Abstract
To functionalize biomaterials for bioconjugation, a chemical vapor deposition (CVD) polymerization technique was utilized to modify material surfaces. Poly [(4-amino-p-xylylene)-co-(p-xylylene)] (PPX-NH2) was deposited on inert polycaprolactone (PCL) surfaces to provide a reactive amine layer on the substrate surfaces. The biocompatibility of PPX-NH2 was evaluated by 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H–tetrazolium (MTS) and lactate dehydrogenase (LDH) assays. The results demonstrated that cells continuously proliferated on CVD treated PCL surfaces with high survival rates. Biotin was conjugated on modified PCL surfaces to immobilize avidin for binding of biotinylated adenovirus. Scanning electron microscopy (SEM) examination illustrated that adenoviruses were evenly bound on both 2-D films and 3-D scaffolds, suggesting CVD was capable of modifying various substrates with different geometries. Using a wax masking technique, the biotin conjugation was controlled to immobilize avidin on specific sites. Due to the virus binding specificity on CVD modified surfaces, cell transduction was restricted to the pattern of immobilized virus on biomaterials, by which transduced and non-transduced cells were controlled in different regions with a distinct interface. Because CVD demonstrated excellent controllability in different hierarchies, this surface modification should be able to tailor bioconjugation and expand the possibility of material application in different fields.
INTRODUCTION
Regenerative gene therapy is the science of transferring genetic material into individuals for therapeutic purposes. Transduced cells that stably express target growth factors function as mini protein reactors during the therapeutic period can lead appropriate cell recruitment and differentiation to recover original tissue function [1]. To avoid possible systemic infection caused by virus dispersion, substrate-mediated gene delivery has been developed to spatially control cell transduction [2–6]. Viral vectors are immobilized on biomaterial substrates for direct exposure in a cellular environment. Because these vectors can only infect cells adhered to the material surfaces, it restricts the transduction region and regulates the therapeutic protein expression to only the substrates [7]. Furthermore, this method may reduce the required amount of virus necessary relative to bolus delivery due to the concentrated vector distribution on biomaterials [4, 8, 9]. Different bioconjugation strategies have been developed to tether viral vectors on substrates via antibody-antigen or biotin-avidin interactions [6, 10–12]. These vector immobilizations have been successfully performed in porcine ventricle [10], collagen-coated stainless steel stents [11], and platinum microcoils [13].
Biomaterial scaffolds are potential substrates to deliver genes in situ. Such scaffolds can provide sufficient space for cell growth and appropriate mechanical strength to support tissue growth during reconstruction. Nevertheless, many biomaterials that satisfying mechanical performance lack reactive functional groups for bioconjugation [14]. To introduce reactive groups with appropriate types and densities on biomaterials, high energy irradiation and thin-film coatings are frequently applied [15].
High energy radiation from sources, such as plasma, laser, and ion beam have been used to generate functional groups on no-reactive material surfaces [16–18]. These surface etching methods provide non-specific modifications for non-reactive biomaterials. These modifications are durable, but may only be applied to 2-D films or very thin 3-D scaffolds because of the penetration limitation of the radiations [19]. To overcome this difficulty, surface coatings have been developed, by which a layer of functionalized film is created on 3-D structures. Dip coating is the simplest approach, but is limited by adhesion, homogeneity, and biocompatibility due to the use of solvents and additives [20]. Chemical vapor deposition (CVD) polymerization is an alternative modification method. Substituted [2.2]paracyclophanes are sublimated, activated, and then deposited to polymerize on the material surface. The CVD process exhibits enhanced features like absolute conformance to substrate topology and the ability to penetrate porous structure for coating complex geometries. Because the conversion from monomer vapor to polymer film is direct, no solvents, plasticizer, or catalysts are used, resulting in a low intrinsic cytotoxicity [20]. CVD polymerization of [2.2]paracyclophanes (PCP) into poly(p-xylylenes) (PPX) follows a well-established protocol. The PCP dimer can be modified with different reactive groups, including amines, alcohols, alkynes, carbonyls, and anhydrides [15, 21]. Functionalized PCPs are then polymerized into reactive PPX coatings, which present the functional groups on the substrate surface. In addition, many of the PPX coatings display excellent biocompatibility properties compared to other polymer coatings, and thus are appropriate for scaffold modification [20]. Amino-[2.2]paracyclophane has been developed for deposition on implant materials surfaces and may be applied to conjugate biomolecules such as proteins, antigens or cell receptors [22]. Due to its reactive properties, it is a potential CVD treatment to allow control of the interactions between biomaterials and live tissues.
To investigate the extent, to which CVD-modified scaffold surfaces may be functionalized for bioconjugation, the virus-biotin-avidin-biotin-materials (VBABM) method was used as a model system [6]. In the VBABM system, material surfaces are conjugated with a layer of biotin for avidin immobilization. These tethered avidin molecules are able to bind biotinylated adenovirus, and thus spatially control gene delivery [6]. Chitosan has been used in this study due to its intrinsic amine groups; however, this VBABM strategy requires biomaterials with reactive functional groups. Consequently, an effective functionalization step should broaden the utilization of this virus immobilization delivery system. Poly (ε-caprolactone) (PCL) is a frequently used scaffold material because of its biocompatibility and biodegradability, but it lacks active functional groups for covalent linkage [23]. Therefore, we sought to apply CVD modification to PCL surfaces to present a layer of amine groups. We hypothesized that biotin conjugation could be performed on these aminated surfaces for avidin immobilization, and thus biotinylated adenovirus could be bound in specific regions of scaffolds to spatially control cell transduction.
MATERIALS AND METHODS
Polycaprolactone film preparation
Polycaprolactone (PCL) (Mn=42500, Mw=65000, Sigma-Aldrich, St. Louis, MO, USA) was dissolved in glacial acetic acid at 0.5% (w:v). After melting the polymer at 60 °C overnight, the PCL solution was filtered through a 0.22 µm membrane (Nalgene, Rochester, NY, USA). The PCL solution (0.5 ml/well) was placed into 24-well culture plates (Corning, Lowell, MA, USA) which were then incubated at 50 °C overnight to evaporate the solvent. Each well was neutralized by 2 M NaOH and then was washed with PBS.
