Abstract
Cancer pain significantly affects the quality of cancer patients, and current treatments for this pain are limited. C-Jun N-terminal kinase (JNK) has been implicated in tumor growth and neuropathic pain sensitization. We investigated the role of JNK in cancer pain and tumor growth in a skin cancer pain model. Injection of luciferase-transfected B16-Fluc melanoma cells into a hindpaw of mouse induced robust tumor growth, as indicated by increase in paw volume and fluorescence intensity. Pain hypersensitivity in this model developed rapidly (<5 days) and reached a peak in 2 weeks, and was characterized by mechanical allodynia and heat hyperalgesia. Tumor growth was associated with JNK activation in tumor mass, dorsal root ganglion (DRG), and spinal cord and a peripheral neuropathy, such as loss of nerve fibers in the hindpaw skin and induction of ATF-3 expression in DRG neurons. Repeated systemic injections of D-JNKI-1 (6 mg/kg, i.p.), a selective and cell permeable peptide inhibitor of JNK, produced an accumulative inhibition of mechanical allodynia and heat hyperalgesia. A bolus spinal injection of D-JNKI-1 also inhibited mechanical allodynia. Further, JNK inhibition suppressed tumor growth in vivo and melanoma cell proliferation in vitro. In contrast, repeated injections of morphine (5 mg/kg), a commonly used analgesic for terminal cancer, produced analgesic tolerance after 1 day and did not inhibit tumor growth. Our data reveal a marked peripheral neuropathy in this skin cancer model and important roles of the JNK pathway in cancer pain development and tumor growth. JNK inhibitors such as D-JNKI-1 may be used to treat cancer pain.
Introduction
Cancer pain significantly affects the diagnosis, quality of life and survival of patients with cancer (Mantyh, 2006). The mechanisms of cancer pain are incompletely understood. Tumor growth may produce inflammation in tumor bearing tissues, which will release inflammatory mediators to stimulate nociceptors. Tumor growth may also compress the peripheral nerves in tumor bearing tissues, inducing nerve injury. Therefore, cancer pain is likely to share mechanisms of inflammatory pain or/and neuropathic pain, although this pain may have distinct mechanisms (Honore, et al., 2000; Mantyh, 2006). Whether inflammatory or neuropathic pain mechanisms dominate during tumor growth may depend on the interactions between tumor cells and surrounding tissues and nerves (Cain, et al., 2001; Wacnik, et al., 2001). In recent years, several laboratories have developed cancer pain models by inoculation of tumor cells into a hindpaw of mouse (Constantin, et al., 2008; Sasamura, et al., 2002), which has mixed nociceptive/neuropathic pain. Since the measurement of tumor growth and cancer pain is relatively easy in hindpaws of rats and mice and spinal cord innervations of hindpaw are well documented, skin cancer pain model provides a useful tool to investigate mechanisms of cancer pain.
Malignant melanoma is a major cause of death from skin cancer and its incidence has increased significantly in the United States (Jemal, et al., 2001). Although pain is not a major symptom of melanoma in clinic, 7% patients still experienced pain (Negin, et al., 2003). Also, metastatic melanoma is associated with pain (in some case neuropathic pain) and more than 50% of the patients require palliative care and morphine treatment (Leach, et al., 2008; Lehembre, et al., 2006). In addition, animals inoculated with melanoma cells into the plantar of the hindpaw show marked pain hypersensitivity (Andoh, et al., 2008; Mao-Ying, et al., 2006; Xia, et al., 2006). Therefore we inoculated luciferase-transfected B16-Fluc melanoma cells into a hindpaw of mouse, which allows us to perform bioluminescent imaging of melanoma growth in live mice (Craft, et al., 2005) and reliably measure pain sensitivity and tumor growth in the hindpaw.
C-Jun N-terminal kinase (JNK) is a member of mitogen-activated protein kinases (MAPK) and responsible for the activation of transcription factor c-Jun. JNK plays an important role in cell mitosis, differentiation and stress (Ji, et al., 2007; Nateri, et al., 2005). C-Jun is critical for tumor progression (Eferl, et al., 2003) and was regarded as a potential target of anticancer therapy (Gurzov, et al., 2008). Interestingly, c-Jun is over-expressed in a large fraction of human melanoma samples (Lopez-Bergami, et al., 2007). The small molecule inhibitor of JNK, SP600125 inhibits cancer cell proliferation in cultures. Further, systemic administration of SP600125 results in the inhibition of DU145 human prostate carcinoma xenografts and murine Lewis lung carcinoma (Ennis, et al., 2005).
Recently, we found that the JNK pathway is activated in the spinal cord after nerve injury and spinal injection of JNK inhibitors can attenuate nerve injury-induced neuropathic pain (Zhuang, et al., 2006). In particular, a cell-permeable peptide inhibitor of JNK, D-JNKI-1 is very selective and inhibits JNK activity by blocking JNK interaction with its substrate (Borsello and Bonny, 2004). In a neuropathic pain model, D-JNKI-1 is 50 times more potent than SP600125 in attenuating mechanical allodynia after intrathecal injection (Zhuang, et al., 2006). Now we report that systemic administration of D-JNKI-1 can suppress both cancer pain and tumor growth in a murine model of melanoma.
Methods
Animals
Experiments were done on adult male C57BL6 mice (8 weeks old, Charles River, MA), weighing 22–24 g. All mice have free access to food and water with a 12/12 light cycle. The Harvard Medical School Animal Care Committee approved all animal procedures in this study.
Cell Culture and Tumor Inoculation
Murine melanoma cell line, B16-Fluc, was kindly provided by Dr. Noah Craft of University of California, Los Angeles. The B16 murine melanoma cells (ATCC, Rockville, Maryland) were transduced with a lentiviral construct containing the Fluc gene and the GFP gene, separated by an encephalomyocarditis virus internal ribosomal entry site, and driven by an internal CMV promoter (Craft, et al., 2005).
B16-Fluc cells were grown in Dulbecco’s modified Eagle medium containing 4,500 mg/l glucose, 100 mg/l penicillin, 100 mg/l streptomycin, and supplemented with 10% fetal bovine serum in 5% CO2/95% air at 37°C. Cells were subcultured or collected following enzymatic digestion using trypsin solution. The melanoma cells (6 × 105 cells/30 μl) suspended in phosphate buffered saline (PBS) were subcutaneously injected into the plantar region of mice left hindpaw.
Behavioral Analysis
Animals were habituated to the testing environment daily for at least two days before baseline testing. For testing mechanical sensitivity, animals were kept in boxes on an elevated metal mesh floor and allowed 30 min for habituation before examination. The plantar surface of left hindpaw was stimulated with a series of von Frey hairs with logarithmically incrementing stiffness (0.02, 0.04, 0.08, 0.16, 0.32, 0.64, 1.28, and 2.56 g, Stoelting), presented perpendicular to the plantar surface. The 50% paw withdrawal threshold was determined using Dixon’s up-down method (Chaplan, et al., 1994). Heat sensitivity was assessed using radiant heat that was applied to the plantar region of left hindpaw and the latency of its withdrawal response was determined, using a plantar anesthesiometer (Ugo Basile, Italy). The intensity of radiant heat was adjusted to elicit a response of around 10 s in normal mice. The cut off time was 20 seconds.
Drug Administration
To evaluate the systemic effect of morphine and D-JNKI-1 on tumor growth and tumor-induced pain, vehicle (PBS), morphine (8 μmol mg/kg or 5 mg/kg), or D-JNKI-1 (2 μmol/kg or 6 mg/kg), in a volume of 100 μl, was given intraperitoneally twice daily (7 AM and 7 PM) from day 5 to 9 after tumor inoculation. Nociceptive behaviors were evaluated before, 3 h and 12 h after the first injection of that day. To evaluate spinal effect of D-JNKI-1 on tumor-induced pain, vehicle or D-JNKI-1 (2 nmol) was delivered to cerebrospinal fluid via a lumbar puncture using a 30G needle, and a volume of 10 μl liquid was given on day 13 after tumor inoculation, and pain behaviors were examined 3 h after the spinal injection. D-JNK-I was kindly provided by Dr. C. Bonny from University of Lausanne, Switzerland.
