Abstract
Pharmacotherapy for epilepsy is limited by high incidence of pharmacoresistance and failure to prevent development and progression of epilepsy. Using the rat hippocampal kindling model, we report on the therapeutic potential of novel silk-based polymers engineered to release the anticonvulsant adenosine. Polymers were designed to release 1000 ng adenosine per day during a time span of ten days. In the first experiment rats were kindled by hippocampal electrical stimulation until all animals reacted with stage 5 seizures. Adenosine-releasing or control polymers were then implanted into the infrahippocampal fissure ipsilateral to the site of stimulation. Subsequently, only recipients of adenosine-releasing implants were completely protected from generalized seizures over a period of ten days corresponding to the duration of sustained adenosine release. To monitor seizure-development in the presence of adenosine, adenosine-releasing or control polymers were implanted prior to kindling. After 30 stimulations – delivered from days 4–8 after implantation – control animals had developed convulsive stage 5 seizures, whereas recipients of adenosine-releasing implants were still protected from convulsive seizures. Kindling was resumed after nine days to allow expiration of adenosine-release. During additional 30 stimulations, recipients of adenosine-releasing implants gradually resumed kindling development at seizure stages corresponding to those when kindling was initially suspended, while control rats resumed kindling development at convulsive seizure stages. Blockade of adenosine A1 receptors did not exacerbate seizures in protected animals. We conclude that silk-based adenosine-delivery exerts potent anti-ictogenic effects, but might also have at least partial anti-epileptogenic effects. Thus, silk-based adenosine augmentation holds promise for the treatment of epilepsy.
Keywords: Adenosine, Epilepsy, Epileptogenesis, Kindling, Focal drug delivery, Silk, Polymer
Introduction
Despite the development of new antiepileptic drugs (AEDs) during recent years, epilepsy – affecting more than 60 million patients worldwide (McNamara, 1999) – continues to be a major health problem, (i) due to pharmacoresistance or intolerable side effects in more than one third of patients (Vajda, 2007), and (ii) due to the limitations of current AEDs to prevent the development of epilepsy (i.e. epileptogenesis) or to modify the progression of epilepsy (Loscher, 2002). Therefore, therapeutic alternatives are urgently needed. Thus, cell and gene therapies, have been explored with the aim to affect epilepsy on a local level (Boison, 2007b; Loscher et al., 2008; Raedt et al., 2007; Shetty and Hattiangady, 2007; Vezzani, 2007). Local, or focal, treatment approaches for epilepsy make sense, since they are regularly well tolerated and avoid systemic side effects (Nilsen and Cock, 2004).
The purine ribonucleoside adenosine is an endogenous inhibitory modulator of brain activity with potent anticonvulsant and neuroprotective properties (Boison, 2007c; Boison, 2008c). Its anticonvulsant properties are largely mediated by activation of adenosine A1 receptors (A1Rs) that mediate most of the protective functions of adenosine (Fredholm et al., 2005a; Fredholm et al., 2005b). Most importantly, A1Rs prevent the spread and generalization of seizures, and limit seizure- or injury-induced cell death (Fedele et al., 2006; Kochanek et al., 2006). Thus, A1Rs constitute an important target for antiepileptic therapy; in fact A1R activation prevented seizures in an animal model that was resistant to conventional antiepileptic drugs (Gouder et al., 2003).Therefore, adenosine augmentation would constitute a promising therapeutic approach for pharmacoresistant epilepsy. However, systemic activation of A1Rs is of limited therapeutic interest due to severe cardiovascular and sedative side effects (Dunwiddie and Masino, 2001).
Most recently, dysfunction of adenosine-based mechanisms in epilepsy, in particular focal adenosine-deficiency due to upregulation of the astrocyte-based adenosine-removing enzyme adenosine kinase (ADK), have been identified as trigger for ictogenesis (Boison, 2008b; Li et al., 2007a; Li et al., 2008). Thus, the astrocyte-specific enzyme ADK has been identified as a molecular link between astrogliosis – a pathological hallmark of the epileptic brain – and neuronal dysfunction in epilepsy (Boison, 2008a). Therefore, adenosine augmentation therapies (AATs) constitute a rational therapeutic approach to prevent seizures by restoring the adenosinergic equilibrium. Based on cell transplantation studies it has been suggested that AATs might combine anti-ictogenic with anti-epileptogenic properties (Li et al., 2007b; Li et al., 2008).
