Abstract
Free fatty acid (FFA) availability increases several-fold during exercise and remains significantly elevated for at least 3–6 h after exercise cessation. Little, however, is known regarding the duration of the postexercise rise in FFA flux. In the present study we used stable isotope-labeled palmitate infusion to examine fatty acid metabolism in 27 healthy untrained men and women (age: 29±7 y, body mass index: 25±4 kg/m2) between 13–16 h and 21–24 h after a single bout of moderate-intensity endurance exercise (1–2 h at 60% of peak oxygen consumption), performed in the evening, and after a time-matched resting trial. Postabsorptive FFA rate of appearance (Ra) and FFA concentration in plasma were significantly greater after exercise than rest throughout the recovery period (P<0.015), but the exercise-induced increases declined from ~40% at 13–16 h to ~10% at 21–24 h postexercise (P=0.001). The magnitude of the exercise-induced increase in plasma FFA concentration was proportional to the increase in FFA Ra. Correlation analysis demonstrated that exercise-induced changes in plasma FFA Ra at 13–16 h are: 1) negatively associated with resting plasma FFA Ra and 2) positively associated with the net energy expenditure of exercise and the exercise-induced changes in whole-body fat oxidation rate (all P<0.05). In multivariate stepwise linear regression analysis, baseline plasma FFA Ra (P ≤ 0.008) and net energy expenditure of exercise (P ≤ 0.005) independently predicted the exercise-induced change in plasma FFA Ra at 13–16 h. We conclude that the exercise-induced increase in FFA mobilization is: 1) long-lived, persisting for 12–24 h after exercise with a progressive decline with time, 2) greater in subjects with low than high resting plasma FFA availability, and 3) greater after exercise with high than low energy demand.
Keywords: exercise, lipolysis, FFA availability, stable isotope, tracer
Introduction
Fatty acids are an important oxidative fuel for humans, both at rest and during exercise [1]. Free fatty acid (FFA) release from adipose tissue is well regulated by the coordinated action of many endocrine, paracrine, and other factors [2,3], allowing appropriate availability of fatty acids to meet the energy requirements of tissues. Thus, adipose tissue lipolytic rate and the release of FFA into the circulation rise ~5-fold above resting values during moderate-intensity exercise to meet the increased fuel demand [4,5]. It is well established that the exercise-induced lipolytic surge persists for at least 3–6 h into recovery [6–10]. And, it has been suggested that increased plasma FFA concentrations [10] and increased adipose tissue FFA release rates [7] in the immediate postexercise period (3–6 h later) are directly related to the intensity and/or duration of prior exercise. However, beyond the immediate postexercise period, it is not known for how long and to what extent lipolysis remains stimulated.
There is evidence of increased plasma FFA concentrations for as long as 12–16 h after exercise [11–17]; yet not all studies measuring plasma FFA concentration as an index of lipolytic activity find them increased by ≥12 h into recovery [18–23]. The reasons for this discrepancy are not known but could be related to differences in the type of exercise performed. Furthermore, exercise is a physiological condition where FFA concentration in plasma may not accurately represent FFA flux rates [24]. In addition, there is evidence that baseline FFA availability at rest may affect the exercise-induced changes in plasma FFA concentration. Experimental elevation of plasma FFA concentration via lipid infusion at rest, immediately before strenuous exercise, markedly blunted (by more than 50% compared with normal saline infusion) the increase in plasma FFA concentration during exercise [25]. Furthermore, studies in animals have presented evidence of feedback down-regulation of adipose tissue FFA release by increased arterial FFA concentration [26]. These observations suggest the existence of a biological ceiling in vivo, similar to the stimulation of human adipocyte lipolysis by various lipolytic agents in vitro [27]. To elucidate the effect of exercise energy expenditure and baseline FFA metabolism on the plasma FFA concentration and kinetics response during the late phase of recovery from exercise, we evaluated FFA concentrations and kinetics on the day after a single bout of moderate-intensity endurance exercise of varying duration, and again after an equivalent period of rest, in healthy but untrained subjects.
Materials and Methods
Subjects and preliminary testing
Twenty-seven men and women (age: 28.9 ± 7.2 yr, body mass index: 24.7 ± 4.0 kg/m2; means ± SD) volunteered for the study; several of them participated in our previous studies examining postexercise lipoprotein metabolism [28–30]. All subjects were considered to be in good health after completing a medical evaluation, which included a history and physical examination and standard blood tests. All were normoglycemic and normolipidemic; none consumed tobacco products or took medications known to affect lipid metabolism. Subjects’ body composition (fat mass and fat-free mass) was assessed by dual-energy X-ray absorptiometry (Delphi-W densitometer, Hologic, Waltham, MA) and peak oxygen consumption (VO2peak) was determined on a bicycle ergometer as previously described [28–30]. Written informed consent was obtained from all subjects before their participation in the study, which was approved by the Human Studies Committee and the General Clinical Research Center (GCRC) Advisory Committee at Washington University School of Medicine in St. Louis, MO.
Experimental protocol
Each subject completed two time-matched stable isotope labeled tracer infusion studies within four weeks, in randomized order: one after resting and one after cycling on the preceding afternoon. Subjects were instructed to adhere to their regular diet and to refrain from exercise for a minimum of three days before being admitted to the GCRC, the afternoon before each isotope infusion study (rest and exercise). For the exercise study, subjects cycled on a semi-recumbent cycle ergometer (EC-C400R Ergometer, Cateye Fitness, Source Distributors, Dallas, TX) for 60 or 120 min between 1700–1900 h. The workload was set to elicit a VO2 equivalent to 60% of VO2peak; VO2 was measured (TrueOne 2400 Metabolic Measurement System, ParvoMedics, Salt Lake City, UT) at regular intervals during exercise, and the workload was adjusted as necessary to maintain the desired VO2 (within ± 5%). For the resting study, subjects lied in bed or sat in a chair. After completion of the exercise or the equivalent period of rest, subjects took a shower and then rested in a chair. At ~1930 h they consumed a standard meal containing ~15 kcal per kg body weight (~55% of total energy from carbohydrate, 30% from fat, and 15% from protein), and then fasted (except for water) and rested in bed until completion of the study the next day.
