Abstract
Introduction
Medicare reimbursement for DXAs performed in non-facility settings (e.g. physician offices) decreased in 2007. With declining reimbursement, some DXA providers may cease providing this service, which would increase travel distance for some people. The impact of travel distance on access to DXA is unclear.
Methods
Using national Medicare data, we identified claims for DXA to evaluate trends in the number and locations of DXAs performed. Travel distance was the distance from beneficiaries’ residence and the nearest DXA provider. Binomial regression evaluated the relationship between travel distance and receipt of DXA.
Results
In 2006, 2.9 million DXAs were performed, a 103% increase since 1999. In 2005–2006, 8.0% of persons were tested at non-facility sites versus 4.2% at facility sites. The remainder (88%) had no DXA. Persons traveling 5–9, 10–24, 25–39, and 40–54, and ≥ 55 miles were less likely to receive DXA (adjusted risk ratios = 0.92, 0.79, 0.43, 0.32, and 0.26, respectively, <5 miles referent). Rural residents were more dependent than urban residents on the availability of DXA from non-facility providers.
Conclusion
Approximately two-thirds of DXAs in 2005–2006 were performed in non-facility settings (e.g. as physician offices). Rural residents would have preferentially reduced access to DXA if there were fewer non-facility sites.
Keywords: bone mineral density, dual-energy xray absorptiometry (DXA), travel distance, osteoporosis
Introduction
Osteoporosis is a common, chronic condition that often results in substantial morbidity and mortality related to fractures. The burden of osteoporosis is expected to grow substantially as the population ages, particularly for non-Caucasians [1]. Although osteoporosis can be identified on the basis of a fragility fracture, it is often defined prior to fracture by measurement of bone mineral density (BMD). Central dual-energy x-ray absorptiometry (DXA) is the preferred testing modality [2]. Once diagnosed, effective therapies to treat osteoporosis are available, and these have been shown to reduce fracture risk [3–6].
Although DXA testing can identify osteoporosis prior to fractures, in the United States (U.S.), fewer than one-third of older women and less than five percent of older men receive such testing [7]. Given a strong relationship between DXA testing and initiation of osteoporosis medications [8], DXA testing provides a potentially important mediating mechanism to reduce fracture-related morbidity and mortality. The indications for DXA testing for Medicare beneficiaries are clearly described in the 1998 Bone Mass Measurement Act [9]. DXA testing is reimbursed by Medicare for five indications, including screening for postmenopausal women, and is recommended in national guidelines for all women over the age of 65 [10]. Some groups also recommend testing for men over the age of 70 [2].
Despite the important role that DXA plays in osteoporosis management, reimbursement for DXA in the U.S. in the non-facility or office/out-patient setting has been reduced by recent legislative and regulatory changes. DXA reimbursement will be cut 60% from approximately $139 in 2006 to approximately $56 in 2010. This reduction is due to changes in the Physician Work and Practice Expense Relative Value Units (RVUs) assigned to central DXA (CPT code 77080) as part of the Five Year Review of the Medicare Physician Fee Schedule (MPFS). Originally intended to be reduced in equal increments over 4 years, DXA reimbursement instead dropped 40% (to $81) from 2006 to 2007 due to the additional effects of Section 5102 of the Deficit Reduction Act which targeted cuts in advanced diagnostic imaging reimbursement to prevent cuts to the Sustained Growth Rate (SGR).
In contrast to non-facility DXA reimbursement which is determined by the MPFS and comes out of Medicare Part B, reimbursement for the hospital or facility setting is derived from Medicare Part A and remains unchanged. For Part A, Medicare uses an entirely different payment system based on a series of diagnostic related groups or DRGs. Payment is unaffected by RVUs or SGR. Moreover, expenses may not be comparable between the non-facility and facility setting as overhead for DXA comprises a far greater proportion of the total overhead of the practice than occurs in the hospital setting. In 2009, reimbursement for DXAs performed in the facility setting is approximately $86.
The effects of declining reimbursement for DXA have yet to be fully characterized, particularly since changes in reimbursement are still being phased-in. However, as a result of these cuts, some DXA providers who operate devices in physician offices and other outpatient settings (non-facility providers) will find it cost prohibitive to provide this service [11]. If so, DXA tests may be ‘shifted’ from non-facility sites to facilities. This may increase travel distances and other factors which could negatively affect access to DXA testing.
Therefore, we evaluated the relationship between travel distance and receipt of DXA among Medicare beneficiaries using longitudinal data from 1999–2006. We hypothesized that travel distance would be an important predisposing factor as to whether patients were tested or not, and that travel distance would most affect persons living in rural areas. We further evaluated the hypothetical impact on travel distance if non-facility providers were to cease providing DXA testing and testing was only available from facility DXA providers.
Methods
Medicare Data Source and Eligible Population
After approval of the study protocol by the University of Alabama at Birmingham institutional review board and the Center for Medicare and Medicaid Services (CMS), we obtained the Chronic Conditions Warehouse (CCW) claims data from Medicare for the years 1999 through 2006. These files provide all Medicare inpatient, outpatient and physician claims data on a 5% random sample of Medicare beneficiaries linked longitudinally based upon the beneficiary’s identification number [12]. Results based on the 5% sample were multiplied by 20 to obtain estimates for the Medicare Fee-for-Service population; beneficiaries enrolled in Medicare HMOs were excluded from the analysis. In accordance with Medicare policies, use of the Medicare data was governed by a Data Use Agreement, and all results were reviewed by CMS for consistency with privacy provisions prior to public release.