Chemical vapor deposition
The PCL surfaces were modified using chemical vapor deposition (CVD) polymerization as documented in previous studies [15, 20, 22]. 4-Amino[2.2]-paracyclophane (1) was synthesized from [2.2] paracyclophane according to previously established protocols [24] (Fig 5.1a). Approximately 30–40 mg of dimer (1) was loaded into the CVD system, and the system working pressure was adjusted to 0.28 mbar. Dimer (1) sublimated at 90–100 °C and was transported through a pyrolysis zone (670 °C) and into the deposition chamber via argon carrier gas (20 sccm). The PCL coated multi-well plates were placed on the sample holder that was cooled down to 15 °C. Sample holder rotation ensured uniform deposition of poly[(4-amino-p-xylylene)-co-(p-xylylene)] (PPX-NH2) (2) . The deposition time lasted 15–20 minutes. The surfaces before and after PPX-NH2 (2) modification were characterized by Fourier transform infrared spectroscopy (FT-IR, Nicolet 6700 Spectrometer, Thermo Fisher, Waltham, MA, USA) utilizing the grazing angle accessory (Smart SAGA, Thermo Fisher) at a angle of 85˚.
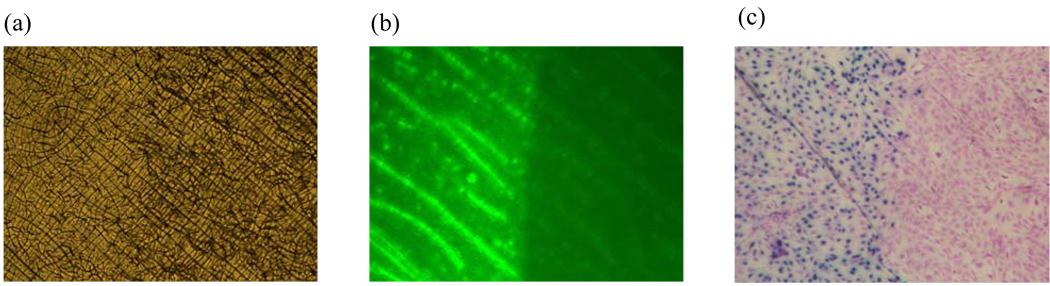
Figure 5. Spatial control of adenovirus immobilization to restrict cell transduction at specific sites.
(a) The image of coated PCL surfaces by phase contrast microscope observation. The wax masking technique was utilized to restrict the CVD treatment to only the left of PCL surfaces, on which Amine-PEO3-Biotin was conjugated to immobilize avidin. (b) Biotin-fluorescein was used to label the surface avidin. Only the exposed area without wax protection expressed green fluorescence. (c) Fibroblasts were cultured on PCL surfaces for 2 days. The transduced cells stained blue after exposure to X-gal staining. Cells grew to confluence on the material surfaces; however, cell transduction was restricted to the non-masked area, which was consistent with the avidin distribution.
The biocompatibility of the C VD modified surface
To investigate the extent of which CVD modification affected the physiological status of cells, 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H–tetrazolium (MTS) and lactate dehydrogenase (LDH) assays were performed to determine cell proliferation and cytotoxicity. C4 fibroblasts (ATCC, Manassas, VA, USA) were seeded at a concentration of 5,000 cells/cm2 on PCL surfaces before and after amination. In the MTS assay, 120 µl of CellTiter 96 AQueous one solution (Promega, Madison, WI, USA) was added per well and incubated at 37°C for 1 hr. The supernatants were sampled in volumes of 150 µl in 96-well plates and were read spectrophotometrically at OD490nm. Additionally, a standard curve of MTS assay with different numbers of fibroblasts was established, and the MTS time-course results were fitted to determine the cell numbers on material surfaces.
The cytotoxicity was determined by using an LDH kit (CytoTox 96 Non-radioactive Cytotoxicity assay, Promega). Fibroblasts were cultured in 24-well plates before and after CVD modification, in the same way as the MTS assay. At different time points, 50 µl of the medium was transfered to a 96-well plate. The reconstituted substrate mix was then added (50 µl/well), and the reaction was incubated at room temperature in darkness for 30 minutes. Finally, 50 µl of stop solution was added to each well, and the sample analyzed spectrophotometrically at a wavelength of 490 nm. Furthermore, a calibration curve of the LDH assay was determined by lysing different numbers of fibroblasts. This was used to estimate the number of dead cells at different time points. The survival rates were then calculated by comparing the cell death numbers to the total cell numbers determined by the MTS assay.
Adenovirus biotinylation
Adenovirus encoding the β-galactosidase gene and localization sequence (AdLacZ) was prepared by Vector Core, University of Michigan. This adenovirus was biotinylated as previously described [6]. Sulfosuccinimidyl-6-[biotinamido] hexanoate (SulfoNHS-LC-biotin) (Pierce, Rockford, IL, USA) was reacted with adenovirus at 4°C for 2 hours. Ultrafiltration was applied to remove unreacted biotin, and the virus was sterilized by passage through 0.2 µm syringe filters.
Adenovirus immobilization on CVD treated PCL surfaces
The CVD-modified plates were washed with water before the conjugation experiments. To immobilize biotinylated adenovirus for in situ transduction, aminated PCL surfaces were conjugated to Amine-PEO3-Biotin (Pierce, Rockford, IL, USA) by glutaraldehyde crosslinking. Avidin was then indirectly docked on biotinylated PCL surfaces to tether biotinylated adenovirus (virus-biotin-avidin-biotin-material, VBABM), as described in previous study [6]. Briefly, Amine-PEO3-Biotin was dissolved in PBS to a concentration of 40 µg/ml and added 250 µl/well to 24well plates. Then, 250 µl glutaraldehyde (0.5% in PBS, v/v) was added in each well and incubated for 2 hours at room temperature. After washing by PBS, 30µg avidin in PBS was immobilized for 2 hours at room temperature. Biotinylated AdLacZ in 0.5% gelatin (w/v in PBS) was incubated at a concentration of 108 pfu /well at 4°C for 2 hours, and unbound viruses were removed by multiple PBS rinses. The same procedure was also performed in 3-D PCL scaffolds which were fabricated by selective laser sintering (SLS) [25].
Virus distribution examined by scanning electron microscope
The immobilized viral particle distribution was determined by scanning electron microscopy (SEM). PCL films or scaffolds with immobilized AdLacZ were fixed by 10% glutaraldehyde in PBS and postfixed by 1% osmium tetroxide (Acros, Geel, Belgium) for 1 hour each. After two washes with distilled water, the samples were incubated at −80 °C for 2 hours and then lyophilized in a freeze dryer (Virtis, Gardiner, NY, USA) at −78.51 °C and 100 mTorr for 24 hours. Samples were gold coated (SPI, West Chester, PA, USA) and examined using a scanning electron microscope (Nova Dual Beam FIB/SEM, FEI, Hillsboro, OR, USA).
