Abstract
Central venous catheter (CVC) use at hemodialysis (HD) initiation remains high, despite reports of CVC-associated morbidity and mortality, and efforts at early arteriovenous fistula placement. In order to determine predictors of CVC use at the start of HD, data from the end-stage renal disease (ESRD) Clinical Performance Measures (CPM) Project was linked to the Centers for Medicare & Medicaid Services Medical Evidence (2728) Form. Of the 4071 incident hemodialysis patients in study years 1999–2003, 71.6% used a CVC at dialysis initiation. After controlling for demographic and co-morbid variables, patients with a CVC were 24% more likely to be female (p = 0.006), and 38% more likely to have ischemic heart disease (p = 0.002), while those with obesity (BMI ≥30) were 24% less likely to start dialysis with a CVC (p = 0.006). Pre-ESRD hypoalbuminemia (< 3.5 g/dl) was associated with a twofold higher risk of CVC use (p = <0.001), while patients with pre-ESRD anemia (hgb < 11 g/dl) were 29% more likely to use a CVC at dialysis initiation (p = 0.006) compared to those with hemoglobin ≥11 g/dl. Patients receiving predialysis erythropoietin had a 41% lower odds of CVC use at dialysis initiation (p = <0.001). Finally, dialysis year was predictive of CVC use; in 2002, 76% of patients initiated dialysis with a CVC compared with 66% in 1998 (p < 0.001). Overall, female gender, ischemic heart disease, lack of obesity, factors suggesting poor pre-ESRD care, and successive year of dialysis initiation were predictive of CVC use at hemodialysis initiation.
The type of hemodialysis access in place at the initiation of hemodialysis among end-stage renal disease (ESRD) patients carries variable risk of infection and mortality. Studies have demonstrated that a central venous catheter (CVC) is associated with increased risk of infection-related and all-cause mortality compared to either the arteriovenous fistula (AVF) or prosthetic graft (1–4). In addition, compared with patients who receive an AVF, patients with a CVC experience poorer clearance of blood toxins secondary to unreliable blood flow, higher rates of bloodstream infections, anemia, central vein scarring with subsequent vein occlusion, and antibiotic resistance (5–10).
Despite these disadvantages, the majority of ESRD patients in the United States initiate dialysis with a CVC. CVC use at dialysis initiation among incident hemodialysis patients ranges from 56.8% to 66% in the United States (2,11). It is unclear why high rates of CVC use persist among hemodialysis patients in the United States in view of these clear disadvantages and evidence-based practice guidelines to the contrary, and few studies have reported on predictors of CVC use at dialysis initiation since the advent of these guidelines. The high use of CVCs may occur for several reasons, including limited access to medical care for many patients in need of chronic hemodialysis, delayed referral to a nephrologist, limited patient education regarding optimal access type, and failure of an AVF to mature. In an effort to curtail CVC use and increase AVF use, the Kidney Foundation’s Kidney Disease Outcomes Quality Initiative (NKF’s K/DOQI) Vascular Access Guidelines recommend the AVF as the vascular access of choice, and have set the goal of AVF use among 50% of incident and 40% of prevalent hemodialysis patients (12). In order to better understand potential causes for the high CVC use, the current study describes factors associated with CVC use at dialysis initiation among a contemporary, nationally representative cohort of incident adult (aged ≤18 years) hemodialysis patients.
Methods
Data for this study were obtained from the Centers for Medicare & Medicaid Services’ (CMS) ESRD Clinical Performance Measures (CPM) Project (known prior to 1999 as the ESRD Core Indicators Project) and from selected U.S. Renal Data System (USRDS) Standard Analytical Files for the years 1999–2003 (data collected during the years 1998–2002). Since 1994, the ESRD CPM Project has annually collected data on a nationally representative random sample of prevalent adult ESRD patients on hemodialysis in the United States. Information on vascular access at hemodialysis initiation has been collected since the study year 1999. Patients are eligible for inclusion in the ESRD CPM Project sample if they are 18 years of age or older on October 1 and alive on December 31 prior to the study year. Data are collected retrospectively for the time period October–December of the year prior to the study year (i.e., October–December 2002 for Study Year 2003).