Here we made use of a novel silk-based time-limited delivery system for adenosine (Wilz et al., 2008) to study antiepileptic effects of focal AAT without any confounds that might be caused by cell-based brain implants. Silk fibroin is a novel biologically derived protein polymer particularly well suited to small molecule drug delivery due to its biocompatibility (Altman et al., 2003) and relatively slow, controllable biodegradation (Horan et al., 2005; Wang et al., 2008). Silk can also be processed under aqueous and ambient conditions (Jin and Kaplan, 2003; Li et al., 2006) into a diverse range of material formats (Hofmann et al., 2006; Sofia et al., 2001; Wang et al., 2005; Wang et al., 2007a). Additional control of drug release from silk biomaterials can be achieved via regulation of beta sheet content (Hofmann et al., 2006) and integration of multiple carrier formats into one implant (Wilz et al., 2008). By combining different time points of polymer implantation with different kindling paradigms we investigated antiepileptic effects via silk-based adenosine delivery.
Materials and Methods
Implant design and fabrication
Implants designed to deliver the target doses 0 (= control) or 1,000 ng adenosine per day were designed and fabricated as described previously (Wilz et al., 2008). Briefly, implants were designed to split the target drug load evenly between microspheres and macroscale films that were integrated into a single implant and capped with silk films. Adenosine containing microspheres were prepared according to the MeOH based protocol described previously (Wang et al., 2007a). Water-based porous scaffolds were prepared as previously described (Kim et al., 2005) using the mixture of microspheres and silk solution to imbed the microspheres in the final porous scaffold. To obtain the desired implant geometry (0.6–0.7 mm diameter, 3 mm length), scaffolds were punched out with a 1 mm Miltex biopsy punch and then trimmed with a razor on either end. The porous scaffolds were next coated with multiple macroscale adenosine-loaded silk films comparable to the films described previously (Hofmann et al., 2006). After drug loading, all implants were coated with multiple silk-based capping layers to delay burst-release of adenosine.
In vitro adenosine release studies
Three implants with a target release dose of 1,000 ng adenosine were characterized for release kinetics in vitro. To evaluate release profiles, implants were immersed in 1 ml of Dulbecco’s phosphate buffer, pH 7.2 (PBS) at 37°C. Every 24 hours (or 48 hours after two weeks) the PBS was removed and replaced. Adenosine content in the PBS samples removed from the system was measured using a modified fluorescence assay as previously described (Wojcik and Neff, 1982). The collected PBS sample was transferred to a 1.5 ml Eppendorf tube and chloroacetaldehyde was added to a final concentration of 220 μM chloroacetaldehyde. Boiling of mixed adenosine and chloroacetaldehyde for 20 min yielded the fluorescent derivative 1,N6- ethenoadenosine. The fluorescence of the sample was measured with a plate reader (excitation = 310 nm, emission = 410 nm (Rosenfeld and Taylor, 1984). For each device at each time point, three fluorescence readings were taken and averaged.
Animals and surgery
All animal procedures were conducted in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) in accordance with protocols approved by the Institutional Animal Care and Use Committee and the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. Adult male Sprague-Dawley rats were used at a body weight of 280 to 300 g. All rats were acclimatized for one week before being used in the experiments. The rats were housed under a 12 h light/dark cycle (lights on from 8:00 A.M) with food and water provided ad libitum. For all procedures described below, anesthesia was induced with 3% isoflurane, 67% N2O, 30% O2 and maintained with 1.5% isoflurane, 68.5% N2O, 30% O2, while rats were placed in a Kopf stereotactic frame.
Kindling