Wax masking to spatially control CVD treatment for virus immobilization on specific sites
To spatially control CVD treatment to specific sites, low-melting polyester wax (EMS, Hatfield, PA, USA) was applied to partially mask defined areas of scaffolds. On PCL films, wax was melted at 40 oC and then added (200 µl/well) to the right side of PCL coated wells. The same process was performed in 3-D PCL scaffolds. 300 µl wax was added to the wells of 24-well plates to mask the lower portion of scaffolds (Fig 7a). After the wax solidified, PPX-NH2 was deposited via CVD onto exposed surfaces. The modified surfaces were biotinylated to immobilize avidin, and the wax was removed by incubation in ethanol at 37 °C for 1 hour.
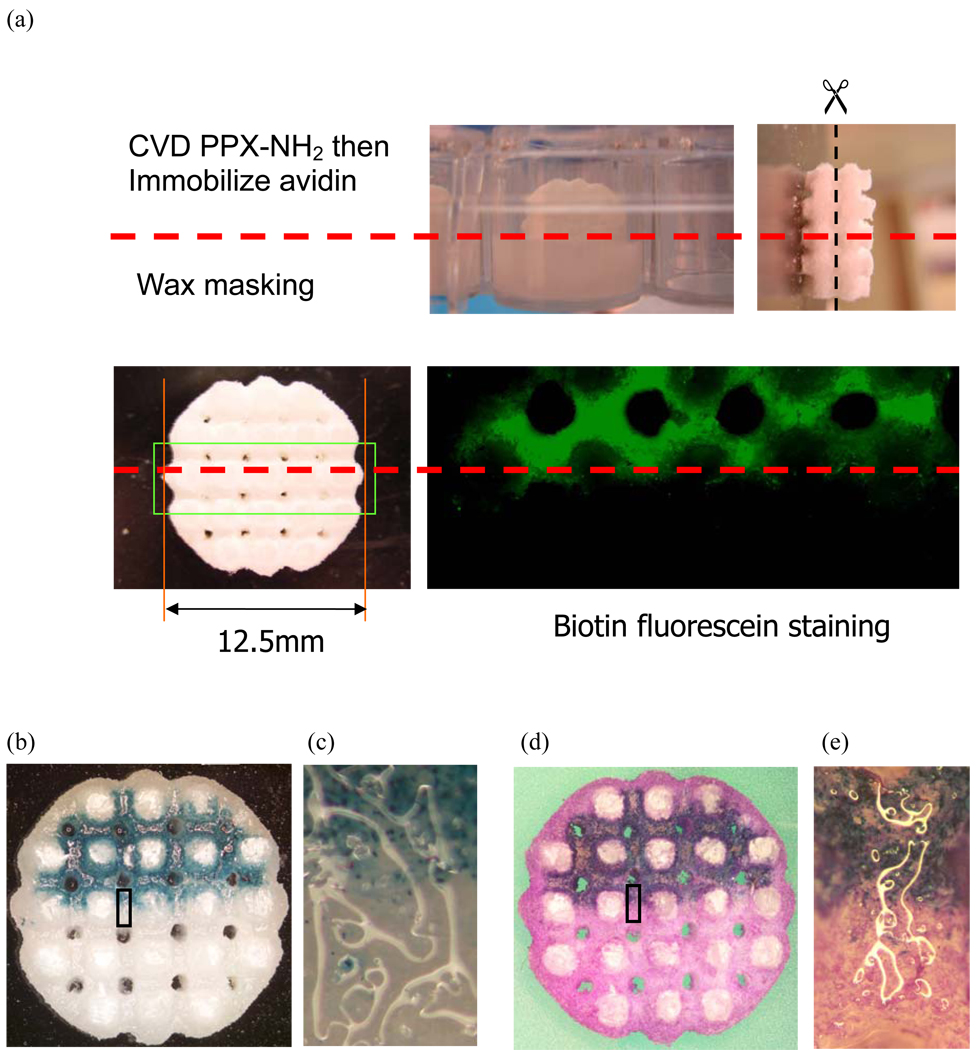
Figure 7. Spatial control of adenovirus immobilization in 3-D scaffolds.
(a) Half of each scaffold was embedded in low-melting wax to restrict the CVD treatment to only the non-masked regions for avidin immobilization. After wax removal, scaffolds were cut halfway down their heights and stained with biotin-fluorescein. The bound avidin was distributed on non-masked regions inside the scaffolds. (b) Biotinylated AdLacZ was immobilized in 3-D scaffolds before seeded fibroblasts. After a 2 day culture period, scaffolds were cut halfway down their height and stained with X-gal. Transduced cells were distributed in the same regions as the bound avidin. (d) Counter staining with crystal violet illustrated that fibroblasts grew to confluence in the scaffolds. Transduced and non-transduced cells were controlled to distribute in two different regions with distinct interfaces. (c) and (e) are high magnitude images of block regions in (b) and (d), respectively.
After avidin immobilization on biotinylated PCL surfaces, Biotin-fluorescein (Thermo Fisher) was used to stain the bound avidin. The labeled region was observed under a fluorescent microscope (Eclipse TE300, Nikon, Melville, NY, USA). To determine the distribution and activity of immobilized virus, in vitro cell culture was performed. For PCL film, fibroblasts were seeded at concentrations of 2.5×104 cells/cm2 for 2 days. The distribution of cell transduced by the immobilized virus was illustrated by X-gal staining followed by crystal violet counter staining. This experiment was also performed for 3-D scaffolds in which 1 million fibroblasts were seeded per scaffold. After 2 days in culture, the scaffolds were dissected at their midpoints and stained by X-gal and crystal violet.