Immunohistochemistry
After appropriate survival times, the animals were deeply anesthetized with isoflurane and perfused through the ascending aorta with saline followed by 4% paraformaldehyde with 1.5% picric in 0.1 M PBS. After the perfusion, the L4-L5 spinal cord segments, L4, L5 dorsal root ganglions (DRGs) and skin with tumor mass were removed and postfixed in the same fixative overnight. Spinal cord sections (30 μm, free-floating sections), DRG sections (14 μm), and skin sections (8 μm) were cut in a cryostat, and processed for immunofluorescence staining. In brief, the sections were blocked with 2% goat serum, and incubated overnight at 4°C with the following primary antibodies: GFAP antibody (mouse, 1:5000, Millipore), Iba-1 antibody (rabbit, 1:5000, Wako), pJNK antibody (rabbit, 1:1000, Neuromics), p-c-Jun antibody (rabbit, 1:500, Cell Signaling), NeuN antibody (mouse, 1:5000, Millipore), prodynorphin antibody (guinea pig, 1:1500, Neuromics), PKCγ antibody (rabbit, 1:1000, Santa Cruz), PGP-9.5 antibody (rabbit, 1:1000, Biogenesis), or ATF-3 antibody (rabbit, 1:500, Millipore). The sections were then incubated for 1 h at room temperature with Cy3- or FITC-conjugated secondary antibodies (1:400, Jackson immunolab). The stained sections were examined with a Nikon fluorescence microscope, and images were captured with a CCD Spot camera. The p-c-Jun immunostaining was quantified by percentage of p-c-Jun positive neurons in the DRG (Zhuang et al., 2006) and by the intensity of p-c-Jun immunofluorescence in the dorsal horn from three animals per group.
Western Blots
To evaluate the JNK activation in tumor mass and spinal cord, tumor mass and spinal cord were harvested on day 9 post-inoculation. The tissues were processed for Western blots. As described previously (Zhuang, et al., 2006), animals were rapidly killed, and the L4-L5 spinal segments were quickly removed and homogenized in a SDS sample buffer containing a mixture of protease and phosphatase inhibitors (Sigma). Protein samples (30 μg) were separated on SDS-PAGE gel and transferred to polyvinylidene difluoride blots. The blots were blocked with 5% milk and incubated overnight at 4°C with antibody against phosphorylated JNK (pJNK) or GAPDH (loading control). These blots were further incubated with HRP-conjugated secondary antibody, developed in ECL solution, and exposed onto Hyperfilm (Amersham Biosciences, Arlington Heights, IL).
Bioluminescence Imaging and Tumor Growth Measurement
Mice were imaged at day 5 and 9 post inoculation by IVIS 100 Bioluminescence Imaging System (Xenogen, Alameda, CA). Mice were anesthetized with a mixture of oxygen and 1.5% of isoflurane and placed in prone position on the imaging platform, with the hindpaws taped to the platform for better exposure of the tumor. Luciferase substrate D-Luciferin (Xenogen Corp., Alameda, CA) in PBS was injected intraperitoneally (150 mg/kg) 5 minutes before imaging. Images were acquired every five minutes for forty minutes with an exposure time ranging from 5 to 10 seconds for every 5 minutes. Bioluminescence signals were quantified using Living ImageR software by drawing regions of interest (ROI) over the tumor region to obtain the normalized photons per second over the regions. The luminescence ratio of Day 5 and Day 9 post-inoculation (Day 9/Day 5) for treatment groups was used as an indicator of tumor growth.
To assess the growth of melanoma in situ, the volume of left hindpaw was measured using the plethysmometer (Ugo Basile, Italy). To further check the histology of tumor cells, hindpaw skin with tumor mass were cut in a cryostat and sections were stained with hematoxylin and eosin (HE).
In Vitro Cell Growth Assay
To determine the in vitro effect of D-JNKI-1 on tumor cell growth, we performed two cell viability assays. B16-Fluc cells (10000, 5000, 2500 cells/well) plated in a 96-well flat bottom plate were grown at 370C in 5% CO2/95% air for 24 h. Then the cells were treated with D-JNKI-1 (0.1–50 μM) for 24 h. For the bioluminescence assay, cells were treated with D-Luciferin (30 μg) at 37°C for 30 min, and the bioluminescence was measured by a Luminometry (Victor 2 1420 Multilabel Counter Plate Reader, PerkinElmer Life Science). The MTT [3-(4,5-dimethyl thiazol-2-yl)-2,5-diphenyl tetrazolium bromide] assay was processed according to the manufacturer’s instructions (Millipore). The ratio of the absorbance of treated cells over the control cells was calculated and used to represent the percentage of cell viability.
Statistical Analysis
Immunohistochemical and behavioral results were analyzed using t-test or one-way ANOVA followed by Newman-Keuls multiple comparison test. Significance level was set at P < 0.05. Data are presented as mean ± SEM.
Results
Hindpaw melanoma inoculation produces profound tumor growth and cancer pain
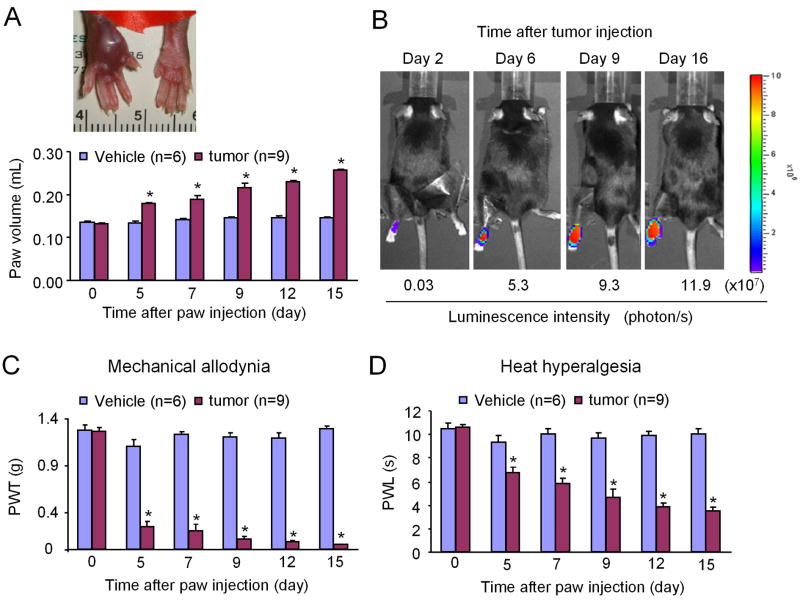
After B16-Fluc melanoma cells were inoculated into the plantar region of a left hindpaw, there was a progressive increase of paw volume, indicating the development of tumor mass (Fig. 1A). On post-inoculation day (PID) 15, the volume of the inoculated paw was increased to 197 ± 5% (n = 9) of that of pre-inoculation (Fig. 1A).
Figure 1.
Time course of tumor growth and cancer pain development following intraplantar injection of luciferase-transfected B16-Fluc melanoma cells. (A) Increase of paw volume after inoculation. Inset shows the tumor-bearing hindpaw (left) 15 days after inoculation. (B) Bioluminescent imaging shows enhanced luminescence intensity over time in an inoculated hindpaw, indicating a continuous growth of melanoma. (C, D) Development of mechanical allodynia (C) and heat hyperalgesia (D) in the inoculated paws. *P < 0.05 vs. vehicle (PBS) control.
Figure 1B shows a time course of consecutive bioluminescence images of a left hindpaw after tumor inoculation. The luminescence intensity increased progressively from day 2 to day 16 post-inoculation, suggesting a continuous growth of tumor mass.
