Abstract
Myocardial tissue lacks the ability to significantly regenerate itself following a myocardial infarction, thus tissue engineering strategies are required for repair. Several injectable materials have been examined for cardiac tissue engineering; however, none have been designed specifically to mimic the myocardium. The goal of this study was to investigate the in vitro properties and in vivo potential of an injectable myocardial matrix designed to mimic the natural myocardial extracellular environment. Porcine myocardial tissue was decellularized and processed to form a myocardial matrix with the ability to gel in vitro at 37°C and in vivo upon injection into rat myocardium. The resulting myocardial matrix maintained a complex composition, including glycosaminoglycan content, and was able to self-assemble to form a nanofibrous structure. Endothelial cells and smooth muscle cells were shown to migrate towards the myocardial matrix both in vitro and in vivo, with a significant increase in arteriole formation at 11 days post-injection. The matrix was also successfully pushed through a clinically used catheter, demonstrating its potential for minimally invasive therapy. Thus, we have demonstrated the initial feasibility and potential of a naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering.
Keywords: angiogenesis, biomimetic material, cardiac tissue engineering, in vivo test, scaffold
1. Introduction
Heart failure (HF) following a myocardial infarction (MI) continues to be the leading cause of death in the United States, and the rest of the western world [1]. While total heart transplantation remains the only successful treatment for end-stage heart failure post-MI, this approach is limited by a lack of donor hearts. In addition, the myocardial tissue that is damaged during MI lacks the ability to significantly regenerate itself, which leads to negative left ventricular (LV) remodeling and eventual HF. Thus, tissue engineering strategies to repair and regenerate the area of infarct are essential to prevent HF post MI. New strategies to treat heart failure, such as the design of scaffold materials for cardiac tissue engineering, show great potential. Injectable materials, in particular, offer the unique advantage of minimally invasive delivery.
Recent trends in designing scaffolds for tissue engineering have focused on materials that are biomimetic and tissue-specific, to provide the appropriate chemical and biological cues that mimic the native microenvironment [2-4]. Injectable materials for myocardial tissue engineering, such as fibrin [5, 6], collagen [7, 8], Matrigel [9, 10], alginate [11], self-assembling peptides [12], and chitosan [13], each delivered alone or with cells, have been explored as potential treatments for MI [14]. However, these materials do not adequately emulate the structural and chemical make up of the natural myocardial extracellular matrix (ECM). Providing cells with the correct environmental cues is essential to the success of a tissue engineering approach [2-4]. Thus, the ideal injectable scaffold for myocardial tissue engineering would be one that mimics the natural myocardial ECM and promotes neovascularization to reduce the ischemic environment.
The use of decellularized tissues, as nature's platform, for a variety of tissue engineering applications has been explored [15, 16]. Recent studies have shown the use of decellularized rat hearts as an intact scaffold for seeding of endothelial cells and cardiomyocytes [17]. Interestingly, rat endothelial cells formed single layers surrounding vessels within the heart construct, suggesting that chemical cues remained in the decellularized ECM to direct the cells. In addition, small intestine submucosa and bladder matrix have resulted in cellular infiltration when utilized as cardiac patch therapies [18, 19]. However, while the ECM of each tissue contains similar components, each individual tissue does have its own unique combination of proteins and proteoglycans [3, 20]. It follows that for myocardial repair, decellularized myocardial matrix would best mimic the native myocardial ECM's biological and chemical cues, providing the most appropriate scaffold material. While a patch of cardiac ECM would retain the natural ECM structure, it would be limited to invasive surgical procedures for implantation and delivery only to the epicardial surface. Therefore we sought to test the hypothesis that an injectable form of decellularized myocardial ECM, which would mimic the biological and chemical cues of native cardiac ECM and be delivered in a minimally invasive procedure, could be utilized as a scaffold for myocardial tissue engineering.
The objectives of this study were to characterize the biochemical composition and structure of an injectable form of decellularized myocardial matrix, demonstrate its ability to form a gel in vivo, and assess its capability for promoting an influx of vasculature. Herein we demonstrate that this form of myocardial matrix retains a complex biochemical composition, re-assembles to form a nanofibrous scaffold, can be injected through a 27 G catheter, and promotes vascular cell migration in vitro and infiltration in vivo. Thus, we have demonstrated that this myocardial matrix has potential to be used for cardiac tissue engineering applications.
2. Materials and methods
All experiments in this study were performed in accordance with the guidelines established by the Committee on Animal Research at the University of California, San Diego and the American Association for Accreditation of Laboratory Animal Care.
2.1. Decellularization of myocardial tissue for matrix preparation
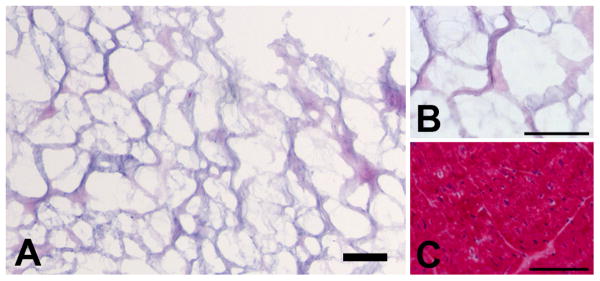
Hearts were harvested from pigs, approximately 30-45 kg, immediately after sedation with a ketamine/xylazine combination (25 mg/kg, 2 mg/kg respectively) and euthanasia with beuthanasia (1 mL/5 kg). The major vessels and atria were removed from the heart, and the ventricular tissue was cut into pieces of about 2 mm in thickness. The cardiac tissue was decellularized using detergents already shown to be effective in decellularizing an entire rat heart, while maintaining its structure [17]. The tissue was briefly rinsed with deionized water and then stirred in 1% (wt/vol) sodium dodecyl sulfate (SDS) in phosphate buffered saline (PBS) for 4-5 days, until the tissue was decellularized. The tissue was then stirred in 1% (vol/vol) Triton X-100 for 30 min for final cell removal. Finally, decellularized cardiac tissue was stirred overnight in deionized water to ensure removal of detergents. A sample of decellularized matrix was frozen in Tissue Tek O.C.T. freezing medium, sectioned into 10 μm slices, and stained with hematoxylin and eosin (H&E) to confirm the absence of cells. Intact ventricular tissue was also frozen, sectioned, and stained as a reference. Following the described decellularization procedure, ventricular ECM was lyophilized and milled using a Wiley Mini Mill to create a powder.