Identification of Central DXA Tests
To identify claims submitted to Medicare for bone mass measurement for the years 1999–2006 we used the Healthcare Current Procedure Classification System (HCPCS) code for central DXA, 76075, in claims from the Medicare carrier and outpatient files. DXA procedures are billed either as a single claim, indicating that the billing provider (e.g. a physician) both performed and interpreted the test, usually in an office-based (non-facility) setting, or alternatively, as two claims, one for the technical charge for the test (which would be assigned to the billing facility such as a hospital) and another for interpretation (which would be assigned to the physician interpreting the DXA). In the latter circumstance, a testing facility (e.g. the outpatient department of a hospital) usually bills for the technical charge, and a physician bills for the interpretation. Because claims for the technical charge and the interpretation are often not submitted by the same provider or on the same day, the use of HCPCS modifiers, TC (technical component only) and −26 (professional component only), were examined to identify facility claims and link these separate components. Claims for DXA occurring within 15 days of one another were aggregated together as a single unit to permit such linkage. Each facility or non-facility site that performed at least one DXA in each calendar year was considered a ‘DXA provider.’
Provider zip codes were identified using the relevant variable in the carrier file for non-facility providers and by linking to the CMS provider file for facility providers. Nine digit zip codes were available for approximately three-quarters of providers; five digit zip codes were used for the remainder. The number of DXAs performed at facility and non-facility sites was evaluated, stratified by calendar year. DXA provider sites then were aggregated by zip code to allow geographic analysis of all U.S. zip codes that contained at least one DXA provider. This process also created the ‘universe’ of zip codes of DXA providers needed to calculate travel distances.
Subject Eligibility Criteria
For the remaining analyses focused on individual subjects, beneficiaries were required to be at least 65 years of age as of 1/1/2003 and living in the 50 United States or the District of Columbia. Medicare beneficiaries younger than 65 are covered by Medicare mainly due to disability or end stage renal disease. We excluded these people because their DXA utilization is likely to differ from that of the general population of Medicare beneficiaries. Eligible subjects must have had 12 months of fee-for-service (FFS) Medicare part A and part B and not be enrolled in a health maintenance organization (HMO) from 2003–2006. Persons receiving care from a Medicare HMO for part or all of a year over this interval were excluded because Medicare data do not include all of their outpatient claims.
Given our hypotheses regarding access to care that might differ based upon rural vs. urban residence, beneficiary residence was classified as urban or rural using two variables developed from zip codes. The first was for the Metropolitan Statistical Area (MSA) obtained from a zip code dataset provided by SAS Institute, Inc. which is based on Census Bureau data. The second variable was the Rural-Urban Commuting Area Codes (RUCA) classification of zip codes as ‘urban core’ or ‘non-urban core’ [12]. We then classified a zip code as urban if both variables indicated urban residence and as rural otherwise. The expected effect of this classification procedure is to improve the specificity of the ‘Urban’ designation. Since receipt of DXA in the prior 2 years is an important determinant of repeat testing [13], we excluded persons who underwent central DXA in either 2003 or 2004.
Travel Distance and Data Analysis
The primary dependent variable of interest was receipt of DXA in either 2005 or 2006, and the primary independent variable of interest was the estimated distance between beneficiaries’ residence and the site of the nearest DXA provider. Because the distance was not directly observable for persons who did not undergo DXA testing, this travel distance was calculated (for all persons) as the distance between the centroid of each beneficiary’s residential zip code and the centroid of every DXA provider’s zip code. The minimum of all these distances was then selected. DXA providers were all sites that performed at least one DXA in each calendar year for any individual in the 5% sample. The validity of this procedure was examined among persons who received DXA in 2005 or 2006; reasonable agreement between estimated and actual distance was observed (Appendix 1).
Appendix.
Actual Versus Estimated Travel Distance for Persons Who Received a DXA in 2006
| Estimated Distance to the Nearest DXA Site in 2006, miles | % of eligible beneficiaries | Actual Distance Traveled to Receive a DXA in 2006, miles | ||||||
|---|---|---|---|---|---|---|---|---|
| < 5 | 5–9 | 10–24 | 25–39 | 40–54 | 55+ | |||
| < 5 | 81.6 | 47.6 | 18.3 | 10.5 | 2.0 | 0.8 | 2.5 | |
| 5–9 | 10.5 | - | 5.2 | 3.9 | 0.6 | 0.2 | 0.5 | |
| 10–24 | 7.4 | - | - | 5.1 | 1.3 | 0.4 | 0.6 | |
| 25–39 | 0.5 | - | - | - | 0.2 | 0.1 | 0.1 | |
| 40–54 | 0.1 | - | - | - | - | 0.0 | 0.1 | |
| 55+ | 0.0 | - | - | - | - | - | 0.0 | |
Numbers in each cell represent the proportion of the total sample size represented in that cell
Categorical variables were presented as frequencies and compared using chi-square tests of association. Multivariable negative binomial regression was used to evaluate the relationship between distance from each beneficiary’s residence and the nearest DXA provider. This approach is a generalization of Poisson regression and corrects for overdispersion; results can be interpreted as risk ratios. Potential confounders were included based upon a-priori interest and included: age, sex, race, geographic region of residence (as a proxy measure of regional variation in DXA reimbursement [13], Charlson comorbidity score [14], median income in 2000 in census block corresponding to beneficiary’s 9-digit zip code, number of physician office visits in 2004, and at least 1 visit in 2004 to a rheumatologist or endocrinologist. Some analyses were stratified for urban or rural place of residence.