Tumor growth was also associated with a progressive development of pain hypersensitivity in the hindpaw, which was characterized by mechanical allodynia (painful response to a normally innocuous mechanical stimulus) and heat hyperalgesia (increased response to a noxious heat stimulus) in the left hindpaws of inoculated mice. However, mice receiving vehicle (PBS) injection did not show changes in paw volume and pain sensitivity (Fig. 1A, C and D). For mechanical sensitivity, the paw withdrawal threshold (PWT) of the ipsilateral paw, in response to von Frey hair stimulation, was decreased from 1.26 ± 0.04 g on day 0 before inoculation to 0.05 ± 0.003 g (n = 9) on PID-15 (Fig. 1C), indicating the development of mechanical allodynia. For heat sensitivity, the paw withdrawal latency (PWL) of the inoculated hindpaw to heat stimulation was decreased from 10.54 ± 0.28 s on day 0 to 3.5 ± 0.29 s (n = 9) on PID-15 (Fig. 1D), indicating the development of heat hyperalgesia. Both mechanical and heat pains developed on PID-5 and reached a peak on PID-15 (Fig. 1C and 1D).
Despite massive tumor growth in hindpaws, the paw skin remained intact, and overall conditions of mice were good in the first 2–3 weeks. After 3 weeks, we found melanoma metastasis to the lung and animal conditions significantly deteriorated (data not shown). This study focused on a period of the first 15 days, especially the first 9 days when animal conditions are generally good but tumor growth and cancer pain are robust.
Hindpaw melanoma inoculation produces nerve degeneration
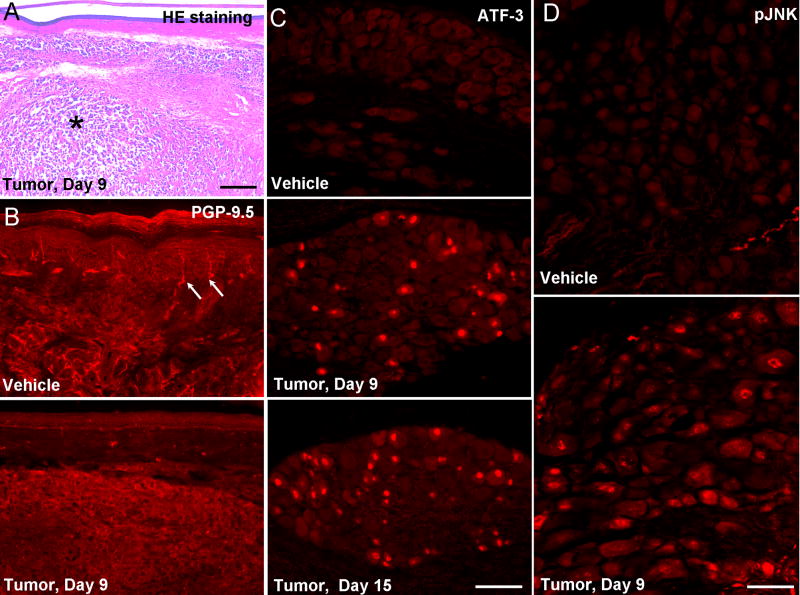
In support of increases in paw volume and luminescence intensity, HE staining demonstrated a massive tumor cell infiltration in the dermis (Fig. 2A). To examine whether tumor growth would cause nerve degeneration, we labeled nerve fibers in the hindpaw skin with PGP-9.5. Tumor growth induced a robust reduction of PGP-9.5-labeled nerve fibers in the epidermis, as well as in the dermis in the central skin area of tumor mass, on PID-9 (Fig. 2B), indicating a nerve degeneration in this model.
Figure 2.
Nerve degeneration in the tumor bearing hindpaws. (A) Hematoxylin-eosin (HE) staining of hindpaw skin (plantar surface) 9 days after tumor inoculation. The tumor tissue (indicated with *) was located in the dermis. Scale bar, 400 μm. (B) PGP-9.5 immunostaining of hindpaw skin (plantar surface) reveals a loss of nerve fibers 9 days after tumor inoculation. Arrows indicated nerve fibers in the epidermis. (C) ATF-3 immunostaining indicates induction of ATF-3 in the nuclei of many DRG neurons after tumor inoculation. Scale bar, 100 μm. (D) pJNK immunostaining shows JNK activation in DRG neurons after tumor inoculation. Scale bar, 50 μm.
To further determine whether tumor growth induces nerve injury, we examined the expression of the transcription factor ATF-3, which is only expressed in DRG neurons with axonal injury (Tsujino, et al., 2000). ATF-3 immunoreactivity was not found in the nucleus of vehicle treated DRG neurons, but progressively increased in the ipsilateral L4/5 DRGs after tumor inoculation (Fig. 2C). Around 20% of neurons in the L4 DRG expressed ATF-3 in the nuclei.
Hindpaw melanoma inoculation produces JNK activation in the DRG and spinal cord
To explore the role of JNK in cancer-associated pain, we examined JNK activation in the DRG and spinal cord using a phosphorylated JNK (pJNK) antibody. As previously shown (Obata et al., 2004; Zhuang et al., 2006), only very few neurons in the DRG exhibited weak pJNK immunoreactivity in non-injured (vehicle-treated) conditions. However, after tumor inoculation, many DRG neurons expressed pJNK (Fig. 2D).
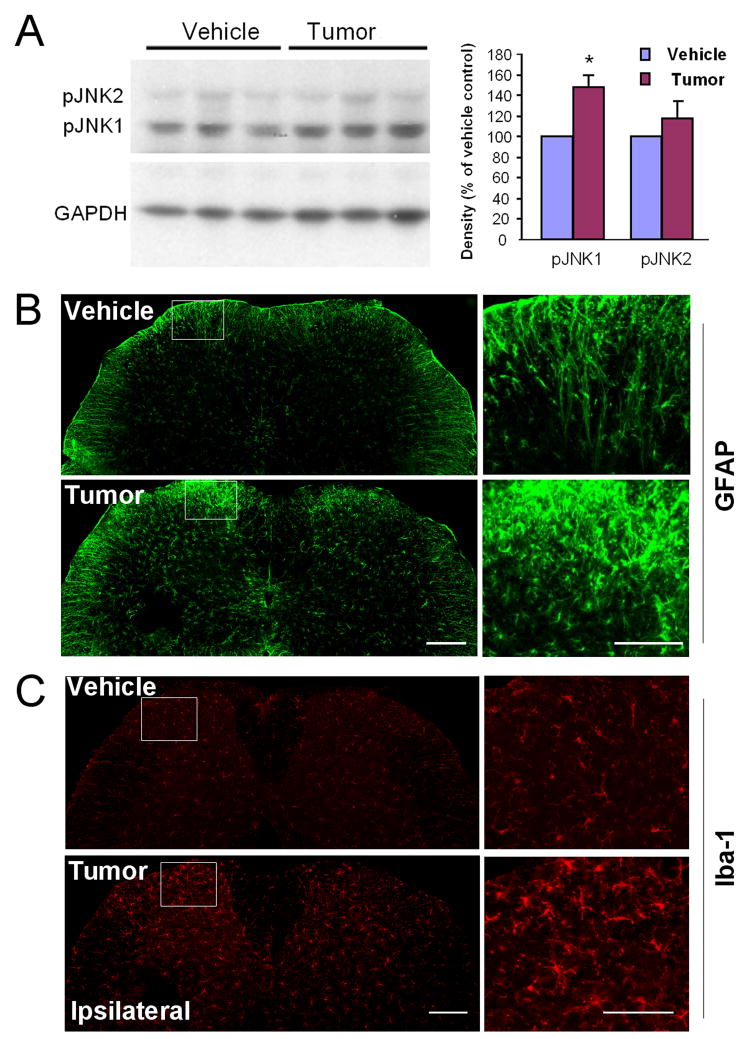
Western blotting showed that the mouse spinal cord mainly expressed pJNK1. In contrast, pJNK2 level in the spinal cord was very low (Fig. 3A). Further, spinal pJNK1 levels were significantly increased in tumor bearing mice on PID-9 (Fig. 3A).
Figure 3.