RESULTS
Characterization of CVD treated PCL surfaces
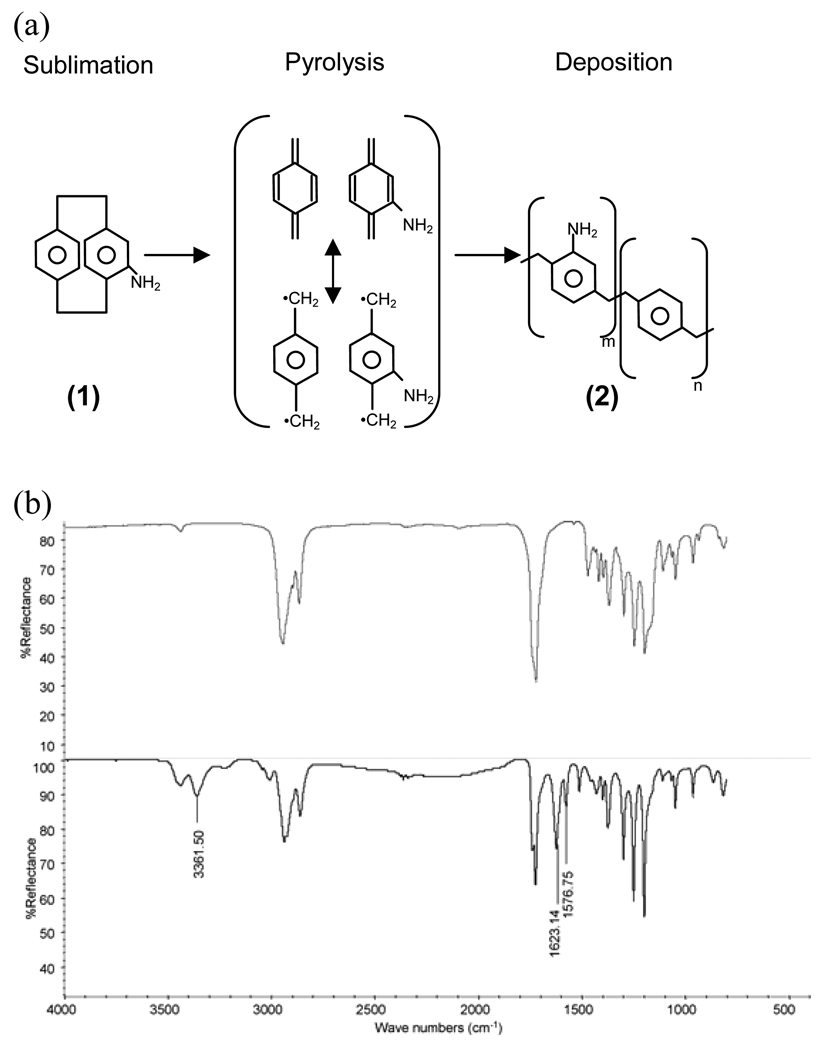
Because PCL lacks reactive functional groups, the CVD technique was used to create a layer of amines on PCL surfaces (Fig 1a). After PPX-NH2 deposition, the treated PCL was examined by FTIR-ATR to confirm appropriate surface modification. When comparing the infrared spectrum before and after modification, several specific absorption peaks demonstrated the newly formed amine groups (Fig 1b). The absorptions at 1576 and 1632 cm−1 were due to the scissoring bending of primary amines. Additionally, the stretching of amines led to specific absorption peaks at 3361 cm−1. These characterizations demonstrated that amine groups were presented on PCL surfaces after CVD surface modification.
Figure 1. Surface modification by chemical vapor deposition (CVD) on polycaprolactone (PCL).
(a) Scheme of Poly [(4-amino-p-xylylene)-co-(p-xylylene)] (PPX-NH2) deposition on PCL surfaces (b) FT-IR spectrums of PCL before (top) and after (bottom) CVD treatment
Biocompatibility of PPX-NH2 deposited PCL films
Because safety is an extremely important issue for the use of biomaterials in humans, it is necessary to determine if a CVD treated surface is appropriate for cell attachment and growth without cytotoxicity. Consequently, fibroblasts were cultured on PPX-NH2-deposited PCL surfaces for 1 week, and cell proliferation rates and survival rates were determined by MTS and LDH assays, respectively (Fig 2a,b).
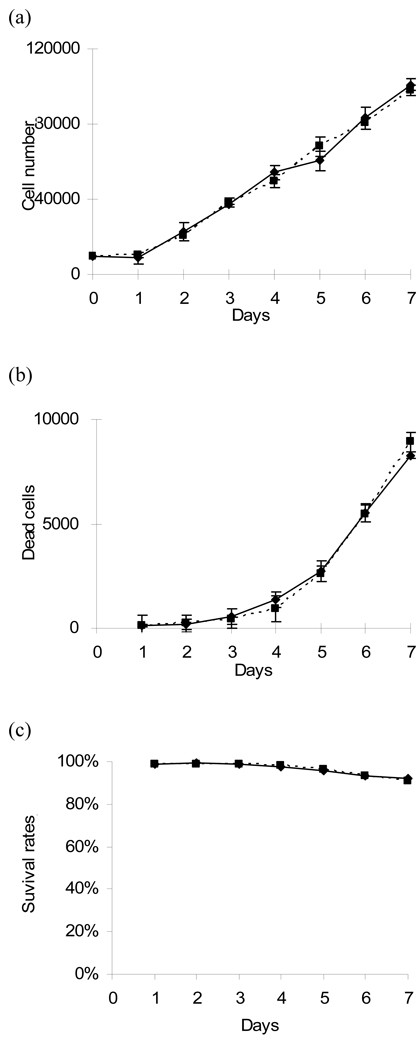
Figure 2. Biocompatibility of PCL with PPX-NH2 treatment.
To evaluate the biocompatibility of CVD treatment on PCL surfaces, fibroblasts were seeded on PCL films with (dashed line) and without (solid line) PPX-NH2 deposition. (a) MTS and (b) LDH assay were performed to investigate the cell proliferation and cell death at 1 week, respectively. (c) The survival rates of cells were normalized by comparing the ratio of lysis cells to the total cell numbers at different time points.
In the MTS assay, the fibroblast numbers steadily increased from 10,000 to approximately 100,000 in 7 days of culture, demonstrating that these surfaces supported cell proliferation (Fig 2a). To investigate potential cytotoxicity of the modified surface, the numbers of dead cells were determined by LDH assay. Cell death was not significant until day 5. This observation may have been caused by high concentrations of waste and toxic protein release from dead cells (Fig 2b). However, a survival rate of greater than 90 % was maintained, indicating that PPX-NH2 treated surfaces did not lead to significant cell death (Fig 2c). In both the MTS and LDH assays, there were no significant differences between the PPX-NH2 deposited and non-deposited PCL groups.
