Abstract
Spinal cord hemisection rostral to the phrenic nucleus leads to paralysis of the ipsilateral hemidiaphragm and respiratory insufficiency. Recovery of the paralyzed hemidiaphragm may be induced by activating a latent respiratory motor pathway in adult rats. Although the pathway is latent in adults, it may not be latent in neonatal rats as shown by the spontaneous expression of activity over this pathway in an earlier in vitro study. Activity mediated over the latent pathway is known as “crossed phrenic activity”. Whether crossed phrenic activity following C2 spinal cord hemisection occurs spontaneously in the neonatal rat in vivo is still unknown. We hypothesized that crossed phrenic activity may be spontaneously expressed in neonates in vivo and may be converted from a spontaneously active state to a latent and nonfunctional state during postnatal development. Thus, a time course study was designed to analyze this activity in rat pups at different ages. The functional status of the ipsilateral and contralateral hemidiaphragm was tested by EMG analysis following hemisection. Crossed phrenic activity was expressed in ventral, lateral, and dorsal parts of the ipsilateral hemidiaphragm in P2 and some P3 and P4 neonatal rats. During postnatal development, the activity was observed only in the ventral area of the ipsilateral hemidiaphragm in P7, P14, P21 and P28 animals. Significant decreases in the extent of ventral crossed phrenic activity were observed from P2 to P28. The pathway generating this activity becomes latent by postnatal day 35. The present results suggest that spontaneous crossed phrenic activity occurs in vivo following C2 hemisection and the activity gradually decreases during the first four postnatal weeks.
Keywords: spinal cord injury, hemisection, respiration, cross phrenic activity, postnatal development
Introduction
Injury of the cervical spinal cord may cause the diaphragm, the primary respiratory muscle in mammals, to become paralyzed and this may significantly impair normal respiratory function (Golder and Mitchell, 2005, Fuller et al., 2008). In rats, the diaphragm is innervated by phrenic motor neurons located at the C3 to C6 level of the cervical spinal cord bilaterally (DeVries and Goshgarian, 1989; Goshgarian and Rafols, 1984). Premotor neurons in the rostral ventral respiratory group (rVRG) of the medulla provide the excitatory drive to phrenic motoneurons during inspiration via crossed and uncrossed descending axon pathways in rats (Dobbins and Feldman, 1994). The descending respiratory pathways on one side of the spinal cord are interrupted by cervical (C2) spinal cord hemisection, resulting in the complete paralysis of the ipsilateral hemidiaphragm (Goshgarian and Guth, 1977; Moreno, et al., 1992). We have previously shown that respiratory function is restored to the paralyzed hemidiaphragm spontaneously 6-16 weeks following spinal cord hemisection without any intervention in adult rats (Nantwi et al., 1999). More recent studies have shown that the spontaneous recovery following C2 hemisection may be augmented by spinal synaptic enhancement with intermittent hypoxia (Golder and Mitchell, 2005; Fuller et al., 2003). Furthermore, respiratory recovery can be induced sooner than 6 weeks after hemisection by the administration of drugs like rolipram and theophyline (Kajana and Goshgarian, 2008; Nantwi and Goshgarian, 1998).
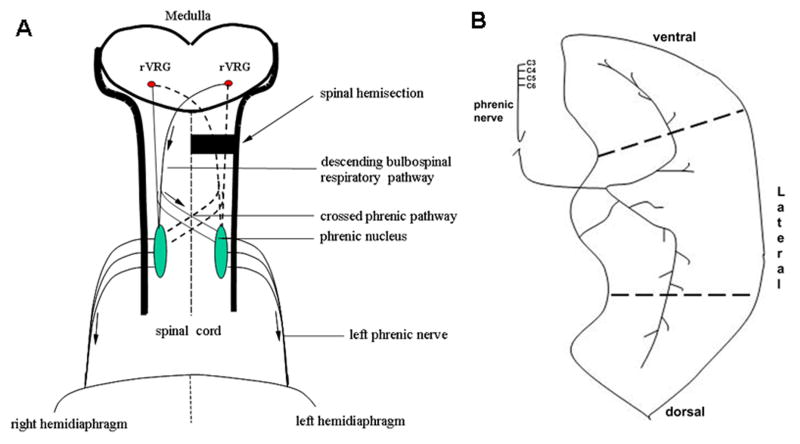
The restored function to the paralyzed hemidiaphragm is mediated by activating a crossed respiratory pathway which has been referred to as “the crossed phrenic pathway” (Goshgarian, 2003; Moreno, et al., 1992). The crossed phrenic pathway has been described as being latent and non-functional in adult rats (Goshgarian, 2003). Furthermore, it has been delineated anatomically as the collaterals from both crossed and uncrossed descending bulbospinal axons that cross the midline of the spinal cord and monosynaptically innervate both left and right phrenic motoneurons (Fig. 1A, see also Moreno, et al., 1992). However, another study found suggested that some propriospinal neurons could relay impulses between medullary respiratory neurons and the phrenic nucleus (Lane et al., 2008).
Figure 1.
A. Diagram of the crossed phrenic pathway in the adult rat. The pathway involves bilateral rVRG respiratory premotor axons with axon collaterals that cross the midline of the spinal cord. Arrows indicate the direction of respiratory impulses to the diaphragm after hemisection. B. Innervation of diaphragm muscle. The phrenic nerve primarily divides into rostral and caudal branches as it enters the diaphragm. EMG recordings were taken from 3 areas (ventral, lateral and dorsal) of the hemidiaphragm to assess the presence of the crossed phrenic activity immediately following C2 hemisection.
Although the crossed phrenic pathway is latent and non-functional in adult rats, we have recently shown that the neural pathway in neonatal brainstem-spinal cord preparations is active and functional (Zimmer and Goshgarian, 2005). This in vitro model was used to demonstrate spontaneous crossed phrenic activity following a complete C2 hemisection from postnatal day 0-4 (P0-P4) (Zimmer and Goshgarian, 2005). Although the peak amplitude of the crossed phrenic activity was significantly lower than the respiratory-like activity observed contralaterally, the activity nevertheless was present after hemisection suggesting that the crossed phrenic pathway may not be latent in neonates (Zimmer and Goshgarian, 2005). Furthermore, we also showed that the spontaneous crossed phrenic activity is age-dependent because younger preparations (P0-P2) exhibited a significantly higher incidence of crossed phrenic activity than older preparations (P3-P4) (Zimmer and Goshgarian, 2005). Since the brainstem-spinal cord preparation has been criticized as not reflecting true respiratory-related activity (Li and Duffin, 2004), there is a possibility that what was observed in the Zimmer and Goshgarian 2005 study may not be observed in vivo and this prompted the present investigation.