Bipolar, coated, stainless steel stimulation/recording electrodes (0.20 mm in diameter, Plastics One, Roanoke, VA) were implanted into the right hippocampus and fixed with a head-set of dental acrylate. Coordinates for the hippocampal electrodes were (tooth bar at 0): 5.0 mm caudal to bregma, 5.0 mm lateral to midline, and 7.5 mm ventral to dura. Experiment 1: Four days after surgery, the animals were stimulated unilaterally 6 times every second or third day with a Grass S-88 stimulator (1-ms square-wave pulses of 5 V at 50-Hz frequency for 10 s, 30-min interval between stimulations; these stimulations corresponded to about 350μA, whereas the afterdischarge thresholds before kindling were in the range of 115μA). Behavioral seizures were scored according to the scale of Racine (Racine, 1978). The electroencephalogram (EEG) was recorded for periods of 1 min before and 5 min after application of each stimulating pulse using a Nervus EEG-recording System connected with a Nervus magnus 32/8 Amplifier with a 2048 Hz digitization rate, and filtered (high-pass filter 0.3 Hz cutoff, low-pass 100 Hz).Differential EEG-recordings were obtained from a montage measuring the potential between the two tips of the intrahippocampal bipolar electrode. Stimulations on each day were discontinued whenever a stage 5 seizure on that day was reached. Eventually, all animals reacted with a stage 5 seizure at the first (and then only) daily stimulation. After three stage 5 seizures elicited by the first stimulation on three subsequent kindling-days animals were considered to be fully kindled (i.e. reproducibility of stage 5 seizure activity after stimulation). Next, responsiveness to adenosine A1R activation was tested by injection of 2-chloro-N(6)-cyclopentyladenosine (CCPA; adenosine A1-receptor subtype selective agonist). Injection of CCPA (3 mg/kg i.p. in saline containing 20% DMSO) delivered the next day 30 min prior to a test stimulation resulted in complete seizure suppression. 24 h later the animals were stimulated again to demonstrate consistency and maintenance of stage 5 seizure activity after stimulation. Only animals that fulfilled these stringent kindling criteria were used for the subsequent experiments. Polymers were implanted (see below) into these fully kindled animals. Implant recipients received one test-stimulation each on day 4, 6, 10, 14, 18, and 21 after polymer implantation and were then subjected to histological analysis. Experiment 2: Polymer implantation (see below) was combined with electrode implantation (see above) in the same surgery. Experiment 2a: Kindling was initiated 4 days after polymer implantation with the animals receiving 6 kindling stimulations (1-ms square-wave pulses of 5 V at 50-Hz frequency for 10 s, 30-min interval between stimulations) each on day 4, 6, 8, and 11 after implantation; this amounted to a total of 24 stimulations. One day after delivery of the 24th stimulus, all animals were treated with 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, adenosine A1-receptor subtype selective antagonist) 30 min prior to stimulation (1mg/kg, i.p. in DMSO). 24 h after this drug test all animals were stimulated again once before being sacrificed for histological analysis. Experiment 2b (Fig. 1): Kindling was initiated 4 days after polymer implantation with the animals receiving 6 kindling stimulations each on day 4, 5, 6, 7, and 8, after implantation; this amounted to a total of 30 stimulations. In these incompletely kindled adenosine-implant recipients further stimulations were suspended until adenosine release from the polymers had expired (from day 18 onwards). Kindling stimulations were resumed on day 18, 19, 20, 21, and 22 after implantation (6 stimulations each day; total of 30 additional stimulations). Afterwards the animals were sacrificed for histological analysis.
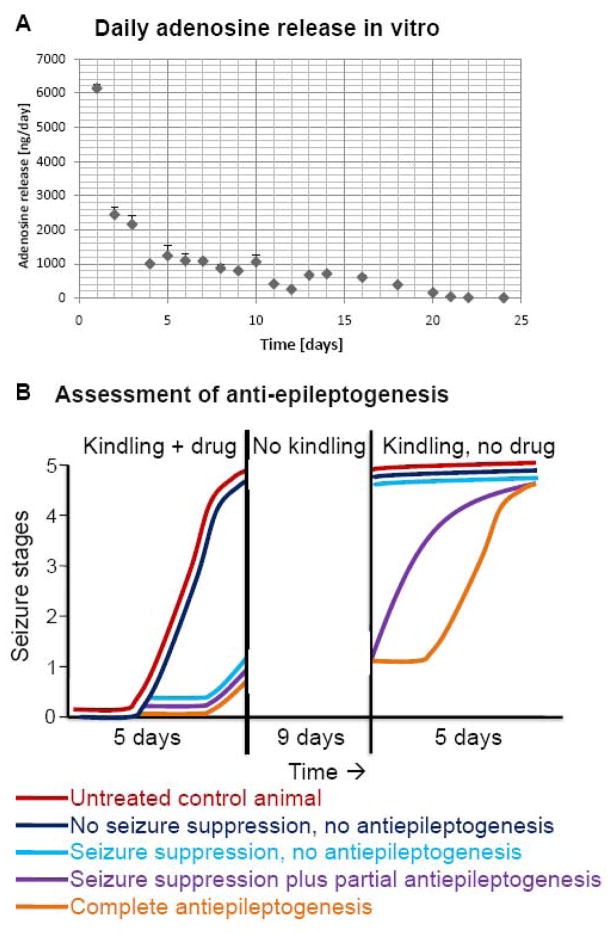
Figure 1.
Daily release of adenosine from silk-based polymers. (A) Adenosine release in vitro was determined for each day shown, based on averaged values from N = 3 polymers. Note the stable release rate of around 1000 ng adenosine per day from day 4 to 10, corresponding to the pre-designed target release rate. Errors are given as ± SD. (B) Assessment of antiepileptogenesis in the rat kindling model according to Silver et al., 1991. Kindling is initiated during drug delivery followed by a washout period of the drug; subsequently, kindling is resumed in the absence of the drug. Five potential kindling outcomes are shown: red, normal kindling development of an untreated or sham-treated animal; dark blue, kindling in the presence of a drug with no effects on seizure expression and epileptogenesis; light blue, kindling development under the influence of a drug that suppresses seizures, but not epileptogenesis; violet, kindling development under the influence of a drug that exerts partial antiepileptogenic effects; orange, kindling under the influence of a drug that completely suppresses epileptogenesis. Note that the criterion for complete suppression of epileptogenesis is a shift of the kindling curve to the right; the number of drug-free kindling stimulations needed to trigger a specific seizure stage should be the same as in control animals.