Specificity of biotinylated adenovirus immobilization
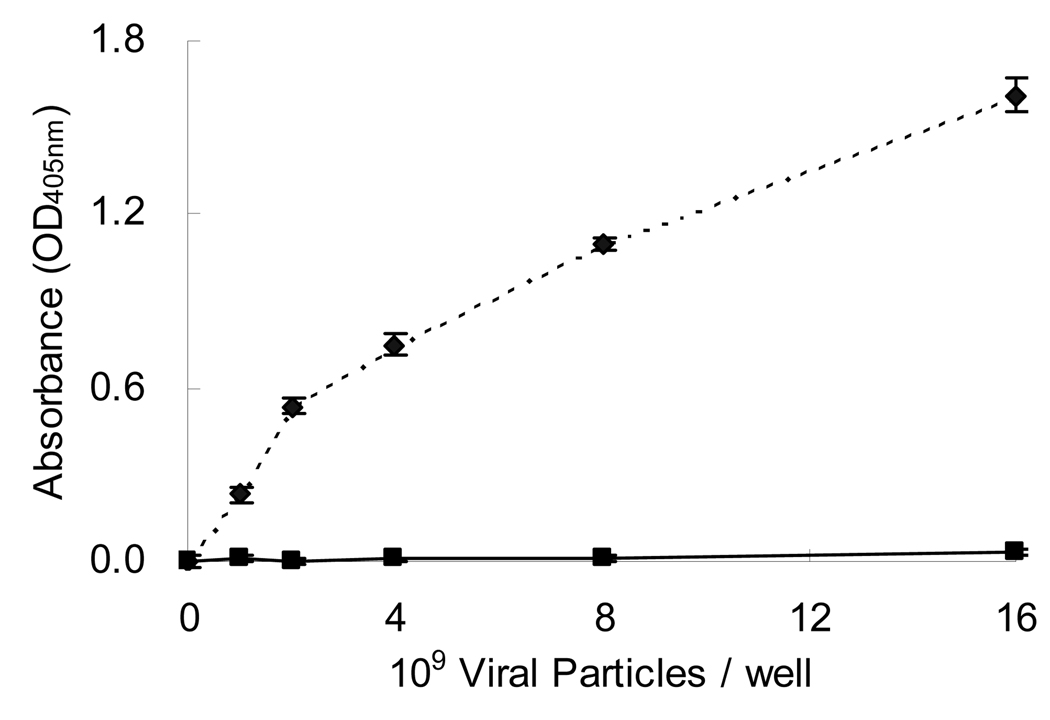
Aminated PCL surfaces were conjugated to Biotin-PEO3-NH2 to form a layer of biotin for avidin immobilization. To investigate if this surface modification specifically tethered biotinylated adenovirus, an ELISA assay was performed. Different concentrations of biotinylated adenoviral vectors were individually incubated on PCL surfaces with or without immobilized avidin. Unbound virus was removed and the surface immobilized adenovirus was detected by an indirect ELISA assay (Fig 3). The binding of biotinylated adenovirus increased with increasing virus concentrations on avidin immobilized surfaces. In contrast, the untreated PCL surfaces had almost no virus adsorption. These results suggested that biotinylated adenovirus could be specifically bound to PPX-NH2 treated PCL surfaces by avidin immobilization using the VBABM method.
Figure 3. Specificity of adenovirus immobilization on PPX-NH2 treated surfaces using the VBABM method.
Biotin was conjugated on PPX-NH2 deposited PCL surfaces to immobilize avidin, and then biotinylated adenoviral vectors in different concentrations were placed on the surfaces (dashed line). The same biotinylated virus solutions were placed on non-modified PCL surfaces as the control group (solid line). The bound adenovirus was determined by ELISA to investigate if these modified surfaces may specifically immobilize virus.
Distribution of adenovirus on CVD treated surfaces
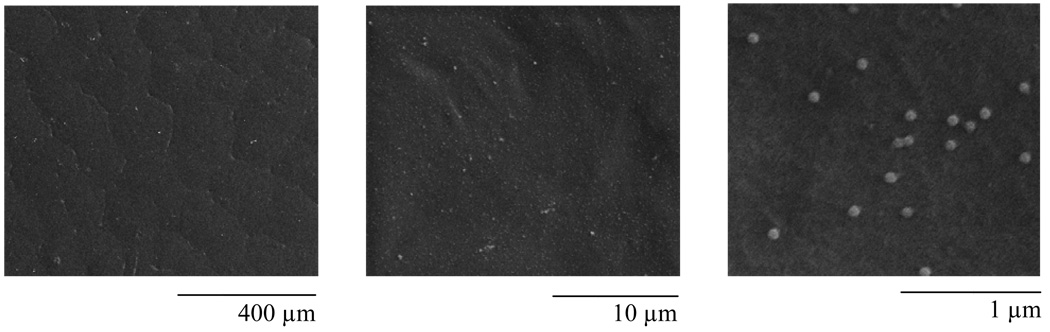
Using the VBABM method, biotinylated adenovirus was bound to modified PCL films. To investigate the distribution pattern of virus, immobilized viral particles were illustrated by SEM examination (Fig 4). Unique spheres with diameters ranging between 70–80 nm were observed on PCL films, suggesting adenovirus (arrange size=70–90 nm) was immobilized on modified PCL surfaces. These adenoviral particles were evenly distributed on material surfaces, indicating well-oriented avidin immobilization.
Figure 4. Adenovirus immobilization on 2-D PCL films.
Biotinylated adenovirus was immobilized on the modified PCL surfaces by the VBABM method [6]. The bound adenoviral particles were illustrated using SEM examination in different magnitudes.
Spatial control of cell transduction
Adenovirus was only tethered on PPX-NH2 treated PCL surfaces by the VBABM method (Fig 3). Therefore, spatial control of virus immobilization to target sites of biomaterials should be possible. To determine the extent to which adenovirus could be bound on distinct regions of biomaterials by controlling the CVD modification, a wax masking technique was used to regulate PPX-NH2 deposition on defined regions of PCL films. After avidin immobilization, the masking wax was removed and biotinylated AdLacZ was bound on materials surfaces.
The coated PCL films on 24-well plates were translucent. Using a phase contrast microscope, the crystals of PCL molecules led to a roughness in the films (Fig 5a). By masking the right site of PCL films during CVD treatments, the unmasked area was functionalized by PPX-NH2 deposition for biotinylation and avidin immobilization. The avidin distribution was examined by biotin-fluorescein staining (Fig 5b). Only exposed PCL surfaces were identified by green fluorescence, suggesting that avidin immobilization was spatially controlled and that only unprotected regions were able to be conjugated.