2.2. Preparation of solubilized myocardial matrix for gelation
To generate an injectable form of myocardial matrix, the decellularized matrix was solubilized, as modified from a previously published protocol for bladder matrix [21]. Optimal concentrations of pepsin and hydrochloric acid (HCl) for ventricular ECM were determined experimentally (data not shown). Briefly, pepsin (Sigma, St. Louis, MO), dissolved in 0.1 M HCl was added so that the pepsin:matrix ratio was 1:10. The matrix was allowed to digest for 48 hours under constant stirring. When completely solubilized, as indicated by the lack of particles in solution, myocardial matrix was brought to pH 8 through the addition of sodium hydroxide (NaOH) and 10× PBS, and diluted to 6 mg/mL with 1× PBS. Final solubilized myocardial matrix was kept on ice until it was used for characterization, in vitro migration experiments, or gelation in vivo.
2.3. Characterization of myocardial matrix
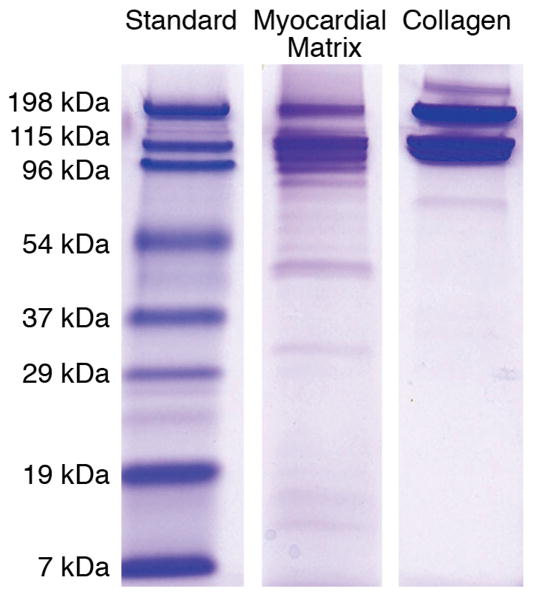
Solubilized myocardial matrix was analyzed by SDS-PAGE and compared to collagen type I (BD Biosciences, San Jose, CA). Solutions were run on a Tris-HCl, 12% polyacrylamide gel (Bio-Rad Laboratories, Inc, Hercules, CA) in Tris/Glycine/SDS buffer (Fisher Scientific Inc, Hanover Park, IL), with 80 mM reducing agent Dithiothreitol (DTT) (Invitrogen, Carlsbad, CA). Gel electrophoresis was performed in an XCell Surelock MiniCell (Invitrogen), compared to a broad range standard, and stained with Imperial Protein Stain (Pierce, Rockfods, IL).
The chemical make up of the myocardial matrix was further characterized for glyocosaminoglycan (GAG) content using the Blyscan assay (Biocolor). GAG content in the native solubilized myocardial matrix from four different digestions, from varied batches of tissue was quantified. Rat tail collagen and collagen from calf skin (Sigma) served as negative controls.
Gels were formed in vitro by allowing solubilized myocardial matrix to incubate at 37°C. Scanning electron microscopy (SEM) was used to visualize the structure of the myocardial matrix gel. Gels were prepared for SEM by fixation with 2.5% glutaraldehyde for two hours, followed by dehydration with a series of ethanol rinses (30-100%), as is common for tissue and natural proteins [17, 21, 22]. Samples were then critical point dried and coated with iridium, using an Emitech K575X Sputter coater. Electron microscopy images were taken using a Phillips XL30 Environmental SEM Field Emission microscope.
2.4. In vitro cell studies
Neonatal rat cardioymyocytes were plated onto collagen coated dishes, or gels of collagen or myocardial matrix to compare cell viability. Cardiomyocytes were harvested from freshly dissected ventricles of 2 day old Sprague-Dawley rats using an isolation kit (Cellutron, Baltimore, MD). The initial supernatant was discarded, but the subsequent 20 min digestions were strained and suspended in Dulbecco's Modified Eagle's Medium (DMEM) (Mediatech Manassas, VA) supplemented with 17% M199, 10% horse serum, 5% fetal bovine serum (FBS) (Gemini Bio-Products, West Sacramento CA), and 1% penicillin/streptomycin. After isolation, the supernatant was pre-plated onto tissue culture polystyrene dishes to increase purity of cardiomyocytes through selective adhesion. 50 μL of myocardial matrix or collagen at 6 mg/mL were allowed to gel by incubation in a 96 well plate at 37 °C for 2-4 hours. Gels were rinsed in sterile PBS for 20 minutes, and seeded with neonatal rat cardiomyocytes at a density of 2 × 104 cells per well. For collagen coated plates, tissue culture dishes were coated with collagen (1 mg/mL) for 1 hr at 37 °C, and rinsed with PBS prior to cardiomyocyte seeding. Media was changed daily to remove any non-adherent cells. Cell viability was assessed with a Live/Dead Cytotoxicity stain (Invitrogen, Carlsbad CA).
Migration of human coronary artery endothelial cells (HCAECs) and rat aortic smooth muscle cells (RASMCs) was assessed, using a Chemotaxis 96-well Cell Migration Assay Kit (Chemicon, Billerica, MA) as previously described [23, 24], to evaluate the chemoattractive properties of the myocardial matrix as compared to collagen, fetal bovine serum, and pepsin. HCAECs were purchased from Cell Applications, Inc (San Diego, CA) and cultured in MesoEndo Endothelial Cell Media (Cell Applications). RASMCs were isolated from 3 month old Harlan Sprague-Dawley rats, as previously described [25]. Briefly, the aorta was isolated, dissected free from adventitia, and opened longitudinally. Endothelial cells were rinsed off, and the aorta was minced into small pieces. These pieces were weighted under sterile cell strainers in DMEM containing 20% FBS and 1% penicillin/streptomycin. RASMCs grew out from the explants after 2 weeks, and were then cultured and passaged.
In preparation for the migration assay, cells were serum starved (per manufacturer's instructions) for 15-17 hours in DMEM, containing 0.5% heat-inactivated FBS with no added growth factors. The cells were then harvested with trypsin, and re-suspended to 4×105 cells/mL in serum-free media. The chemoattractant, myocardial matrix, was solubilized and neutralized as described above. Collagen was also neutralized for the study to be used as comparison to the myocardial matrix. 10% FBS in DMEM, a known chemoattractant for both endothelial cells [26] and smooth muscle cells [27], was used as a positive control. As pepsin was used to digest the decellularized ECM, it was tested alone as a control. Thus, pepsin was concurrently stirred in 0.1 M HC1 for 48 hours, neutralized, and diluted to match the myocardial matrix concentration. Manufacturer instructions were followed for the assay, where 150 μL of the chemoattractant was added into the feeder tray, and 100 μL of cell suspension was added to the top insert that was separated from the bottom chamber by a 8 μm pore filter. After a 4 hour incubation at 37°C with 5% CO2, the cells that migrated toward the chemoattractant remained on the underside of the 8 μm porous membrane. These cells were detached, lysed, stained, and quantified by fluorescence. Fluorescent measurements were performed on a SpectraMax Plate Reader at 483/535 nm. An increase in fluorescence intensity correlates to an increase in the number of cells that migrated through the filter.
