is a complex interplay among a variety of factors that influence meniscal healing, including zonal characteristics, inflammatory cytokines, growth factors, mechanical loading, and cellular effects. In an effort to enhance the intrinsic meniscal repair mechanisms, future studies need to address these complex interactions in order to increase our understanding of meniscal metabolism and the importance of different factors in meniscal healing. In particular, the degradative effects of the inflammatory cytokines that are upregulated following joint injury must be overcome in order to promote repair. The use of anti-cytokine therapies, such as IL-1ra and TNF mAb, has shown promising results in vitro [34], but still must be evaluated in animal models. TGF-β1 enhances meniscal repair, even in the presence of IL-1, but is not able to completely overcome the IL-1 mediated degradation alone (Figure 6A). Additionally, the effects of IL-1 and/or TGF-β1 on meniscal repair during joint loading have yet to be assessed. Such pharmacologic or physical therapies, identified in the in vitro meniscal repair model system, can be translated to animal models to provide in vivo data on novel therapies that promote meniscal repair.
Acknowledgments
Supported in part by the Arthritis Foundation and NIH grants AR50245, AG15768, and AR48852. We would like to thank J. Brice Weinberg, Alfred Hennerbichler, and Rebecca Wilusz for their contributions to this work.
References
- 1.Markolf KL, Bargar WL, Shoemaker SC, Amstutz HC. The role of joint load in knee stability. J Bone Joint Surg Am. 1981;63:570–585. [PubMed] [Google Scholar]
- 2.Wojtys EM, Chan DB. Meniscus Structure and Function. AAOS Instructional Course Lectures. 2005;54:323–330. [PubMed] [Google Scholar]
- 3.Ahmed AM, Burke DL. In-vitro measurement of static pressure distribution in synovial joints--Part I: Tibial surface of the knee. J Biomech Eng. 1983;105:216–225. doi: 10.1115/1.3138409. [DOI] [PubMed] [Google Scholar]
- 4.Eyre DR, Muir H. The distribution of different molecular species of collagen in fibrous, elastic and hyaline cartilages of the pig. Biochem J. 1975;151:595–602. doi: 10.1042/bj1510595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDevitt CA, Webber RJ. The ultrastructure and biochemistry of meniscal cartilage. Clin Orthop Relat Res. 1990:8–18. [PubMed] [Google Scholar]
- 6.Hellio Le Graverand MP, Ou Y, Schield-Yee T, Barclay L, Hart D, Natsume T, Rattner JB. The cells of the rabbit meniscus: their arrangement, interrelationship, morphological variations and cytoarchitecture. J Anat. 2001;198:525–535. doi: 10.1046/j.1469-7580.2000.19850525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung HS. Distribution of type I, II, III and V in the pepsin solubilized collagens in bovine menisci. Connect Tissue Res. 1987;16:343–356. doi: 10.3109/03008208709005619. [DOI] [PubMed] [Google Scholar]
- 8.Kambic HE, McDevitt CA. Spatial organization of types I and II collagen in the canine meniscus. Journal of Orthopaedic Research. 2005;23:142–149. doi: 10.1016/j.orthres.2004.06.016. [DOI] [PubMed] [Google Scholar]
- 9.Arnoczky SP, Warren RF. Microvasculature of the human meniscus. Am J Sports Med. 1982;10:90–95. doi: 10.1177/036354658201000205. [DOI] [PubMed] [Google Scholar]
- 10.Arnoczky SP, Warren RF. The microvasculature of the meniscus and its response to injury. An experimental study in the dog. Am J Sports Med. 1983;11:131–141. doi: 10.1177/036354658301100305. [DOI] [PubMed] [Google Scholar]