In vitro cell culture was also performed to determine if this spatially controlled avidin could be applied to transduce cells that adhere to and proliferate on biomaterial surfaces in situ. After incubating for 2 days, the transduced cells were identified by X-gal staining and non-infected cells were identified by crystal violet counter staining (Fig 5c). The distribution of cell transduction was consistent with the immobilized avidin, resulting in blue stained cells only being presented on the side of the PCL film that was functionalized. This demonstrates that cell transduction can be controlled by CVD modification and virus immobilization on specific sites of biomaterials.
Adenovirus immobilization in 3-D scaffolds
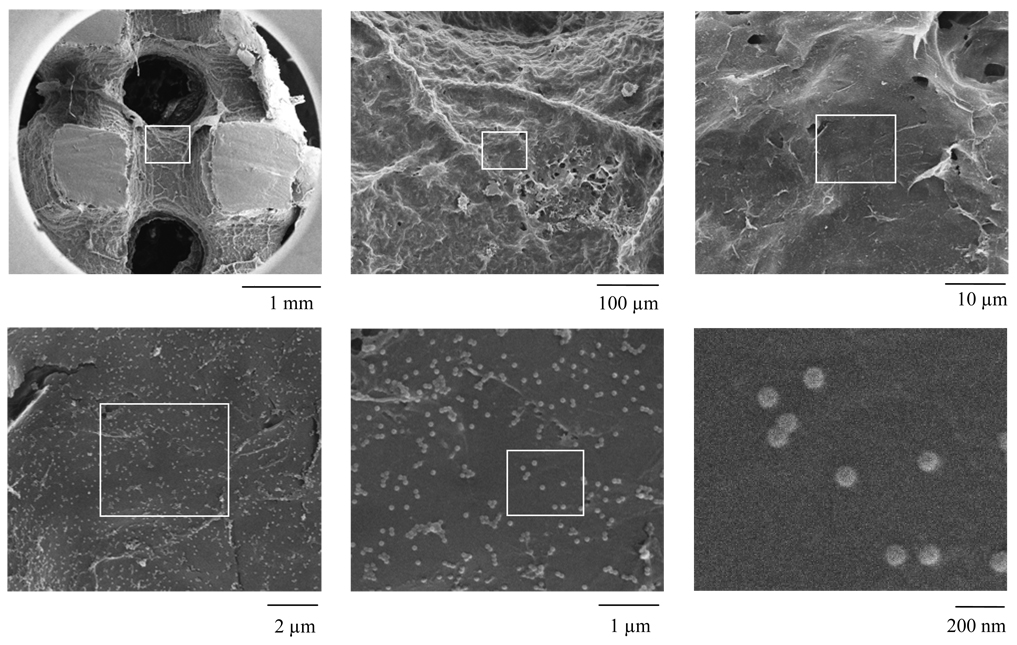
Because adenovirus was successfully immobilized on 2-D PCL films, we considered it important to investigate the feasibility of this technique in 3-D scaffolds that may be used for tissue regeneration. Cylindrical PCL scaffolds 12 mm in diameter and 5mm in height were treated by PPX-NH2 deposition and adenovirus was bound by the VBABM method. After adenovirus immobilization, the scaffolds were cross-sectioned at the mid-line of their heights before SEM examination.
Immobilized adenovirus was identified in the inner surfaces of the scaffolds (Fig 6). This indicated that CVD treatment was able to modify a complex 3-D structure and that functionalized surfaces could be applied for virus immobilization. These results demonstrated that CVD modification was not only feasible for functionalizing 2-D surfaces, but also complex 3-D structures for bioconjugation.
Figure 6. Adenovirus immobilization on CVD-modified PCL scaffolds.
To determine if CVD could functionalize 3-D complex structures, PPX-NH2 was deposited on PCL scaffolds and biotinylated adenovirus was immobilized using the VBABM method. The scaffolds were cut halfway down their height and examined by SEM. Images in different magnitudes were captured, and the blocks were used to indicate the regions in high magnitudes.
Using wax masking, PPX-NH2 deposition was spatially controlled to functionalize one half of the 3-D scaffold surfaces. The scaffolds were then sectioned to examine the inner regions for bioconjugation. Biotin-fluorescein staining illustrated that avidin was only immobilized in non-masking regions of the scaffolds (Fig 7a). Fibroblast culture in PCL scaffolds also demonstrated that AdLacZ was consistently bound to avidin in the 3-D structures (Fig 7b,c). Counter staining by crystal violet illustrated that fibroblasts proliferated to confluence with the scaffolds. Transduced and non-transduced cells were restricted to two different regions with a distinct interface (Fig 7d,e). These results were consistent with 2-D experiments, suggesting that conjugation can be controlled by CVD treatment combined with wax masking, and can be applied to complex 3-D structures.
DISCUSSION
Surface modification is an emerging technique to functionalize materials for many useful applications due to the tunability of their properties by varying the chemical composition. Biomaterials with appropriate mechanical properties, but lacking a desired chemical reactivity, are capable of being improved by introducing a range of functional groups. Thus, these reactive moieties expand the function of biomaterials, especially in the application of bioconjugation [26].
In our previous studies, adenovirus was successfully immobilized on chitosan surfaces to transduce cells in situ using the biotin-avidin interaction [6]. Using the VBABM method, immobilized viral particles were evenly distributed on biomaterials, and the transduction efficiency was significantly improved. Although these results promisingly suggested that bioconjugation maintained virus binding on biomaterial surfaces to control gene delivery within scaffolds, the strategy was limited to the availability of reactive functional groups on biomaterials. Therefore, in this study, we utilized surface modification to generalize our established viral delivery to an inert biomaterial such as PCL. PPX-NH2 was deposited on PCL surfaces to present a layer of amines. The CVD coating was characterized by FT-IR analysis, suggesting that this modification could be performed on polymer materials (Fig 1b).
Biocompatibility is an important issue for biomaterials, therefore a safety evaluation of surface modification is necessary before human studies are performed. The assessment of MTS and LDH indicated that cells proliferated on PPX-NH2 modified surfaces with extremely low death rates, suggesting that modified surfaces maintained cell proliferation without causing cytotoxicity (Fig 2a–c). Prior studies also indicated that other PPX derivatives do not induce cell lysis [15]. These results support concept that surface modification by PPX deposition is a feasible strategy for biological applications.