2.5. In vivo feasibility of myocardial matrix
Prior to proceeding with in vivo studies, the solubilized myocardial matrix was tested for its ability to be pushed through the 27 gauge nitinol tubing of a Myostar catheter (Cordis), which is used in clinical trials for cell transplantation into myocardium [28]. Solubilized myocardial matrix (0.5 mL) at 6 mg/mL was drawn up in a 1 mL Luer Lok syringe and allowed to sit at room temperature for approximately 20 min before being pushed through the catheter at a rate of 0.2 mL in 30 seconds.
For in vivo assessment, solubilized myocardial matrix was injected into the left ventricular (LV) wall of male Harlan Sprague-Dawley rats (375-450 g), as previously described [5, 6, 29, 30]. Briefly, rats were anesthetized using isoflurane at 5%, intubated, and maintained at 2.5% isoflurane during surgery. Animals were placed in supine position, an incision was made from the xyphoid process along the abdomen, and a minimal incision was then made in the diaphragm to expose the apex of the heart, leaving the sternum intact. A single injection of 90 μL of solubilized myocardial matrix was then made directly into the LV free wall through a 30 gauge needle. The needle was held at an angle to ensure injection into the free wall and reduce the possibility of injecting into the lumen. Injection was confirmed by a lightening of the myocardium at the location of injection. Prior to recovery from anesthesia, animals were given 0.05 mg/kg of buprenorphine hydrochloride (Reckitt Benckiser Healthcare (UK) Ltd., Hull, England), an analgesic. Animals were also given 3 mL of Lactated Ringers (Hospira Inc. Lake Forest IL) solution for hydration during surgery. A total of eleven rats were injected with myocardial matrix for this study. All animals survived the injection surgery. To determine how quickly the myocardial matrix gelled in vivo, rats were euthanized at 30 min (n=2), and 1 hr (n=2). For assessment of cellular infiltration, rats were euthanized at either 4 hr (n=2) or 11 days (n=5) post-injection.
2.6. Histology and immunohistochemistry
At varied time points after injection surgery, animals were euthanized with an overdose of sodium pentobarbital (200 mg/kg). Hearts were immediately removed, fresh frozen in Tissue Tek O.C.T. freezing medium, and sectioned into 10 μm slices. Slides spaced every 0.5 mm were stained with H&E. To assess the inflammatory response a blinded pathologist compared myocardial matrix injections to hearts injected with fibrin (11 days post-injection), which is a well-established and characterized biomaterial. Adjacent slides from the regions with the largest sections of matrix injection, as identified by H&E, were then used for immunohistochemistry (IHC). Two slides from each heart removed at 4 hours and 11 days were examined. Sections were fixed for 1.5 minutes in acetone and blocked with staining buffer for 25 minutes (0.3% Triton X-100 and 2% goat serum in PBS). Hearts were stained with an anti-smooth muscle actin antibody (Dako, Carpinteria, CA; 1:75 dilution) and FITC labeled isolectin (Vector Laboratories, Burlingame, CA; 1:100 dilution), to label smooth muscle cell and endothelial cell infiltration, respectively, into the injected region. Alexa Fluor 568 anti-mouse (Invitrogen; 1:2000 dilution) was used as a secondary for the smooth muscle actin. Sections that were stained with only the primary antibody or only the secondary antibody were used as negative controls. All sections were mounted with Fluoromount (Sigma). Images were taken using Carl Zeiss Observer D.1 and analyzed with AxioVision software and Photoshop. Arterioles were quantified using the following criteria: 1) positive smooth muscle staining, 2) within the myocardial matrix injection region, 3) having a visible lumen, and 4) diameter ≥ 10μm. Long axis and short axis dimensions were averaged to find vessel diameter.
2.7. Statistical analysis
All data is presented as the mean ± standard deviation. For the Blyscan assay, samples were run in triplicate and results averaged. In the migration assay, each experimental condition was tested across 3-4 wells. Significance was determined using one-way analysis of variance (ANOVA) with a Holm's correction for the migration assay data, a two-tailed student's t-test for all other data, and reported as p < 0.05 and p < 0.001.
3. Results
3.1. Gelation and characterization of myocardial matrix in vitro
The myocardial matrix material was derived through decellularization of porcine ventricular tissue. Upon completion of decellularization, ventricular ECM was frozen, sectioned, and stained with H&E, in order to confirm the complete removal of cells. The absence of nuclei demonstrated the complete decellularization of the tissue, as compared to intact ventricular porcine tissue (Figure 1). The decellularized ECM was solubilized using pepsin, and after visual confirmation of the lack of particles in solution, solubilized myocardial matrix was further characterized to determine the presence of several extracellular matrix components. Gel electrophoresis of solubilized myocardial matrix demonstrated corresponding bands to collagen, as well as the presence of lower molecular weight bands. Thus, gel electrophoresis revealed additional peptides or ECM fragments, demonstrating the complexity of the myocardial matrix, as compared to strictly collagen (Figure 2). Moreover, using a Blyscan assay, the GAG content of the solubilized myocardial matrix was determined to be 23.2 ± 4.63 μg per mg of matrix, while no GAG content was present in collagen.
Figure 1.
Hematoxylin and eosin stained sections of the decellularized myocardial matrix, as compared to non-decellularized ventricular pig tissue. (A,B) Intact decellularized matrix prior to lyophilization and milling, at 10× and 40× respectively. (C) Intact porcine ventricular tissue, prior to decellularization. Scale bars are 100 μm. Note the absence of cells of the decellularized matrix, as compared to native tissue.
Figure 2.
PAGE of solubilized myocardial matrix and collagen type I. Note that the matrix is a more complex mixture of ECM components than collagen, as indicated by several lower molecular weight bands.