At 0530 h the following morning, one catheter was inserted into a forearm vein to administer stable isotope labeled tracers, and a second catheter was inserted into a vein in the contralateral hand, which was heated to 55°C with a thermostatically controlled box, to obtain arterialized blood samples [31]. Catheters were kept open with slow, controlled infusion of 0.9% NaCl solution (30 ml/h). At 0700 h (time = 0; ~12 h after the cessation of exercise or the equivalent period of rest on the previous evening), blood samples were obtained for the determination of background palmitate tracer-to-tracee ratio (TTR) in plasma, and a constant infusion of [2,2-2H2]palmitate (0.03 μmol/kg min), dissolved in 25% human albumin solution, was started and maintained for 12 h. Additional blood samples were collected at 60, 90, 120, 180, and 240 min and again at 9, 10, 11, and 12 h to determine palmitate TTR and FFA concentrations in plasma. Resting metabolic rate (RMR) and whole-body fat oxidation rate were measured by using indirect calorimetry (Deltatrac Metabolic Monitor, SensorMedics, Yorba Linda, CA) between 2.0 h to 2.5 h after beginning the isotope infusion [32].
Sample collection and analyses
Blood samples were collected in chilled tubes containing sodium EDTA. Samples were placed on ice, and plasma was separated by centrifugation within 30 min of collection and stored at −80°C until final analyses were performed. Plasma insulin concentration was measured by radioimmunoassay (RIA; Linco Research, St. Louis, MO). Plasma FFA concentrations were quantified by gas chromatography (HP 5890 Series II GC, Hewlett-Packard, Palo Alto, CA) after adding heptadecanoic acid to plasma as an internal standard [33]. Plasma free palmitate TTR was determined by analyzing the methyl ester derivative with gas chromatography – mass spectrometry (Agilent Technologies/HP 6890 Series GC System – 5973 Mass Selective Detector, Hewlett-Packard, Palo Alto, CA) [33].
Calculations
Palmitate rate of appearance (Ra) in plasma was calculated by dividing the palmitate tracer infusion rate by the average plasma palmitate TTR value between 1–4 h and 9–12 h into the infusion (i.e., 13–16 and 21–24 h postexercise, respectively) during both physiologic and isotopic steady state [34–37]; total FFA Ra (in μmol/min) was derived by dividing palmitate Ra by the proportional contribution of palmitate to total plasma FFA concentration [37]. Exercise-induced changes were calculated as differences from respective time-matched resting values.
The gross energy expenditure of exercise was determined from respiratory measurements during exercise; net energy expenditure of exercise was calculated by subtracting the RMR during the equivalent period of rest from the corresponding gross energy expenditure during the exercise session.
Statistical analysis
All data sets were normally distributed according to the Kolmogorov-Smirnov test, and are presented as means ± SD. Differences between exercise and rest and between time into recovery (13–16 h and 21–24 h) were evaluated by using analysis of variance with repeated measurements for trial and time. Relationships between exercise-induced changes in FFA metabolism and other parameters of interest were assessed with linear correlation and multiple regression analyses. A P-value ≤ 0.05 was considered statistically significant. All analyses were carried out with SPSS 16 for Windows (SPSS Inc., Chicago, IL).
Results
Average heart rate during exercise was 134 ± 10 bpm (70 ± 4% of age-predicted maximum heart rate). VO2 remained constant during exercise at 1.74 ± 0.60 l/min, corresponding to 60 ± 6% of subjects’ VO2peak. Absolute power output during the exercise session ranged from 60 to 194 watts (113 ± 36 watts) and net energy expenditure from 244 to 2021 kcal (665 ± 442 kcal).
Plasma insulin concentration tended to be lower after exercise than rest (P = 0.088) and was significantly lower (P < 0.001), during both the exercise and resting trials, at 21–24 h (rest: 3.2 ± 2.2 mU/l, exercise: 2.8 ± 1.2 mU/l) than at 13–16 h (rest: 5.5 ± 3.0 mU/l, exercise: 5.1 ± 2.9 mU/l). RMR tended to be greater at 13–16 h after exercise than rest (1.11 ± 0.18 and 1.07 ± 0.17 kcal/min, respectively, P = 0.051) and whole-body fat oxidation rate was ~20% greater after exercise than rest (61 ± 23 and 52 ± 19 mg/min, respectively, P = 0.020).