- 11.Wyland DJ, Guilak F, Elliott DM, Setton LA, Vail TP. Chondropathy after meniscal tear or partial meniscectomy in a canine model. J Orthop Res. 2002;20:996–1002. doi: 10.1016/S0736-0266(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 12.Christoforakis J, Pradhan R, Sanchez-Ballester J, Hunt N, Strachan RK. Is there an association between articular cartilage changes and degenerative meniscus tears? Arthroscopy-the Journal of Arthroscopic and Related Surgery. 2005;21:1366–1369. doi: 10.1016/j.arthro.2005.08.031. [DOI] [PubMed] [Google Scholar]
- 13.Roos H, Lauren M, Adalberth T, Roos EM, Jonsson K, Lohmander LS. Knee osteoarthritis after meniscectomy: prevalence of radiographic changes after twenty-one years, compared with matched controls. Arthritis Rheum. 1998;41:687–693. doi: 10.1002/1529-0131(199804)41:4<687::AID-ART16>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 14.Henning CE, Lynch MA, Clark JR. Vascularity for healing of meniscus repairs. Arthroscopy. 1987;3:13–18. doi: 10.1016/s0749-8063(87)80004-x. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Ochi M, Shu N, Uchio Y. Meniscal rasping for repair of meniscal tear in the avascular zone. Arthroscopy. 1999;15:281–286. doi: 10.1016/s0749-8063(99)70035-6. [DOI] [PubMed] [Google Scholar]
- 16.Jitsuiki J, Ochi M, Ikuta Y. Meniscal repair enhanced by an interpositional free synovial autograft: an experimental study in rabbits. Arthroscopy. 1994;10:659–666. doi: 10.1016/s0749-8063(05)80065-9. [DOI] [PubMed] [Google Scholar]
- 17.Ghadially FN, Wedge JH, Lalonde JM. Experimental methods of repairing injured menisci. The Journal of bone and joint surgery. 1986;68:106–110. doi: 10.1302/0301-620X.68B1.3753606. [DOI] [PubMed] [Google Scholar]
- 18.Gershuni DH, Skyhar MJ, Danzig LA, Camp J, Hargens AR, Akeson WH. Experimental models to promote healing of tears in the avascular segment of canine knee menisci. J Bone Joint Surg Am. 1989;71:1363–1370. [PubMed] [Google Scholar]
- 19.Arnoczky SP, Warren RF, Spivak JM. Meniscal repair using an exogenous fibrin clot. An experimental study in dogs. J Bone Joint Surg Am. 1988;70:1209–1217. [PubMed] [Google Scholar]
- 20.Henning CE, Lynch MA, Yearout KM, Vequist SW, Stallbaumer RJ, Decker KA. Arthroscopic meniscal repair using an exogenous fibrin clot. Clin Orthop Relat Res. 1990:64–72. [PubMed] [Google Scholar]
- 21.Hashimoto J, Kurosaka M, Yoshiya S, Hirohata K. Meniscal repair using fibrin sealant and endothelial cell growth factor. An experimental study in dogs. Am J Sports Med. 1992;20:537–541. doi: 10.1177/036354659202000509. [DOI] [PubMed] [Google Scholar]
- 22.Reindel ES, Ayroso AM, Chen AC, Chun DM, Schinagl RM, Sah RL. Integrative Repair of Articular Cartilage In Vitro: Adhesive Strength of the Interface Region. Journal of Orthopaedic Research. 1995;13:751–760. doi: 10.1002/jor.1100130515. [DOI] [PubMed] [Google Scholar]
- 23.Ahsan T, Lottman LM, Harwood F, Amiel D, Sah RL. Integrative Cartilage Repair: Inhibition of B-Aminopropionitrile. Journal of Orthopaedic Research. 1999;17:850–857. doi: 10.1002/jor.1100170610. [DOI] [PubMed] [Google Scholar]
- 24.Ahsan T, Sah RL. Biomechanics of integrative cartilage repair. Osteoarthritis & Cartilage. 1999;7:29–40. doi: 10.1053/joca.1998.0160. [DOI] [PubMed] [Google Scholar]