Polymer implantation
Polymers with a target release rate of 1000 ng adenosine per day or respective control polymers (0 ng adenosine) were implanted using a stereotactic implantation device (internal diameter 0.7 mm, external diameter 1 mm) as described (Boison et al., 2002). The polymers were either implanted after completion of kindling (Experiment 1) or before the onset of kindling (Experiment 2). The polymer-loaded device was stereotactically inserted into the brain using a drill hole above the left hemisphere 2 mm rostral to bregma and 1.6 mm lateral to the midline. Using this drill hole the loaded device was inserted into the brain using an angle of 47° from vertical and an angle of 47° from midline. Thus, a diagonal injection tract was created aiming at a coordinate of 5.0 mm caudal to bregma, 5.0 mm to the right of the midline and 7.5 mm below the dura. Upon reaching the target site the 3 mm long polymer was released and deposited within the infrahippocampal fissure by slowly retracting the outer tube of the device. Finally, the device was fully retracted as described previously (Boison et al., 2002). Thus, the implanted polymers were deposited within a formed cavity of 3 mm length within the right infrahippocampal fissure and adjacent to the electrode implantation site. The diagonal implantation approach pursued here and previously (Li et al., 2007b; Li et al., 2008; Wilz et al., 2008) is characterized by a number of important advantages: (i) Coverage of 3 mm of the dorso-ventral extent of the hippocampus by placement of the implants into the infra-hippocampal fissure; (ii) Minimization of damage to the ipsilateral hippocampus; (iii) Compatibility with the electrode-containing headset of the animals.
Histology and sample degradation
Rats were transcardially perfused with 4% paraformaldehyde in phosphate buffer (0.15 M, pH 7.4). To characterize the gross anatomy of the brain at the site of implantation and to confirm implant location, whole rat brains were sectioned (10 to 40 μm) either in the coronal or in the sagittal plane and stained with either Cresyl violet, or with hematoxylin and eosin. Scaffold morphology was determined post-implantation by retrieval from the brains with tweezers and sectioned, or the scaffolds were sectioned while still imbedded in the brain tissue. Scaffolds pre-implantation and samples harvested after implantation were washed in PBS, and fixed in 10% neutral buffered formalin before histological analysis. Samples were dehydrated through a series of graded alcohols, embedded in paraffin and sectioned at 5 μm thickness. Sections were stained with either hematoxylin and eosin (H&E) or Cresyl violet (methyl violet 10B). Samples of implants before and after implantation were compared for degradation. Sections were examined under a Zeiss Axiovert S100 light microscope with a Sony Exwave HAD 3CCD color video camera. The ratio of total surface area of pores (in pixels) to total surface area of the implant (in pixels) was evaluated using Image J image processing software. Data were analyzed with a two sample t-test: t = 5.08, df = 10, p < 0.001.
Statistics
In the in vitro studies standard deviations were calculated for the three repetitions per group by averaging three fluorescence readings per implant. In vivo seizure data are based on N = 5 to 8 rats depending on experimental design and experimental group. Individual seizure scores were pooled and averaged for each stimulation in each experimental group. Errors are given as ± SD and data were analyzed using two way ANOVA on ranks followed by a Bonferroni test.