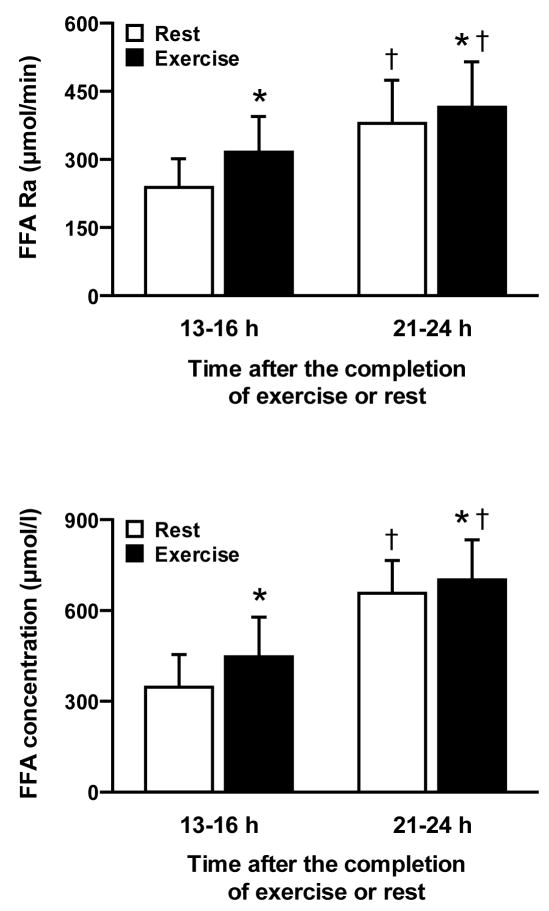
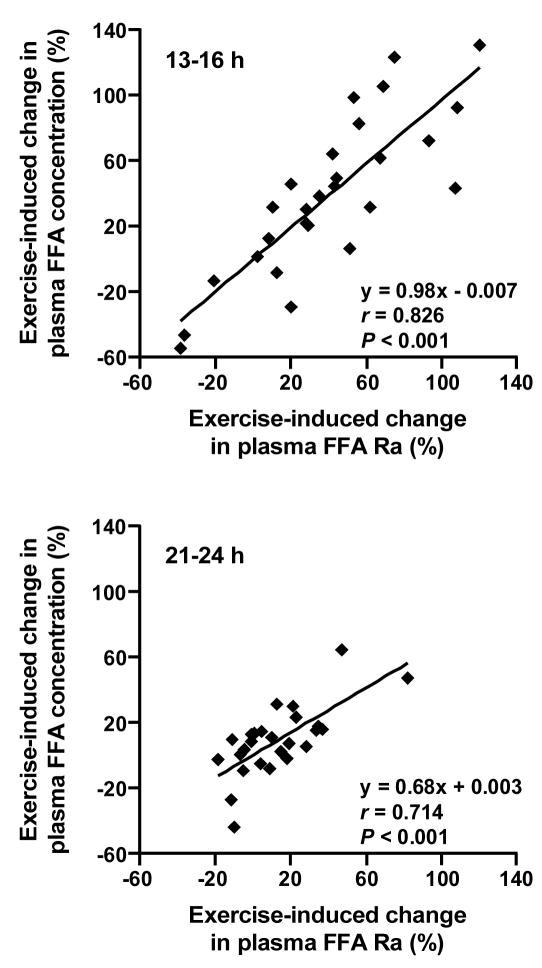
FFA Ra and FFA concentration in plasma were significantly greater after exercise than rest (P < 0.015) and both were greater (P < 0.001) at 21–24 h than 13–16 h after exercise or rest (Figure 1). The magnitude of the differences between exercise and rest in FFA Ra and FFA concentration in plasma was significantly greater at 13–16 h than at 21–24 h (~40% and ~10%, respectively, P = 0.001). Exercise-induced changes in plasma FFA Ra and FFA concentration (expressed as percent increase above resting values) were strongly and positively correlated with each other both at 13–16 h, when the magnitude of change was almost entirely proportional, and at 21–24 h (Figure 2).
FIGURE 1.
Free fatty acid (FFA) rate of appearance (Ra) (top) and FFA concentration (bottom) in plasma 13–16 h and 21–24 h after a single bout of exercise or an equivalent period of rest. Values are means ± SD. *Value after exercise is significantly different from time-matched value after rest (P < 0.015), †Value at 21–24 h is significantly different from trial-matched value at 13–16 h (P < 0.001).
FIGURE 2.
Relationship between exercise-induced changes in free fatty acid (FFA) rate of appearance (Ra) and FFA concentration in plasma at 13–16 h (top) and 21–24 h (bottom).
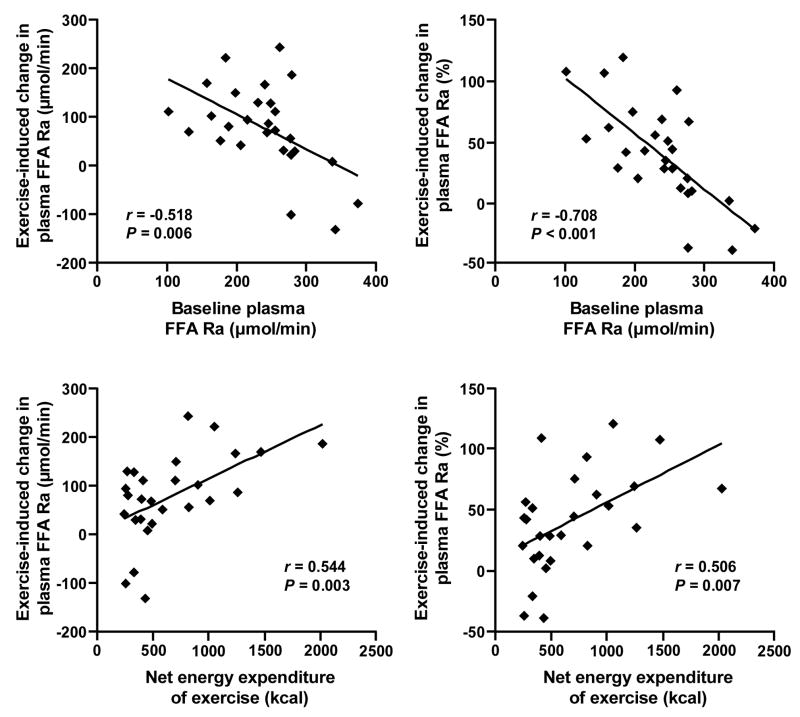
Exercise-induced changes in plasma FFA Ra at 13–16 h, both when expressed in absolute (μmol/min) and relative (%) terms, were negatively associated with baseline plasma FFA Ra during the resting trial and positively with the net energy expenditure of exercise (Figure 3); these relationships were no longer apparent by 21–24 h (all P-values > 0.1). At 13–16 h, changes in FFA Ra were positively associated with changes in whole-body fat oxidation rate, both when expressed in absolute (r = 0.567, P = 0.002) and relative (r = 0.535, P = 0.004) terms, but did not correlate with respective changes in plasma insulin concentration (P-values > 0.8) and RMR (P-values > 0.5).