- 25.DiMicco MA, Sah RL. Integrative cartilage repair: adhesive strength is correlated with collagen deposition. Journal of Orthopaedic Research. 2001;19:1105–1112. doi: 10.1016/S0736-0266(01)00037-7. [DOI] [PubMed] [Google Scholar]
- 26.DiMicco MA, Waters SN, Akeson WH, Sah RL. Integrative articular cartilage repair: dependence on developmental stage and collagen metabolism. Osteoarthritis & Cartilage. 2002;10:218–225. doi: 10.1053/joca.2001.0502. [DOI] [PubMed] [Google Scholar]
- 27.Ahsan T, Sah RL. Fracture Mechanics Characterization of Integrative Cartilage Repair using the T-Peel Test. Transactions of the Orthopaedic Research Society. 1996;21:537. [Google Scholar]
- 28.Webber RJ, York JL, Vanderschilden JL, Hough AJ., Jr An organ culture model for assaying wound repair of the fibrocartilaginous knee joint meniscus. Am J Sports Med. 1989;17:393–400. doi: 10.1177/036354658901700314. [DOI] [PubMed] [Google Scholar]
- 29.Ochi M, Mochizuki Y, Deie M, Ikuta Y. Augmented meniscal healing with free synovial autografts: an organ culture model. Archives of orthopaedic and trauma surgery. 1996;115:123–126. doi: 10.1007/BF00434537. [DOI] [PubMed] [Google Scholar]
- 30.Bhargava MM, Hidaka C, Hannafin JA, Doty S, Warren RF. Effects of hepatocyte growth factor and platelet-derived growth factor on the repair of meniscal defects in vitro. In vitro cellular & developmental biology. 2005;41:305–310. doi: 10.1290/0503018.1. [DOI] [PubMed] [Google Scholar]
- 31.Kobayashi K, Fujimoto E, Deie M, Sumen Y, Ikuta Y, Ochi M. Regional differences in the healing potential of the meniscus-an organ culture model to eliminate the influence of microvasculature and the synovium. Knee. 2004;11:271–278. doi: 10.1016/j.knee.2002.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Interleukin-1 and tumor necrosis factor alpha inhibit repair of the porcine meniscus in vitro. Osteoarthritis & Cartilage. 2007;15:1053–1060. doi: 10.1016/j.joca.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hennerbichler A, Moutos FT, Hennerbichler D, Weinberg JB, Guilak F. Repair response of the inner and outer regions of the porcine meniscus in vitro. American Journal of Sports Medicine. 2007;35:754–762. doi: 10.1177/0363546506296416. [DOI] [PubMed] [Google Scholar]
- 34.McNulty AL, Moutos FT, Weinberg JB, Guilak F. Enhanced integrative repair of the porcine meniscus in vitro by inhibition of interleukin-1 or tumor necrosis factor-alpha. Arthritis Rheum. 2007;56:3033–3043. doi: 10.1002/art.22839. [DOI] [PubMed] [Google Scholar]
- 35.Wilusz RE, Weinberg JB, Guilak F, McNulty AL. Inhibition of Integrative Repair of the Meniscus Following Acute Exposure to Interleukin-1 in vitro. Journal of Orthopaedic Research. doi: 10.1002/jor.20538. (in review) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Upton ML, Chen J, Setton LA. Region-specific constitutive gene expression in the adult porcine meniscus. J Orthop Res. 2006;24:1562–1570. doi: 10.1002/jor.20146. [DOI] [PubMed] [Google Scholar]
- 37.Kobayashi K, Mishima H, Hashimoto S, Goomer RS, Harwood FL, Lotz M, Moriya H, Amiel D. Chondrocyte apoptosis and regional differential expression of nitric oxide in the medial meniscus following partial meniscectomy. J Orthop Res. 2001;19:802–808. doi: 10.1016/S0736-0266(01)00023-7. [DOI] [PubMed] [Google Scholar]