Conventionally, high energy radiation has been broadly applied to generate reactive surfaces for bioconjugation. For example, allylamine plasma has been developed to modify polydimethylsiloxane (PDMS) substrates [27]. Epidermal growth factor with homobifunctional N-hydroxysuccinimide (NHS) ester of poly (ethylene glycol) -butanoic acid (SBA2-PEG) was subsequently covalently conjugated on modified surfaces to investigate the nature of the bound bioactive factor and to optimize the conditions for the reaction. Poly (lactic acid) films have been modified using the same process to conjugate poly (L-lysine) and RGD peptide [28]. Although these treatments may create amines for functionalizing flat substrates, the application to non-planar structures is still not possible due to penetration limitations. Chemical vapor deposition is an improved strategy in which monomers were delivered to a reaction chamber and then polymerized on biomaterial surfaces. Intrinsically, sublimated monomers can penetrate complex geometries to evenly deposit in 3-D structures. Therefore, we sought to apply this surface modification to the immobilization of adenovirus in 3-D inert PCL scaffolds. We found that viral particles were evenly bound on the surfaces of the central regions of the scaffolds, suggesting that reactive amines were successfully created by the CVD treatment and the bioconjugation was capable of immobilizing virus on the surfaces of entire scaffolds (Fig 6).
Because inert biomaterials lack reactive functional groups, bioconjugation may be controlled through CVD on specific sites in scaffolds. The specificity of biotinylated adenovirus on modified PCL surfaces suggested that biotinylated adenovirus was only immobilized on PPX-NH2 modified biomaterials (Fig 3). Therefore, we further investigated the feasibility of spatially controlling virus delivery on specific sites of scaffolds. Using wax masking, avidin was specifically immobilized on non-masked regions (Fig 5b and Fig 7a). Adenoviral vectors were thus bound on the desired surfaces to infect cells in situ (Fig 5c and Fig 7b,c). These results demonstrated that the surface modification could be tailored through physical protection, and that bioconjugation could be controlled on target sites.
For tissue engineering of complex tissues, regeneration in tissue interfaces is a challenge because the surrounding environment complicates the healing process. However, this may be improved by precise biofactor induction [29]. In this study, scaffolds were applied as gene carriers to investigate the feasibility of guiding appropriate tissue growth in implants. Using wax masking, the CVD technique was shown to result in polymer coatings present only in unmasked areas. This resulted in spatially control in situ transduction, by which transduced and non-transduced cells distributed in different regions of scaffolds with a distinct interface. This controllable gene delivery system should be beneficial in manipulating tissue regeneration. Using this strategy, surface modification may be used to regulate appropriate bioactive factor expression through the pattern of immobilized viral vectors and to engineer specific tissue formations in target sites of scaffolds.
CONCLUSIONS
In this study, a surface modification was developed to functionalize biomaterial surfaces, by which viral particles were bound on materials and that gene expression was spatially controlled with a distinct interface. Although our strategy can effectively tether viral vectors on material surfaces to control gene delivery, this technique should not be restricted to this application only. Because PPX derivatives are capable of distributing not only on 2-D substrates but also in complex 3-D structures, this surface functionalization has been broadly applied to different biomaterial devices [22, 30–33]. Using this CVD technique, some proteins such as extracellular matrix (ECM), inductive growth factors, or plasmid DNA could also potentially be bound in 3-D biomaterials scaffolds to facilitate tissue regeneration. Consequently, we project that this surface modification may customize biomaterial surface properties to tailor bioconjugation to expand the possibility of material application in different fields.
ACKNOWLEDGEMENTS
The research was supported by a grant from the National Institutes of Health grant RO1 DE018890 (PHK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Franceschi RT. Biological approaches to bone regeneration by gene therapy. J Dent Res. 2005;84(12):1093–1103. doi: 10.1177/154405910508401204. [DOI] [PubMed] [Google Scholar]
- 2.Bengali Z, Rea JC, Shea LD. Gene expression and internalization following vector adsorption to immobilized proteins: dependence on protein identity and density. J Gene Med. 2007;9(8):668–678. doi: 10.1002/jgm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jang JH, Houchin TL, Shea LD. Gene delivery from polymer scaffolds for tissue engineering. Expert Rev Med Devices. 2004;1(1):127–138. doi: 10.1586/17434440.1.1.127. [DOI] [PubMed] [Google Scholar]
- 4.Hu WW, Wang Z, Hollister SJ, Krebsbach PH. Localized viral vector delivery to enhance in situ regenerative gene therapy. Gene Ther. 2007;14(11):891–901. doi: 10.1038/sj.gt.3302940. [DOI] [PubMed] [Google Scholar]
- 5.Hu WW, Lang MW, Krebsbach PH. Digoxigenin modification of adenovirus to spatially control gene delivery from chitosan surfaces. J Control Release. 2009;135(3):250–258. doi: 10.1016/j.jconrel.2009.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hu WW, Lang MW, Krebsbach PH. Development of adenovirus immobilization strategies for in situ gene therapy. J Gene Med. 2008;10(10):1102–1112. doi: 10.1002/jgm.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen H, Tan J, Saltzman WM. Surface-mediated gene transfer from nanocomposites of controlled texture. Nat Mater. 2004;3(8):569–574. doi: 10.1038/nmat1179. [DOI] [PubMed] [Google Scholar]