Results
Adenosine release profile and design of experiments
The daily dose of adenosine released from the adenosine-loaded polymers (ADO-polymers) was determined by fluorescence analysis of adenosine after derivatization to 1,N6- ethenoadenosine. After an initial burst in adenosine-release (> 2000 ng/day) during the first three days of incubation, the polymers were characterized by a stable release rate of around 1000 ng per day (1019 ± 197) between day 4 and day 10 (Fig. 1A). After this stable release period, daily rates of adenosine release rapidly dropped to 414 ± 59 ng adenosine at day 11 and 256 ± 45 ng at day 12. Polymers ceased to release adenosine within a time frame of 21 days of incubation. In contrast, no adenosine was detectable in supernatants from cultured control polymers. This unique release profile of high and stable initial release rates (1000 ng/day) followed by gradual expiration of adenosine release allowed us to confirm the adenosine-dependence of implant-mediated therapeutic effects. In Experiment 1 we studied the antiictogenic effects of polymer-based adenosine release, after the polymers were implanted into fully kindled rats. Given the high initial adenosine-release rate from the ADO-polymers we expected initial complete protection from seizures followed by a gradual recurrence of seizure activity in parallel with the decline in therapeutic adenosine release. In Experiment 2 (schematically depicted in Fig. 1B) the unique release profile of adenosine allowed us to assess antiictogenic and anti-epileptogenic effects of polymer-based adenosine release. Here polymers were implanted four days prior to kindling initiation. The expectation is reduced kindling development in the ADO-polymer treated group of rats. Suppression of kindling development can either be due to true suppression of epileptogenesis, or “just” to suppression of seizures. In the latter case adenosine-mediated seizure suppression would “mask” any anti-epileptogenic effects. To distinguish among these two possibilities we adopted an experimental paradigm first described by McNamara (Silver et al., 1991): ADO-polymer and control-polymer recipients were kindled only until the ADO-group showed first signs of kindling (Fig. 1B); during that same time frame animals from the control group are expected to be fully kindled; this period of initial kindling during a period of constant adenosine release in the ADO-group (1000 ng adenosine per day during days 4–8 after polymer implantation) was followed by a 9-day gap in kindling. During this gap in kindling, the polymers ceased to release significant amounts of adenosine. Kindling was then resumed in both groups. In case that adenosine in the ADO-polymer group had merely suppressed seizures but not epileptogenesis, we expect a “jump” in seizure expression, i.e. seizure stages should be similar to those observed in the control group (Fig. 1B, light blue line). In contrast, if polymer-based adenosine-release had suppressed epileptogenesis, we expect that kindling development resumes, where it was discontinued previously, i.e. the seizure stage curve in the second kindling phase of the ADO-polymer group, should be parallel to the seizure stage curve in the initial kindling phase of the control group. Using this paradigm, the number of drug-free afterdischarges to reach a particular kindling stage should be the same in case of complete antiepileptogenesis (Fig. 1B, orange line); if the number of drug-free afterdischarges in the drug-treated group is less than in the control group (Fig. 1B, violet line), some epileptogenesis took place during drug administration (Silver et al., 1991).
Experiment 1: Suppression of kindled seizures by polymer-based adenosine release
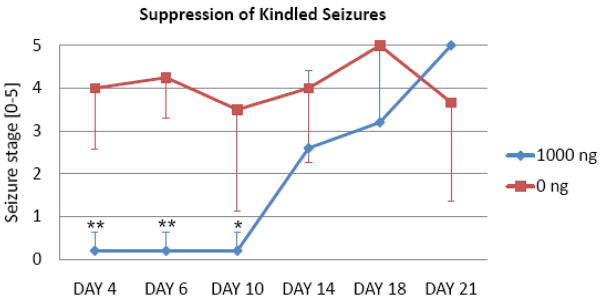
To establish the anti-ictogenic potential of silk-polymer based release of adenosine, we implanted adenosine releasing polymers (Fig. 1A) (n = 5) or corresponding control polymers (n = 4) that were not loaded with adenosine into the infrahippocampal fissure of fully kindled rats. Before polymer implantation all rats reproducibly reacted with stage 4 or 5 seizures following stimulation and thus met our stringent kindling criteria. Animals received one test stimulus each at day 4, 6, 10, 14, 18, and 21 after polymer implantation. While recipients of control polymers maintained the expression of convulsive seizures (averaged seizure stage of 3.9 ± 1.6) during the course of the experiments, recipients of implants releasing 1000 ng adenosine per day were initially almost completely protected from any seizures (averaged seizures stage of 0.2 ± 0.5 until day 10 after implantation) (Fig. 2). During this time, 4 out of 5 rats did not express any seizures (stage 0), while the remaining rat expressed non-convulsive stage 1 seizures. In line with reduced levels of adenosine released from the polymers from day 10 onwards (Fig. 1A), seizure activity in the adenosine group gradually resumed (Fig. 2) indicating that seizure suppression was due to implant-dependent adenosine release. To further confirm that seizure suppression is due to implant-derived adenosine an ADO-polymer treated rat received an intraperitoneal injection of the adenosine A1R antagonist DPCPX (1 mg/kg, i.p.) at day 3. When tested before or after DPCPX on days 2 and 4 the rat was protected from seizures (stage 0). However, 30 min after injection of DPCPX on day 3 a stage-5 seizure was elicited indicating adenosine-dependence of seizure suppression. Together, these results suggest a powerful anti-ictogenic activity of focal implant derived-adenosine release in the range of 1000 ng per day.
Figure 2.
Suppression of fully kindled seizures by implant-derived adenosine. Fully kindled rats (criterion: at least three consecutive stage 5 seizures) received infrahippocampal implants of silk-based polymers with a daily target release rate for adenosine of 0 ng (N = 4, red), or 1000 ng (N = 5, blue). Individual test stimulations were delivered at days 4, 6, 10, 14, 18, and 21. Seizure stages, averaged across animals from each group, are shown for every stimulus. Note that recipients of a target dose of 1000 ng adenosine per day are completely protected from any seizures during the 10 days after implantation corresponding to sustained release of adenosine during that time period. Errors are given as ± SD. Data were analyzed by two way ANOVA followed by a Bonferroni test; the significance of interaction between groups was determined as F=2.390; P<0.05; significance levels of individual tests is indicated: * P<0.05, ** P<0.01.