A “shift table” was then constructed with the rows showing the distance in 2006 that each beneficiary needed to travel to the nearest DXA provider. For the persons represented on each row, the columns show the new distance that these individuals would have to travel if the non-facility DXA providers (e.g. physician offices) stopped performing DXAs and only the facilities remained. These results were stratified by urban versus rural beneficiary residence. All analyses were conducted using SAS 9.1 (SAS Institute, Cary, NC).
Results
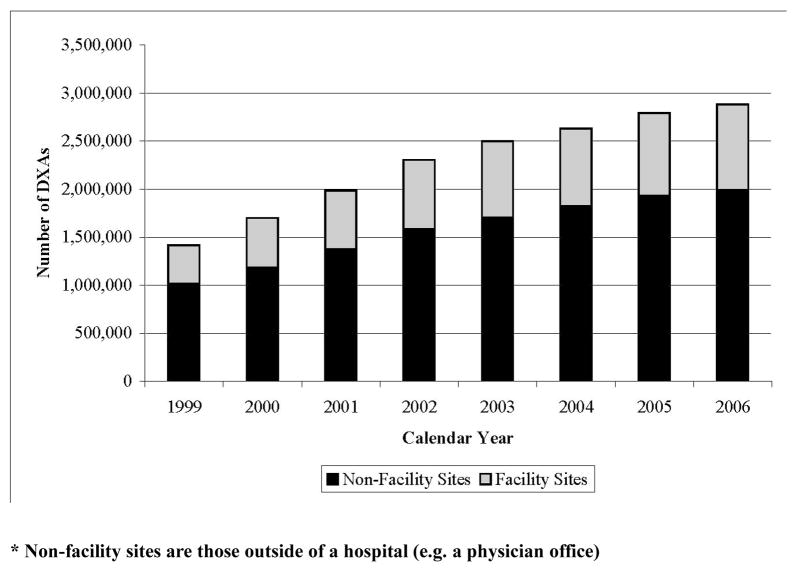
As shown in Figure 1, the number of central DXAs performed over the 8-year interval from 1999 to 2006 approximately doubled. The rate of increase tended to decline over time and this pattern was similar for both facility and non-facility sites. Applying the beneficiary eligibility criteria previously described, the characteristics of the persons who received a DXA in 2005 or 2006 at non-facility and facility sites were compared with individuals who did not receive a DXA (Table 1). As shown, 8.0% of the eligible population (approximately two-thirds of those tested) received a DXA at a non-facility site compared to 4.2% who received a DXA at a facility site. Individuals who underwent DXA at a non-facility site had somewhat shorter travel distances compared to those receiving DXAs at facility sites or who did not undergo DXA testing. They also had higher incomes and more physician office visits. One notable difference is shown in the last row of the table; individuals receiving DXAs at non-facility sites were more likely to live in urban areas than individuals who had DXAs performed at facility sites or who did not undergo DXA.
Figure 1.
Change in Number of Central DXAs performed at Non-Facility* and Facility Sites, 1999–2006
* Non-facility sites are those outside of a hospital (e.g. a physician office)
Table 1.