Patients were defined as incident for this study if the onset of ESRD was from January 1 to August 31 of the year prior to the Study Year (1998–2002). A priori exclusions included patients with a port or “other” as vascular access at initiation, and race other than black or white. Of the 6692 patients eligible for inclusion in the study, 972 were missing information on vascular access at initiation of dialysis, 31 with a “port” or “other” access type were excluded, leaving 5689 patients. Of these, 2132 were missing data on one or more covariates, leaving 3557 patients who were included in the logistic regression analysis. Multivariate models with “missing” categories for body mass index (BMI) class, serum albumin, serum hemoglobin, and pre-ESRD erythropoietin (EPO) use were considered, and odds ratio (OR) estimates were similar to the models presented.
Demographic and co-morbid characteristics used in our analysis included race (black, white), gender, tobacco use (yes, no), age (< 53.5, 53.5 to < 65.6, 65.6 to < 74.6, ≥74.6), BMI (kg/m2) was classified according to the World Health Organization classification system as underweight (BMI < 18.5) normal (BMI 18.5 to < 25), overweight (BMI 25 to < 30), and obese (BMI ≥30), diabetes (yes, no), hypertension (yes, no), ischemic heart disease (yes, no), myocardial infarction (yes, no), peripheral vascular disease (PVD; yes, no), inability to ambulate (yes, no), pre-ESRD EPO (yes, no) pre-ESRD serum albumin (< 3.5 mg/dl, ≥3.5 mg/dl), pre-ESRD hemoglobin (< 11 g/dl, ≥11 g/dl), pre-ESRD medical coverage (yes, no), and year of incidence (1998–2002).
Chi-squared tests and Mantel–Haenszel ORs were used to examine crude associations between patient characteristics and vascular access at initiation of hemodialysis. A p-value < 0.05 was considered to be statistically significant. Adjusted analyses were performed using logistic regression to compare CVC to permanent access methods and using polytomous logistic regression to compare CVC and AVG with AVF as the reference group. Exploratory interaction assessment was performed with cross-product terms in multivariable models.
Results
At initiation of hemodialysis, 4071 (71.6%) of the 5689 patients were using a CVC, while the remainder (28.4%) were using an AVF or AVG. Overall, 694 (12.2%) patients were using an AVF, while 924 (16.2%) used an AVG. One-third of patients were black, and nearly one half were female. 48.8% had diabetes, 77.4% had hypertension, and 13.7% had PVD. Of the CVC subgroup, 66.9% were white, 51.6% male, and the mean age was 62.8 years (SD 15.7).
Women were 20% more likely than men to use a CVC at dialysis initiation (OR = 1.20; 95% CI 1.07–1.34; p = 0.002) (Table 1). Overweight and obese patients were 15% (OR = 0.85; 95% CI 0.73–0.99; p = 0.010) and 20% less likely (OR = 0.80; 95% CI 0.69–0.93; p = 0.010) to use a CVC, respectively, compared with normal or underweight patients. The presence of hypertension was associated with a 15% lower likelihood of CVC use (OR = 0.85; 95% CI 0.73–0.98; p = 0.029), while patients with ischemic heart disease were 23% more likely to have a CVC (OR = 1.23; 95% CI 1.07–1.41; p = 0.004) than were patients without ischemic heart disease. Patients with an inability to ambulate were 58% more likely to use a CVC (OR = 1.58; 95% CI 1.08–2.32; p = 0.018) than were patients who could ambulate.
TABLE 1.