Numerous studies have suggested that the organization of the central respiratory network in perinatal rats and neonatal rats is different from adult rats and some nonfunctional neural projections are eliminated during development (Cameron, et al., 1991; Cameron and Nunez-Abades, 2000; Greer, et al., 1999; Rekling, et al., 2000). Since the latent crossed phrenic pathway still exists and has not been eliminated in adult rats (Moreno, et al., 1992), the crossed phrenic pathway may be functional in neonatal rats, otherwise it would likely have been eliminated. Based on the above, we hypothesized that crossed phrenic activity might be spontaneously expressed in neonates in vivo and we set out to determine when functional crossed phrenic activity converts to a latent state during postnatal development.
In the present investigation, a time course study was conducted to assess crossed phrenic activity in postnatal rats from P2 to P35. Our results showed that crossed phrenic activity is expressed spontaneously in vivo in postnatal rats following C2 hemisection. Furthermore, the extent of the crossed respiratory activity gradually decreases and eventually disappears by postnatal day 35. This differs significantly from what is seen in adult rats which show a completely quiescent ipsilateral phrenic nerve and paralyzed hemidiaphragm immediately after C2 hemisection (Goshgarian, et al., 1986; Moreno, et al., 1992).
Materials and methods
Animal surgery protocol
Timed pregnant female Sprague-Dawley rats were purchased from Harlan Rodent Laboratories and allowed to give birth in the animal care facilities at Wayne State University, School of Medicine. Litters of rat pups were housed with mothers together and individual postnatal rats were brought to the laboratory before each experiment. Postnatal rats from different litters were separated into 6 groups: P2, P7, P14, P21, P28, and P35 (N=16 /group, both female and male). 10 of the rats in each age group were subjected to a C2 hemisection, while the other 6 served as non-hemisected controls. An additional 6 hemisected rats per group at P3, P4, P5, and P6 were used to monitor the day to day changes in crossed phrenic activity observed during the first postnatal week. The average body weight of the postnatal rats in the different age groups is shown in Table 1. P2 was selected as the initial postnatal time to be analyzed because rat pups younger than P2 are too small to undergo diaphragmatic EMG recordings.
Table 1.
Body weight (grams) in different age groups on the day of hemisection surgery.
| P2 | P7 | P14 | P21 | P28 | P35 | |
|---|---|---|---|---|---|---|
| Control | 8.4±0.7 | 18.6± 1.7 | 31.9± 2.2 | 66.4± 5.4 | 87.8± 7.9 | 115.2±8.7 |
| Hemisected | 8.3±0.6 | 18.7± 1.2 | 30.2±1.9 | 64.7± 4.3 | 91.0±5.8 | 112.2±7.5 |
All values are presented as means±SE. Rat weight increased gradually during postnatal development.
Postnatal rats were prepared for aseptic hemisection surgery of the left C2 spinal cord. Rat pups were anesthetized with ketamine (30-40mg/kg, ip) and xylazine (10mg/kg, ip) and placed on a warming pad (37 °C) during surgery. A dorsal midline incision (1cm) was made through the cervical skin and the paravertebral muscles above the first three cervical vertebrate to expose the second vertebra. A laminectomy of the C2 vertebral bone and durotomy were performed to expose the cervical spinal cord. A left C2 spinal cord hemisection was performed with microscissors just caudal to the C2 dorsal roots under magnification. Then, the paravertebral muscles were sutured at the midline with 5.0 absorbable sutures and the skin was closed with tissue glue.
Electrophysiology
Immediately after hemisection, the pups were placed in the supine position and a horizontal incision was made through the abdominal skin and muscle (1-2cm) on the ventral surface caudal to the rib edge. A gauze pad soaked in sterile saline was placed over the exposed abdominal viscera to prevent desiccation. By retracting the ventral cut edge of the abdominal muscle rostrally, the abdominal surface of the diaphragm was exposed. Bipolar Grass E2B platinum wire recording electrodes were carefully inserted into the right and left side of the hemidiaphragm and a test for respiratory activity was made. The hemidiaphragm was divided into ventral, lateral, and dorsal areas (Fig. 1B). The ventral area was adjacent to the sternum, the lateral area was adjacent to the lateral edges of ribs 8-10, and the dorsal area was adjacent to the lateral edges of ribs 11-13. Respiratory activity on both sides of the diaphragm was recorded from ventral, lateral, and dorsal areas in that order by EMG. Body temperature was maintained at 37 °C with a thermostatic pad (Harvard). The EMG signals were filtered (0.1-3 Hz) and amplified (10 K) by using a Tektronix 502 amplifier. Animals were not ventilated and were allowed to breath spontaneously throughout the experiment.
Histology
Histological verification of a complete C2 hemisection was carried out in each animal. All rat pups were sacrificed by bilateral pneumothorax under anesthetization at the end of the experiment. The spinal cord tissue from C1 to C3 including the hemisection site (C2) was collected and fixed by immersion with 2.5% glutaraldehyde and 1% paraformaldehyde for 3 days at 4 °C and subsequently cryoprotected with 30% sucrose in 0.1 M PBS buffer for 3 days at 4 °C. The spinal cord tissue was then sectioned transversely at 50 μm on a cryostat and was dehydrated and stained with neutral red. Sections through the hemisection zone were mounted on glass slides and covered with permount.
Data analysis
EMG recordings were analyzed offline for respiratory frequency and peak amplitude of the diaphragmatic contraction for a period of 30 seconds. The 30 second window for analysis was selected approximately 10 min after recording in order to establish the baseline diaphragmatic activity. Right and left hemidiaphragm EMG signals were rectified and integrated (time constant=100 ms) by using Spike2 data analysis software (v 4.13, CED Cambridge, UK). Crossed phrenic activity was identified as the activity of the ipsilateral hemidiaphragm following C2 hemisection. Although the primary purpose of this study was to test a “yes/no” presence of crossed phrenic activity, the extent of crossed phrenic activity in the left hemidiaphragm was also expressed quantitatively as the percentage of the peak amplitude of the right hemidiaphragm activity in the same animal following left C2 hemisection. It needs to be emphasized that because of the small size of the animals employed in this study, standardized recording conditions including the regulation of blood pressure, end tidal CO2 and oxygen levels in the blood could not be established and there was significant variability between animals. Thus, the quantitative data presented is semi-quantitative at best, and is meant to provide only a general idea of the extent of crossed phrenic activity following hemisection in postnatal rats.