FIGURE 3.
Relationships between absolute (left) and relative (right) exercise-induced changes in free fatty acid (FFA) rate of appearance (Ra) in plasma at 13–16 h, and baseline FFA Ra during the resting trial (top) and net energy expenditure of exercise (bottom).
In multivariate stepwise linear regression analysis, baseline plasma FFA Ra (P ≤ 0.008) and net energy expenditure of exercise (P ≤ 0.005) independently predicted the exercise-induced change in plasma FFA Ra at 13–16 h, both when expressed in absolute (μmol/min) and relative (%) terms, together accounting for 48% and 65% of the total variance, respectively. Sex, age, fat mass and fat-free mass (in kg and % of body weight), VO2peak (in l/min and ml/kg min), power output, baseline and exercise-induced changes (absolute and relative) in insulin concentration, RMR, and whole-body fat oxidation rate did not enter the prediction models.
Discussion
Exercise is a very potent lipolytic stimulus to meet the increased energy requirements due to muscle work. During exercise, whole-body lipolytic rate and plasma FFA availability increase by ~5-fold above resting values [4,5]. Here we show that the exercise-induced lipolytic surge is: i) long-lived but diminishes with time until it is nearly vanished by 24 h after the exercise, and ii) dependent on baseline (i.e., resting) fatty acid metabolism and the acute exercise-induced energy expenditure. Our findings are consistent with observations during the immediate postexercise recovery. First, increased plasma FFA concentrations [10] and increased adipose tissue FFA release [7] some 3–6 h after exercise cessation are directly related to the intensity and/or duration of prior exercise. Higher-intensity exercise results in greater adipose tissue FFA release rates and plasma FFA concentrations in the immediate postexercise period (3–6 h) than lower-intensity exercise [7,10], and longer-duration exercise elicits greater increases in plasma FFA concentrations during the immediate (~6 h) [10] and late (the next day) [38] phases of the recovery than shorter-duration exercise, likely due to the greater energy deficit induced by higher-intensity and longer-duration exercise. In fact, manipulating the intensity of exercise while keeping total energy expenditure constant does not modify the FFA concentration response to exercise some 16 h later [39]. Secondly, experimental elevation of plasma FFA concentrations at rest, before commencing exercise, results in a markedly blunted increase (~75% compared with ~200%) in plasma FFA concentrations at the end of exercise [25]. Our findings indicate that these phenomena extend until late into the recovery from exercise, probably because of a gradual return to resting values after exercise.
Our findings help explain the inconsistent results from previous studies in which the prolonged effect of exercise on plasma FFA concentration was evaluated. Some investigators found increased fasting plasma FFA concentrations the morning after a single bout of moderate-intensity exercise lasting 1–2 h, compared to a time-matched resting trial [11–17] whereas others found no differences [18–23]. For example, in healthy young men, the same exercise bout (90 min at 60–65% of VO2peak; total energy cost ~1100 kcal) did not alter fasting plasma FFA concentrations the next day in subjects whose average resting FFA concentrations were ~0.8 mM [38] but caused a ~50% increase in subjects whose average resting FFA concentrations were ~0.4 mM [15]. Our data suggest that what might be considered discrepant findings is in fact a normal physiological response to different resting FFA availability. In addition, our results may help explain differences in the postexercise lipid metabolism response between men and women. For instance, we have recently reported a significant increase in plasma FFA availability (by ~55%) on the morning after a single 1-h exercise bout in men [29] but not in women [28], who had ~50% higher resting FFA availability than men [28,29]. On the other hand, the exercise-induced increase in FFA Ra during the immediate postexercise recovery period (3 h after exercise cessation) was reported to be comparable in men and women who had similar resting FFA Ra [40]. In the present study, data for men and women fell onto the same regression lines indicating that previously observed differences in the FFA metabolism response to exercise in men and women are most likely secondary to sex differences in FFA metabolism at rest [28,29,36,41,42] and differences in net energy expenditure of exercise when men and women perform exercise at the same relative intensity [28,29,40,43].
The results from our study along with the data reported in the literature indicate that the increase in plasma FFA availability at rest several hours after exercise in trained compared with untrained subjects [44,45] likely represents an acute effect of exercise only, rather than a cumulative effect in response to repeated exercise sessions (i.e., training). For example, in cross-sectional studies, it was found that postabsorptive FFA Ra in plasma is 50–100% higher in endurance-trained than in untrained subjects when measurements are made the day after a typical training session [44,45], but not different when exercise is withheld for 48 h [46,47]. Likewise, longitudinal studies report that several weeks of endurance training in previously sedentary subjects increase postabsorptive FFA Ra and FFA concentration in plasma by 40–50% compared to pre-training values when measured within one day from the last training session [48], but not 36–72 h later [49–54]. Furthermore, detraining for ~1 week leads to a significant reduction in fasting plasma FFA concentration by 40–50% in endurance-trained individuals, compared to the day after their last training session [55,56], and this effect is already evident after ~60 h without exercise [55]. The acute lipolytic effect of exercise is likely not due to the negative energy balance induced by exercise. Supplementary food intake to compensate for the exercise-induced energy deficit does not abolish the exercise-induced increase in basal plasma FFA concentration ~14 h postexercise [57]. Furthermore, reducing dietary energy intake through calorie restriction to match the energy expended during exercise does not lead to a significant change in fasting plasma FFA 5concentration the next morning [13].