Activation of JNK, microglia, and astrocytes in the spinal cord after tumor inoculation. (A) Western blot shows increased pJNK1 but not pJNK2 levels in the spinal cord 9 days after inoculation. pJNK level in the spinal cord was quantified by the density of specific Western bands that are normalized by GAPDH loading control. *, P<0.05, n=3. (B, C) Immunohistochemistry shows microglia activation (up-regulation of Iba-1, B) and astrocyte activation (up-regulation of GFAP, C) in the ipsilateral spinal cord 9 days after tumor inoculation. Right panels: high power views of the ipsilateral dorsal horn insets in the laminae I-II. Scale bar, 200 μm (left panels), 100 μm (right panels).
Hindpaw melanoma inoculation produces glial activation and neurochemical changes in the spinal cord
To further characterize this skin cancer pain model, we also examined glial activation and neurochemical changes in the spinal cord that are associated with the development of chronic pain. We have previously shown that spinal nerve ligation induces substantial glial activation in the spinal cord such as up-regulation of GFAP, an astrocyte marker (Zhuang et al., 2006), and Iba-1, a microglia marker (Jin et al., 2003). Intraplantar tumor inoculation also induced marked upregulation of GFAP and Iba-1 in the spinal cord (Fig. 3B, C).
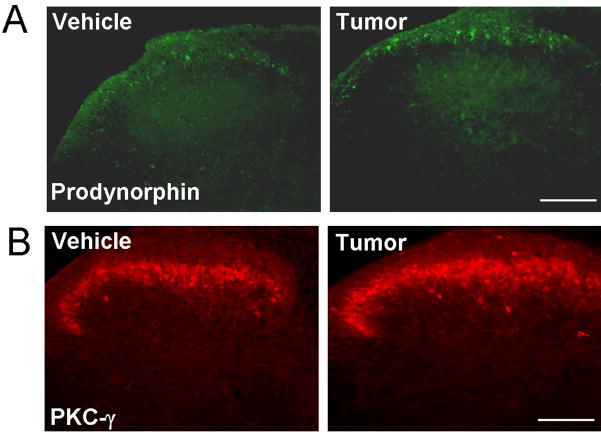
Further, nerve injury has been shown to produce neurochemical changes, such as up-regulation of prodynorphin and PKCγ in dorsal horn neurons, and these changes are important for chronic pain sensitization (Honore, et al., 2000; Malmberg, et al., 1997; Wang, et al., 2001). Similarly, tumor inoculation induced a marked upregulation of prodynorphin (Fig. 4A) and PKCγ (Fig. 4B) in superficial dorsal horn neurons.
Figure 4.
Immunohistochemistry shows up-regulation of prodynorphin (B) and PKCγ in the ipsilateral superficial dorsal horn 9 days after tumor inoculation. Scale bar, 100 μm.
Semi-quantification of immunofluorescence indicated that all these glial and neural changes in the spinal cord were significant in tumor-bearing mice (Table I).
Table I.
Quantitative analysis of neurochemical changes and glial changes in the spinal cord of melanoma bearing mice and the effects of the JNK inhibitor D-JNKI-1. GFAP and Iba-1 levels in the dorsal horn were quantified by the density of immunostaining. Prodynorphin and PKCγ levels in the superficial dorsal horn were quantified by counting the number of positive neurons. The data of GFAP and Iba-1 are presented as percent of vehicle (PBS) control. D-JNKI-1 (6 mg/kg) was injected intraperitoneally twice a day from day 5 to 9 after tumor inoculation.
| Vehicle | Tumor + Vehicle | Tumor + D-JNKI-1 | |
|---|---|---|---|
| GFAP | 100 ± 13.2 | 231.8 ± 15.2* | 220.2 ± 22.6 |
| Iba-1 | 100 ± 8.7 | 247.2 ± 24.4 * | 196.2 ± 13.3 |
| Prodynorphin | 7.0 ± 0.6 | 11.4 ± 0.6* | 7.7± 0.4 # |
| PKCγ | 34.1 ± 2.9 | 50.3 ± 2.3* | 50.1 ± 5.4 |
P< 0.05, compared with vehicle control;
P<0.05, compared with tumor; n=3–5.
Systemic and intrathecal inhibition of JNK with D-JNKI-1 attenuates cancer pain
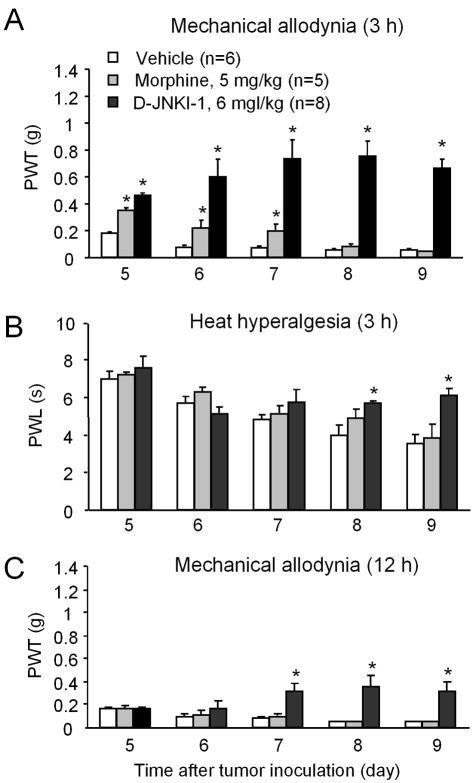
We used two different protocols to test the effects of peptide inhibitor of JNK, D-JNKI-1, on cancer-induced pain. In the first protocol, we gave repeated intraperitoneal injections of D-JNKI-1, twice a day, 12 h apart, for 5 days, starting from PID-5, when cancer pain began to develop. We tested cancer pain at 3 h and 12 h after the first daily injection on that day. D-JNKI-1 markedly inhibited mechanical allodynia at 3 h (Fig. 5A). Interestingly, the anti-allodynic effect of D-JNKI-1 was progressively increased after repeated injections, from PID-5 to PID-9 (Fig. 5A), suggesting an accumulative effect of the drug.
Figure 5.
Inhibition of melanoma-induced cancer pain by systemic injection of the JNK inhibitor D-JNKI-1. (A, B) Repeated injections of D-JNKI-1 (2 μmol or 6 mg/kg, i.p., twice a day) inhibit melanoma-induced mechanical allodynia as measured by paw withdrawal threshold (A) and heat hyperalgesia as measured by paw withdrawal latency (B). As comparison, repeated injections of morphine (8 μmol or 5 mg/kg, i.p., twice a day) only produce mild inhibition of mechanical allodynia with rapid development of tolerance. Pain behavior was tested 3 hours after the previous injection. (C) Repeated injections of D-JNKI-1 (6 mg/kg, i.p., twice a day) but not morphine (5 mg/kg, i.p., twice a day) also inhibit melanoma-induced mechanical allodynia when tested 12 hours after the previous injection. *P<0.05 vs. vehicle (PBS) control. Drugs were injected intraperitoneally twice daily from day 5 to 9 after tumor inoculation. Note accumulating effects of D-JNKI-1 on cancer pain after repeated injections.
To confirm that these behavioral effects of D-JNKI-1 result from specific inhibition of the JNK pathway, we examined the phosphorylation of the transcription factor c-Jun, a critical downstream target of JNK. In normal conditions, only few neurons in the DRG expressed p-c-Jun (Zhuang et al., 2006). However, after tumor implantation, 47.5 ± 0.6% DRG neurons expressed p-c-Jun. Importantly, this tumor-induced increase in p-c-Jun levels was suppressed by DJNKI-1 (daily injections from day 5 to day 9). Thus, only 23.4 ± 0.5% DRG neurons expressed p-c-Jun after the treatment (P<0.01, n=3). Further, p-c-Jun levels in the spinal cord dorsal horn in tumor-bearing mice were reduced by D-JNKI-1; and the intensity of p-c-Jun staining in tumor-bearing mice decreased from 43.5±1.0 (vehicle-treated) to 38.4±1.1 (D-JNKI-1-treated, P<0.01, n=3).