- 38.Hennerbichler A, Fermor B, Hennerbichler D, Weinberg JB, Guilak F. Regional differences in prostaglandin E2 and nitric oxide production in the knee joint meniscus in response to dynamic mechanical compression. Biochemical and Biophysical Research Communications. 2007;358:1047–1053. doi: 10.1016/j.bbrc.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Upton ML, Guilak F, Laursen TA, Setton LA. Finite element modeling predictions of region-specific cell-matrix mechanics in the meniscus. Biomechanics and modeling in mechanobiology. 2006;5:140–149. doi: 10.1007/s10237-006-0031-4. [DOI] [PubMed] [Google Scholar]
- 40.Vangsness CT, Jr, Burke WS, MacPhee RD, Narvy S. Human Knee Synovial Fluid Analysis. International Cartilage Repair Society; San Diego, CA. 2006. p. 87. [Google Scholar]
- 41.Lotz M. Cytokines in cartilage injury and repair. Clinical Orthopaedics & Related Research. 2001:S108–115. doi: 10.1097/00003086-200110001-00011. [DOI] [PubMed] [Google Scholar]
- 42.Fernandes JC, Martel-Pelletier J, Pelletier JP. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–246. [PubMed] [Google Scholar]
- 43.van den Berg WB, Joosten LA, van de Loo FA. TNF alpha and IL-1 beta are separate targets in chronic arthritis. Clin Exp Rheumatol. 1999;17:S105–114. [PubMed] [Google Scholar]
- 44.Lotz M, Blanco FJ, von Kempis J, Dudler J, Maier R, Villiger PM, Geng Y. Cytokine regulation of chondrocyte functions. J Rheumatol Suppl. 1995;43:104–108. [PubMed] [Google Scholar]
- 45.LeGrand A, Fermor B, Fink C, Pisetsky DS, Weinberg JB, Vail TP, Guilak F. Interleukin-1, tumor necrosis factor alpha, and interleukin-17 synergistically up-regulate nitric oxide and prostaglandin E2 production in explants of human osteoarthritic knee menisci. Arthritis Rheum. 2001;44:2078–2083. doi: 10.1002/1529-0131(200109)44:9<2078::AID-ART358>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 46.Shin SJ, Fermor B, Weinberg JB, Pisetsky DS, Guilak F. Regulation of matrix turnover in meniscal explants: role of mechanical stress, interleukin-1, and nitric oxide. Journal of Applied Physiology. 2003;95:308–313. doi: 10.1152/japplphysiol.00131.2003. [DOI] [PubMed] [Google Scholar]
- 47.Ferretti M, Madhavan S, Deschner J, Rath-Deschner B, Wypasek E, Agarwal S. Dynamic biophysical strain modulates proinflammatory gene induction in meniscal fibrochondrocytes. Am J Physiol Cell Physiol. 2006;290:C1610–1615. doi: 10.1152/ajpcell.00529.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cao M, Stefanovic-Racic M, Georgescu HI, Miller LA, Evans CH. Generation of nitric oxide by lapine meniscal cells and its effect on matrix metabolism: stimulation of collagen production by arginine. J Orthop Res. 1998;16:104–111. doi: 10.1002/jor.1100160118. [DOI] [PubMed] [Google Scholar]
- 49.Hashimoto S, Takahashi K, Ochs RL, Coutts RD, Amiel D, Lotz M. Nitric oxide production and apoptosis in cells of the meniscus during experimental osteoarthritis. Arthritis Rheum. 1999;42:2123–2131. doi: 10.1002/1529-0131(199910)42:10<2123::AID-ANR12>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 50.Hardy MM, Seibert K, Manning PT, Currie MG, Woerner BM, Edwards D, Koki A, Tripp CS. Cyclooxygenase 2-dependent prostaglandin E2 modulates cartilage proteoglycan degradation in human osteoarthritis explants. Arthritis & Rheumatism. 2002;46:1789–1803. doi: 10.1002/art.10356. [DOI] [PubMed] [Google Scholar]
- 51.Lemke AK, Lee J, Sandy J, Grodzinsky A, Mentlein R, Fay J, Schunke M, Kurz B. IL-1 Induced Matrix Degradation and Gene Expression in the Meniscus Depends on the Anatomical Location. 52nd Annual Orthopaedic Research Society Meeting; Chicago, IL: . 2006. p. 1043. [Google Scholar]