A one-way multiple analysis of variance (ANOVA) was used to compare the age-group difference of the peak amplitude and respiratory frequency and the successive age group difference was tested by a Tukey test of Post-Hoc analysis (SPSS 11). A Chi-Square Test was performed to test the incidence of crossed phrenic activity in different age groups in the first postnatal week. Group differences were considered statistically significant when P<0.05. All data is expressed as means± standard error (SE).
Results
Crossed phrenic activity in different age groups
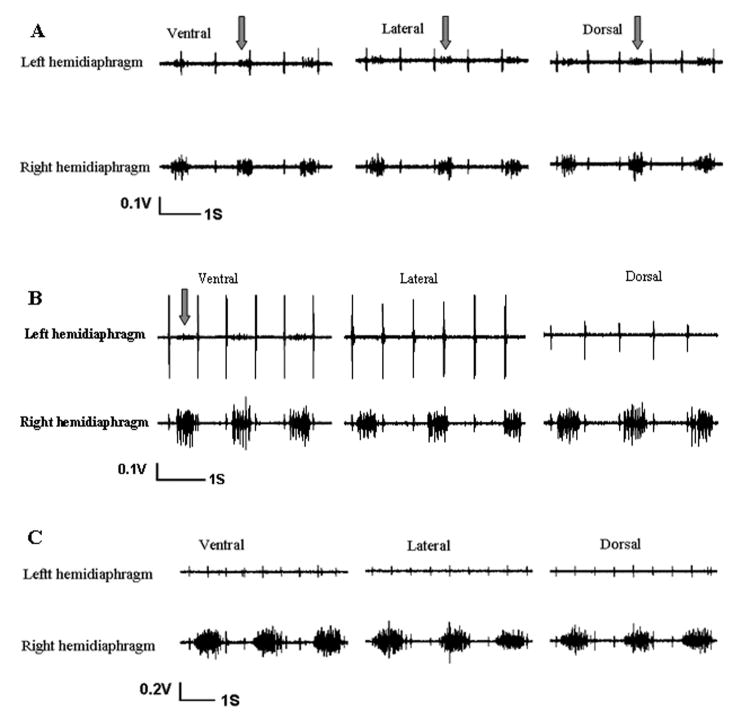
Every P2 neonatal rat showed a persistent, albeit weak expression of respiratory activity immediately following left C2 spinal cord hemisection in all three regions of the left hemidiaphragm as shown by EMG recordings (Fig. 2A). The respiratory activity of the left hemidiaphragm was coincident with activity on the right non-hemisected side. However, the activity ipsilateral to hemisection was markedly lower in amplitude qualitatively compared to the activity of the contralateral hemidiaphragm. The crossed phrenic activity in the left hemidiaphragm was spontaneously expressed in ventral, lateral, and dorsal areas of the left hemidiaphragm in P2 neonatal rats (Fig. 2A).
Figure 2.
Electromyographic (EMG) recordings from the left and right hemidiaphragm of postnatal rats after a complete left C2 spinal cord hemisection showing activity on both sides of the diaphragm. A) The spontaneous crossed phrenic activity which drives the left hemidiaphram after left C2 hemisection was expressed in a P2 neonatal rat. Arrows show the crossed activity in ventral, lateral, and dorsal parts of the left hemidiaphragm. B) The spontaneous crossed phrenic activity (arrow) is only expressed in the ventral part of the left hemidiaphragm of a P7 hemisected rat. No crossed phrenic activity was expressed at the lateral, or dorsal parts of the left hemidiaphragm. This pattern of crossed phrenic activity was also observed at P14, P21 and P28. C) EMG recordings from the left and right hemidiaphragm of a P35 hemisected rat. No spontaneous crossed activity was observed in the ventral, lateral, and dorsal parts of the left hemidiaphragm. This reflects the adult rat pattern in which no crossed phrenic activity is expressed after ipsilateral spinal cord hemisection.
All the postnatal rats at P7, P14, P21 and P28 (N=10/group) expressed spontaneous respiratory activity only in the ventral area of the ipsilateral hemidiaphragm immediately after left C2 spinal hemisection (Fig. 2B). Again, the ipsilateral EMG activity was lower in amplitude as compared to the non-hemisection side. The crossed phrenic activity corresponded to the respiratory activity contralateral to the hemisection in every discharge. However, the spontaneous crossed phrenic activity which was present in the lateral and dorsal parts of the left hemidiaphragm in P2 neonate rats was not found in the older neonatal rats after the first postnatal week (Fig. 2B).
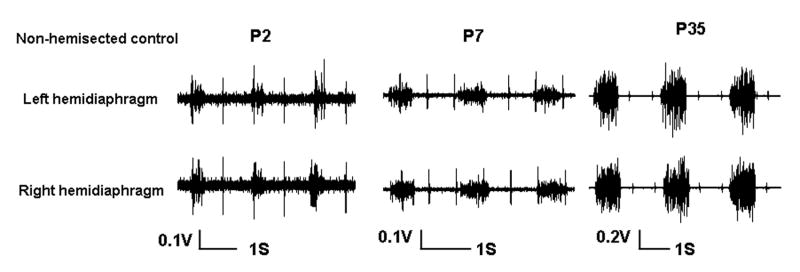
In P35 postnatal rats, EMG recording failed to show any spontaneous respiratory activity in the ipsilateral hemidiaphragm immediately after spinal hemisection (Fig. 2C). The respiratory discharges in ventral, lateral, and dorsal areas of the contralateral hemidiaphragm were persistent and rhythmic following hemisection. All areas of the left hemidiaphragm were completely paralyzed by hemisection in the fifth postnatal week of development (Fig. 2C). As expected, the EMG activity of the left and right hemidiaphragm was relatively symmetrical in the non-hemisected control animals at different ages although there was considerable variability between animals (Fig. 3).
Figure 3.
Electromyographic (EMG) recordings from the left and right hemidiaphragm in P2, P7 and P35 non-hemisected control rats. Note that although there was variability between animals, the left and right diaphragmatic activity was relatively symmetric in the same rat.
Since we noted that spontaneous crossed phrenic activity disappeared in the lateral and dorsal areas of the ipsilateral hemidiaphragm when animals were hemisected during the first postnatal week, we carried out studies on additional animals at P3, P4, P5 and P6 to carefully assess the time course of this change. One half of the P3 rats and one third of the P4 animals showed a persistent weak expression of crossed phrenic activity immediately following hemisection in all three regions of the left hemidiaphragm (Table 2). The other P3 and P4 rats and all of the P5 and P6 rats expressed the spontaneous crossed phrenic activity only in the ventral hemidiaphragm after C2 hemisection (Table 2). A Chi-Square test revealed a significant age-related difference in the expression of crossed phrenic activity (P<0.01).
Table 2.