Unadjusted results: CVC use at dialysis initiation vs. permanent access at dialysis initiation
| CVC use at HD initiation |
||||
|---|---|---|---|---|
| Predicator | Yes (n = 4071) | No (n = 1618) | OR (95% CI) | Chi-square p value |
| Race | ||||
| Black | 1349 (33.1) | 551 (34.1) | 0.96 (0.85–1.08) | 0.508 |
| White | 2722 (66.9) | 1067 (65.9) | Reference | |
| Gender | ||||
| Female | 1971 (48.4) | 711 (44.0) | 1.20 (1.07–1.34) | 0.002 |
| Male | 2099 (51.6) | 906 (56.0) | Reference | |
| Smoke | ||||
| Yes | 221 (5.6) | 89 (5.6) | 0.99 (0.77–1.28) | 0.944 |
| No | 3751 (94.4) | 1497 (94.4) | Reference | |
| Age (years) | ||||
| Q4 (≥74.6) | 1073 (26.4) | 399 (24.7) | 0.99 (0.84–1.16) | |
| Q3 (65.6 to <74.6) | 941 (23.1) | 413 (25.5) | 0.84 (0.71–0.99) | |
| Q2 (53.5 to <65.6) | 959 (23.6) | 402 (24.9) | 0.88 (0.75–1.03) | |
| Q1 (<53.5) | 1098 (27.0) | 404 (25.0) | Reference | 0.083 |
| BMI class (kg/m2) | ||||
| Underweight (<18.5) | 228 (6.0) | 74 (4.9) | 1.09 (0.82–1.44) | |
| Normal (18.5 to <25) | 1455 (38.1) | 513 (34.2) | Reference | |
| Overweight (25 to <30) | 1087 (28.4) | 450 (30.0) | 0.85 (0.73–0.99) | |
| Obese (≥30) | 1053 (27.5) | 463 (30.9) | 0.80 (0.69–0.93) | 0.010 |
| Diabetes | ||||
| Yes | 1951 (49.1) | 827 (52.1) | 0.89 (0.79–1.00) | |
| No | 2021 (50.9) | 759 (47.9) | Reference | 0.042 |
| Hypertension | ||||
| Yes | 3119 (78.5) | 1287 (81.1) | 0.85 (0.73–0.98) | 0.029 |
| No | 853 (21.5) | 299 (18.9) | ||
| Ischemic heart disease | ||||
| Yes | 1012 (25.5) | 346 (21.8) | 1.23 (1.07–1.41) | 0.004 |
| No | 2960 (74.5) | 1240 (78.2) | ||
| Myocardial infarction | ||||
| Yes | 372 (9.4) | 124 (7.8) | 1.22 (0.99–1.51) | 0.068 |
| No | 3600 (90.6) | 1462 (92.2) | ||
| Peripheral vascular disease | ||||
| Yes | 559 (14.1) | 225 (14.2) | 0.99 (0.84–1.17) | 0.913 |
| No | 3413 (85.9) | 1361 (85.8) | ||
| Inability to ambulate | ||||
| Yes | 133 (3.4) | 34 (2.1) | 1.58 (1.08–2.32) | 0.018 |
| No | 3839 (96.7) | 1552 (97.9) | ||
| Pre-ESRD EPO use | ||||
| Yes | 1064 (26.7) | 623 (39.1) | 0.57 (0.50–0.64) | <0.001 |
| No | 2924 (73.3) | 969 (60.9) | ||
| Pre-ESRD albumin (g/dl) | ||||
| Low (< 3.5 g/dl) | 2102 (72.1) | 631 (53.3) | 2.26 (1.97–2.60) | <0.001 |
| Normal (≥3.5 g/dl) | 812 (27.9) | 552 (46.7) | Reference | |
| Pre-ESRD hemoglobin (g/dl) | ||||
| Low (< 11 g/dl) | 2802 (79.8) | 1032 (74.7) | 1.33 (1.15–1.54) | <0.001 |
| Normal (≥11 g/dl) | 711 (20.2) | 349 (25.3) | Reference | |
| Pre-ESRD medical coverage | ||||
| Yes | 3632 (91.0) | 1499 (94.1) | Reference | <0.001 |
| No | 360 (9.0) | 94 (5.9) | 1.58 (1.25–2.00) | |
| Year of incidence | ||||
| 1998 | 820 (20.1) | 426 (26.3) | Reference | <0.001 |
| 1999 | 581 (14.3) | 299 (18.5) | 1.01 (0.84–1.21) | |
| 2000 | 841 (20.7) | 303 (18.7) | 1.44 (1.21–1.72) | |
| 2001 | 919 (22.6) | 301 (18.6) | 1.59 (1.33–1.89) | |
| 2002 | 910 (22.4) | 289 (17.9) | 1.64 (1.37–1.95) | |
Values expressed as mean and number (%). To convert albumin and hemoglobin in g/dl to g/l, multiply by 10. CVC, central venous catheter; HD, hemodialysis; OR, odds ratio; CI, confidence interval; BMI, body mass index; ESRD, end-stage renal disease.