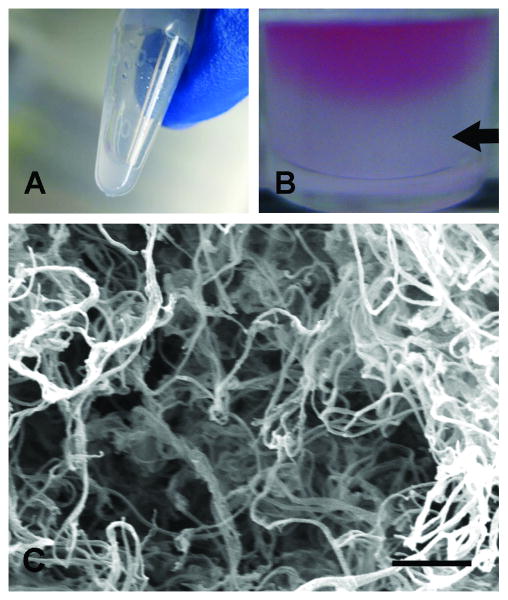
The myocardial matrix was further characterized, as a gel form in vitro. Solubilized myocardial matrix remained a viscous liquid on ice or at room temperature (Figure 3A), until gelation was induced at 37°C (Figure 3B). The resulting gel was a soft solid that required gentle handling. SEM analysis revealed that the myocardial matrix consisted of a nanofibrous and mesoporous structure. The re-assembled nanofibers were approximately 40-100 nm in diameter (Figure 3C).
Figure 3.
Myocardial matrix gelation and characterization. (A) At room temperature the solubilized matrix was a liquid. (B) At 37 °C, the myocardial matrix self-assembled into a hydrogel, as indicated by the arrow. Pink media is shown on top as a contrast to the solidified gel. (C) Scanning electron micrograph of a cross-section of the myocardial matrix gel with nanofibers approximately 40-100 nm. Scale bar is 1 μm.
3.2. In vitro cell studies
As a first step towards assessing biocompatibility, we examined the ability of cardiomyocytes to be cultured on the myocardial matrix gel. We found that cardiomyocytes were able to adhere and survive on the myocardial matrix for up to 5 days (longest time point tested). Cell viability was comparable to cardiomyocytes cultured on collagen coated dishes and collagen gels (data not shown).
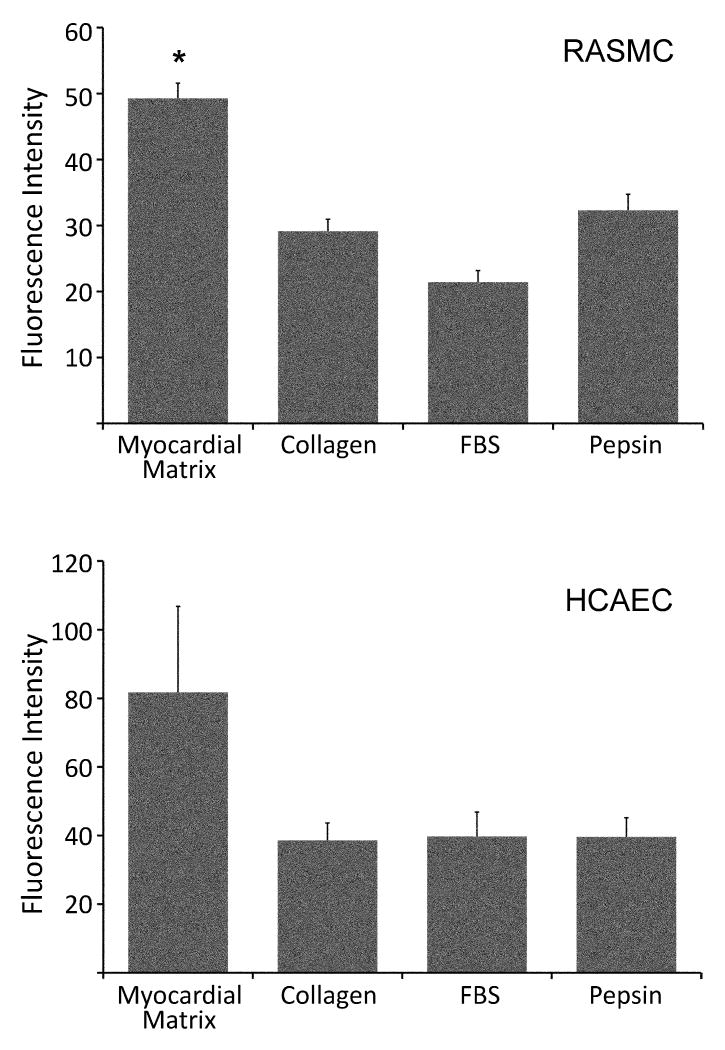
Since vascular cell infiltration in vivo will be essential to neovascularization, migration of vascular cells towards the myocardial matrix was first tested in vitro. A Chemotaxis 96-well Cell Migration Assay Kit was used to quantify the attraction of HCAECs and RASMCs towards the solubilized myocardial matrix, as compared to collagen, FBS, and pepsin. The migration of RASMCs towards the myocardial matrix was significantly greater than all other groups: collagen, FBS, and pepsin (p < 0.001) (Figure 4). While HCAECs migrated toward the myocardial matrix, the migration was not statistically greater than the other groups per ANOVA. However, there is a noticeable trend similar to that of RASMCs (Figure 4). Thus, the matrix was shown to promote migration of both cell types in vitro.
Figure 4.
Vascular cell migration in vitro. Rat aortic smooth muscle cell (RASMC) and human coronary artery endothelial cell (HCAEC) migration towards solubilized myocardial matrix, collagen, fetal bovine serum (FBS), and pepsin. Fluorescence Intensity is proportional to the number of cells that migrated toward each chemoattractant. Both cell types show migration towards the myocardial matrix. Significance from other groups, as determined by an ANOVA with Holm's correction, is indicated (*p < 0.001).
3.3 Injection and gelation in vivo
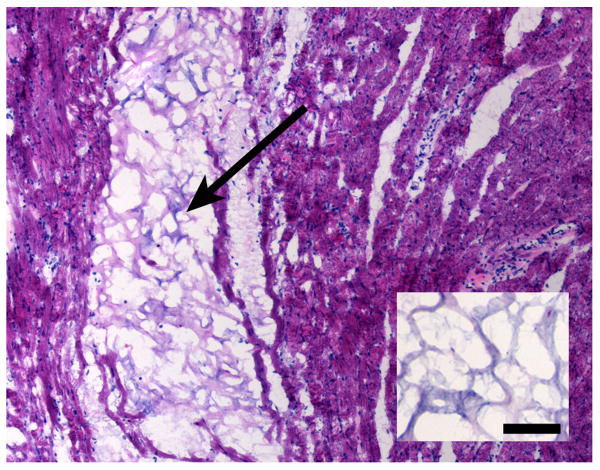
To be a clinically relevant injectable material for minimally invasive delivery, the matrix must be able to pass through a small gauge catheter. Thus, before beginning in vivo studies in a rodent model, we confirmed that the solubilized myocardial matrix could be pushed through a catheter that used clinically for endocardial injection into the myocardium. The myocardial matrix was tested after being maintained at room temperature for 20 minutes, and was successfully able to be pushed with minimal resistance. After confirming that the solubilized myocardial matrix was compatible with the Cordis catheter, we demonstrated that it could also be injected into the LV free wall of a rat through a 30 gauge needle. Animals were euthanized at various time points post-injection to determine the gelation time in vivo. A fibrous gel was observed as early as 30 min post-injection (Figure 5), indicating that the myocardial matrix can be successfully injected into the LV free wall and that it gels in situ. Each of the short time points confirmed that the matrix was able to gel in vivo (n = 6), in addition to preliminary studies in test hearts. It is also interesting to note that the structure and porosity of the injected gel resemble that of the decellularized intact ventricular ECM (Figure 5, inset). At 11 days post-injection, a blinded pathologist confirmed that the response to the myocardial matrix was similar to fibrin (TISSEEL sealant) injections. Fibrin sealant was chosen as a control since it is widely used in various surgeries and tissue engineering applications (including the myocardium), and is well known to be biocompatible and non-toxic, without inducing chronic inflammation, foreign body reactions, tissue necrosis or extensive fibrosis [31].