- 52.Fermor B, Jeffcoat D, Hennerbichler A, Pisetsky DS, Weinberg JB, Guilak F. The effects of cyclic mechanical strain and tumor necrosis factor alpha on the response of cells of the meniscus. Osteoarthritis Cartilage. 2004;12:956–962. doi: 10.1016/j.joca.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 53.Collier S, Ghosh P. Effects of transforming growth factor beta on proteoglycan synthesis by cell and explant cultures derived from the knee joint meniscus. Osteoarthritis Cartilage. 1995;3:127–138. doi: 10.1016/s1063-4584(05)80045-7. [DOI] [PubMed] [Google Scholar]
- 54.Goto H, Shuler FD, Niyibizi C, Fu FH, Robbins PD, Evans CH. Gene therapy for meniscal injury: enhanced synthesis of proteoglycan and collagen by meniscal cells transduced with a TGFbeta(1)gene. Osteoarthritis Cartilage. 2000;8:266–271. doi: 10.1053/joca.1999.0300. [DOI] [PubMed] [Google Scholar]
- 55.Spindler KP, Mayes CE, Miller RR, Imro AK, Davidson JM. Regional mitogenic response of the meniscus to platelet-derived growth factor (PDGF-AB) J Orthop Res. 1995;13:201–207. doi: 10.1002/jor.1100130208. [DOI] [PubMed] [Google Scholar]
- 56.Lietman SA, Hobbs W, Inoue N, Reddi AH. Effects of selected growth factors on porcine meniscus in chemically defined medium. Orthopedics. 2003;26:799–803. doi: 10.3928/0147-7447-20030801-19. [DOI] [PubMed] [Google Scholar]
- 57.Kasemkijwattana C, Menetrey J, Goto H, Niyibizi C, Fu FH, Huard J. The use of growth factors, gene therapy and tissue engineering to improve meniscal healing. Materials Science & Engineering C-Biomimetic and Supramolecular Systems. 2000;13:19–28. [Google Scholar]
- 58.Bhargava MM, Attia ET, Murrell GA, Dolan MM, Warren RF, Hannafin JA. The effect of cytokines on the proliferation and migration of bovine meniscal cells. Am J Sports Med. 1999;27:636–643. doi: 10.1177/03635465990270051601. [DOI] [PubMed] [Google Scholar]
- 59.Pangborn CA, Athanasiou KA. Effects of growth factors on meniscal fibrochondrocytes. Tissue Eng. 2005;11:1141–1148. doi: 10.1089/ten.2005.11.1141. [DOI] [PubMed] [Google Scholar]
- 60.Pangborn CA, Athanasiou KA. Growth factors and fibrochondrocytes in scaffolds. J Orthop Res. 2005;23:1184–1190. doi: 10.1016/j.orthres.2005.01.019. [DOI] [PubMed] [Google Scholar]
- 61.Imler SM, Doshi AN, Levenston ME. Combined effects of growth factors and static mechanical compression on meniscus explant biosynthesis. Osteoarthritis Cartilage. 2004;12:736–744. doi: 10.1016/j.joca.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 62.Tumia NS, Johnstone AJ. Regional regenerative potential of meniscal cartilage exposed to recombinant insulin-like growth factor-I in vitro. The Journal of bone and joint surgery. 2004;86:1077–1081. doi: 10.1302/0301-620x.86b7.13747. [DOI] [PubMed] [Google Scholar]
- 63.Steinert AF, Palmer GD, Capito R, Hofstaetter JG, Pilapil C, Ghivizzani SC, Spector M, Evans CH. Genetically Enhanced Engineering of Meniscus Tissue Using Ex Vivo Delivery of Transforming Growth Factor-beta1 Complementary Deoxyribonucleic Acid. Tissue Eng. 2007 doi: 10.1089/ten.2006.0270. [DOI] [PubMed] [Google Scholar]
- 64.Blaney Davidson EN, van der Kraan PM, van den Berg WB. TGF-beta and osteoarthritis. Osteoarthritis Cartilage. 2007;15:597–604. doi: 10.1016/j.joca.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Hui W, Rowan AD, Cawston T. Modulation of the expression of matrix metalloproteinase and tissue inhibitors of metalloproteinases by TGF-beta1 and IGF-1 in primary human articular and bovine nasal chondrocytes stimulated with TNF-alpha. Cytokine. 2001;16:31–35. doi: 10.1006/cyto.2001.0950. [DOI] [PubMed] [Google Scholar]