As a comparison, we also tested the effects of morphine, a commonly used analgesic for patients with terminal cancer. Like JNK, morphine was injected twice a day for 5 days, at the dose of 8 μmol/kg (5 mg/kg, i.p.). This does is 4 times higher than that of D-JNKI-1 at mole scale. After the first injection, morphine significantly attenuated tumor-induced mechanical allodynia at 3 h (Fig. 5A). However, repeated injections of morphine produced a very rapid analgesic tolerance, a reduction in analgesic efficacy, which appeared on the second day. Morphine completely lost its anti-allodynic effect after 3 days (Fig. 5A).
Initial injection of D-JNKI-1 on day 5 did not attenuate tumor-induced heat hyperalgesia. However, repeated injections of D-JNKI-1 attenuated tumor-induced heat hyperalgesia on PID-8 and PID-9 (Fig. 5B), again supporting an accumulating effect of D-JNKI-1 on heat hyperalgesia. However, repeated morphine injections did not inhibit heat hyperalgesia from day 5 to 9, when tested 3 h after injections (Fig. 5B). To investigate long lasting and accumulating effects of D-JNKI-1, we also tested tumor-induced mechanical allodynia at 12 h after the first daily drug injection. Repeated injections of D-JNKI-1 (6 mg/kg) but not morphine (5 mg/kg) also attenuated tumor-induced mechanical allodynia from day PID-7 to PID-9 in an accumulative manner (Fig. 5C).
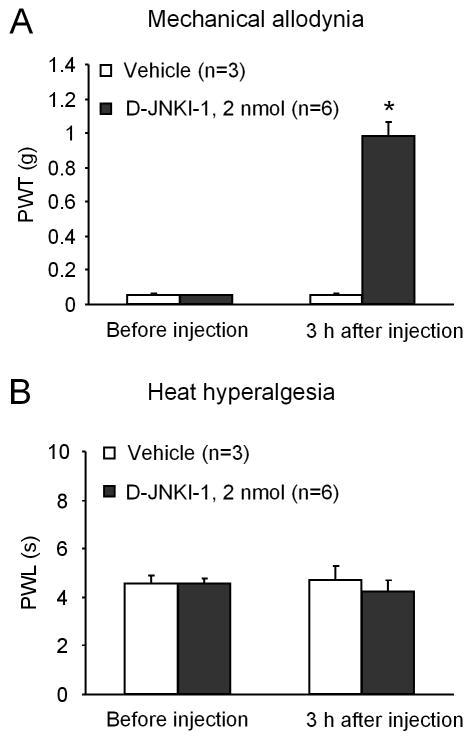
To further determine the role of spinal cord JNK in cancer pain, we performed a single bolus injection of D-JNKI-1 (2 nmol) via an intrathecal route on PID-13. A single spinal injection of D-JNKI-1 suppressed tumor-induced mechanical allodynia but not heat hyperalgesia at 3 h (Fig. 6).
Figure 6.
Inhibition of melanoma-induced mechanical allodynia (A) but not heat hyperalgesia (B) after spinal injection of D-JNKI-1 (2 nmol). The drug was injected on day 13 after tumor inoculation and pain behavior was tested 3 hours after the drug injection. *P < 0.05 vs. vehicle control (normal saline).
We also examined the effects of D-JNKI-1 on melanoma-induced glial activation and neurochemical changes in the spinal cord on PID-9 after repeated intraperitoneal injections. Interestingly, D-JNKI-1 had different effects on these changes. While melanoma-induced up-regulation of prodynorphin was almost completely blocked by D-JNKI-1, melanoma-induced up-regulation of Iba-1, GFAP, and PKCγ was not significantly reduced by the JNK inhibitor (Table I).
D-JNKI-1 suppresses tumor growth both in vivo and in vitro
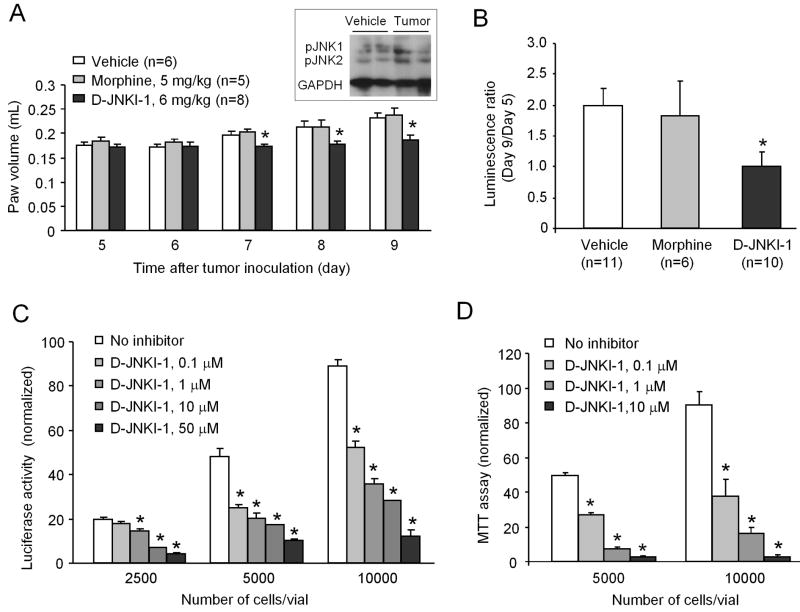
To determine whether JNK inhibition would affect tumor growth in vivo, we measured hindpaw volume from PID-5 to PID-9. Tumor growth (i.e., increase in the volume of the inoculated hindpaw) was significantly inhibited by D-JNKI-1, but not by morphine, on PID 7–9, as compared with vehicle control group (Fig. 7A). We also measured tumor growth by luminescence ratio (Day 9/Day 5). In vehicle-treated animals, the ratio increased to 1.99 ± 0.27 (n = 11). But in D-JNKI-1-treated animals, the ratio remained unchanged (1.01 ± 0.22, n = 10), indicating an inhibition of tumor growth after D-JNKI-1 treatment (Fig. 7B). In contrast, morphine had no effect on tumor growth when measured by luminescence ratio (Fig. 7B).
Figure 7.
Inhibition of melanoma tumor growth by D-JNKI-1 both in vivo and in vitro. (A) Repeated injections of D-JNKI-1 (6 mg/kg, i.p., twice a day) but not morphine (5 mg/kg, i.p., twice a day) inhibit melanoma growth as measured by paw volume. Drugs were injected intraperitoneally twice daily from day 5 to 9 after tumor inoculation. *P < 0.05 vs. vehicle control group. Inset shows increases in pJNK1 and pJNK2 levels in hindpaw tumor mass 9 days after inoculation of melanoma cells. (B) Luminescence ratio (Day 9/Day 5) also shows suppression of tumor growth in D-JNKI-1-treated mice. *P < 0.05 vs. vehicle control group. (C, D) In vitro bioluminescence assay (C) and MTT viability assay (D) reveal that D-JNKI-1 dose-dependently inhibit melanoma cell proliferation (C) and viability (D). *P < 0.05 vs. vehicle control group. Each condition was repeated in 3–5 different cultures.
Finally, we examined the effects of the JNK inhibitor in cultured B16-Fluc melanoma cells. Both the bioluminescence and MTT viability assay revealed that D-JNKI-1, at the concentrations of 0.1–50 μM, dose-dependently inhibited tumor cell proliferation and viability (Fig. 7C and 7D).
Discussion
The melanoma model of cancer pain
Animal models of cancer pain have been developed to test mechanisms and treatments of this pain (Mantyh, 2006). Intramedullary inoculation of tumor cells was used to induce bone cancer pain (Schwei, et al., 1999), which is the most frequently encountered type of cancer pain in patients (Mercadante, 1997). In this model, the neurochemical changes are different from that in inflammatory and neuropathic pain models (Honore, et al., 2000). For example, in the primary afferents, there is no up-regulation of the neuropeptide substance P, which is seen in inflammatory pain conditions, or down-regulation of substance P, which is seen in neuropathic pain conditions (Honore, et al., 2000). However, up-regulation of prodynorphin and activation of astrocytes (GFAP up-regulation) were found in all three pain conditions (Honore, et al., 2000; Ji, et al., 2002; Wang, et al., 2001; Zhuang, et al., 2006). Microglia activation in the spinal cord was also found in a bone cancer pain model (Zhang, et al., 2005).