Figure 5.
Hematoxylin and eosin stained section of the myocardial matrix gel within the rat myocardium 30 min post-injection, indicating gelation time in vivo. Arrow denotes area of injected myocardial matrix. Inserted image is decellularized intact myocardial ECM. Scale bar is 100 μrn. Note the similar structure of the decellularized tissue to the self-assembled gel in vivo.
3.4 Cellular infiltration and neovascularization
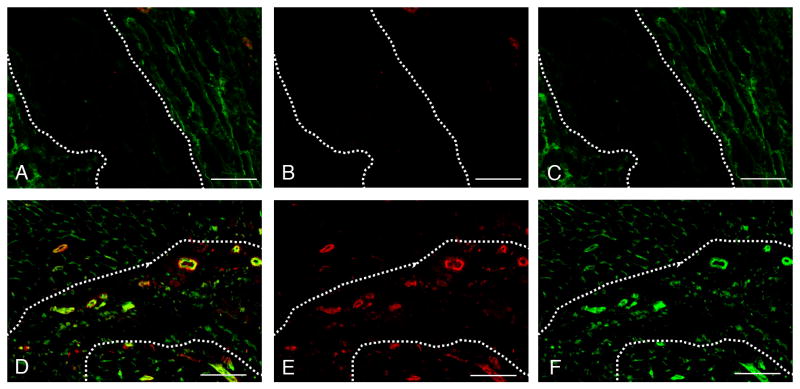
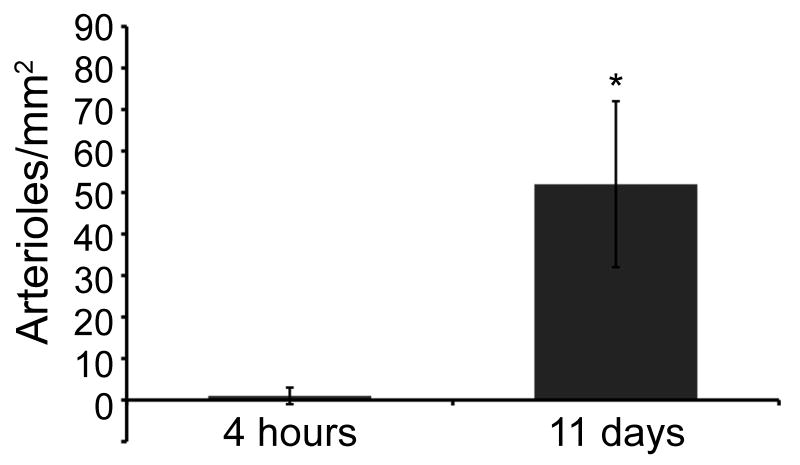
After demonstrating migration of vascular cells to the myocardial matrix in vitro and the ability of the solubilized matrix to form a gel in situ, we sought to determine whether the myocardial matrix would support vascular cell infiltration and new vessel formation in vivo. Immunohistochemistry was performed on several sections from the hearts of animals euthanized at 4 hours and at 11 days post injection, in order to observe cellular infiltration and potential neovascularization in the myocardial matrix region. Slides were stained for endothelial cells and smooth muscle cells, the two cell types already demonstrated to preferentially migrate towards the myocardial matrix in vitro. Each stain revealed little to no cellular infiltration immediately following injection (up to 4 hours post-injection) (Figure 6A-C). Endothelial and smooth muscle cell staining indicated the infiltration of both vascular cell types within the matrix region at 11 days (Figure 6D-F). In addition, arteriole density at 11 days post-injection (52 ± 20 arterioles/mm2) was significantly higher than at 4 hours post-injection (1 ± 2 arterioles/ mm2), p = 0.02 (Figure 7). As 4 hours is too soon to for a mature vessel to develop, it is likely that the matrix formed a gel around an existing vessel. Arterioles with a visible lumen, and diameter larger than 10 μm, were observed at 11 days, indicating maturity. In addition, several arterioles were >25 μm in diameter. Inaccurate segmentation prevented reliable analysis of endothelial cells, and thus capillary density was not quantified. It can be visually noted, however, that capillaries infiltrated the matrix at 11 days (Figure 6F).
Figure 6.
Endothelial and smooth muscle cell infiltration into the myocardial matrix gel, within the rat myocardium. (A-C) 4 hr post-injection. Note the lack of cells within the matrix. (D-F) 11 days post-injection. (A,D) Merged images showing endothelial cells (green) and smooth muscle cells (red). Area of myocardial matrix is denoted by arrows and is surrounded by the dotted line. (B, E) arterioles only. (C, F) endothelial cells only. Scale bars are 100 μm.
Figure 7.
Quantification of arteriole density within myocardial matrix at 4 hours and 11 days post injection into the rat myocardium. Arterioles were counted if they stained positive for smooth muscle cell actin and had a visible lumen greater than 10 μm. Arteriole density at 11 days post injection was shown to significantly increase from 4 hours post injection. ( *p = 0.02)
4. Discussion
Recent trends in tissue engineering have focused on scaffold materials that are biomimetic, meaning that they mimic the structure, morphology, chemical cues, and biologic cues of the natural environment [2-4]. The ECM is a complex combination of fibrous proteins (such as collagen, laminin, and fibronectin) and hydrophilic proteoglycans that serves to guide cellular attachment, survival, migration, proliferation, and differentiation [2, 3, 16, 20, 32, 33]. Thus, to properly regenerate tissue, an engineered scaffold will have the distinct role of influencing how cells will organize, proliferate, and differentiate in vivo [2, 32]. To fulfill this role, the ideal scaffold for tissue engineering is one that will provide the proper structural and chemical cues to allow for cell-matrix interactions that mimic those of the natural microenvironment [2-4]. Materials currently being explored for in situ cardiac tissue engineering do not provide a cardiac specific extracellular environment [14]. For example, materials have been single protein or peptide scaffolds such as collagen [7, 8], fibrin [5, 6], and synthetic self-assembling peptides [12], which do not mimic the complex ECM. Others have been derived from non-mammalian sources such as alginate [11], and chitosan [13]. Matrigel [9, 10], which provides the most complex mixture of ECM components, is derived from mouse sarcoma cells and does not contain the proper mixture of ECM components for any natural tissue. Herein, we demonstrate the feasibility of a biomimetic injectable myocardial matrix that has been designed specifically for cardiac tissue engineering.