In accordance with the results from previous studies in which subjects performed similar exercise as in our study and measurements were made 12–24 h postexercise [15,39,58], we observed that exercise brought about a minor increase in RMR (~4%) and a ~20% increase in whole-body fat oxidation rate. Unlike FFA Ra, the postexercise increases in RMR and fat oxidation rate are probably due to the exercise-induced energy deficit. In studies which did not adjust for the exercise-induced energy deficit (i.e., negative energy balance after exercise; as is the case in our study), RMR and lipid oxidation rates were increased during the immediate (~3 h) postexercise period [40,43] and the late phase of the recovery (12–48 h) from exercise [15,39,58]. However, these responses were absent in studies where the exercise-induced energy deficit was compensated with additional energy intake [59–61]. In addition, although RMR has been suggested to be a major determinant of basal FFA Ra [41], our results clearly demonstrate a dissociation of this relationship in response to exercise and suggest that the increase in FFA mobilization late into the recovery from exercise is not merely the result of increased energy demand. Similar observations, i.e., increased plasma FFA availability without simultaneous changes in RMR and lipid oxidation have been made previously by us [29] and other investigators [12]. Therefore, after exercise there appears to be no direct link between the availability of plasma FFA and RMR and fat oxidation.
The mechanisms responsible for the increase in plasma FFA availability the morning after a single bout of exercise remain obscure. It is unlikely that the accelerated FFA flux ≥13 h after exercise observed in the present study is related to changes in fatty acid metabolism occurring during exercise because complete blockade of the normal lipolytic response during exercise by pharmacological means (acipimox) does not abolish the exercise-induced increase in fasting plasma FFA concentration ~15 h later [16]. Increased plasma catecholamine and decreased plasma insulin concentrations are thought to be the most important hormonal signals mediating the lipolytic surge during exercise [62]. However, catecholamine concentrations fall sharply immediately after exercise cessation, and return to pre-exercise levels within ~2 h of recovery and do not change thereafter [10,63]. And, the greater FFA Ra and FFA concentration in trained athletes (the day after a typical training session) compared with untrained subjects have been observed without any differences in resting catecholamine and insulin concentrations [45]. Likewise, increased fasting plasma FFA Ra [29] and FFA concentrations [13,15,16] the morning after a single bout of exercise have been shown without any accompanying changes in plasma insulin concentration. This is consistent with the absence of a relationship between exercise-induced changes in plasma insulin and those in plasma FFA Ra in the present study. It has been suggested that the “slower” exercise-induced increase in growth hormone concentration, albeit not involved in the lipolytic response during exercise, is an important factor mediating the postexercise (~4 h into recovery) increase in adipose tissue lipolysis [6,63]. Enhanced adipose tissue blood flow is unlikely to be responsible for the late exercise-induced increase in plasma FFA availability because, although it is acutely increased during exercise and remains elevated for ~4 h after its cessation [8], there is no evidence that this effect persists into the late phase of postexercise recovery (12–16 h later) [21]. The mechanisms for the increase in FFA availability late into the recovery from exercise warrant further investigation.
In summary, exercise is a potent stimulus for the mobilization of FFA, not only during but for up to 24 h after exercise, in a manner that depends directly on the energy expenditure of exercise sand inversely on resting plasma FFA availability. The blunted exercise-induced FFA release during the late phase of the recovery in subjects with high fasting plasma FFA concentration probably prevents fatty acid cytotoxicity at a time when energy requirements are not substantially increased.
Acknowledgments
We wish to thank Megan Steward for subject recruitment, Junyoung Kwon and Adewole Okunade for technical assistance, and the study subjects for their participation.
This study was supported by National Institutes of Health grants AR 49869, HD 057796, DK 56341 (Clinical Nutrition Research Unit), RR 00954 (Biomedical Mass Spectrometry Resource), grant number UL1 RR024992 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and NIH Roadmap for Medical Research, and grants from the American Heart Association (0365436Z and 0510015Z).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jensen MD. Fate of fatty acids at rest and during exercise: regulatory mechanisms. Acta Physiol Scand. 2003;178:385–90. doi: 10.1046/j.1365-201X.2003.01167.x. [DOI] [PubMed] [Google Scholar]