Patients receiving pre-ESRD EPO were 43% less likely to use a CVC than those who did not receive pre-ESRD EPO (OR = 0.57; 95% CI 0.50–0.64; p < 0.001) and patients with pre-ESRD diabetes were less likely to use a CVC than those without pre-ESRD diabetes (OR = 0.89; 95% CI 0.79–1.00; p = 0.042). In contrast, patients with pre-ESRD hypoalbuminemia (OR = 2.26; 95% CI 1.97–2.60; p < 0.001), pre-ESRD anemia (OR = 1.33; 95% CI 1.15–1.54; p < 0.001), and lack of medical coverage prior to ESRD (OR = 1.58; 95% CI 1.25–2.0; p = 0.001) were significantly more likely to use a CVC at dialysis initiation compared with patients with normalized serum albumin, hemoglobin, and medical coverage, respectively.
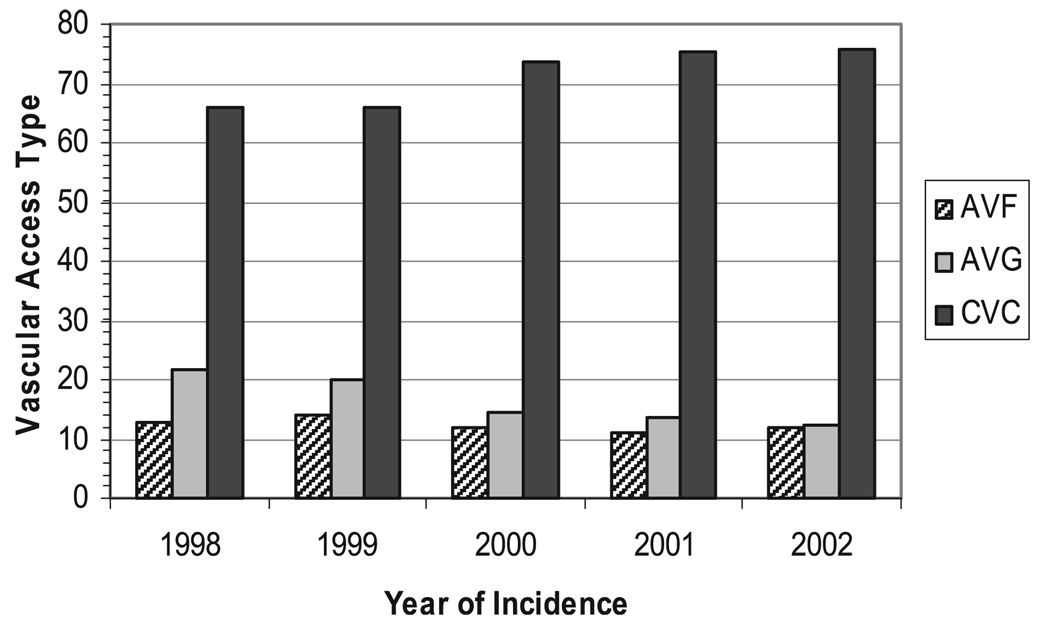
With each consecutive year, beginning in 1998, the odds of CVC use increased, and by 2002, a patient was 64% more likely to initiate dialysis using a catheter compared to a patient in 1998 (OR = 1.64; 95% CI 1.37–1.95; p < 0.001) (Fig. 1). Race, smoking, age, history of myocardial infarction, and the presence of PVD were not associated with CVC use. After adjusting for demographic and co-morbid conditions, only female gender, lack of obesity, the presence of ischemic heart disease, indicators of pre-ESRD care (lack of pre-ESRD EPO, pre-ESRD hypoalbuminemia, and pre-ESRD anemia), and successive year of dialysis initiation remained predictive of CVC use (Table 2).
Fig. 1.
Vascular access type at initiation of hemodialysis, by year of incidence.
TABLE 2.