Experiment 2: Suppression of epileptogenesis by polymer-based adenosine release
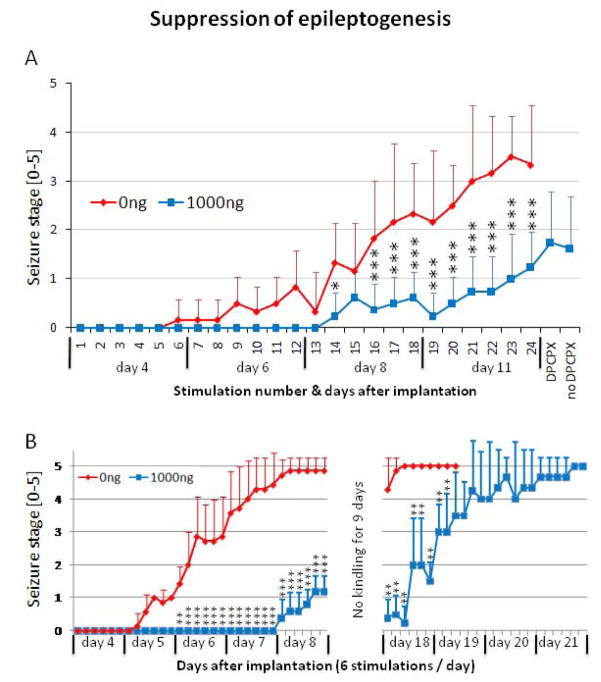
To investigate the possibility for anti-epileptogenic effects of polymer-based adenosine release we performed two separate experiments. In Experiment 2a we implanted kindling electrodes and polymers (target release rate of 1000 ng adenosine per day, n = 8; and control polymers, n = 6) into adult male SD-rats at day 0. The animals received 4 times 6 kindling stimulations delivered on day 4, 6, 8, and 11 after polymer implantation; according to our adenosine release data (Fig. 1), the first 18 stimulations were given during a time frame of almost constant release of 1000 ng adenosine per day, while adenosine release at day 11 (stimulation 19 to 24) had dropped to about 400 ng adenosine per day. On day 12, each of the adenosine-implant recipients received a single injection of the A1R antagonist DPCPX (1 mg/kg, i.p.), followed after 30 minutes by a single test stimulation. On day 13, each of these animals was tested again in the absence of DPCPX. Our results (Fig. 3A) demonstrate complete suppression of kindling development during the first 13 stimulations in the adenosine group, despite the regular presence of afterdischarges and wet dog shakes, elicited by the test stimulations. Compared to control implant recipients, the adenosine implant recipients continued to display a significant suppression of kindling epileptogenesis during the course of this experiment. Only at day 11, corresponding to a drop of implant-derived adenosine-release, kindling development started to progress in the ADO-group. At stimulation 24 the averaged seizure response of the adenosine implant recipients (stage 1.25 ± 0.7) was significantly (P<0.001) lower than the seizure response observed in the control group (stage 3.3 ± 1.2). To determine whether seizure suppression was due to suppression of ictogenesis (by implant-derived adenosine activating A1Rs) or due to suppression of epileptogenesis, stimulation #25 was given in the presence of DPCPX. The resulting seizure response (stage 1.8 ± 1.0) was only slightly different from the preceding seizure response #24 (stage 1.25 ± 0.7; P=0.05) and not different from the subsequent seizure response #26 elicited on day later (stage 1.6 ± 1.1; P=0.3). Whereas DPCPX, paired with kindling stimulations, readily elicits stage 5 seizures in fully kindled rats that are otherwise protected from adenosine releasing brain implants (Boison et al., 2002; Güttinger et al., 2005; Huber et al., 2001), in the present study test stimulations in the presence of DPCPX failed to elicit control group-like seizures. These results indicate that reduced seizure scores in adenosine-releasing implant recipients might be related to anti-epileptogenic effects of the adenosine-releasing brain implants.
Figure 3.