Characteristics of Eligible* Medicare Beneficiaries undergoing Central DXA in 2005 or 2006, by Type of Site Performing DXA (Facility vs. Non-Facility)
| DXA at a Non-Facility Site | DXA at a Facility | No DXA | ||||
|---|---|---|---|---|---|---|
| Number of Persons | % | Number of Persons | % | Number of Persons | % | |
| Total number of persons | 1,157,380 | 600,880 | 12,685,400 | |||
| Distance to Nearest Site Performing DXA, miles | ||||||
| < 5 | 930,860 | 80.4 | 456,880 | 76.0 | 9,227,320 | 72.7 |
| 5–9 | 132,540 | 11.5 | 81,260 | 13.5 | 1,634,880 | 12.9 |
| 10–24 | 87,020 | 7.5 | 58,780 | 9.8 | 1,552,920 | 12.2 |
| 25–39 | 5,460 | 0.5 | 3,160 | 0.5 | 191,340 | 1.5 |
| 40–54 | 980 | 0.1 | 480 | 0.1 | 45,120 | 0.4 |
| 55+ | 520 | 0.0 | 320 | 0.1 | 33,820 | 0.3 |
| Age, years | ||||||
| 65–69 | 220,640 | 19.1 | 119,420 | 19.5 | 2,259,800 | 17.8 |
| 70–74 | 346,320 | 29.9 | 185,220 | 30.8 | 3,426,920 | 27.0 |
| 75–79 | 300,200 | 25.9 | 158,260 | 26.3 | 3,012,460 | 23.7 |
| 80–84 | 197,620 | 17.1 | 96,260 | 16.0 | 2,251,880 | 17.8 |
| 85+ | 92,600 | 8.0 | 43,720 | 7.3 | 1,734,340 | 13.7 |
| Sex | ||||||
| Women | 990,720 | 85.6 | 537,920 | 89.5 | 6,029,360 | 47.5 |
| Men | 166,660 | 14.4 | 62,960 | 10.5 | 6,656,040 | 52.5 |
| Race/ethnicity | ||||||
| Caucasian | 1,049,280 | 90.7 | 549,220 | 91.4 | 11,190,100 | 88.2 |
| African American | 60,080 | 5.2 | 32,780 | 5.5 | 985,900 | 7.8 |
| Other | 48,020 | 4.1 | 18,880 | 3.1 | 509,400 | 4.0 |
| Annual Income, dollars | ||||||
| < 29,999 | 197,160 | 17.0 | 109,600 | 18.2 | 2,636,980 | 20.8 |
| 30,000–44,999 | 416,200 | 36.0 | 230,980 | 38.4 | 4,910,900 | 38.7 |
| 45,000–59,999 | 262,840 | 22.7 | 137,880 | 22.9 | 2,723,840 | 21.5 |
| 60,000–74,999 | 144,520 | 12.5 | 66,620 | 11.1 | 1,313,500 | 10.4 |
| 75,000+ | 136,660 | 11.8 | 55,800 | 9.3 | 1,100,180 | 8.7 |
| U.S. Geographic Region | ||||||
| East North Central | 173,100 | 12.0 | 147,280 | 8.6 | 2,402,940 | 10.4 |
| East South Central | 79,840 | 6.9 | 33,320 | 5.5 | 906,160 | 7.1 |
| Middle Atlantic | 151,520 | 11.8 | 84,440 | 8.2 | 1,768,020 | 10.5 |
| Mountain | 58,460 | 5.1 | 23,620 | 3.9 | 610,540 | 4.8 |
| New England | 58,020 | 5.0 | 48,960 | 8.1 | 716,000 | 5.6 |
| Pacific | 138,700 | 24.3 | 51,520 | 18.2 | 1,315,980 | 20.1 |
| South Atlantic | 280,740 | 6.9 | 109,440 | 8.9 | 2,553,800 | 8.5 |
| West North Central | 80,240 | 15.0 | 53,260 | 24.5 | 1,073,980 | 18.9 |
| West South Central | 136,760 | 13.1 | 49,040 | 14.1 | 1,337,980 | 13.9 |
| Charlson Cormorbidity Score | ||||||
| 0 | 500,720 | 43.3 | 269,780 | 44.9 | 5,461,360 | 43.1 |
| 1 | 158,880 | 13.7 | 78,320 | 13.0 | 1,743,520 | 13.7 |
| 2 | 201,240 | 17.4 | 106,720 | 17.8 | 1,956,220 | 15.4 |
| 3 | 111,580 | 9.6 | 55,460 | 9.2 | 1,245,000 | 9.8 |
| 4+ | 184,960 | 16.0 | 90,600 | 15.1 | 2,279,300 | 18.0 |
| Number of Annual Physician Office Visits | ||||||
| 0 Visits | 44,300 | 3.8 | 28,420 | 4.7 | 1,563,240 | 12.3 |
| 1–4 Visits | 286,360 | 24.7 | 167,060 | 27.8 | 3,810,900 | 30.0 |
| 5–9 Visits | 385,780 | 33.3 | 202,300 | 33.7 | 3,835,840 | 30.2 |
| 10–14 Visits | 224,940 | 19.4 | 108,420 | 18.0 | 1,925,860 | 15.2 |
| 15+ Visits | 216,000 | 18.7 | 94,680 | 15.8 | 1,549,560 | 12.2 |
| At least 1 office visit to a rheumatologist or endocrinologist in 2004 | 98,420 | 8.5 | 36,420 | 6.1 | 408,580 | 3.2 |
| Beneficiary Residence Geography | ||||||
| Urban Metropolitan | 782,040 | 67.6 | 344,220 | 57.3 | 7,428,360 | 58.6 |
| Rural | 375,340 | 32.4 | 256,660 | 42.7 | 5,257,040 | 41.4 |
Data are shown as n (%). All comparisons were significant at p < 0.0001.
eligible persons must have had full year Medicare Part A + Part B for 2003–2006, not have had a central DXA study done in 2003 or 2004, and be age ≥ 65 on 1/1/2003. These estimates reflect data from the Medicare national 5% sample.
The adjusted relationship between travel distance and receipt of DXA is shown in Table 2. There was a strong relationship between travel distance and receipt of DXA. Other factors also associated with receiving a DXA included Caucasian race, female sex, more physician office visits, and previous medical care provided by a rheumatologist or an endocrinologist. Greater comorbidity had an inverse relationship with receipt of DXA.
Table 2.