Adjusted results: CVC use at dialysis initiation vs. permanent access at dialysis initiation
| Predictor | CVC vs. permanent access OR (95% CI) |
Wald chi-square p value |
|---|---|---|
| Race | ||
| Black | 0.94 (0.80–1.12) | 0.506 |
| White | – | |
| Gender | ||
| Female | 1.24 (1.06–1.46) | 0.006 |
| Male | – | |
| Smoke | ||
| Yes | 0.97 (0.70–1.34) | 0.835 |
| No | – | |
| Age (years) | ||
| Q4 (≥74.5) | 0.92 (0.73–1.16) | 0.487 |
| Q3 (65.8 to < 74.5) | 0.87 (0.69–1.09) | 0.231 |
| Q2 (54.6 to < 65.8) | 0.84 (0.67–1.04) | 0.111 |
| Q1 (< 54.6) | – | |
| BMI class (kg/m2) | ||
| Underweight (<18.5) | 1.27 (0.88–1.84) | 0.196 |
| Normal (18.5 to <25) | – | |
| Overweight (25 to <30) | 0.90 (0.75–1.09) | 0.290 |
| Obese (≥30) | 0.76 (0.62–0.92) | 0.006 |
| Diabetes | ||
| Yes | 0.86 (0.74–1.02) | 0.078 |
| No | – | |
| Hypertension | ||
| Yes | 0.87 (0.72–1.06) | 0.175 |
| No | – | |
| Ischemic heart disease | ||
| Yes | 1.38 (1.12–1.69) | 0.002 |
| No | – | |
| Myocardial infarction | ||
| Yes | 1.03 (0.77–1.38) | 0.824 |
| No | – | |
| Peripheral vascular disease | ||
| Yes | 1.01 (0.80–1.27) | 0.945 |
| No | – | |
| Inability to ambulate | ||
| Yes | 1.00 (0.64–1.55) | 0.990 |
| No | – | |
| Pre-ESRD EPO use | ||
| Yes | 0.59 (0.50–0.70) | <0.001 |
| No | – | |
| Pre-ESRD albumin (g/dl) | ||
| Low (<3.5 g/dl) | 2.13 (1.82–2.50) | <0.001 |
| Normal (≥3.5 g/dl) | – | |
| Pre-ESRD hemoglobin (g/dl) | ||
| low (<11 g/dl) | 1.29 (1.08–1.55) | 0.006 |
| normal (> = 11 g/dl) | – | |
| Pre-ESRD medical coverage | ||
| Yes | 1.27 (0.94–1.72) | 0.119 |
| No | – | |
| Year of incidence | ||
| 1998 | – | |
| 1999 | 1.15 (0.90–1.48) | 0.253 |
| 2000 | 1.65 (1.31–2.10) | <0.001 |
| 2001 | 1.68 (1.36–2.11) | <0.001 |
| 2002 | 1.90 (1.50–2.39) | <0.001 |
To convert albumin and hemoglobin in g/dl to g/l, multiply by 10. CVC, central venous catheter; OR, odds ratio; BMI, body mass index; ESRD, end-stage renal disease; EPO, erythropoietin.
After controlling for other risk factors, women were 24% more likely to use a CVC at the start of dialysis than were men (OR = 1.24; 95% CI 1.06–1.46; p = 0.006), while obese patients were 24% less likely to use a CVC at dialysis initiation (OR = 0.76; 95% CI 0.62–0.92; p = 0.006) compared with nonobese patients. Patients with ischemic heart disease were 38% more likely to use a CVC at dialysis initiation (OR = 1.38; 95% CI 1.12–1.69; p = 0.002) compared to patients without ischemic heart disease. Patients receiving EPO prior to ESRD were 41% less likely to use a CVC (OR = 0.59; 95% CI 0.50–0.70; p < 0.001), while hypoalbuminemia prior to dialysis initiation was associated with a greater than twofold higher risk of CVC use (OR = 2.13, 95% CI 1.82–2.50; p < 0.001) compared to patients with serum albumin ≥3.5 g/dl, and patients with pre-ESRD anemia were 29% more likely to use a CVC (OR = 1.29; 95% CI 1.08–1.55; p = 0.006) compared to patients with pre-ESRD hemoglobin ≥11 g/dl.