- 2.Coppack SW, Jensen MD, Miles JM. In vivo regulation of lipolysis in humans. J Lipid Res. 1994;35:177–93. [PubMed] [Google Scholar]
- 3.Langin D. Control of fatty acid and glycerol release in adipose tissue lipolysis. C R Biol. 2006;329:598–607. doi: 10.1016/j.crvi.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 4.Romijn JA, Coyle EF, Sidossis LS, et al. Regulation of endogenous fat and carbohydrate metabolism in relation to exercise intensity and duration. Am J Physiol. 1993;265:E380–91. doi: 10.1152/ajpendo.1993.265.3.E380. [DOI] [PubMed] [Google Scholar]
- 5.Wolfe RR, Klein S, Carraro F, Weber JM. Role of triglyceride-fatty acid cycle in controlling fat metabolism in humans during and after exercise. Am J Physiol. 1990;258:E382–9. doi: 10.1152/ajpendo.1990.258.2.E382. [DOI] [PubMed] [Google Scholar]
- 6.Enevoldsen LH, Polak J, Simonsen L, et al. Post-exercise abdominal, subcutaneous adipose tissue lipolysis in fasting subjects is inhibited by infusion of the somatostatin analogue octreotide. Clin Physiol Funct Imaging. 2007;27:320–6. doi: 10.1111/j.1475-097X.2007.00754.x. [DOI] [PubMed] [Google Scholar]
- 7.Mulla NA, Simonsen L, Bulow J. Post-exercise adipose tissue and skeletal muscle lipid metabolism in humans: the effects of exercise intensity. J Physiol. 2000;524:919–28. doi: 10.1111/j.1469-7793.2000.00919.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Van Hall G, Bulow J, Sacchetti M, Al Mulla N, Lyngso D, Simonsen L. Regional fat metabolism in human splanchnic and adipose tissues; the effect of exercise. J Physiol. 2002;543:1033–46. doi: 10.1113/jphysiol.2002.022392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bahr R, Hansson P, Sejersted OM. Triglyceride/fatty acid cycling is increased after exercise. Metabolism. 1990;39:993–9. doi: 10.1016/0026-0495(90)90313-2. [DOI] [PubMed] [Google Scholar]
- 10.Bahr R, Hostmark AT, Newsholme EA, Gronnerod O, Sejersted OM. Effect of exercise on recovery changes in plasma levels of FFA, glycerol, glucose and catecholamines. Acta Physiol Scand. 1991;143:105–15. doi: 10.1111/j.1748-1716.1991.tb09205.x. [DOI] [PubMed] [Google Scholar]
- 11.Gill JM, Al-Mamari A, Ferrell WR, et al. Effects of prior moderate exercise on postprandial metabolism and vascular function in lean and centrally obese men. J Am Coll Cardiol. 2004;44:2375–82. doi: 10.1016/j.jacc.2004.09.035. [DOI] [PubMed] [Google Scholar]
- 12.Gill JM, Frayn KN, Wootton SA, Miller GJ, Hardman AE. Effects of prior moderate exercise on exogenous and endogenous lipid metabolism and plasma factor VII activity. Clin Sci (Lond) 2001;100:517–27. [PubMed] [Google Scholar]
- 13.Gill JM, Hardman AE. Postprandial lipemia: effects of exercise and restriction of energy intake compared. Am J Clin Nutr. 2000;71:465–71. doi: 10.1093/ajcn/71.2.465. [DOI] [PubMed] [Google Scholar]
- 14.Gill JM, Murphy MH, Hardman AE. Postprandial lipemia: effects of intermittent versus continuous exercise. Med Sci Sports Exerc. 1998;30:1515–20. doi: 10.1097/00005768-199810000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Herd SL, Kiens B, Boobis LH, Hardman AE. Moderate exercise, postprandial lipemia, and skeletal muscle lipoprotein lipase activity. Metabolism. 2001;50:756–62. doi: 10.1053/meta.2001.24199. [DOI] [PubMed] [Google Scholar]
- 16.Malkova D, Hardman AE, Bowness RJ, Macdonald IA. The reduction in postprandial lipemia after exercise is independent of the relative contributions of fat and carbohydrate to energy metabolism during exercise. Metabolism. 1999;48:245–51. doi: 10.1016/s0026-0495(99)90042-2. [DOI] [PubMed] [Google Scholar]
- 17.Mougios V, Ring S, Petridou A, Nikolaidis MG. Duration of coffee- and exercise-induced changes in the fatty acid profile of human serum. J Appl Physiol. 2003;94:476–84. doi: 10.1152/japplphysiol.00624.2002. [DOI] [PubMed] [Google Scholar]
- 18.Gill JM, Mees GP, Frayn KN, Hardman AE. Moderate exercise, postprandial lipaemia and triacylglycerol clearance. Eur J Clin Invest. 2001;31:201–7. doi: 10.1046/j.1365-2362.2001.00799.x. [DOI] [PubMed] [Google Scholar]
- 19.Gill JM, Herd SL, Hardman AE. Moderate exercise and post-prandial metabolism: issues of dose-response. J Sports Sci. 2002;20:961–7. doi: 10.1080/026404102321011715. [DOI] [PubMed] [Google Scholar]
- 20.Holm G, Bjorntorp P, Jagenburg R. Carbohydrate, lipid and amino acid metabolism following physical exercise in man. J Appl Physiol. 1978;45:128–31. doi: 10.1152/jappl.1978.45.1.128. [DOI] [PubMed] [Google Scholar]
- 21.Malkova D, Evans RD, Frayn KN, Humphreys SM, Jones PR, Hardman AE. Prior exercise and postprandial substrate extraction across the human leg. Am J Physiol Endocrinol Metab. 2000;279:E1020–8. doi: 10.1152/ajpendo.2000.279.5.E1020. [DOI] [PubMed] [Google Scholar]
- 22.Kolifa M, Petridou A, Mougios V. Effect of prior exercise on lipemia after a meal of moderate fat content. Eur J Clin Nutr. 2004;58:1327–35. doi: 10.1038/sj.ejcn.1601968. [DOI] [PubMed] [Google Scholar]