Crossed phrenic activity following hemisection surgery in the neonatal rats.
| P2 | P3 | P4* | P5 | P6 | P7 | |
|---|---|---|---|---|---|---|
| Crossed phrenic activity (ventral, lateral, dorsal areas) | 10 | 3 | 2 | 0 | 0 | 0 |
| Crossed phrenic activity (ventral area only) | 0 | 3 | 4 | 6 | 6 | 10 |
| Total Number | 10 | 6 | 6 | 6 | 6 | 10 |
All P2 group rats (10) spontaneously expressed crossed phrenic activity at ventral, lateral, and dorsal areas of the left hemidiaphragm. 3 out of 6 P3 rats and 2 out of 6 P4 rats completely expressed crossed phrenic activity at three hemidiaphragmatic areas.
denotes the last day (P4) that the neonatal rats showed crossed phrenic activity in the ventral, lateral and dorsal areas of the left hemidiaphragm. After P4, activity was only expressed in the ventral area of the left hemidiaphragm.
Histological analysis of the cervical spinal cord tissue in all animals confirmed a complete hemisection at the C2 spinal cord level. Most sections showed a complete hemisection only (Fig. 4C), but necrotic tissue of the ipsilateral spinal cord was often seen in some of the hemisected rats (Fig. 4B, E). All of the ventral horn, dorsal horn, ventral and lateral funiculus were completely removed with the exception of slight sparing of the dorsal funiculus or lamina X following hemisection surgery in a few animals (Fig. 4A, F). An over-hemisection (Fig. 4D) with the lesion slightly crossing the midline of the C2 spinal cord was also observed in a few animals. Since the descending respiratory pathways from the brainstem lie in the lateral and ventral funiculus of spinal cord, the histological analysis suggested that EMG activity in the left hemidiaphragm was being transmitted via a neural pathway that crossed the midline of the spinal cord below the site of injury. The slight sparing of the ipsilateral dorsal funiculus or lamina X after hemisection would not include fibers mediating the descending respiratory drive to the left hemidiaphragm.
Figure 4.
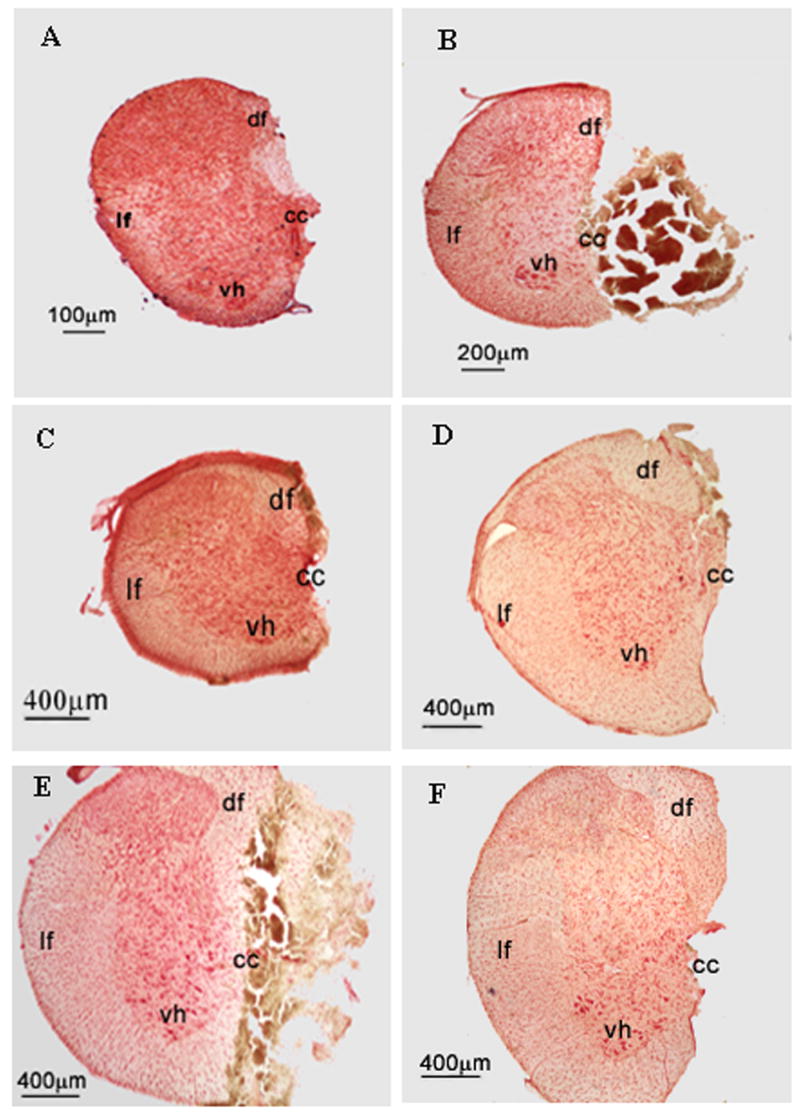
Representative photomicrographs of transverse sections showing six examples of the extent of the C2 hemisection in six rats from different age groups. A)P2 rat; B) P7 rat; C) P14 rat; D) P21 rat; E) P28 rat; F) P35 rat. Note that one half of the C2 spinal cord is missing with the exception of some slight sparing of the dorsal funiculus or lamina X in A, D, and F. Same necrotic tissue of the hemisected spinal cord is shown in B and E. df=dorsal funiculus; lf = lateral funiculus; vh = ventral horn; cc = central canal.
Altered crossed phrenic activity in postnatal development
The respiratory frequency of the different age groups of postnatal rats is shown in Figure 5. The highest frequency (101±13 breaths/min) was expressed in P7 rats and the lowest frequency (56±9 breaths/min) was expressed in P35 postnatal rats. Interestingly, the respiratory frequency significantly increased during the first postnatal week from 75±15 breaths/min (P2 neonatal rats) to 101±13 breaths/min (P7 neonatal rats) (P<0.01, Fig. 5A). After that, the mean respiratory frequency declined in P14 rats; however, this reduction in frequency was not statistically significant. The respiratory rate in P7 and P14 rat pups were significantly higher compared to the frequency of P21 as well as P28 and P35 animals (P<0.01, P<0.05 respectively, Fig. 5A). Furthermore, P21 rats had a much higher mean respiratory rate compared to the frequency of P35 animals (P<0.05, Fig. 5A). However, no significant difference was found in the respiratory frequency between the P21 and P28 group. In addition, the respiratory frequency was significant different among all different age groups of uninjured rats. In the non-hemisected control animals, the highest frequency (82±11 breaths/min) was found in P7 rats and the lowest frequency (46±5 breaths/min) was found in P35 postnatal rats (Fig. 5B). During the first postnatal week, the respiratory frequency of uninjured controls increased significantly and then significantly decreased after the P7 postnatal day (Fig. 5B). Therefore, the developmental change in the respiratory frequency in the control animals was similar to the hemisected animals. The respiratory frequency was significantly increased after hemisection compared to non-hemisected controls (P<0.05, Fig. 5C).