The association of dialysis vintage and CVC use grew stronger in the adjusted analysis, and increased with each year of enrollment; 66% of subjects initiated dialysis with a CVC in 1998 compared with 74% in 2000 (OR = 1.65; 95% CI 1.31–2.10; p < 0.001), and 76% in 2002 (OR = 1.90; 95% CI 1.50–2.39; p < 0.001) (Table 2). Race, smoking, age, diabetes, hypertension, myocardial infarction, PVD, inability to ambulate, and pre-ESRD medical coverage were not significantly associated with CVC use at initiation of dialysis in the final adjusted model.
Discussion
We conducted a cross-sectional study evaluating predictors of CVC use at dialysis initiation among incident adult hemodialysis patients. CVC use was highly prevalent and 71.6% of our patient cohort initiated dialysis with a CVC, while 12.2% patients used an AVF, and 16.2% used an AVG. Patients using a CVC at the start of dialysis tended to be female, were less likely to be obese or receive pre-ESRD EPO, and more likely to have ischemic heart disease, hypoalbuminemia, and anemia prior to ESRD than those starting dialysis with a permanent form of vascular access (AVF or AVG). Furthermore, CVC use was strongly associated with the year of dialysis initiation; patients initiating dialysis in more recent years (2000–2002) were significantly more likely to use a CVC than an AVF or AVG at dialysis initiation than were patients in 1998 and1999.
Our data are consistent with prior studies which demonstrate a high rate of CVC use among patients initiating hemodialysis (2,13). Stehman-Breen et al. reported that 66% of patients in the USRDS Wave II cohort used a CVC at dialysis initiation, while Astor et al. found that 68% of patients in their patient cohort initiated dialysis using a CVC (11,14). Findings similar to ours were reported among incident ESRD patients by Xue et al., who found that hypoalbuminemic patients were more likely to initiate dialysis with a CVC rather than an AVF or AVG, and are supported by Stehman-Breen et al., who reported that hypoalbuminemia and lack of pre-ESRD EPO use were associated with a lack of permanent access use at dialysis initiation (2,11). Similarly, Pastan et al. found that female gender and hypoalbuminemia were independently associated with the CVC use among prevalent ESRD patients (1). However, our study utilizes contemporary data reflecting a nationally representative, incident cohort of patients, and incorporates quality of care indicators. Our findings differ from Stehman-Breen et al., who did not find a relationship between gender and CVC use, and from Avorn et al., who found no relationship between gender, BMI, and CVC use (11,15). Both studies had smaller study populations than the current study, but a similar gender distribution.
The reasons for CVC use at dialysis initiation are multifactorial and likely include few nephrology visits prior to dialysis initiation, the presence of an immature AVF, patient refusal of permanent access, and late referral to a nephrologist (15). However, even when referral time is as great as 1 year prior to dialysis initiation, Lee et al. found that a majority of patients (65%) do not start dialysis with a permanent vascular access, largely owing to repeatedly missed access-related appointments, despite adequate provision of patient transportation. Only the presence of diabetes was predictive of permanent access rather than CVC use at dialysis initiation, possibly due to greater nephrology referral by endocrinologists (13). This suggests a role for early predialysis vascular access education among chronic kidney disease patients.
Women were more likely to use a CVC at dialysis initiation. Reasons for this are unclear, but may include a perceived disparity in vein caliber compared to men. Caplin et al. addressed this issue, and found no difference of vein diameter at 17 venous sites between men and women, regardless of age, the presence of diabetes, or race (16).
We found an association between obesity and decreased CVC use at dialysis initiation. Previous reports suggest that obese patients are more likely to use an AVG than a CVC at dialysis initiation. Dhingra et al. reported no association between body weight and CVC use, but reported that patients with an AVG tended to have a higher BMI (3). Xue et al. reported CVC use to be higher among underweight patients, while graft use was greater among obese patients (2). Nonobese patients may be more likely to have poorer overall health than obese patients, and therefore deemed poor candidates for permanent access creation. One hypothesis for greater AVG use among obese patients is that obese patients have large arms and thus a fistula may be considered too deep to cannulate with dialysis needles. Thus, obese patients may be referred late and get an AVG that matures in 2 weeks while nonobese patients receive an AVF that requires months to mature. Despite greater use of AVGs, obese patients have been shown to have similar vasculature to nonobese patients when vein mapping is employed, and an equal number of functional AVFs, possibly due to preserved vasculature from greater avoidance of blood draws secondary to vessel depth (17).