Intraplantar inoculation of lung carcinoma cells (Constantin, et al., 2008) or melanoma cells (Sasamura, et al., 2002) into hindpaws of mice was used to induce skin cancer pain, because cancer pain and tumor growth can be easily measured in the hindpaws. Inoculation of luciferase-transfected bioluminescent melanoma cells into a hindapw has provided a model for real-time longitudinal analyses of tumor growth in live mice (Craft, et al., 2005). Importantly, aggressive skin cancer or metastatic melanoma is associated with pain (Leach, et al., 2008; Lehembre, et al., 2006). We showed that intraplantar inoculation of melanoma cells induced robust pain hypersensitivity including mechanical allodynia and heat hyperalgesia. In particular, this model showed marked peripheral neuropathy, as indicated by a loss of PGP-9.5-lableld nerve fibers in the hindpaw skin, up-regulation of ATF-3 in DRG neurons, and profound activation of microglia and astrocytes in the spinal cord. Thus, our skin cancer pain model may share mechanisms with peripheral neuropathic pain. Nerve degeneration in the skin was also found after implantation of fibrosarcoma cells in and around the calcaneus bone (Cain, et al., 2001), but not evident in another skin cancer pain model induced by intraplantar inoculation of lung carcinoma cells (Constantin, et al., 2008). Interestingly, in another melanoma model, PGP-9.5-labeled nerve fibers disappear in the center of tumor mass but increase in the periphery of the tumor (Zhang, et al., 2003). Thus, different skin cancer pain models may have different features, depending on types of tumor cells, stages of tumor growth, and interaction between tumor cells and surrounding tissues and nerves.
JNK and cancer pain
We previously showed that spinal nerve ligation induced JNK activation in the spinal cord, and spinal injection of the peptide inhibitor D-JNKI-1 and small molecule inhibitor SP600125 could attenuate nerve ligation-induced mechanical allodynia (Zhuang, et al., 2006). pJNK1 appears to be the predominant JNK isoform activated in the spinal cord of both rat and mouse. JNK1 is known to express in spinal cord astrocytes (Obata, et al., 2004; Zhuang, et al., 2006). pJNK1 also increased in the spinal cord after melanoma inoculation and spinal injection of D-JNKI-1 attenuated melanoma-induced mechanical allodynia. We further demonstrated that systemic injections of D-JNKI-1 persistently inhibited melanoma-induced mechanical allodynia. Because D-JNKI-1 with TAT sequence is cell-permeable, it can be taken up by cells in the central nervous system after systemic injection (Borsello and Bonny, 2004). Interestingly, repeated injections of D-JNKI-1 showed an accumulative anti-allodynic effect without producing tolerance. For example, three days after repeated injections, D-JNKI-1 not only inhibited allodynia at 3 h but also at 12 h after the previous injection (Fig. 4C). Furthermore, melanoma-induced heat hyperalgesia was not inhibited by single injection of D-JNKI-1 via spinal and systemic route, but inhibited 3 days after repeated injections of D-JNKI-1 (Figs. 4, 5).
We observed marked up-regulation of Iba-1 (microglia marker) and GFAP (astrocyte marker) in the spinal cord after melanoma inoculation. But these glial changes were not significantly inhibited by D-JNKI-1, in agreement with our previous study (Zhuang, et al., 2006). Thus, the anti-allodynic effect of D-JNKI-1 is not associated with reversal of these spinal glial changes. However, D-JNKI-1 suppressed melanoma-induced up-regulation of prodynorphin in dorsal horn neurons. Prodynorphin is essential for the development of neuropathic pain development (Wang, et al., 2001). Our recent study also shows that spinal JNK activation produces the chemokine CCL2 for neuropathic pain sensitization (Gao et al., 2009). JNK may also increase cancer pain via peripheral mechanism, since tumor inoculation and nerve injury also activate JNK in DRG neurons (Fig. 2D, Obata, et al., 2004; Zhuang, et al., 2006) and the spinal nerve. Further, inhibition of tumor growth by D-JNKI-1 (see below) could indirectly alleviate cancer pain.
JNK and tumor growth
The American Cancer Society has estimated that approximately 9,000 people die each year from skin cancer and about 7,000 of these deaths are from melanoma. Activation of JNK is associated with cell proliferation and shorter relapse-free period for patients with superficial spreading melanomas, serving as a potential marker for malignant melanoma (Jorgensen, et al., 2006). JNK inhibition was found to induce cell cycle arrest and apoptosis in human melanoma cells (Alexaki, et al., 2008). The major effector of JNK, c-Jun, is a potential target for anticancer cell therapy (Gurzov, et al., 2008). JNK inhibitor SP600125 inhibits tumor growth and interferes with tumor angiogenesis, a critical process for tumor growth (Ennis, et al., 2005). In gastrointestinal cancer cells, SP600125 inhibits cell proliferation and induces apoptosis and cell cycle arrest (Xia, et al., 2006). We have shown that repeated injections of D-JNKI-1 inhibited melanoma growth in the hindpaw as measured both by paw volume and luminescence intensity. Further, D-JNKI-1 inhibited proliferation of melanoma in cultured melanoma cells, indicating a direct effect of D-JNKI-1 on melanoma cells. JNK activation is also important for the expression of vascular endothelial growth factor (VEGF) in malignant cells (Krejsgaard, et al., 2006; Yoshino, et al., 2006), an essential molecule for angiogenesis (Einspahr, et al., 2007). The tumor suppressing effect of D-JNKI-1 may also be associated with its inhibition on angiogenesis.
Morphine and cancer pain
Morphine is the major drug of choice in the terminal stage of cancer pain. Humans suffering from bone cancer pain generally require significantly higher doses of morphine as compared to individuals with inflammatory pain. The doses of morphine required to block bone cancer pain in mouse are ten times that required to block peak inflammatory pain behaviors (Luger, et al., 2002; Menendez, et al., 2003; Wacnik, et al., 2001). Sasamura et al. reported that subcutaneous morphine, at the dose of 5 mg/kg, inhibits melanoma-induced heat hyperalgesia (Sasamura, et al., 2002). In our study, this dose of morphine inhibited melanoma-induced mechanical allodynia but not heat hyperalgesia when tested after 3 hours. Repeated injections of morphine (5 mg/kg, twice a day) induced a rapid development of analgesic tolerance in the second day, which is faster than that seen in another skin cancer model (Andoh, et al., 2008). Morphine-induced tolerance leads to increased drug consumption and incidence of unwanted side effects, such as sedation, constipation, itching, nausea, vomiting and respiratory depression (Mao, 2002; McNicol, et al., 2003). Morphine also induces rapid tolerance in neuropathic pain models (Bulka, et al., 2002; Raghavendra, et al., 2002; Tawfik, et al., 2005). The rapid development of morphine tolerance in melanoma bearing mice further supports a neuropathic involvement in this cancer pain model. Our data suggest that morphine only has limited role in controlling the pain symptoms in aggressive skin cancer states.
Morphine was shown to suppress tumor growth in a melanoma model. This anti-tumor effect of morphine may be associated with the analgesic effect of morphine, because cancer pain results in psychological stress that will suppress immune functions and enhance tumor growth (Sasamura, et al., 2002). In contrast, morphine at high doses enhances tumor growth due to the suppression of immune system (Lewis, et al., 1983). In this study, morphine had no effect on the growth of melanoma, which is correlated with limited analgesic effect of morphine in the melanoma model.