Several groups have shown the potential of decellularizing tissues such as small intestine, liver, urinary bladder, blood vessels, dermis, and heart valves through physical, chemical, and enzymatic methods for a variety of tissue engineering applications [15, 16]. For myocardial applications, urinary bladder matrix and small intestine submucosa (SIS) have been examined for treating cardiac wall defects and MI [18, 19]. However, they have been shown to form undesirable tissue such as cartilage in the myocardium [19], potentially because they do not provide cardiac specific cues for cell-matrix interactions in the myocardium. Each tissue has its own ECM, designed for and secreted by the cells of that tissue, suggesting that there are tissue-specific interactions between cells and the ECM [16, 34]. Recently, a comparison of decellularized dermal, fat, and sarcoma tissues revealed that ECM composition varies among tissue and disease [20]. This finding demonstrated that while ECM components may be similar across a range of tissues, each tissue has its own distinct combination of macromolecules that provide the necessary cellular cues [20]. Thus, when designing a scaffold to mimic the ECM of a particular tissue, it follows logically to utilize the ECM from that tissue when possible.
In this study, we explored the feasibility of an injectable form of decellularized ventricular myocardial matrix for myocardial tissue repair. While some of the three dimensional structure is admittedly lost when preparing an injectable form of myocardial matrix, the solubilized matrix still retains ECM proteins and peptide fragments, as well as glycosaminoglycan content. These findings demonstrate that the myocardial matrix retained its biochemical components, as is necessary for cell-matrix interactions [2-4].
In addition to the biochemical composition of a scaffold material, the structural, mechanical, and degradation properties are of importance [2-4]. Herein, we have focused on the structural properties. A nanofibrous scaffold allows for a high surface area to volume ratio, thus allowing for increased cell attachment [35]. To this end, various research groups have focused on the development of synthetic, natural, and combination materials, fabricated via electrospinning and other techniques [3, 36-38], to use as scaffolds that appropriately mimic the nanofibrous structure of the natural ECM. While electrospun materials and scaffolds developed ex vivo provide the nanostructure desired for tissue engineering applications, they often do not lend themselves to injectable delivery. The myocardial matrix gel self-assembles with nanofibers of similar diameter to collagen [22], and can be injected directly into the myocardial tissue. It should also be noted that the nanofibrous structure of the myocardial matrix gel closely mimics that of a recently developed urinary bladder matrix gel [21]. In addition to a nanofibrous structure, pore sizes should be of adequate size (> 10 μm) to allow for cellular infiltration and migration in vivo [3, 4]. The pores of the matrix upon injection in vivo are ∼ 30 μm, with a similar porosity and structure to the decellularized tissue shown here. Although the mechanical and degradation properties remain to be elucidated, it should be noted that cross-linking techniques similar to those used for collagen gels [39] can be explored to help strengthen the mechanical properties of the material, as well control degradation mechanisms and rates. What we have demonstrated is that while a variety of techniques are being explored to design the ideal biomimetic material for myocardial tissue engineering, the decellularized myocardial tissue explored here is able to self-assemble into a nanofibrous and mesoporous structure.
The biochemical properties and structural composition of a tissue engineered scaffold are important in guiding cell-matrix interaction, including cell migration into the matrix material [2-4]. The ability of the scaffold to support and promote vascular infiltration is considered one of the most important requirements of a tissue engineering scaffold material [3, 4, 40]. This is of particular importance in the ischemic post-MI environment. A biomaterial itself, or soluble signals such as degradation products, should thus facilitate repair via recruitment of vascular cells [3, 4]. Herein, we have shown that endothelial cells and smooth muscle cells migrate towards the myocardial matrix in vitro and form mature vessels with the matrix in vivo. This result is consistent with studies that have demonstrated cellular migration towards pepsin-digested fragments of other matrix materials in vitro [3, 23, 41, 42], the migration of endothelial cells into decellularized matrices ex vivo [43, 44], as well as in vivo neovascularization into cardiac patches made from SIS, bladder matrix, and fibroblast matrix [18, 19, 45]. While these patches serve only the epicardial surface of the ventricle, an injectable material has the potential to promote neovascularization throughout the LV wall. Although several other materials have demonstrated the ability to promote angiogenesis when injected into the myocardium alone or with cells [6, 12, 30], not all materials do this at 1 -2 weeks [12], as is observed with myocardial matrix. Neovascularization potential of a material is important for both cellular and acellular approaches, as cells rely on new vessels to provide oxygen and nutrients for survival. Even in an acellular approach, where a material is injected on its own, neovascularization is essential to support the cells that infiltrate into the material and for eventual regeneration of the region.
This study demonstrates the ability of our myocardial matrix gel to attract vascular cells both in vitro and in a rat model. While a rat model provides an effective means of studying new materials and their capabilities, before beginning in vivo studies, it was critical to look ahead to the clinical relevance of developed strategies to treat MI. Cellular cardiomyoplasty is a technique used clinically [28, 46, 47], whereby cells are suspended in liquid solutions of saline or media and are delivered minimally invasively through a catheter into the myocardial tissue. Therefore, developing scaffolds that are compatible with catheter delivery would eliminate the need for invasive surgical procedures required for cardiac patch therapies. Several other materials have been tested in a rat model, but may not translate into large animal models or human trials because of their inability to be used in a small gauge catheter. Thus, before beginning in vivo studies, we felt it necessary to confirm that the matrix could be potentially translated to the clinic for minimally invasive catheter delivery. Solubilized myocardial matrix remains liquid at room temperature, preventing possible clogging of the catheter, which may occur with more rapidly gelling materials, such as collagen and fibrin. Thus, the success of our myocardial matrix material to be pushed through the tubing of a 27 gauge catheter with minimal resistance, after 20 minutes at room temperature, indicates its potential for clinical translation as a minimally invasive scaffold for in situ myocardial tissue engineering.