Multivariable-Adjusted Relationship between Receipt of Central DXA in 2005 or 2006 and Distance to the Nearest DXA Provider*
| Factor | Risk Ratio (95% Confidence Interval) |
|---|---|
| Distance to Nearest DXA Provider, miles | |
| < 5 | Referent |
| 5–9 | 0.92 (0.91 – 0.94) |
| 10–24 | 0.79 (0.85 – 0.78) |
| 25–39 | 0.43 (0.40 – 0.48) |
| 40–54 | 0.32 (0.25 – 0.40) |
| 55+ | 0.26 (0.19 – 0.34) |
| Age, years | |
| 65–69 | Referent |
| 70–74 | 0.97 (0.96– 0.99) |
| 75–79 | 0.87 (0.86 – 0.89) |
| 80–84 | 0.70 (0.68 – 0.71) |
| 85+ | 0.40 (0.38 – 0.40) |
| Sex | |
| Women | 6.39 (6.27 – 6.52) |
| Men | Referent |
| Race/ethnicity | |
| Caucasian | Referent |
| African American | 0.65 (0.63 – 0.67) |
| Other | 0.90 (0.88 – 0.93) |
| Annual Income, dollars | |
| < 29,999 | Referent |
| 30,000–44,999 | 1.09 (1.07 – 1.11) |
| 45,000–59,999 | 1.16 (1.14 – 1.18) |
| 60,000–74,999 | 1.23 (1.20 – 1.26) |
| 75,000+ | 1.34 (1.31 – 1.38) |
| U.S. Geographic Region | |
| East North Central | Referent |
| East South Central | 0.99 (0.96 – 1.02) |
| Middle Atlantic | 0.96 (0.94 – 0.98) |
| Mountain | 1.16 (1.13 – 1.20) |
| New England | 1.06 (1.03 – 1.08) |
| Pacific | 1.05 (1.03 – 1.07) |
| South Atlantic | 1.15 (1.13 – 1.17) |
| West North Central | 1.08 (1.05 – 1.10) |
| West South Central | 1.14 (1.12 – 1.17) |
| Charlson Cormorbidity Score | |
| 0 | Referent |
| 1 | 0.85 (0.83 – 0.86) |
| 2 | 0.95 (0.94 – 0.97) |
| 3 | 0.81 (0.79 – 0.82) |
| 4+ | 0.72 (0.70 – 0.73) |
| Number of Physician Office Visits | |
| < 2 Visits | Referent |
| 2–4 Visits | 1.85 (1.80 – 1.90) |
| 5–9 Visits | 2.26 (2.20 – 2.31) |
| 10–14 Visits | 2.55 (2.48 – 2.61) |
| 15+ Visits | 2.90 (2.82 – 2.98) |
| At least 1 visit to a rheumatologist or endocrinologist in 2004 | 1.48 (1.45 – 1.51) |
DXA providers included both non-facility and facility sites
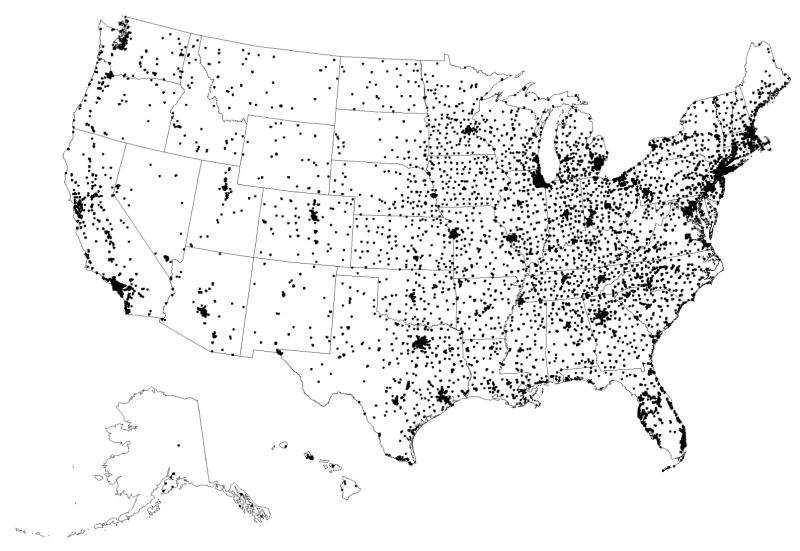
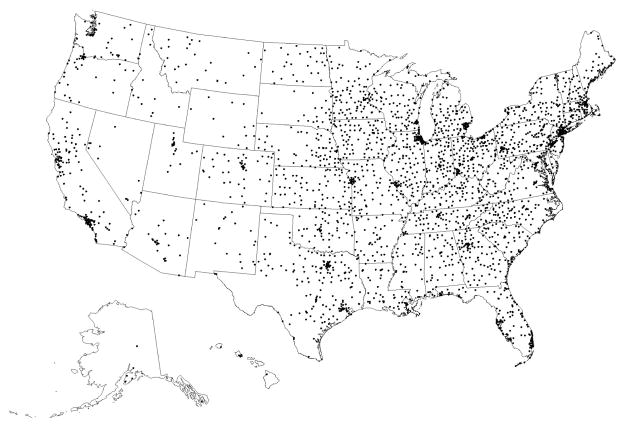
The distance between beneficiary residence in 2006 and the nearest DXA provider is shown in Table 3, before and after removing the non-facility DXA providers. As shown in the first column comparing urban and rural residence and including all DXA providers, the travel distance in 2006 was greater for persons living in rural rather than urban areas. The travel distance to the nearest DXA provider was more than 10 miles for approximately 11% of rural residents, whereas it was more than 10 miles for less than 1% of urban residents. The additional columns in Table 3 show how travel distances changed after removing non-facility DXA providers. The change in travel distance varied most for rural residents. For example, for rural residents who initially had to travel less than 5 miles to the nearest DXA provider (75.4% of all rural residents), only 61.5% of them still had to travel less than 5 miles after removing the non-facility DXA providers. Overall, more than one-quarter of urban residents and more than one-third of rural residents who initially had to travel less than 10 miles had to travel more than 10 miles once the non-facility sites were removed. Figure 2a shows the geographic locations of the DXA providers in 2006 including both non-facility and facility sites. Figure 2b shows the hypothetical effect in the geographic availability of DXA after removing the non-facility providers. The most visually apparent changes were observed in the least dense areas on the first map.