Influence on epileptogenesis by adenosine releasing polymers. (A) Experiment 2A: Four days after infrahippocampal implantation of silk-based polymers with daily target release rates for adenosine of 0 ng (N = 5, red), or 1000 ng (N = 8, blue) kindling stimulations were delivered at a rate of 6 stimulations per day on days 4, 6, 8, and 11 following implantation. A total of 24 kindling stimulations were delivered. On day 12 DPCPX (1 mg/kg, i.p.) was injected 30 min prior to stimulation. Each animal was tested again on day 13 (no DPCPX). Seizure stages were averaged across animals from each group for each individual stimulus. Note that recipients of a target dose of 1000 ng adenosine per display significant protection from kindling development, while DPCPX does not increase the seizure score. Errors are given as ± SD. Data were analyzed by two way ANOVA followed by a Bonferroni test; the significance of interaction between groups was determined as F=6.704, P<0.0001; significance levels of individual tests are indicated: * P<0.05, ** P<0.01, *** P<0.001. (B) Experiment 2B: Four days after infrahippocampal implantation of silk-based polymers with daily target release rates for adenosine of 0 ng (N = 7, red), or 1000 ng (N = 5, blue) kindling stimulations were delivered at a rate of 6 stimulations per day on days 4, 5, 6, 7, and 8 following implantation. A total of 30 kindling stimulations were delivered. Please note the increased kindling frequency compared to (A). After the 30th kindling stimulation, kindling was discontinued for 9 days. Kindling stimulations were resumed at day 18. Seizure stages were averaged across animals from each group for each individual stimulus. Note that recipients of a target dose of 1000 ng adenosine per day resumed kindling at day 18 at a level at which kindling was discontinued at day 8. After 7 consecutive stage 5 seizures kindling was discontinued in control animals due to animal welfare considerations. Errors are given as ± SD. Data were analyzed by two way ANOVA followed by a Bonferroni test; the significance of interaction between groups was determined as F=19.36, P<0.0001; significance levels of individual tests are indicated: ** P<0.01, *** P<0.001.
To further study possible anti-epileptogenic effects of adenosine, we performed Experiment 2b (Fig. 1B), in which we paired kindling stimulations with the specific adenosine-release profile of the polymers. Two groups of rats were implanted with adenosine-releasing (n = 5) or control polymers (n = 7) at the day of electrode implantation. The first set of 30 kindling stimulations was delivered during a time-window during which the polymers afforded a stable release rate of 1000 ng adenosine per day (day 4 to 8, 6 stimulations per day). In these pre-kindled rats kindling was resumed after a gap of 9 days, with the aim to deliver additional stimulations during the expiration phase of the polymers (day 18 to 21) until all animals were fully kindled. In line with our findings from Experiment 2a recipients of adenosine-releasing implants showed robust suppression of kindling development (Fig. 3B) during the first 5 days of stimulation. Even after 30 stimulations these animals continued to be protected from convulsive seizures and reacted with an average seizure score of 1.3 ± 0.5 at the 8th day after polymer implantation. In contrast, recipients of control implants kindled the same way reacted reproducibly with stage 4 or 5 seizures at that time point (average seizure score of 4.9 ± 0.4 at stimulation 30 at the 8th day after polymer implantation). After this initial seizure assessment, the animals were not subjected to any further stimulation during the following 9 days. Kindling was resumed at day 18, when all control animals continued to display stage 4 and 5 seizures. In contrast, kindling in adenosine-releasing polymer recipients resumed with stage 0 to 1 scores (average score of 0.5 ± 0.6) at a level similar to the seizure scores when kindling was discontinued (Fig. 3B). Subsequently, these animals responded with gradual increases in seizure severity until they reached stage 4 to 5 scores at day 20 and 21 after polymer implantation. This kindling curve was parallel – albeit shifted to the right – to the kindling curve of recipients of control implants. However, the number of drug-free afterdischarges in ADO-treated rats to elicit seizure stages comparable to those of control animals was lower than in those control rats, indicating that some epileptogenesis had occurred during the phase of adenosine delivery (Silver et al., 1991). Together, experiments 2A and 2B suggest, that focal adenosine delivery exerts partial antiepileptogenic effects.
The focal release of adenosine does not affect the expression of afterdischarges
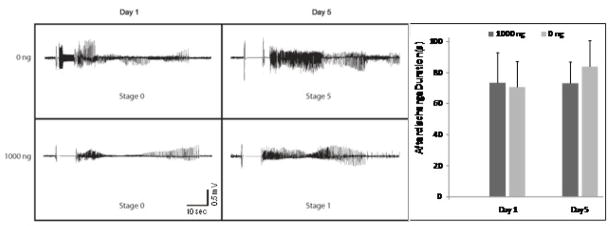
To rule out the possibility that the stimulus delivered to adenosine-implant recipients was insufficient to trigger epileptogenesis in the presence of this inhibitory modulator, we quantified electrographic afterdischarges in recipients of adenosine-releasing or control implants at the onset of kindling and during the 5th day of kindling (i.e. day 8 after polymer implantation), a time point at which control animals were almost fully kindled, while adenosine-implant recipients did not proceed beyond stage 1 seizures. Our data (Fig. 4) demonstrate that during day 1 of kindling (= day 4 after polymer implantation) afterdischarge durations in both groups of animals were initially almost identical (71±16 sec in control animals vs. 73±20 sec in recipients of ADO polymers; P>0.05). These data indicate that adenosine-release from the polymers did not affect the expression of epileptogenic afterdischarges. Afterdischarge durations in ADO polymer recipients remained fairly constant during the first 5 days of kindling. Averaged afterdischarge durations during the fifth day of kindling amounted to 73± 14 seconds (Fig. 4) in the ADO group, whereas the afterdischarge duration in the control group had increased to 84±17 seconds.