In this study, we set out to characterize an injectable myocardial matrix gel as a myocardial ECM mimic and determine whether it would meet the criteria of an injectable scaffold for myocardial tissue engineering. Similar to a previous report [12], we examined this material first in healthy myocardium, to allow for easy visualization and identification of the re-assembled matrix in situ. This allowed for distinct identification of the borders of the myocardial matrix, which facilitated assessment of cell infiltration. Now that we have characterized this material, and demonstrated its feasibility as an injectable scaffold, with the ability to gel in situ and promote vascular cell infiltration, future studies will allow us to assess the potential of the myocardial matrix in post MI rats, as a cellular or acellular scaffold for myocardial tissue engineering.
5. Conclusion
The results of this study show the potential of an injectable form of myocardial matrix for use as an in situ gelling scaffold for myocardial tissue engineering. We have successfully demonstrated the ability of the solubilized myocardial matrix to form a nanofibrous gel in vitro and in situ, the migration of vascular cells towards the myocardial matrix in vitro, and the ability of the matrix to promote vascular cell infiltration, including arteriole formation at 11 days post-injection in vivo. In addition, we have shown the clinical potential of this material for minimally invasive delivery, by pushing the solubilized material through a 27 gauge Myostar catheter. This myocardial matrix has the potential to advance cardiac tissue engineering therapies by providing the appropriate biomimetic environment. This study demonstrates the in vivo feasibility of a naturally derived injectable material that has been designed specifically to mimic the natural myocardial ECM.
Acknowledgments
The authors would like to acknowledge the following members of the Christman lab: Adam Young for assistance with gel electrophoresis, Cynthia Cam for assistance with the migration assay, and Alvin Cabrera for histology. In addition, we would like to thank Richard Sievers, of the University of California, San Francisco, for surgical and technical guidance, and Nabil Dib, MD for assistance with initial catheter compatibility tests. We would also like to acknowledge Ryan Anderson, Michael Clark, and the CalIT2 Nano3 Facility for the use of the Scanning Electron Microscope, Cordis for providing the nitinol tubing from their Myostar catheter, and Dr. Nissi Varki for histopathological analysis. J.A.D. would also like to thank the California Institute for Regenerative Medicine (CIRM) under the UCSD Interdisciplinary Stem Cell Research and Training Program for a pre-doctoral fellowship.
Funding Sources: This research was supported in part by the NIH Director's New Innovator Award Program, part of the NIH Roadmap for Medical Research, through grant number 1-DP2-OD004309-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts to disclose.
References
- 1.Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, et al. Heart disease and stroke statistics -- 2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Leor J, Amsalem Y, Cohen S. Cells, scaffolds, and molecules for myocardial tissue engineering. Pharmacol Ther. 2005;105:151–163. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 3.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23:47–55. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 4.Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. Myocardial tissue engineering: a review. J Tissue Eng Regen Med. 2007;1:327–342. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- 5.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Fibrin glue alone and skeletal myoblasts in a fibrin scaffold preserve cardiac function after myocardial infarction. Tissue Eng. 2004;10:403–409. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 6.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. Injectable fibrin scaffold improves cell transplant survival, reduces infarct expansion, and induces neovasculature formation in ischemic myocardium. J Am Coll Cardiol. 2004;44:654–660. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 7.Dai W, Wold LE, Dow JS, Kloner RA. Thickening of the infarcted wall by collagen injection improves left ventricular function in rats: a novel approach to preserve cardiac function after myocardial infarction. J Am Coll Cardiol. 2005;46:714–719. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 8.Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, et al. Tissue-engineered injectable collagen-based matrices for improved cell delivery and vascularization of ischemic tissue using CD133+ progenitors expanded from the peripheral blood. Circulation. 2006;114:I138–144. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- 9.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nature Biotechnology. 2007;25:1015–1024. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 10.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Novel injectable bioartificial tissue facilitates targeted, less invasive, large-scale tissue restoration on the beating heart after myocardial injury. Circulation. 2005;112:I173–177. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 11.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008;117:1388–1396. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 12.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, et al. Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells. Circulation. 2005;111:442–450. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lu WN, Lu SH, Wang HB, Li DX, Duan CM, Liu ZQ, et al. Functional Improvement of Infarcted Heart by Co-Injection of Embryonic Stem Cells with Temperature-Responsive Chitosan Hydrogel. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0143. [DOI] [PubMed] [Google Scholar]
- 14.Christman KL, Lee RJ. Biomaterials for the treatment of myocardial infarction. J Am Coll Cardiol. 2006;48:907–913. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 15.Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–3683. doi: 10.1016/j.biomaterials.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Badylak SF. The extracellular matrix as a biologic scaffold material. Biomaterials. 2007;28:3587–3593. doi: 10.1016/j.biomaterials.2007.04.043. [DOI] [PubMed] [Google Scholar]
- 17.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI, et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat Med. 2008;14:213–221. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 18.Robinson KA, Li J, Mathison M, Redkar A, Cui J, Chronos NAF, et al. Extracellular matrix scaffold for cardiac repair. Circulation. 2005;112:I-135–143. doi: 10.1161/CIRCULATIONAHA.104.525436. [DOI] [PubMed] [Google Scholar]