The presence of ischemic heart disease was associated with greater CVC use at dialysis initiation. This finding may suggest greater vascular disease among patients with ischemic heart disease, resulting in poor quality vasculature for AVF creation, or an expected shorter life expectancy compared to patients without ischemic heart disease. Overall, it appears that underlying cardiovascular status may influence the choice of access type.
We found that certain quality of care indicators were predictive of CVC use at dialysis initiation. Patients who lacked pre-ESRD EPO, or who had low pre-ESRD serum albumin or low hemoglobin were more likely to use a CVC at the start of dialysis, likely reflecting delayed pre-ESRD care, or a less healthy patient population unable to wait for the requisite time required for a permanent access to mature. Finally, we show a time trend in CVC use at dialysis initiation from 1998 to 2002, while during the same period, AVG use decreased. The rise in CVC use at the start of dialysis may reflect an increased number of patients being referred late to a nephrologist, poor vascular access education during the pre-ESRD period, or the increasing use of a CVC as a bridge to AVF maturation.
A number of limitations to this study need to be considered. We lack information regarding time to nephrology referral, which has been shown to predict vascular access type at dialysis initiation (11,14,15). We also lack data regarding AVF placement prior to dialysis initiation, as it is possible that some patients using a CVC at the start of dialysis have an AVF which has not yet matured fully. However, this is unlikely to comprise a large group, as we have observed that 90 days after dialysis initiation, a majority of those starting dialysis with a CVC are still using a CVC.
Our study is the first to analyze multiple years of ESRD CPM data with regard to vascular access type and reflects a nationally representative sample. We were able to examine time trends in vascular access use, and incorporate quality of care indicators with vascular access type using a contemporary cohort of incident adult hemodialysis patients.
In conclusion, we have identified factors predictive of CVC use at dialysis initiation among a nationally representative population of incident ESRD patients, and found greater CVC use among females, patients with ischemic heart disease, those with poor pre-ESRD quality of care indicators, and less CVC use among obese patients. Moreover, we have identified an important pattern of increasing CVC use at dialysis initiation as the ESRD cohort becomes more contemporary. It is unlikely that the high rate of CVC use found here, and reported by others, is due solely to late nephrology referral; of patients with nephrology care > 30 days prior to ESRD, only 48% of U.S. patients use a permanent access compared with 79% of European patients (18). Additional investigation is needed to determine the impact of specific factors such as predialysis vascular access education that emphasizes AVF use at dialysis initiation, timely access to surgery, and early assessment of nonmaturing AVFs.
Acknowledgment
This work was supported in part by a National Institutes of Health Career Development Award K23 DK65634 (H.W.).