Table 3.
Distance to the Nearest DXA Site in 2006, and Change in Distance after Removing the Non-Facility Sites*
| Actual Distance to the Nearest DXA Site in 2006, miles | % of beneficiaries | New Distance necessary to travel to nearest DXA site after removing Non-Facility DXA Sites, miles | ||||||
|---|---|---|---|---|---|---|---|---|
| < 5 | 5–9 | 10–24 | 25–39 | 40–54 | 55+ | |||
| URBAN | < 5 | 80.5 | 72.8 | 18.3 | 6.5 | 1.9 | 0.2 | 0.1 |
| 5–9 | 19.1 | 0 | 68.6 | 27.6 | 2.9 | 0.6 | 0.1 | |
| 10–24 | 0.3 | 0 | 0 | 95.1 | 3.5 | 0.4 | 0.9 | |
| 25–39 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 40–54 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 55+ | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| RURAL | < 5 | 75.4 | 61.5 | 10.3 | 20.3 | 5.7 | 1.2 | 0.8 |
| 5–9 | 13.2 | 0 | 61.3 | 33.7 | 3.9 | 0.6 | 0.3 | |
| 10–24 | 10.4 | 0 | 0 | 88.8 | 8.9 | 1.4 | 0.8 | |
| 25–39 | 0.7 | 0 | 0 | 0 | 79.4 | 14.4 | 6.2 | |
| 40–54 | 0.2 | 0 | 0 | 0 | 0 | 71.2 | 28.8 | |
| 55+ | 0.1 | 0 | 0 | 0 | 0 | 0 | 100 | |
Non-facility sites are those outside of a hospital (e.g. a physician office)
Data in each cell are the proportion of persons in each row who have to drive the distance specified in each of the columns after removing the non-facility DXA sites
Figure 2.
Figure 2a. Geographic Distribution of All Sites Performing Central DXAs in 2006
Figure 2b. Geographic Distribution of Facility Sites Performing Central DXAs in 2006, After Excluding Non-Facility Sites
Discussion
Using data from a national sample of Medicare beneficiaries, we showed that travel distance is strongly associated with receipt of DXA in 2005–2006. This relationship persisted even after accounting for differences in age, sex, comorbidity, health services utilization, and a number of other important potentially confounding factors. Over an 8-year period through 2006, there was a two-fold increase in the number of central DXAs performed, and non-facility DXA providers performed approximately two-thirds of these tests. If due to national reduction in DXA reimbursements the non-facility sites were to stop performing DXAs, then based on travel distance, persons living in rural areas would have reduced DXA access. Overall, our data suggest that the availability of DXA is likely to be an important enabling factor [15, 16] in whether at-risk persons undergo testing or not. This is an important finding since fewer than one-third of older women and only 5% of older men have undergone testing [7], and thus an unmet need exists based on recommendations from national guidelines [2, 10]. Moreover, receipt of DXA has been previously shown to be an important mediating factor to promote osteoporosis medication use among high risk persons [8] that has been linked to a reduced rate of fractures [17].
The potential implications of our results are best understood in the context of changes in reimbursement for central DXAs that selectively affected non-facility DXA providers. DXAs performed in 2006 were previously reimbursed by the Medicare program at $139 (including both technical and professional components). After a series of progressive cuts to Medicare reimbursement rates in the non-facility setting, central DXA payments are scheduled to drop to approximately $76 in 2009 and $56 in 2010 [18, 19]. The net effect of these changes may be to shift a substantial portion of the tests currently being performed in non-facility settings to facility settings or to high-volume non-facility settings. A contraction in the number of DXA providers has the potential to limit patient access to a service that is recommended for a substantial portion of the population.