- 19.Badylak SF, Obermiller J, Geddes L, Matheny R. Extracellular matrix for myocardial repair. The Heart Surgery Forum. 2002;6:E20–26. doi: 10.1532/hsf.917. [DOI] [PubMed] [Google Scholar]
- 20.Uriel S, Labay E, Francis-Sedlak M, Moya ML, Weichselbaum RR, Ervin N, et al. Extraction and Assembly of Tissue-Derived Gels for Cell Culture and Tissue Engineering. Tissue Eng Part C Methods. 2008;15 doi: 10.1089/ten.tec.2008.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Preparation and rheological characterization of a gel form of the porcine urinary bladder matrix. Biomaterials. 2008;29:1630–1637. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Stuart K, Panitch A. Influence of chondroitin sulfate on collagen gel structure and mechanical properties at physiologically relevant levels. Biopolymers. 2008;89:841–851. doi: 10.1002/bip.21024. [DOI] [PubMed] [Google Scholar]
- 23.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, et al. Degradation Products of Extracellular Matrix Affect Cell Migration and Proliferation. Tissue Eng Part A. 2009;15(3):605–614. doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 24.Akahane T, Akahane M, Shah A, Connor CM, Thorgeirsson UP. TIMP-1 inhibits microvascular endothelial cell migration by MMP-dependent and MMP-independent mechanisms. Exp Cell Res. 2004;301:158–167. doi: 10.1016/j.yexcr.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 25.San Antonio JD, Karnovsky MJ, Ottlinger ME, Schillig R, Pukac LA. Isolation of heparin-insensitive aortic smooth muscle cells. Growth and differentiation. Arterioscler Thromb. 1993;13:748–757. doi: 10.1161/01.atv.13.5.748. [DOI] [PubMed] [Google Scholar]
- 26.Lau YT, Ma WC. Nitric oxide inhibits migration of cultured endothelial cells. Biochem Biophys Res Commun. 1996;221:670–674. doi: 10.1006/bbrc.1996.0654. [DOI] [PubMed] [Google Scholar]
- 27.Di Luozzo G, Pradhan S, Dhadwal AK, Chen A, Ueno H, Sumpio BE. Nicotine induces mitogen-activated protein kinase dependent vascular smooth muscle cell migration. Atherosclerosis. 2005;178:271–277. doi: 10.1016/j.atherosclerosis.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Steendijk P, Smits PC, Valgimigli M, van der Giessen WJ, Onderwater EE, Serruys PW. Intramyocardial injection of skeletal myoblasts: long-term follow-up with pressure-volume loops. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S94–100. doi: 10.1038/ncpcardio0416. [DOI] [PubMed] [Google Scholar]
- 29.Huang NF, Sievers RE, Park JS, Fang Q, Li S, Lee RJ. A rodent model of myocardial infarction for testing the efficacy of cells and polymers for myocardial reconstruction. Nat Protoc. 2006;1:1596–1609. doi: 10.1038/nprot.2006.188. [DOI] [PubMed] [Google Scholar]
- 30.Huang NF, Yu J, Sievers R, Li S, Lee RJ. Injectable biopolymers enhance angiogenesis after myocardial infarction. Tissue Eng. 2005;11:1860–1866. doi: 10.1089/ten.2005.11.1860. [DOI] [PubMed] [Google Scholar]
- 31.Radosevich M, Goubran HI, Burnouf T. Fibrin sealant: scientific rationale, production methods, properties, and current clinical use. Vox Sang. 1997;72:133–143. doi: 10.1046/j.1423-0410.1997.7230133.x. [DOI] [PubMed] [Google Scholar]
- 32.Macfelda K, Kapeller B, Wilbacher I, Losert UM. Behavior of cardiomyocytes and skeletal muscle cells on different extracellular matrix components--relevance for cardiac tissue engineering. Artif Organs. 2007;31:4–12. doi: 10.1111/j.1525-1594.2007.00334.x. [DOI] [PubMed] [Google Scholar]
- 33.Brown L. Cardiac extracellular matrix: a dynamic entity. Am J Physiol Heart Circ Physiol. 2005;289:H973–974. doi: 10.1152/ajpheart.00443.2005. [DOI] [PubMed] [Google Scholar]
- 34.Rosso F, Giordano A, Barbarisi M, Barbarisi A. From cell-ECM interactions to tissue engineering. J Cell Physiol. 2004;199:174–180. doi: 10.1002/jcp.10471. [DOI] [PubMed] [Google Scholar]
- 35.Matthews JA, Wnek GE, Simpson DG, Bowlin GL. Electrospinning of collagen nanofibers. Biomacromolecules. 2002;3:232–238. doi: 10.1021/bm015533u. [DOI] [PubMed] [Google Scholar]
- 36.Huang ZM, Zhang YZ, Kotaki M, Ramakrishna S. A review on polymer nanofibers by electrospinning and their applications in nanocomposites. Composites Science and Technology. 2003;63:2223–2253. [Google Scholar]
- 37.Pham QP, Sharma U, Mikos AG. Electrospinning of polymeric nanofibers for tissue engineering applications: a review. Tissue Eng. 2006;12:1197–1211. doi: 10.1089/ten.2006.12.1197. [DOI] [PubMed] [Google Scholar]
- 38.Stankus JJ, Freytes DO, Badylak SF, Wagner WR. Hybrid nanofibrous scaffolds from electrospinning of a synthetic biodegradable elastomer and urinary bladder matrix. J Biomater Sci Polym Ed. 2008;19:635–652. doi: 10.1163/156856208784089599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheu MT, Huang JC, Yeh GC, Ho HO. Characterization of collagen gel solutions and collagen matrices for cell culture. Biomaterials. 2001;22:1713–1719. doi: 10.1016/s0142-9612(00)00315-x. [DOI] [PubMed] [Google Scholar]
- 40.Patel ZS, Mikos AG. Angiogenesis with biomaterial-based drug- and cell-delivery systems. J Biomater Sci Polym Ed. 2004;15:701–726. doi: 10.1163/156856204774196117. [DOI] [PubMed] [Google Scholar]
- 41.Beattie AJ, Gilbert TW, Guyot JP, Yates AJ, Badylak SF. Chemoattraction of Progenitor Cells by Remodeling Extracellular Matrix Scaffolds. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brennan EP, Tang XH, Stewart-Akers AM, Gudas LJ, Badylak SF. Chemoattractant activity of degradation products of fetal and adult skin extracellular matrix for keratinocyte progenitor cells. J Tissue Eng Regen Med. 2008;2:491–498. doi: 10.1002/term.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li F, Li W, Johnson S, Ingram D, Yoder M, Badylak S. Low-molecular-weight peptides derived from extracellular matrix as chemoattractants for primary endothelial cells. Endothelium. 2004;11:199–206. doi: 10.1080/10623320490512390. [DOI] [PubMed] [Google Scholar]
- 44.Bader A, Schilling T, Teebken OE, Brandes G, Herden T, Steinhoff G, et al. Tissue engineering of heart valves--human endothelial cell seeding of detergent acellularized porcine valves. Eur J Cardiothorac Surg. 1998;14:279–284. doi: 10.1016/s1010-7940(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 45.Kellar RS, et al. Scaffold-based three-dimensional human fibroblast culture provides a structural matrix that supports angiogenesis in infarcted heart tissue. Circulation. 2001;104:2063–2068. doi: 10.1161/hc4201.097192. [DOI] [PubMed] [Google Scholar]
- 46.Menasche P, Hagege AA, Scorsin M, Pouzet B, Desnos M, Duboc D, et al. Myoblast transplantation for heart failure. Lancet. 2001;357:279–280. doi: 10.1016/S0140-6736(00)03617-5. [DOI] [PubMed] [Google Scholar]
- 47.Chachques JC, Acar C, Herreros J, Trainini JC, Prosper F, D'Attellis N, et al. Cellular cardiomyoplasty: clinical application. Ann Thorac Surg. 2004;77:1121–1130. doi: 10.1016/j.athoracsur.2003.09.081. [DOI] [PubMed] [Google Scholar]