- 23.Tsekouras YE, Yanni AE, Bougatsas D, Kavouras SA, Sidossis LS. A single bout of brisk walking increases basal very low-density lipoprotein triacylglycerol clearance in young men. Metabolism. 2007;56:1037–43. doi: 10.1016/j.metabol.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Wahren J, Hagenfeldt L, Felig P. Splanchnic and leg exchange of glucose, amino acids, and free fatty acids during exercise in diabetes mellitus. J Clin Invest. 1975;55:1303–14. doi: 10.1172/JCI108050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pilegaard H, Birk JB, Sacchetti M, et al. PDH-E1alpha dephosphorylation and activation in human skeletal muscle during exercise: effect of intralipid infusion. Diabetes. 2006;55:3020–7. doi: 10.2337/db06-0152. [DOI] [PubMed] [Google Scholar]
- 26.Madsen J, Bulow J, Nielsen NE. Inhibition of fatty acid mobilization by arterial free fatty acid concentration. Acta Physiol Scand. 1986;127:161–6. doi: 10.1111/j.1748-1716.1986.tb07889.x. [DOI] [PubMed] [Google Scholar]
- 27.Hellstrom L, Langin D, Reynisdottir S, Dauzats M, Arner P. Adipocyte lipolysis in normal weight subjects with obesity among first-degree relatives. Diabetologia. 1996;39:921–8. doi: 10.1007/BF00403911. [DOI] [PubMed] [Google Scholar]
- 28.Magkos F, Patterson BW, Mohammed BS, Mittendorfer B. Basal adipose tissue and hepatic lipid kinetics are not affected by a single exercise bout of moderate duration and intensity in sedentary women. Clin Sci (Lond) 2009;116:327–34. doi: 10.1042/CS20080220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Magkos F, Patterson BW, Mohammed BS, Mittendorfer B. A single 1-h bout of evening exercise increases basal FFA flux without affecting VLDL-triglyceride and VLDL-apolipoprotein B-100 kinetics in untrained lean men. Am J Physiol Endocrinol Metab. 2007;292:E1568–74. doi: 10.1152/ajpendo.00636.2006. [DOI] [PubMed] [Google Scholar]
- 30.Magkos F, Wright DC, Patterson BW, Mohammed BS, Mittendorfer B. Lipid metabolism response to a single, prolonged bout of endurance exercise in healthy young men. Am J Physiol Endocrinol Metab. 2006;290:E355–62. doi: 10.1152/ajpendo.00259.2005. [DOI] [PubMed] [Google Scholar]
- 31.Jensen MD, Heiling VJ. Heated hand vein blood is satisfactory for measurements during free fatty acid kinetic studies. Metabolism. 1991;40:406–9. doi: 10.1016/0026-0495(91)90152-m. [DOI] [PubMed] [Google Scholar]
- 32.Frayn KN. Calculation of substrate oxidation rates in vivo from gaseous exchange. J Appl Physiol. 1983;55:628–34. doi: 10.1152/jappl.1983.55.2.628. [DOI] [PubMed] [Google Scholar]
- 33.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–24. [PubMed] [Google Scholar]
- 34.Horowitz JF, Coppack SW, Paramore D, Cryer PE, Zhao G, Klein S. Effect of short-term fasting on lipid kinetics in lean and obese women. Am J Physiol. 1999;276:E278–84. doi: 10.1152/ajpendo.1999.276.2.E278. [DOI] [PubMed] [Google Scholar]
- 35.Klein S, Young VR, Blackburn GL, Bistrian BR, Wolfe RR. Palmitate and glycerol kinetics during brief starvation in normal weight young adult and elderly subjects. J Clin Invest. 1986;78:928–33. doi: 10.1172/JCI112682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mittendorfer B, Horowitz JF, Klein S. Gender differences in lipid and glucose kinetics during short-term fasting. Am J Physiol Endocrinol Metab. 2001;281:E1333–9. doi: 10.1152/ajpendo.2001.281.6.E1333. [DOI] [PubMed] [Google Scholar]
- 37.Mittendorfer B, Liem O, Patterson BW, Miles JM, Klein S. What does the measurement of whole-body fatty acid rate of appearance in plasma by using a fatty acid tracer really mean? Diabetes. 2003;52:1641–8. doi: 10.2337/diabetes.52.7.1641. [DOI] [PubMed] [Google Scholar]
- 38.Annuzzi G, Jansson E, Kaijser L, Holmquist L, Carlson LA. Increased removal rate of exogenous triglycerides after prolonged exercise in man: time course and effect of exercise duration. Metabolism. 1987;36:438–43. doi: 10.1016/0026-0495(87)90040-0. [DOI] [PubMed] [Google Scholar]
- 39.Tsetsonis NV, Hardman AE. Reduction in postprandial lipemia after walking: influence of exercise intensity. Med Sci Sports Exerc. 1996;28:1235–42. doi: 10.1097/00005768-199610000-00005. [DOI] [PubMed] [Google Scholar]
- 40.Henderson GC, Fattor JA, Horning MA, et al. Lipolysis and fatty acid metabolism in men and women during the postexercise recovery period. J Physiol. 2007;584:963–81. doi: 10.1113/jphysiol.2007.137331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nielsen S, Guo Z, Albu JB, Klein S, O’Brien PC, Jensen MD. Energy expenditure, sex, and endogenous fuel availability in humans. J Clin Invest. 2003;111:981–8. doi: 10.1172/JCI16253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Magkos F, Patterson BW, Mohammed BS, Klein S, Mittendorfer B. Women produce fewer but triglyceride-richer very low-density lipoproteins than men. J Clin Endocrinol Metab. 2007;92:1311–8. doi: 10.1210/jc.2006-2215. [DOI] [PubMed] [Google Scholar]
- 43.Kuo CC, Fattor JA, Henderson GC, Brooks GA. Lipid oxidation in fit young adults during postexercise recovery. J Appl Physiol. 2005;99:349–56. doi: 10.1152/japplphysiol.00997.2004. [DOI] [PubMed] [Google Scholar]