Travel distance has been previously shown to be an important determinant of the timing of and receipt of important medical services such as cardiac catheterization and radiation treatment for breast cancer [20]. Unlike the acute or life threatening nature of these conditions, however, DXA is often performed as a screening test and thus the impetus to perform it may be less compelling. Correspondingly, given less perceived need to obtain testing, barriers to access may be even more important [15, 16], A previous report that evaluated the relationship between travel distance and receipt of DXA in New Jersey found only a weak relationship [21]. However, as the authors pointed out, New Jersey is a relatively small, urban state and travel distance were likely shorter than would be expected in other states with a higher proportion of rural residents. Indeed, the median travel distance in that report was only 3 miles. In our study, we found that > 99% of urban dwelling persons had travel distances < 10 miles, and the likelihood of receipt of DXA was quite similar for persons who had to travel less than 5 compared with 5–9 miles.
We are cautious in the interpretation of our results of Table 3 that described the hypothetical scenario of the travel distance after removal of the non-facility sites. Even with declining DXA reimbursement, particularly affecting non-facility DXA providers, it is unlikely that all of them would stop providing this service. A recent analysis that evaluated the relationship between travel distance and receipt of chemotherapy as a result of the Medicare modernization act in 2003 that shifted some oncology services from physician offices to facility settings showed only modest effects of this policy change in terms of travel distance and wait times [22]. However, a subgroup analysis showed that those most affected were individuals living in rural areas, which is consistent with observations in our study. These results may or may not be generalizable to DXA utilization since chemotherapy services are typically ordered by oncologists, not primary care physicians who account for the majority of DXA ordering [7]. Moreover, besides travel distance, there likely are a number of factors that would be affected if DXA tests are shifted to facility providers. Possible outcomes/consequences that also may be impediments to access to care include the inconvenience of obtaining testing in a facility that is often not proximate to physicians’ offices, less continuity of care, and higher patient co-payments (e.g. 40% for facility DXAs compared to 20% for non-facility DXAs). Because the life-threatening nature of cancer treatment may provide more impetus to overcome these barriers compared to obtaining screening for osteoporosis, these factors may have a greater impact on receipt of DXA than receipt of chemotherapy.
Our study must be understood in light of some potential limitations. Our distance calculations were based on zip code centroids. Although a majority of our data was based upon 9 digit zip codes, these distances do not necessarily reflect actual driving distance. However, this method has been previously observed to correlate well with driving distance [23]. Moreover, we showed high agreement between estimated distance and actual distance for those persons who did receive testing (Appendix). Our population represented the Medicare Fee for Service population and did not include commercially-insured individuals or those participating in government-sponsored managed care plans (e.g. Medicare Advantage). Travel distance relationships for these individuals may or may not be similar. Finally, we had no measure of DXA capacity or density (e.g. number of DXA scanners per number of persons in a geographic region). Attempting to estimate capacity or density in an administrative claims datasource would likely have limited validity given the large but variable numbers of persons age < 65 and with other types of insurance that ‘compete’ for DXA capacity with the population we studied. For this reason, the hypothetical scenario presented in Table 3 showing only modest effects on travel distance if non-facility providers are excluded might be overly optimistic. The reasoning is that this analysis assumes an adequate capacity of facility DXA providers to perform tests that would otherwise have been performed by non-facility DXA providers. However, the true impact on travel distance resulting from the reduction in non-facility DXA providers depends upon both the capacity of new or existing facility providers and the acceptability to patients and ordering physicians of obtaining DXAs in these facility settings.
In conclusion, travel distance to DXA provider is an important factor associated with receipt of DXA. Although recent legislative and regulatory changes affecting reimbursement of DXAs performed by non-facility providers may have only a limited impact on travel distance for persons living in urban areas, the effects are likely to be more substantial for rural residents. Additional work to evaluate the impact of these recent and ongoing cuts in DXA reimbursement is needed to determine the extent to which access to this important service has been affected.
Acknowledgments
Funding acknowledgements: This research was supported by a contract between UAB and Amgen, Inc. Only the authors from UAB had access to the Medicare data used. The analysis, presentation and interpretation of the results were solely the responsibility of the authors. Some of the investigators (JRC, KGS) also receive salary support from the National Institutes of Health (AR053351, AR052361), the Agency for Healthcare Research and Quality (U18 HS016956), and the Arthritis Foundation (JRC).