Figure 5.
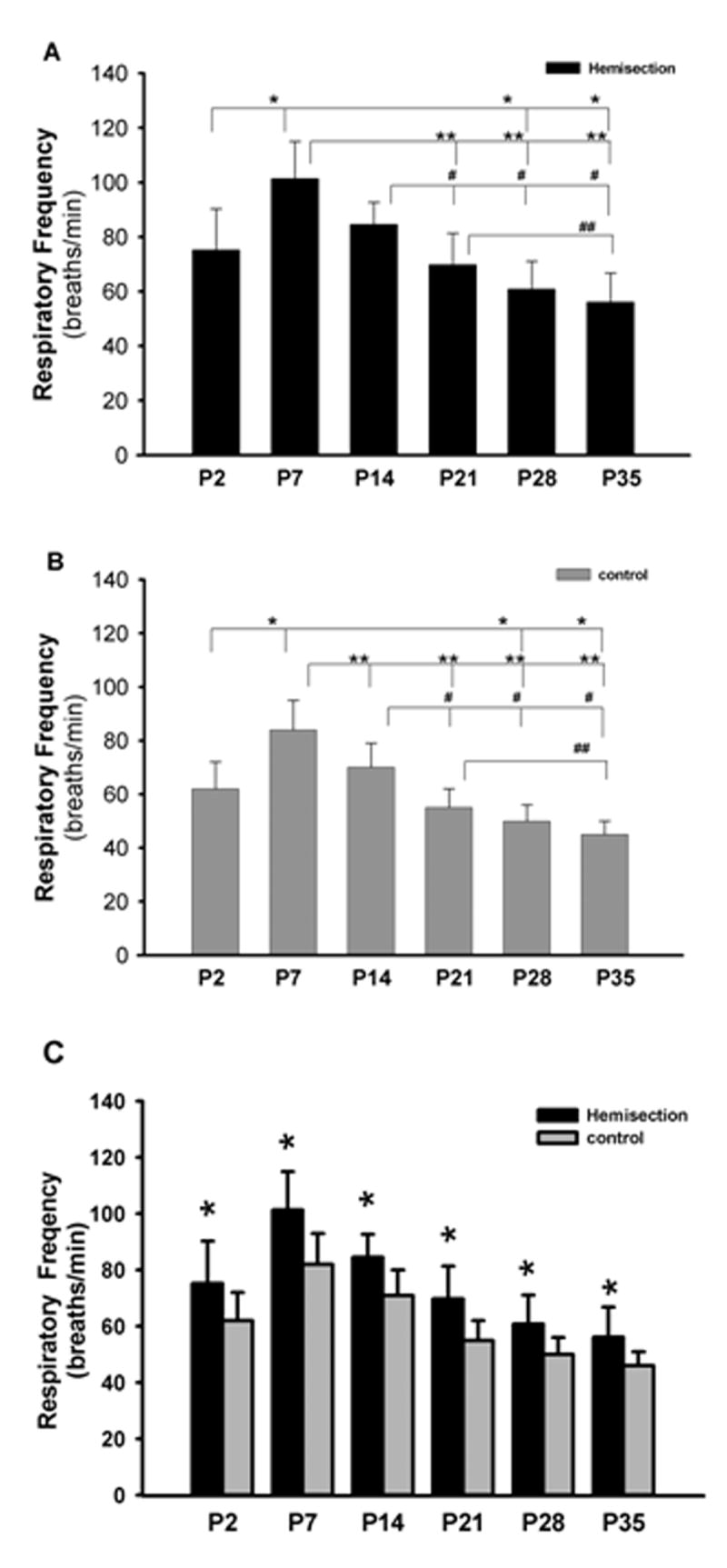
Graph of the respiratory burst frequency of the hemisected and non-hemisected postnatal rats in the P2, P7, P14, P21, P28 and P35 age groups. A) The respiratory frequency of the hemisected postnatal rats in different age groups. * represents a significant difference between P2 and P7 as well as P28 and P35. ** represents significant differences between P7 and all other older age groups except P14. # represents significant decreased respiratory frequency of P21, P28 and P35 as compared to P14. ## represents a significant decreased respiratory frequency of P35 compared to P21. B) Respiratory frequency of the non-hemisected postnatal rats in different age groups. Frequency changes observed in the hemisected rats were also observed in the controls except there was a significant decreased frequency in P14 as compared to P7. C) Graph showing significant increases of respiratory frequency after hemisection compared to controls in every age group.