- 44.Klein S, Coyle EF, Wolfe RR. Fat metabolism during low-intensity exercise in endurance-trained and untrained men. Am J Physiol. 1994;267:E934–40. doi: 10.1152/ajpendo.1994.267.6.E934. [DOI] [PubMed] [Google Scholar]
- 45.Romijn JA, Klein S, Coyle EF, Sidossis LS, Wolfe RR. Strenuous endurance training increases lipolysis and triglyceride-fatty acid cycling at rest. J Appl Physiol. 1993;75:108–13. doi: 10.1152/jappl.1993.75.1.108. [DOI] [PubMed] [Google Scholar]
- 46.Coggan AR, Raguso CA, Gastaldelli A, Sidossis LS, Yeckel CW. Fat metabolism during high-intensity exercise in endurance-trained and untrained men. Metabolism. 2000;49:122–8. doi: 10.1016/s0026-0495(00)90963-6. [DOI] [PubMed] [Google Scholar]
- 47.Turcotte LP, Richter EA, Kiens B. Increased plasma FFA uptake and oxidation during prolonged exercise in trained vs. untrained humans. Am J Physiol. 1992;262:E791–9. doi: 10.1152/ajpendo.1992.262.6.E791. [DOI] [PubMed] [Google Scholar]
- 48.Phillips SM, Green HJ, Tarnopolsky MA, Heigenhauser GF, Hill RE, Grant SM. Effects of training duration on substrate turnover and oxidation during exercise. J Appl Physiol. 1996;81:2182–91. doi: 10.1152/jappl.1996.81.5.2182. [DOI] [PubMed] [Google Scholar]
- 49.Horowitz JF, Leone TC, Feng W, Kelly DP, Klein S. Effect of endurance training on lipid metabolism in women: a potential role for PPARalpha in the metabolic response to training. Am J Physiol Endocrinol Metab. 2000;279:E348–55. doi: 10.1152/ajpendo.2000.279.2.E348. [DOI] [PubMed] [Google Scholar]
- 50.Friedlander AL, Casazza GA, Horning MA, Usaj A, Brooks GA. Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J Appl Physiol. 1999;86:2097–105. doi: 10.1152/jappl.1999.86.6.2097. [DOI] [PubMed] [Google Scholar]
- 51.Friedlander AL, Casazza GA, Horning MA, Buddinger TF, Brooks GA. Effects of exercise intensity and training on lipid metabolism in young women. Am J Physiol. 1998;275:E853–63. doi: 10.1152/ajpendo.1998.275.5.E853. [DOI] [PubMed] [Google Scholar]
- 52.Sial S, Coggan AR, Hickner RC, Klein S. Training-induced alterations in fat and carbohydrate metabolism during exercise in elderly subjects. Am J Physiol. 1998;274:E785–90. doi: 10.1152/ajpendo.1998.274.5.E785. [DOI] [PubMed] [Google Scholar]
- 53.Horowitz JF, Braudy RJ, Martin WH, 3rd, Klein S. Endurance exercise training does not alter lipolytic or adipose tissue blood flow sensitivity to epinephrine. Am J Physiol. 1999;277:E325–31. doi: 10.1152/ajpendo.1999.277.2.E325. [DOI] [PubMed] [Google Scholar]
- 54.Friedlander AL, Jacobs KA, Fattor JA, et al. Contributions of working muscle to whole body lipid metabolism are altered by exercise intensity and training. Am J Physiol Endocrinol Metab. 2007;292:E107–16. doi: 10.1152/ajpendo.00148.2006. [DOI] [PubMed] [Google Scholar]
- 55.Hardman AE, Lawrence JE, Herd SL. Postprandial lipemia in endurance-trained people during a short interruption to training. J Appl Physiol. 1998;84:1895–901. doi: 10.1152/jappl.1998.84.6.1895. [DOI] [PubMed] [Google Scholar]
- 56.Gill JM, Caslake MJ, McAllister C, et al. Effects of short-term detraining on postprandial metabolism, endothelial function, and inflammation in endurance-trained men: dissociation between changes in triglyceride metabolism and endothelial function. J Clin Endocrinol Metab. 2003;88:4328–35. doi: 10.1210/jc.2003-030226. [DOI] [PubMed] [Google Scholar]
- 57.Harrison M, O’Gorman DJ, McCaffrey N, et al. Influence of acute exercise with and without carbohydrate replacement on postprandial lipid metabolism. J Appl Physiol. 2008 doi: 10.1152/japplphysiol.91367.2008. [DOI] [PubMed] [Google Scholar]
- 58.Jamurtas AZ, Koutedakis Y, Paschalis V, et al. The effects of a single bout of exercise on resting energy expenditure and respiratory exchange ratio. Eur J Appl Physiol. 2004;92:393–8. doi: 10.1007/s00421-004-1156-8. [DOI] [PubMed] [Google Scholar]
- 59.Melanson EL, Sharp TA, Seagle HM, et al. Resistance and aerobic exercise have similar effects on 24-h nutrient oxidation. Med Sci Sports Exerc. 2002;34:1793–800. doi: 10.1097/00005768-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 60.Melanson EL, Sharp TA, Seagle HM, et al. Effect of exercise intensity on 24-h energy expenditure and nutrient oxidation. J Appl Physiol. 2002;92:1045–52. doi: 10.1152/japplphysiol.00706.2001. [DOI] [PubMed] [Google Scholar]
- 61.Melanson EL, Donahoo WT, Grunwald GK, Schwartz R. Changes in 24-h substrate oxidation in older and younger men in response to exercise. J Appl Physiol. 2007;103:1576–82. doi: 10.1152/japplphysiol.01455.2006. [DOI] [PubMed] [Google Scholar]
- 62.Horowitz JF. Fatty acid mobilization from adipose tissue during exercise. Trends Endocrinol Metab. 2003;14:386–92. doi: 10.1016/s1043-2760(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 63.Wee J, Charlton C, Simpson H, et al. GH secretion in acute exercise may result in post-exercise lipolysis. Growth Horm IGF Res. 2005;15:397–404. doi: 10.1016/j.ghir.2005.08.003. [DOI] [PubMed] [Google Scholar]