References
- 1.Pastan S, Soucie JM, McClellan WM. Vascular access and increased risk of death among hemodialysis patients. Kidney Int. 2002;62:620–626. doi: 10.1046/j.1523-1755.2002.00460.x. [DOI] [PubMed] [Google Scholar]
- 2.Xue JL, Dahl D, Ebben JP, Collins AJ. The association of initial hemodialysis access type with mortality outcomes in elderly Medicare ESRD patients. Am J Kidney Dis. 2003;42:1013–1019. doi: 10.1016/j.ajkd.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Dhingra RK, Young EW, Hulbert-Shearon TE, Leavey SF, Port FK. Type of vascular access and mortality in U.S. hemodialysis patients. Kidney Int. 2001;60:1443–1451. doi: 10.1046/j.1523-1755.2001.00947.x. [DOI] [PubMed] [Google Scholar]
- 4.Astor BC, Eustace JA, Powe NR, Klag MJ, Fink NE, Coresh J. Type of vascular access and survival among incident hemodialysis patients: the Choices for Healthy Outcomes in Caring for ESRD (CHOICE) Study. J Am Soc Nephrol. 2005;16:1449–1455. doi: 10.1681/ASN.2004090748. [DOI] [PubMed] [Google Scholar]
- 5.Taylor GD, McKenzie M, Buchanan-Chell M, Caballo L, Chui L, Kowalewska-Grochowska K. Central venous catheters as a source of hemodialysis-related bacteremia. Infect Control Hosp Epidemiol. 1998;19:643–646. doi: 10.1086/647891. [DOI] [PubMed] [Google Scholar]
- 6.Kairaitis LK, Gottlieb T. Outcome and complications of temporary haemodialysis catheters. Nephrol Dial Transplant. 1999;14:1710–1714. doi: 10.1093/ndt/14.7.1710. [DOI] [PubMed] [Google Scholar]
- 7.Sandroni S, McGill R, Brouwer D. Hemodialysis catheter-associated endocarditis: clinical features, risks, and costs. Semin Dial. 2003;16:263–265. doi: 10.1046/j.1525-139x.2003.16050.x. [DOI] [PubMed] [Google Scholar]
- 8.Oguzkurt L, Tercan F, Torun D, Yildirim T, Zumrutdal A, Kizilkilic O. Impact of short-term hemodialysis catheters on the central veins: a catheter venographic study. Eur J Radiol. 2004;52:293–299. doi: 10.1016/j.ejrad.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 9.Berns JS. Infection with antimicrobial-resistant microorganisms in dialysis patients. Semin Dial. 2003;16:30–37. doi: 10.1046/j.1525-139x.2003.03009.x. [DOI] [PubMed] [Google Scholar]
- 10.Roberts TL, Obrador GT, St Peter WL, Pereira BJ, Collins AJ. Relationship among catheter insertions, vascular access infections, and anemia management in hemodialysis patients. Kidney Int. 2004;66:2429–2436. doi: 10.1111/j.1523-1755.2004.66020.x. [DOI] [PubMed] [Google Scholar]
- 11.Stehman-Breen CO, Sherrard DJ, Gillen D, Caps M. Determinants of type and timing of initial permanent hemodialysis vascular access. Kidney Int. 2000;57:639–645. doi: 10.1046/j.1523-1755.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 12.Clinical practice guidelines for vascular access. Am J Kidney Dis. 2006;48 Suppl. 1:S176–S247. doi: 10.1053/j.ajkd.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 13.Lee T, Barker J, Allon M. Associations with predialysis vascular access management. Am J Kidney Dis. 2004;43:1008–1013. doi: 10.1053/j.ajkd.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Astor BC, Eustace JA, Powe NR, Klag MJ, Sadler JH, Fink NE, Coresh J. Timing of nephrologist referral and arteriovenous access use: the CHOICE Study. Am J Kidney Dis. 2001;38:494–501. doi: 10.1053/ajkd.2001.26833. [DOI] [PubMed] [Google Scholar]
- 15.Avorn J, Winkelmayer WC, Bohn RL, Levin R, Glynn RJ, Levy E, Owen W., Jr Delayed nephrologist referral and inadequate vascular access in patients with advanced chronic kidney failure. J Clin Epidemiol. 2002;55:711–716. doi: 10.1016/s0895-4356(02)00415-8. [DOI] [PubMed] [Google Scholar]
- 16.Caplin N, Sedlacek M, Teodorescu V, Falk A, Uribarri J. Venous access: women are equal. Am J Kidney Dis. 2003;41:429–432. doi: 10.1053/ajkd.2003.50052. [DOI] [PubMed] [Google Scholar]
- 17.Vassalotti JA, Falk A, Cohl ED, Uribarri J, Teodorescu V. Obese and non-obese hemodialysis patients have a similar prevalence of functioning arteriovenous fistula using pre-operative vein mapping. Clin Nephrol. 2002;58:211–214. doi: 10.5414/cnp58211. [DOI] [PubMed] [Google Scholar]
- 18.Pisoni RL, Young EW, Dykstra DM, et al. Vascular access use in Europe and the United States: results from the DOPPS. Kidney Int. 2002;61:305–316. doi: 10.1046/j.1523-1755.2002.00117.x. [DOI] [PubMed] [Google Scholar]