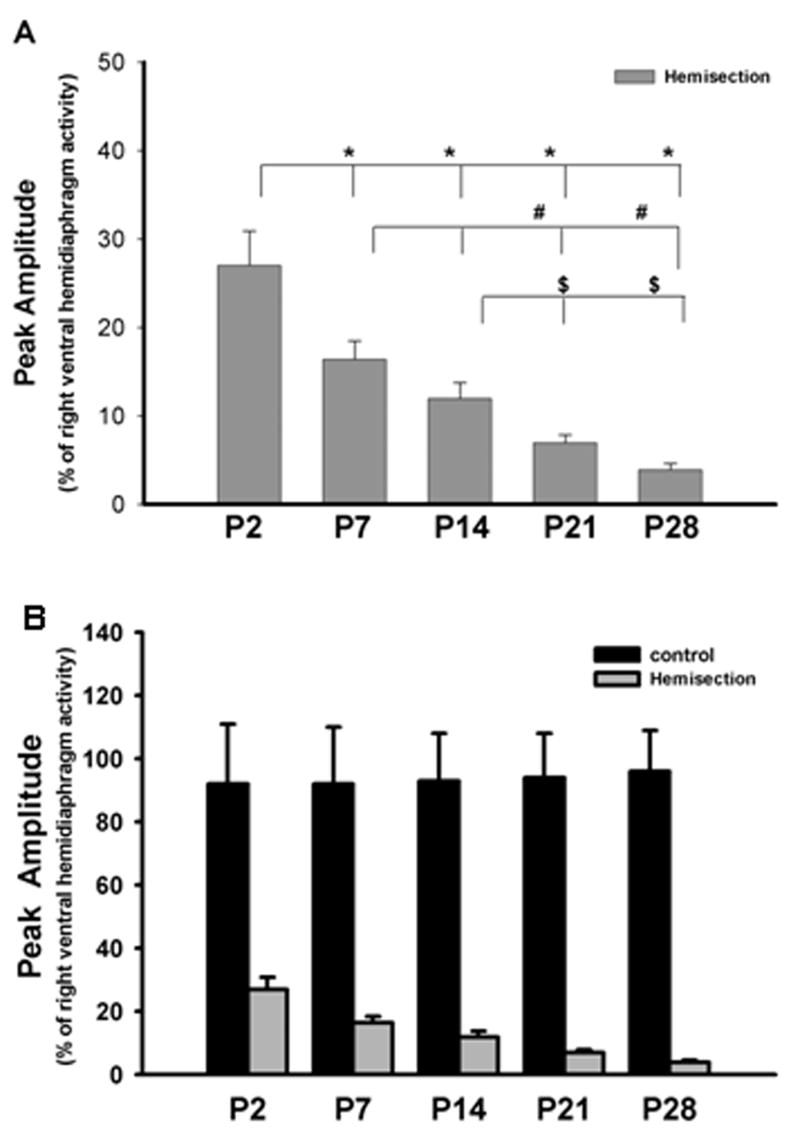
In order to provide a semi-quantitative estimate of the expression of spontaneous crossed phrenic activity during postnatal development, the extent of crossed phrenic activity was expressed as a percentage of the peak amplitude of the right hemidiaphragm activity following left hemisection in different age group rats. Only the P2 group and some of the P3 and P4 animals showed crossed phrenic activity in all three parts of the left hemidiaphragm while the other groups expressed spontaneous crossed phrenic activity only in the ventral parts. For this reason, estimates of differences were made only in the ventral area of the hemidiaphragm between different groups. An ANOVA statistical analysis revealed there was a significant difference in the peak amplitude of the ventral crossed phrenic activity among the different groups (P<0.01, Fig. 6A). The burst amplitude of the ventral crossed phrenic activity in the P2 neonatal rats was significantly higher compared to that of P7 as well as the other age group rats (P<0.01 respectively, Fig. 6A). Furthermore, the extent of the ventral crossed phrenic activity in the P7 group was significantly higher than the activity recorded in the P21 and P28 groups (P<0.01 for both, Fig. 6A). During postnatal development, the peak amplitude of crossed phrenic activity was significantly lower in P21 and P28 when compared to the P14 group (P<0.05 and P<0.01 respectively, Fig. 6A). However, there was no significant difference in the peak amplitude of crossed phrenic activity between P7 and P14 groups, and between P21 and P28 groups. In addition, the peak amplitude of the left and right ventral hemidiaphragm activity was also quantified in uninjured rat pups (Fig. 6B). The ratio of the left and right ventral hemidiaphragm activity ranged from 0.92 to 0.96 in non-hemisected controls and no significant difference was found in these activity ratios in different age controls (Fig. 6B).
Figure 6.
Peak amplitude of crossed phrenic activity in P2, P7, P14, P21 and P28 groups. A) Peak amplitude of crossed phrenic activity in hemisection groups * represents significant difference between P2 and all the other postnatal groups respectively, # represents a significant difference between P7 and all the older age groups except P14, $ represents a significant difference between P14 and P21 as well as P28. Note the progressive decrease in the mean peak amplitude as the animals get older. B) No significant difference in peak amplitude among the non-hemisected groups.
In summary, the semi-quantitative estimate of the extent of respiratory activity in the ipsilateral hemidiaphragm after C2 hemisection in different age group postnatal rats showed a gradual reduction in spontaneous crossed phrenic activity during postnatal development. At the P35 developmental stage, there was no crossed phrenic activity expressed spontaneously immediately after C2 hemisection. Thus, crossed phrenic activity during postnatal development changed from a spontaneously active to a latent state.
Discussion
We have previously reported that crossed phrenic activity is spontaneously expressed in the in vitro brainstem-spinal cord preparation of P0-P2 neonatal rats and completely latent in adult hemisected rats (Goshgarian, 1979; Zimmer and Goshgarian, 2005). In the present study, by using an in vivo model, we demonstrated for the first time the conversion of crossed phrenic activity from a spontaneously active to latent status during postnatal development. Crossed phrenic activity was spontaneously expressed in all three parts of the ipsilateral hemidiaphragm of P2 rats following C2 hemisection injury. The time window for the conversion of spontaneous crossed phrenic activity from three areas of the hemidiaphragm to one area (ventral) occurred between P3 and P5. The activity persisted in the ventral area of the ipsilateral hemidiaphragm in rats for the first four postnatal weeks, but was not present in the lateral or dorsal areas of the hemidiaphragm. By the end of the fifth postnatal week (P35), the expression of the crossed respiratory activity was completely suppressed. It is not known if the crossed phrenic pathway is expressed spontaneously in uninjured rats. The current study only investigated the expression of this pathway in injured rats.
Interestingly, in both hemisected and non-hemisected animals, we found that respiratory frequency was significantly increased during the first postnatal week from P2 to P7 and gradually reduced in P14 and older age groups. This finding is supported by a previous report which shows that the basic frequency of respiratory rhythm increases and becomes faster from P0 to P3 in rat brainstem-spinal cord preparations (Shvarev and Lagercrantz, 2006). Since there are significant changes in frequency between injured and non-injured animals within specific time groups, this suggests that the hemisection injury induced the change of respiratory frequency. In addition, a significant difference in frequency was not observed between P7 and P14 hemisected rats, but was found between the two non-injured control groups (P7 frequency was significantly higher than P14). This suggests that the respiratory frequency in P14 rats increased more following hemisection injury as compared to that in P7 animals. However, the developmental pattern of respiratory frequency in the hemisected animals is the same as the non-hemisection controls. In general, the developmental change of the respiratory frequency suggests that the respiratory rhythm generation and the transformation by the motoneurons of rhythmic inspiratory drive into appropriate patterns of respiratory output continue to mature postnatally (Greer and Funk, 2005; Shvarev and Lagercrantz, 2006).
The exact mechanism underlying the conversion of the crossed phrenic activity from a spontaneously active to latent state is not known. Several factors may contribute to this change. One possibility is that there is a change in the anatomical organization of the descending bulbospinal respiratory pathways during postnatal development. In normal adult rats, ventilation is controlled by respiratory bulbospinal premotor neurons which arise bilaterally in the rVRG. The axons of rVRG neurons travel in the lateral and ventral funiculi of the spinal cord to synapse with phrenic motoneurons (Dobbins and Feldman, 1994). The latent crossed phrenic pathway consists of axon collaterals of the rVRG neurons which run down the spinal cord and cross the midline of spinal cord below the C2 hemisection site to innervate the phrenic motor neurons ipsilateral to hemisection (Boulenguez, et al., 2007; Goshgarian, 2003; Moreno, et al., 1992).