Concluding remarks
We have characterized a skin cancer pain model induced by intraplantar inoculation of melanoma cells into a hindpaw. This model is characterized by robust tumor growth and rapid development of mechanical and heat hypersensitivity and exhibits marked peripheral neuropathy. Given the low incidence of pain in melanoma patients, this model may not be very clinically relevant compared to other models, such as bone cancer pain models. However, this model is very convenient to study mechanisms of cancer pain and tumor growth and to test new treatment. Future studies will be needed to test the role of the JNK pathway in other cancer pain models. Our data have shown that repeated administration of the peptide inhibitor of JNK, D-JNKI-1, not only attenuates melanoma-induced mechanical allodynia but also suppresses tumor growth both in vivo and in vitro. In contrast, repeated administration of morphine produces rapid analgesic tolerance and shows no effect on tumor growth.
It is worthwhile to compare JNK with its family member p38. Both MAPKs are pronociceptive (Obata and Noguchi, 2004; Ji, et al., 2007). Spinal administration of p38 inhibitors was shown to attenuate inflammatory pain and neuropathic pain in different models (Jin, et al., 2003; Schafers, et al., 2003; Svensson, et al., 2003; Obata et al., 2004). However, oral delivery of the p38 inhibitor SCIO-469 shows no effect on osteosarcoma-induced cancer pain (Svensson, et al., 2008). In contrast to D-JNKI-1, SCIO-469 has poor CNS penetration after systemic administration. It is also possible that p38 plays limited role in cancer pain. Our data have shown that inhibition of the JNK pathway can directly suppress the proliferation of melanoma cells. Notably, most deaths from skin cancer result from melanoma (Jemal, et al., 2001) and aggressive skin cancer is associated with pain (Leach, et al., 2008; Lehembre, et al., 2006). Therefore, inhibition of the JNK pathway with one stone can hit two birds: cancer pain and tumor growth. Finally, a recent clinical study suggests that the peptide inhibitor D-JNKI-1 can be well tolerated by patients and shows efficacy in treating acute acoustic trauma (Suckfuell, et al., 2007). Thus, D-JNKI-1 may be a promising therapeutic agent for the treatment of melanoma and cancer-related pain.
Acknowledgments
We thank Dr. Noah Craft of University of California, Los Angeles for providing us with the B16-Fluc cell line and Dr. Christophe Bonny of University of Lausanne, Switzerland for providing us with the JNK peptide inhibitor D-JNKI-1. This work was supported by NIH grants DE17794, NS54932, TW7180, and National Natural Science Foundation of China (NSFC) 30528019 to RRJ, and NSFC 30500153 and Natural Science Research Program of Jiangsu Province 05KJB180100 to YJG. JKC was supported by a John J. Bonica Trainee Fellowship from the International Association for the Study of Pain (IASP) and by a fellowship from Mackay Memorial Hospital (Taipei, Taiwan). QZ and XX were supported by the Department of Radiology of Brigham and Women’s Hospital. ID was supported by Swiss National Science Foundation and Pierre Mercier science foundation. This study was also partly supported by an IASP collaboration grant to RRJ and ID.
References
- Alexaki VI, Javelaud D, Mauviel A. JNK supports survival in melanoma cells by controlling cell cycle arrest and apoptosis. Pigment Cell Melanoma Res. 2008;21:429–438. doi: 10.1111/j.1755-148X.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- Andoh T, Sugiyama K, Fujita M, Iida Y, Nojima H, Saiki I, Kuraishi Y. Pharmacological evaluation of morphine and non-opioid analgesic adjuvants in a mouse model of skin cancer pain. Biol Pharm Bull. 2008;31:520–522. doi: 10.1248/bpb.31.520. [DOI] [PubMed] [Google Scholar]
- Borsello T, Bonny C. Use of cell-permeable peptides to prevent neuronal degeneration. Trends Mol Med. 2004;10:239–244. doi: 10.1016/j.molmed.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bulka A, Plesan A, Xu XJ, Wiesenfeld-Hallin Z. Reduced tolerance to the anti-hyperalgesic effect of methadone in comparison to morphine in a rat model of mononeuropathy. Pain. 2002;95:103–109. doi: 10.1016/s0304-3959(01)00382-7. [DOI] [PubMed] [Google Scholar]
- Cain DM, Wacnik PW, Turner M, Wendelschafer-Crabb G, Kennedy WR, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve: changes in excitability and morphology of primary afferent fibers in a murine model of cancer pain. J Neurosci. 2001;21:9367–9376. doi: 10.1523/JNEUROSCI.21-23-09367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Constantin CE, Mair N, Sailer CA, Andratsch M, Xu ZZ, Blumer MJ, Scherbakov N, Davis JB, Bluethmann H, Ji RR, Kress M. Endogenous tumor necrosis factor alpha (TNFalpha) requires TNF receptor type 2 to generate heat hyperalgesia in a mouse cancer model. J Neurosci. 2008;28:5072–5081. doi: 10.1523/JNEUROSCI.4476-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft N, Bruhn KW, Nguyen BD, Prins R, Liau LM, Collisson EA, De A, Kolodney MS, Gambhir SS, Miller JF. Bioluminescent imaging of melanoma in live mice. J Invest Dermatol. 2005;125:159–165. doi: 10.1111/j.0022-202X.2005.23759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eferl R, Ricci R, Kenner L, Zenz R, David JP, Rath M, Wagner EF. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- Einspahr JG, Thomas TL, Saboda K, Nickolof BJ, Warneke J, Curiel-Lewandrowski C, Ranger-Moore J, Duckett L, Bangert J, Fruehauf JP, Alberts DS. Expression of vascular endothelial growth factor in early cutaneous melanocytic lesion progression. Cancer. 2007;110:2519–2527. doi: 10.1002/cncr.23076. [DOI] [PubMed] [Google Scholar]
- Ennis BW, Fultz KE, Smith KA, Westwick JK, Zhu D, Boluro-Ajayi M, Bilter GK, Stein B. Inhibition of tumor growth, angiogenesis, and tumor cell proliferation by a small molecule inhibitor of c-Jun N-terminal kinase. J Pharmacol Exp Ther. 2005;313:325–332. doi: 10.1124/jpet.104.078873. [DOI] [PubMed] [Google Scholar]
- Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, Park JY, Lind AL, Ma Q, Ji RR. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–4108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurzov EN, Bakiri L, Alfaro JM, Wagner EF, Izquierdo M. Targeting c-Jun and JunB proteins as potential anticancer cell therapy. Oncogene. 2008;27:641–652. doi: 10.1038/sj.onc.1210690. [DOI] [PubMed] [Google Scholar]
- Honore P, Rogers SD, Schwei MJ, Salak-Johnson JL, Luger NM, Sabino MC, Clohisy DR, Mantyh PW. Murine models of inflammatory, neuropathic and cancer pain each generates a unique set of neurochemical changes in the spinal cord and sensory neurons. Neuroscience. 2000;98:585–598. doi: 10.1016/s0306-4522(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Jemal A, Devesa SS, Hartge P, Tucker MA. Recent trends in cutaneous melanoma incidence among whites in the United States. J Natl Cancer Inst. 2001;93:678–683. doi: 10.1093/jnci/93.9.678. [DOI] [PubMed] [Google Scholar]
- Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Zhang YQ. Protein kinases as potential targets for the treatment of pathological pain. Handb Exp Pharmacol. 2007:359–389. doi: 10.1007/978-3-540-33823-9_13. [DOI] [PubMed] [Google Scholar]
- Jin SX, Zhuang ZY, Woolf CJ, Ji RR. p38 mitogen-activated protein kinase is activated after a spinal nerve ligation in spinal cord microglia and dorsal root ganglion neurons and contributes to the generation of neuropathic pain. J Neurosci. 2003;23:4017–4022. doi: 10.1523/JNEUROSCI.23-10-04017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen K, Davidson B, Florenes VA. Activation of c-jun N-terminal kinase is associated with cell proliferation and shorter relapse-free period in superficial spreading malignant melanoma. Mod Pathol. 2006;19:1446–1455. doi: 10.1038/modpathol.3800662. [DOI] [PubMed] [Google Scholar]