Footnotes
Disclosures
JRC: Consulting: Roche, UCB, Procter & Gamble; speakers bureau: Merck, Procter & Gamble, Eli Lilly, Roche, Novartis; research grants: Merck, Procter & Gamble, Eli Lilly, Amgen, Novartis
AL: Speakers bureau: Eli Lily, Novartis, Roche, GSK
DJB: Research grants: Amgen
LC: Consulting and speaker’s bureau: Merck, Procter & Gamble, Aventis, Novartis
LG: Research grants: Amgen
MLK: Consulting and Research grants: Amgen, Eli Lilly
RM: Research grants: Amgen
MM: Research grants: Amgen
KGS: Consulting: Merck, Novartis; speakers bureau: Merck, Procter & Gamble, Aventis, Eli Lilly, Roche, Novartis, Amgen; research grants: Novartis, Amgen, Eli Lilly, Roche
SBT: Consulting and research grants: Merck, Procter & Gamble, Eli Lilly, Roche, GSK, Novartis, Wyeth, Amgen
ED: Research grants: Amgen
References
- 1.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22(3):465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 2.Lewiecki EM, Watts NB, McClung MR, et al. Official positions of the international society for clinical densitometry. J Clin Endocrinol Metab. 2004;89(8):3651–5. doi: 10.1210/jc.2004-0124. [DOI] [PubMed] [Google Scholar]
- 3.Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Lancet. 1996;348:1535–41. doi: 10.1016/s0140-6736(96)07088-2. [DOI] [PubMed] [Google Scholar]
- 4.Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356(18):1809–22. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 5.Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. JAMA. 1999;282:1344–1352. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 6.Ettinger B, Black D, Mitlak B, Knickerbocker R, Nickelsen T, Genant HK, Christiansen C, Delmas PD, Zanchetta JR, Stakkestad J, Gluer CC, Krueger K, Cohen FJ, Eckert S, Ensrud KE, Avioli LV, Lips P, Cummings SR. Reduction of vertebral fracture risk in postmenopausal women with osteoporosis treated with raloxifene: results from a 3-year randomized clinical trial. Multiple Outcomes of Raloxifene Evaluation (MORE) Investigators. JAMA. 1999;282(7):637–645. doi: 10.1001/jama.282.7.637. [DOI] [PubMed] [Google Scholar]
- 7.Curtis JR, Carbone L, Cheng H, et al. Longitudinal trends in use of bone mass measurement among older americans, 1999–2005. J Bone Miner Res. 2008;23(7):1061–7. doi: 10.1359/JBMR.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cadarette SM, Gignac MA, Jaglal SB, Beaton DE, Hawker GA. Access to osteoporosis treatment is critically linked to access to dual-energy x-ray absorptiometry testing. Med Care. 2007;45(9):896–901. doi: 10.1097/MLR.0b013e318054689f. [DOI] [PubMed] [Google Scholar]
- 9.Watts NB. Understanding the bone mass measurement act. J Clin Densitometry. 1999;2:211–217. doi: 10.1385/jcd:2:3:211. [DOI] [PubMed] [Google Scholar]
- 10.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. 2008 [Google Scholar]
- 11.The Lewin Group. Assessing the Total Cost of Providing DXA in the Office-Based Setting and Scoring a Reimbursement Alternative. Presented to CMS. 2007 August 28; [Google Scholar]
- 12. [accessed April 25th, 2008]; http://www.resdac.umn.edu/CCW/CCWFAQ.asp.
- 13.Curtis JR, Laster A, Becker D, et al. Regional Variation in the Denial of Reimbursement for Bone Mineral Density Testing Among U.S. Medicare Beneficiaries. Journal of Clinical Densitometry. doi: 10.1016/j.jocd.2008.07.004. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–9. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 15.Andersen RM. Revisiting the behavioral model and access to medical care: does it matter? J Health Soc Behav. 1995;36(1):1–10. [PubMed] [Google Scholar]
- 16.Aday LA, Andersen R. A framework for the study of access to medical care. Health Serv Res. 1974;9(3):208–20. [PMC free article] [PubMed] [Google Scholar]
- 17.Kern LM, Powe NR, Levine MA, et al. Association between screening for osteoporosis and the incidence of hip fracture. Ann Intern Med. 2005;142(3):173–81. doi: 10.7326/0003-4819-142-3-200502010-00007. [DOI] [PubMed] [Google Scholar]
- 18.Federal Register: CMS 1321-FC. November 2006. “Medicare Program: Revisions to Payment Policies Five Year Review of Work Relative Value Units, Changes to the Practice Expense Methodology Under the Physician Fee Schedule and other Changes to Payment Under Part B; Revisions to the Payment Policies of Ambulance Services Under the Fee Schedule for Ambulance Services; and Ambulance Inflation Factor Update for CY 2007.
- 19.Federal Register: CMS 1385-FC. November 2007. Medicare Program; Revisions to Payment Policies Under the Physician Fee Schedule, and Other Part B Payment Policies for CY 2008; Revisions to the Payment Policies of Ambulance Services Under the Ambulance Fee Schedule for CY 2008; and the Amendment of the E-Prescribing Exemption for Computer-Generated Facsimile Transmissions.
- 20.McClellan M, McNeil BJ, Newhouse JP. Does more intensive treatment of acute myocardial infarction in the elderly reduce mortality? Analysis using instrumental variables. Jama. 1994;272(11):859–66. [PubMed] [Google Scholar]
- 21.Solomon DH, Polinski JM, Truppo C, et al. Access to bone mineral density testing in patients at risk for osteoporosis. Osteoporos Int. 2006;17(12):1749–54. doi: 10.1007/s00198-006-0180-4. [DOI] [PubMed] [Google Scholar]
- 22.Shea AM, Curtis LH, Hammill BG, DiMartino LD, Abernethy AP, Schulman KA. Association between the Medicare modernization act of 2003 and patient wait times and travel distance for chemotherapy. JAMA. 2008;300(2):189–96. doi: 10.1001/jama.300.2.189. [DOI] [PubMed] [Google Scholar]
- 23.Phibbs CS, Luft HS. Correlation of travel time on roads versus straight line distance. Med Care Res Rev. 1995;52(4):532–42. doi: 10.1177/107755879505200406. [DOI] [PubMed] [Google Scholar]