By using cross-correlation analyses, Duffin and his colleagues have shown that left and right phrenic nerve outputs in adult rats as well as in neonatal rats are synchronized (Li and Duffin, 2004; Li, et al., 2003). However, the cross-correlation analyses indicated that the mechanisms of respiratory transmission and synchronization are different between neonatal rats and adult rats (Li and Duffin, 2004; Li, et al., 2003). In adult rats, respiratory pre-motor neurons project bifurcating axons to excite phrenic motoneurons located on both sides of the spinal cord. These bifurcating axons are primarily responsible for the synchronized output of both the right and left phrenic discharge (Li and Duffin, 2004). In neonatal rats, the synchronized activity of the right and left phrenic output is different as compared to the activity in adult rats and may be attributed to both sides of the rVRG which interconnect by gap junctions within the medulla (Li and Duffin, 2004). Therefore, it is possible that the above developmental difference of the organization of central respiratory transmission may contribute to the developmental change in the expression of spontaneous crossed phrenic activity which we found in the present study.
In addition, studies have shown that some dendrites of phrenic motoneurons cross the midline of the spinal cord in neonatal rats and the percentage of crossing phrenic dendrites is significantly higher in very young rats as compared to adult rats (Allan and Greer, 1997; Song, et al., 2000). Crossed phrenic dendrites exist in neonatal rats younger than 21 postnatal days and contralaterally projecting phrenic dendrites retract back to their cell body area during postnatal maturation (Prakash, et al., 2000). These crossed dendrites may be involved in the expression of the crossed phrenic pathway at early postnatal periods as revealed in the present study and suggested elsewhere (Zimmer and Goshgarian, 2005; Zimmer, et al., 2008).
It is also possible that the spontaneous expression of crossed phrenic activity may be related to the transient high expression of certain glutamate receptor subunits in the spinal cord during postnatal development. This hypothesis is also supported by our previous reports. The spontaneous functional recovery of the ipsilateral hemidiaphragm has been correlated with an upregulation of the NR2A and GluR1 subunits of the NMDA and AMPA receptors respectively in the adult rats with chronic hemisection injury (Alilain and Goshgarian, 2008). Glutamate is the major excitatory neurotransmitter in the central nervous system and in the bulbospinal respiratory pathways, including the latent crossed phrenic pathway (Ge and Feldman, 1998; Tai and Goshgarian, 1996; McCrimmon, et al., 1989). Pharmacological upregulation of NR2A and GluR1 expression induced functional recovery of the paralyzed ipsilateral hemidiaphragm following acute C2 hemisection injury in adult rats (Alilain and Goshgarian, 2007). These observations strongly indicate that NR2A and GluR1 may be critical components underlying the activation of the latent crossed phrenic pathway in adults. During development, there is evidence indicating that the protein levels of the NMDA receptor subunit NR2A and the AMPA receptor subunit GluR1 in the spinal cord are transiently high during the first two weeks after birth and then these levels gradually decrease over the next 35 days to adult levels (Brown, et al., 2002; Verhovshek, et al., 2005).
In addition, a previous study has demonstrated that the phrenic motor neuron pool establishes a rostrocaudal somatotopic organization on the diaphragm muscle during early development (Laskowski and Owens, 1994; Laskowski and Sanes, 1987). Rostral motor neurons tend to innervate the ventral (rostral) part of diaphragm and caudal motor neurons tend to innervate the dorsal (caudal) part of diaphragm. In the present study, we made the interesting finding that the spontaneous crossed phrenic activity started to become latent in the lateral and dorsal parts of the hemidiaphragm firstly after the first neonatal week and then the extent of crossed respiratory input gradually decreased in the ventral parts of the ipsilateral hemidiaphragm until the pathway became completely inactive at P35. As discussed above, this finding might be correlated to the different expression of some glutamate receptor subunits at the different levels of the spinal cord or to other developmental changes of the respiratory neural pathway (e.g. retraction of phrenic dendrites) that may occur first in the caudal and middle regions of the phrenic nucleus before occurring rostrally (Prakash, et al., 2000). Currently, we are investigating some of the putative mechanisms that may be related to the differential expression of crossed phrenic activity during postnatal development.
Summary
Spontaneous expression of crossed phrenic activity following cervical spinal cord hemisection occurred during the first four postnatal weeks, but this activity became latent by the fifth postnatal week. This developmental change of respiratory activity may play an important role in understanding central respiratory control mechanisms during development and may be also beneficial for development of strategies for promoting respiratory recovery after spinal cord injury in humans.
Acknowledgments
This study was supported by NIH grant HD 31550 (Dr. H.G. Goshgarian).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alilain WJ, Goshgarian HG. MK-801 upregulates NR2A protein levels and induces functional recovery of the ipsilateral hemidiaphragm following acute C2 hemisection in adult rats. J Spinal Cord Med. 2007;30:346–354. doi: 10.1080/10790268.2007.11753950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alilain WJ, Goshgarian HG. Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol. 2008 doi: 10.1016/j.expneurol.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan DW, Greer JJ. Development of phrenic motoneuron morphology in the fetal rat. J Comp Neurol. 1997;382:469–479. doi: 10.1002/(sici)1096-9861(19970616)382:4<469::aid-cne4>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- Boulenguez P, Gauthier P, Kastner A. Respiratory neuron subpopulations and pathways potentially involved in the reactivation of phrenic motoneurons after C2 hemisection. Brain Res. 2007;1148:96–104. doi: 10.1016/j.brainres.2007.02.060. [DOI] [PubMed] [Google Scholar]
- Brown KM, Wrathall JR, Yasuda RP, Wolfe BB. Quantitative measurement of glutamate receptor subunit protein expression in the postnatal rat spinal cord. Brain Res Dev Brain Res. 2002;137:127–133. doi: 10.1016/s0165-3806(02)00435-2. [DOI] [PubMed] [Google Scholar]
- Cameron WE, He F, Kalipatnapu P, Jodkowski JS, Guthrie RD. Morphometric analysis of phrenic motoneurons in the cat during postnatal development. J Comp Neurol. 1991;314:763–776. doi: 10.1002/cne.903140409. [DOI] [PubMed] [Google Scholar]