- Krejsgaard T, Vetter-Kauczok CS, Woetmann A, Lovato P, Labuda T, Eriksen KW, Zhang Q, Becker JC, Odum N. Jak3- and JNK-dependent vascular endothelial growth factor expression in cutaneous T-cell lymphoma. Leukemia. 2006;20:1759–1766. doi: 10.1038/sj.leu.2404350. [DOI] [PubMed] [Google Scholar]
- Leach BC, Kulbersh JS, Day TA, Cook J. Cranial neuropathy as a presenting sign of recurrent aggressive skin cancer. Dermatol Surg. 2008;34:483–497. doi: 10.1111/j.1524-4725.2007.34094.x. [DOI] [PubMed] [Google Scholar]
- Lehembre S, Carvalho P, Young P, Josset V, Hacpille L, Joly P. [Palliative care management of patients in a dermatology department] Ann Dermatol Venereol. 2006;133:967–970. doi: 10.1016/s0151-9638(06)71079-1. [DOI] [PubMed] [Google Scholar]
- Lewis JW, Shavit Y, Terman GW, Gale RP, Liebeskind JC. Stress and morphine affect survival of rats challenged with a mammary ascites tumor (MAT 13762B) Nat Immun Cell Growth Regul. 1983;3:43–50. [PubMed] [Google Scholar]
- Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M, Smalley KS, Mahale A, Eroshkin A, Aaronson S, Ronai Z. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11:447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luger NM, Sabino MA, Schwei MJ, Mach DB, Pomonis JD, Keyser CP, Rathbun M, Clohisy DR, Honore P, Yaksh TL, Mantyh PW. Efficacy of systemic morphine suggests a fundamental difference in the mechanisms that generate bone cancer vs inflammatory pain. Pain. 2002;99:397–406. doi: 10.1016/S0304-3959(02)00102-1. [DOI] [PubMed] [Google Scholar]
- Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCgamma. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- Mantyh PW. Cancer pain and its impact on diagnosis, survival and quality of life. Nat Rev Neurosci. 2006;7:797–809. doi: 10.1038/nrn1914. [DOI] [PubMed] [Google Scholar]
- Mao-Ying QL, Cui KM, Liu Q, Dong ZQ, Wang W, Wang J, Sha H, Wu GC, Wang YQ. Stage-dependent analgesia of electro-acupuncture in a mouse model of cutaneous cancer pain. Eur J Pain. 2006;10:689–694. doi: 10.1016/j.ejpain.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, Lau J, Carr D. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. J Pain. 2003;4:231–256. doi: 10.1016/s1526-5900(03)00556-x. [DOI] [PubMed] [Google Scholar]
- Menendez L, Lastra A, Fresno MF, Llames S, Meana A, Hidalgo A, Baamonde A. Initial thermal heat hypoalgesia and delayed hyperalgesia in a murine model of bone cancer pain. Brain Res. 2003;969:102–109. doi: 10.1016/s0006-8993(03)02284-4. [DOI] [PubMed] [Google Scholar]
- Mercadante S. Malignant bone pain: pathophysiology and treatment. Pain. 1997;69:1–18. doi: 10.1016/s0304-3959(96)03267-8. [DOI] [PubMed] [Google Scholar]
- Nateri AS, Spencer-Dene B, Behrens A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature. 2005;437:281–285. doi: 10.1038/nature03914. [DOI] [PubMed] [Google Scholar]
- Negin BP, Riedel E, Oliveria SA, Berwick M, Coit DG, Brady MS. Symptoms and signs of primary melanoma: important indicators of Breslow depth. Cancer. 2003;98:344–348. doi: 10.1002/cncr.11513. [DOI] [PubMed] [Google Scholar]
- Obata K, Noguchi K. MAPK activation in nociceptive neurons and pain hypersensitivity. Life Sci. 2004;74:2643–2653. doi: 10.1016/j.lfs.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–10222. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Rutkowski MD, DeLeo JA. The role of spinal neuroimmune activation in morphine tolerance/hyperalgesia in neuropathic and sham-operated rats. J Neurosci. 2002;22:9980–9989. doi: 10.1523/JNEUROSCI.22-22-09980.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasamura T, Nakamura S, Iida Y, Fujii H, Murata J, Saiki I, Nojima H, Kuraishi Y. Morphine analgesia suppresses tumor growth and metastasis in a mouse model of cancer pain produced by orthotopic tumor inoculation. Eur J Pharmacol. 2002;441:185–191. doi: 10.1016/s0014-2999(02)01450-4. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwei MJ, Honore P, Rogers SD, Salak-Johnson JL, Finke MP, Ramnaraine ML, Clohisy DR, Mantyh PW. Neurochemical and cellular reorganization of the spinal cord in a murine model of bone cancer pain. J Neurosci. 1999;19:10886–10897. doi: 10.1523/JNEUROSCI.19-24-10886.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suckfuell M, Canis M, Strieth S, Scherer H, Haisch A. Intratympanic treatment of acute acoustic trauma with a cell-permeable JNK ligand: a prospective randomized phase I/II study. Acta Otolaryngol. 2007;127:938–942. doi: 10.1080/00016480601110212. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86:1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- Svensson CI, Medicherla S, Malkmus S, Jiang Y, Ma JY, Kerr I, Brainin-Mattos J, Powell HC, Luo ZD, Chakravarty S, Dugar S, Higgins LS, Protter AA, Yaksh TL. Role of p38 mitogen activated protein kinase in a model of osteosarcoma-induced pain. Pharmacol Biochem Behav. 2008 doi: 10.1016/j.pbb.2008.05.016. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, LaCroix-Fralish ML, Nutile-McMenemy N, DeLeo JA. Transcriptional and translational regulation of glial activation by morphine in a rodent model of neuropathic pain. J Pharmacol Exp Ther. 2005;313:1239–1247. doi: 10.1124/jpet.104.082420. [DOI] [PubMed] [Google Scholar]
- Tsujino H, Kondo E, Fukuoka T, Dai Y, Tokunaga A, Miki K, Yonenobu K, Ochi T, Noguchi K. Activating transcription factor 3 (ATF3) induction by axotomy in sensory and motoneurons: A novel neuronal marker of nerve injury. Mol Cell Neurosci. 2000;15:170–182. doi: 10.1006/mcne.1999.0814. [DOI] [PubMed] [Google Scholar]
- Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: morphology, algogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21:9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Gardell LR, Ossipov MH, Vanderah TW, Brennan MB, Hochgeschwender U, Hruby VJ, Malan TP, Jr, Lai J, Porreca F. Pronociceptive actions of dynorphin maintain chronic neuropathic pain. J Neurosci. 2001;21:1779–1786. doi: 10.1523/JNEUROSCI.21-05-01779.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia HH, He H, De Wang J, Gu Q, Lin MC, Zou B, Yu LF, Sun YW, Chan AO, Kung HF, Wong BC. Induction of apoptosis and cell cycle arrest by a specific c-Jun NH2-terminal kinase (JNK) inhibitor, SP-600125, in gastrointestinal cancers. Cancer Lett. 2006;241:268–274. doi: 10.1016/j.canlet.2005.10.031. [DOI] [PubMed] [Google Scholar]
- Yoshino Y, Aoyagi M, Tamaki M, Duan L, Morimoto T, Ohno K. Activation of p38 MAPK and/or JNK contributes to increased levels of VEGF secretion in human malignant glioma cells. Int J Oncol. 2006;29:981–987. [PubMed] [Google Scholar]
- Zhang HW, Iida Y, Andoh T, Nojima H, Murata J, Saiki I, Kuraishi Y. Mechanical hypersensitivity and alterations in cutaneous nerve fibers in a mouse model of skin cancer pain. J Pharmacol Sci. 2003;91:167–170. doi: 10.1254/jphs.91.167. [DOI] [PubMed] [Google Scholar]
- Zhang RX, Liu B, Wang L, Ren K, Qiao JT, Berman BM, Lao L. Spinal glial activation in a new rat model of bone cancer pain produced by prostate cancer cell inoculation of the tibia. Pain. 2005;118:125–136. doi: 10.1016/j.pain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, Decosterd I, Ji RR. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–3560. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]