- 8.Bajaj B, Lei P, Andreadis ST. High efficiencies of gene transfer with immobilized recombinant retrovirus: kinetics and optimization. Biotechnol Prog. 2001;17(4):587–596. doi: 10.1021/bp010039n. [DOI] [PubMed] [Google Scholar]
- 9.Hanenberg H, Xiao XL, Dilloo D, Hashino K, Kato I, Williams DA. Colocalization of retrovirus and target cells on specific fibronectin fragments increases genetic transduction of mammalian cells. Nat Med. 1996;2(8):876–882. doi: 10.1038/nm0896-876. [DOI] [PubMed] [Google Scholar]
- 10.Levy RJ, Song C, Tallapragada S, DeFelice S, Hinson JT, Vyavahare N, et al. Localized adenovirus gene delivery using antiviral IgG complexation. Gene Ther. 2001;8(9):659–667. doi: 10.1038/sj.gt.3301452. [DOI] [PubMed] [Google Scholar]
- 11.Klugherz BD, Song C, DeFelice S, Cui X, Lu Z, Connolly J, et al. Gene delivery to pig coronary arteries from stents carrying antibody-tethered adenovirus. Hum Gene Ther. 2002;13(3):443–454. doi: 10.1089/10430340252792576. [DOI] [PubMed] [Google Scholar]
- 12.Mei L, Jin X, Song C, Wang M, Levy RJ. Immobilization of gene vectors on polyurethane surfaces using a monoclonal antibody for localized gene delivery. J Gene Med. 2006;8(6):690–698. doi: 10.1002/jgm.912. [DOI] [PubMed] [Google Scholar]
- 13.Abrahams JM, Song C, DeFelice S, Grady MS, Diamond SL, Levy RJ. Endovascular microcoil gene delivery using immobilized anti-adenovirus antibody for vector tethering. Stroke. 2002;33(5):1376–1382. doi: 10.1161/01.str.0000014327.03964.c0. [DOI] [PubMed] [Google Scholar]
- 14.Lee KB, Kim DJ, Lee ZW, Woo SI, Choi IS. Pattern generation of biological ligands on a biodegradable poly(glycolic acid) film. Langmuir. 2004;20(7):2531–2535. doi: 10.1021/la036209i. [DOI] [PubMed] [Google Scholar]
- 15.Elkasabi Y, Yoshida M, Nandivada H, Chen HY, Lahann J. Towards multipotent coatings: Chemical vapor deposition and biofunctionalization of carbonyl-substituted copolymers. Macromolecular Rapid Communications. 2008;29(11):855–870. [Google Scholar]
- 16.Yang J, Bei J, Wang S. Enhanced cell affinity of poly (D,L-lactide) by combining plasma treatment with collagen anchorage. Biomaterials. 2002;23(12):2607–2614. doi: 10.1016/s0142-9612(01)00400-8. [DOI] [PubMed] [Google Scholar]
- 17.Heitz J, Niino H, Yabe A. Chemical surface modification on polytetrafluoroethylene films by vacuum ultraviolet excimer lamp irradiation in ammonia gas atmosphere. Applied Physics Letters. 1996;68(19):2648–2650. [Google Scholar]
- 18.Celina M, Kudoh H, Renk TJ, Gillen KT, Clough RL. Surface modification of polymeric materials by pulsed ion beam irradiation. Radiation Physics and Chemistry. 1998;51(2):191–194. [Google Scholar]
- 19.Liu X, Ma PX. Polymeric scaffolds for bone tissue engineering. Ann Biomed Eng. 2004;32(3):477–486. doi: 10.1023/b:abme.0000017544.36001.8e. [DOI] [PubMed] [Google Scholar]
- 20.Lahann J, Klee D, Hocker H. Chemical vapour deposition polymerization of substituted [2.2]paracyclophanes. Macromolecular Rapid Communications. 1998;19(9):441–444. [Google Scholar]
- 21.Lahann J, Langer R. Novel poly(p-xylylenes): Thin films with tailored chemical and optical properties. Macromolecules. 2002;35(11):4380–4386. [Google Scholar]
- 22.Lahann J, Klee D, Pluester W, Hoecker H. Bioactive immobilization of r-hirudin on CVD-coated metallic implant devices. Biomaterials. 2001;22(8):817–826. doi: 10.1016/s0142-9612(00)00244-1. [DOI] [PubMed] [Google Scholar]
- 23.Kurella A, Dahotre NB. Review paper: surface modification for bioimplants: the role of laser surface engineering. J Biomater Appl. 2005;20(1):5–50. doi: 10.1177/0885328205052974. [DOI] [PubMed] [Google Scholar]
- 24.Waters JF, Sutter JK, Meador MAB, Baldwin LJ, Meador MA. Addition Curing Thermosets Endcapped with 4-Amino [2.2] Paracyclophane. J Polym Sci Pol Chem. 1991;29(13):1917–1924. [Google Scholar]
- 25.Williams JM, Adewunmi A, Schek RM, Flanagan CL, Krebsbach PH, Feinberg SE, et al. Bone tissue engineering using polycaprolactone scaffolds fabricated via selective laser sintering. Biomaterials. 2005;26(23):4817–4827. doi: 10.1016/j.biomaterials.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 26.Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci. 2007;32(7):698–725. [Google Scholar]
- 27.Klenkler BJ, Sheardown H. Characterization of EGF coupling to aminated silicone rubber surfaces. Biotechnol Bioeng. 2006;95(6):1158–1166. doi: 10.1002/bit.21083. [DOI] [PubMed] [Google Scholar]
- 28.Hu Y, Winn SR, Krajbich I, Hollinger JO. Porous polymer scaffolds surface-modified with arginine-glycine-aspartic acid enhance bone cell attachment and differentiation in vitro. J Biomed Mater Res A. 2003;64(3):583–590. doi: 10.1002/jbm.a.10438. [DOI] [PubMed] [Google Scholar]
- 29.Phillips JE, Burns KL, Le Doux JM, Guldberg RE, Garcia AJ. Engineering graded tissue interfaces. Proc Natl Acad Sci U S A. 2008;105(34):12170–12175. doi: 10.1073/pnas.0801988105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen HY, Lahann J. Fabrication of discontinuous surface patterns within microfluidic channels using photodefinable vapor-based polymer coatings. Anal Chem. 2005;77(21):6909–6914. doi: 10.1021/ac050964e. [DOI] [PubMed] [Google Scholar]
- 31.Lahann J, Klee D, Thelen H, Bienert H, Vorwerk D, Hocker H. Improvement of haemocompatibility of metallic stents by polymer coating. J Mater Sci Mater Med. 1999;10(7):443–448. doi: 10.1023/a:1008939400812. [DOI] [PubMed] [Google Scholar]
- 32.Lahann J, Balcells M, Lu H, Rodon T, Jensen KF, Langer R. Reactive polymer coatings: a first step toward surface engineering of microfluidic devices. Anal Chem. 2003;75(9):2117–2122. doi: 10.1021/ac020557s. [DOI] [PubMed] [Google Scholar]
- 33.Chen HY, Elkasabi Y, Lahann J. Surface modification of confined microgeometries via vapor-deposited polymer coatings. J Am Chem Soc. 2006;128(1):374–380. doi: 10.1021/ja057082h. [DOI] [PubMed] [Google Scholar]