- Cameron WE, Nunez-Abades PA. Physiological changes accompanying anatomical remodeling of mammalian motoneurons during postnatal development. Brain Res Bull. 2000;53:523–527. doi: 10.1016/s0361-9230(00)00385-3. [DOI] [PubMed] [Google Scholar]
- DeVries KL, Goshgarian HG. Spinal cord localization and characterization of the neurons which give rise to the accessory phrenic nerve in the adult rat. Exp Neurol. 1989;104:88–90. doi: 10.1016/0014-4886(89)90013-7. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol. 1994;347:64–86. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Doperalski NJ, Dougherty BJ, Sandhu MS, Bolser DC, Reier PJ. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008;211(1):97–106. doi: 10.1016/j.expneurol.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Johnson SM, Olson EB, Jr, Mitchell GS. Synaptic pathways to phrenic motoneurons are enhanced by chronic intermittent hypoxia after cervical spinal cord injury. J Neurosci. 2003;23(7):2993–3000. doi: 10.1523/JNEUROSCI.23-07-02993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge Q, Feldman JL. AMPA receptor activation and phosphatase inhibition affect neonatal rat respiratory rhythm generation. J Physiol. 1998;509(1):255–66. doi: 10.1111/j.1469-7793.1998.255bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder FJ, Mitchell GS. Spinal synaptic enhancement with acute intermittent hypoxia improves respiratory function after chronic cervical spinal cord injury. J Neurosci. 2005;25(11):2925–2932. doi: 10.1523/JNEUROSCI.0148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Allan DW, Martin-Caraballo M, Lemke RP. An overview of phrenic nerve and diaphragm muscle development in the perinatal rat. J Appl Physiol. 1999;86:779–786. doi: 10.1152/jappl.1999.86.3.779. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Funk GD. Perinatal development of respiratory motoneurons. Respir Physiol Neurobiol. 2005;149(13):43–61. doi: 10.1016/j.resp.2005.03.017. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. Developmental plasticity in the respiratory pathway of the adult rat. Exp Neurol. 1979;66:547–555. doi: 10.1016/0014-4886(79)90201-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG. The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94:795–810. doi: 10.1152/japplphysiol.00847.2002. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Guth L. Demonstration of functionally ineffective synapses in the guinea pig spinal cord. Exp Neurol. 1977;57:613–621. doi: 10.1016/0014-4886(77)90093-0. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Moran MF, Prcevski P. Effect of cervical spinal cord hemisection and hemidiaphragm paralysis on arterial blood gases, pH, and respiratory rate in the adult rat. Exp Neurol. 1986;93:440–445. doi: 10.1016/0014-4886(86)90206-2. [DOI] [PubMed] [Google Scholar]
- Goshgarian HG, Rafols JA. The ultrastructure and synaptic architecture of phrenic motor neurons in the spinal cord of the adult rat. J Neurocytol. 1984;13:85–109. doi: 10.1007/BF01148320. [DOI] [PubMed] [Google Scholar]
- Kajana S, Goshgarian HG. Administration of phosphodiesterase inhibitors and an adenosine A1 receptor antagonist induces phrenic nerve recovery in high cervical spinal cord injured rats. Exp Neurol. 2008;210:671–680. doi: 10.1016/j.expneurol.2007.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane MA, White TE, Coutts MA, Jones AL, Sandhu MS, Bloom DC, Bolser DC, Yates BJ, Fuller DD, Reier PJ. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008;511(5):692–709. doi: 10.1002/cne.21864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskowski MB, Owens JL. Embryonic expression of motoneuron topography in the rat diaphragm muscle. Dev Biol. 1994;166:502–508. doi: 10.1006/dbio.1994.1333. [DOI] [PubMed] [Google Scholar]
- Laskowski MB, Sanes JR. Topographic mapping of motor pools onto skeletal muscles. J Neurosci. 1987;7:252–260. doi: 10.1523/JNEUROSCI.07-01-00252.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YM, Duffin J. Developmental changes in transmission of respiratory rhythm in the rat. Respir Physiol Neurobiol. 2004;142:153–163. doi: 10.1016/j.resp.2004.06.013. [DOI] [PubMed] [Google Scholar]
- Li YM, Shen L, Peever JH, Duffin J. Connections between respiratory neurones in the neonatal rat transverse medullary slice studied with cross-correlation. J Physiol. 2003;549:327–332. doi: 10.1113/jphysiol.2003.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrimmon DR, Smith JC, Feldman JL. Involvement of excitatory amino acids in neurotransmission of inspiratory drive to spinal respiratory motoneurons. J Neurosci. 1989;9(6):1910–21. doi: 10.1523/JNEUROSCI.09-06-01910.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. doi: 10.1016/0014-4886(92)90001-7. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, Goshgarian HG. Effects of chronic systemic theophylline injections on recovery of hemidiaphragmatic function after cervical spinal cord injury in adult rats. Brain Res. 1998;789:126–129. doi: 10.1016/s0006-8993(98)00024-9. [DOI] [PubMed] [Google Scholar]
- Nantwi KD, El-Bohy AA, Schrimsher GW, Reier PJ, Goshgarian HG. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal injury in adult rats. Neurorehabilitation Neural Repair. 1999;13:225–234. [Google Scholar]
- Prakash YS, Mantilla CB, Zhan WZ, Smithson KG, Sieck GC. Phrenic motoneuron morphology during rapid diaphragm muscle growth. J Appl Physiol. 2000;89:563–572. doi: 10.1152/jappl.2000.89.2.563. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic Control of Motoneuronal Excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shvarev YN, Lagercrantz H. Early postnatal changes in respiratory activity in rat in vitro and modulatory effects of substance P. Eur J Neurosci. 2006;24:2253–2263. doi: 10.1111/j.1460-9568.2006.05087.x. [DOI] [PubMed] [Google Scholar]
- Song A, Ashwell KWS, Tracey DJ. Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Ana Embryol. 2000;202:159–177. doi: 10.1007/s004290000096. [DOI] [PubMed] [Google Scholar]
- Tai Q, Goshgarian HG. Ultrastructural quantitative analysis of glutamatergic and GABAergic synaptic terminals in the phrenic nucleus after spinal cord injury. J Comp Neurol. 1996;372(3):343–55. doi: 10.1002/(SICI)1096-9861(19960826)372:3<343::AID-CNE2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Verhovshek T, Wellman CL, Sengelaub DR. NMDA receptor binding declines differentially in three spinal motor nuclei during postnatal development. Neurosci Lett. 2005;384:122–126. doi: 10.1016/j.neulet.2005.04.080. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Goshgarian HG. Spontaneous crossed phrenic activity in the neonatal respiratory network. Exp Neurol. 2005;194:530–540. doi: 10.1016/j.expneurol.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Zimmer MB, Nantwi K, Goshgarian HG. Effect of spinal cord injury on the neural regulation of respiratory function. Exp Neurol. 2008;209:399–406. doi: 10.1016/j.expneurol.2007.05.015. [DOI] [PubMed] [Google Scholar]