- 66.Guilak F, Sah RL, Setton LA. Physical regulation of cartilage metabolism. In: Mow VC, Hayes WC, editors. Basic Orthopaedic Biomechanics. Lippincott-Raven; Philadelphia: 1997. pp. 179–207. [Google Scholar]
- 67.Burton-Wurster N, Vernier-Singer M, Farquhar T, Lust G. Effect of compressive loading and unloading on the synthesis of total protein, proteoglycan, and fibronectin by canine cartilage explants. J Orthop Res. 1993;11:717–729. doi: 10.1002/jor.1100110514. [DOI] [PubMed] [Google Scholar]
- 68.Guilak F, Meyer BC, Ratcliffe A, Mow VC. The effects of matrix compression on proteoglycan metabolism in articular cartilage explants. Osteoarthritis Cartilage. 1994;2:91–101. doi: 10.1016/s1063-4584(05)80059-7. [DOI] [PubMed] [Google Scholar]
- 69.Gray ML, Pizzanelli AM, Grodzinsky AJ, Lee RC. Mechanical and physiochemical determinants of the chondrocyte biosynthetic response. J Orthop Res. 1988;6:777–792. doi: 10.1002/jor.1100060602. [DOI] [PubMed] [Google Scholar]
- 70.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7:619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 71.Fink C, Fermor B, Weinberg JB, Pisetsky DS, Misukonis MA, Guilak F. The effect of dynamic mechanical compression on nitric oxide production in the meniscus. Osteoarthritis Cartilage. 2001;9:481–487. doi: 10.1053/joca.2001.0415. [DOI] [PubMed] [Google Scholar]
- 72.Upton ML, Chen J, Guilak F, Setton LA. Differential effects of static and dynamic compression on meniscal cell gene expression. J Orthop Res. 2003;21:963–969. doi: 10.1016/S0736-0266(03)00063-9. [DOI] [PubMed] [Google Scholar]
- 73.Deschner J, Wypasek E, Ferretti M, Rath B, Anghelina M, Agarwal S. Regulation of RANKL by biomechanical loading in fibrochondrocytes of meniscus. J Biomech. 2006;39:1796–1803. doi: 10.1016/j.jbiomech.2005.05.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Agarwal S, Long P, Gassner R, Piesco NP, Buckley MJ. Cyclic tensile strain suppresses catabolic effects of interleukin-1beta in fibrochondrocytes from the temporomandibular joint. Arthritis Rheum. 2001;44:608–617. doi: 10.1002/1529-0131(200103)44:3<608::AID-ANR109>3.0.CO;2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen AC, Sah RL. Effect of static compression on proteoglycan biosynthesis by chondrocytes transplanted to articular cartilage in vitro. J Orthop Res. 1998;16:542–550. doi: 10.1002/jor.1100160504. [DOI] [PubMed] [Google Scholar]
- 76.Jackson DW, Whelan J, Simon TM. Cell survival after transplantation of fresh meniscal allografts. DNA probe analysis in a goat model. Am J Sports Med. 1993;21:540–550. doi: 10.1177/036354659302100411. [DOI] [PubMed] [Google Scholar]
- 77.Kambic HE, Futani H, McDevitt CA. Cell, matrix changes and alpha-smooth muscle actin expression in repair of the canine meniscus. Wound Repair Regen. 2000;8:554–561. doi: 10.1046/j.1524-475x.2000.00554.x. [DOI] [PubMed] [Google Scholar]
- 78.Andriacchi TP, Alexander EJ. Studies of human locomotion: past, present and future. J Biomech. 2000;33:1217–1224. doi: 10.1016/s0021-9290(00)00061-0. [DOI] [PubMed] [Google Scholar]
- 79.Hurwitz DE, Sharma L, Andriacchi TP. Effect of knee pain on joint loading in patients with osteoarthritis. Curr Opin Rheumatol. 1999;11:422–426. doi: 10.1097/00002281-199909000-00017. [DOI] [PubMed] [Google Scholar]