Figure 4.
Afterdischarges during kindling acquisition. Representative EEG recordings are shown from control polymer recipients (0 ng) and adenosine implant recipients (1000 ng) on the first and fifth day of kindling (corresponding to day 4 and day 8 after polymer implantation). The scale bar represents the 10 second stimulation interval. Note the presence of electrographic afterdischarges (2 – 3 Hz frequency) in the adenosine implant recipients. The afterdischarge duration (ADD) on day 1 and 5 of kindling was determined after each kindling-stimulation by analyzing the respective EEG recordings. ADDs were averaged for each day (n = 6 stimulations) and treatment type: implants releasing target doses of 0 ng adenosine (N=7) or 1000 ng (N=5) adenosine per day. Errors are given as ± SD. Data were analyzed by ANOVA; ADDs were not statistically different, P > 0.05.
Degradation of silk-based brain implants after 4 weeks in vivo
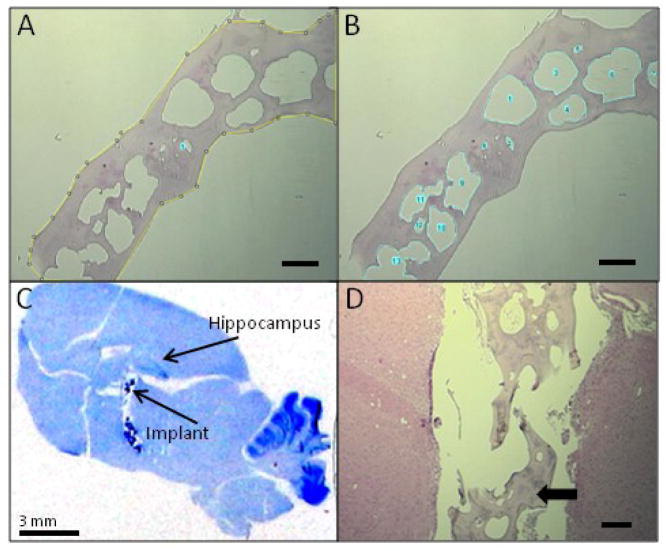
The adenosine release kinetic described above and the time-restricted therapeutic efficacy of the implants used in the current study suggested high and consistent initial release rates of adenosine coupled to degradation of the implants over time. We therefore subjected the adenosine-releasing silk-based polymers to a rigorous analysis to assess possible degradation processes. 5 μm sections of adenosine-loaded polymers before transplantation, or retrieved after 4 weeks in vivo (N = 6, each), were subjected to an Image J analysis to calculate the ratio of the total surface area of pores (in pixels) to the total surface area of the implant (in pixels) (Fig. 5A,B). Prior to implantation, the average silk implant porosity based on surface area analysis was 41.1%, and this increased to 50.9% after implantation (Table 1). The silk implants on average exhibited 9.8% more pore surface area after 4 weeks in vivo, suggesting that degradation of the polymers was occuring in the rat brain. After completion of the in vivo experiments all rat brains were subjected to histological analysis to verify electrode and polymer location. 4 weeks after implantation, the partly degraded polymers were still located in close proximity to the stimulated hippocampus (Fig. 5C). Closer inspection of the implants (Fig. 5D) revealed signs of degradation based on the loss of structural integrity of the scaffolds.
Figure 5.
Characterization of implants before and after implantation. (A, B) Example of Image J degradation analysis (pre-implantation sample #1, Table 1): The total surface area (yellow line) in pixels is measured (A), then the sum of the surface areas of all the pores (blue lines) is measured (B). The percentage porosity is calculated by taking the ratio of pore surface area over total implant area. Scale bars in images A and B = 300 μm. (C) Cresyl violet stain of a representative sagittal rat brain section 4 weeks post implantation. The polymer implant is dark blue and indicated with arrow. Scale bar = 3 mm. (D) Hematoxylin & eosin stain showing the morphology of representative infrahippocampal aqueous-derived adenosine-loaded silk fibroin implant after 4 weeks. Scale bar = 300 μm. Solid arrow = remaining scaffold.
Table 1. Sample porosity before and after implantation.
Sample porosity as determined by Image J analysis reflects the ratio of the total surface area of pores to the total surface area of the implants. Data presented are from 6 representative adenosine-loaded polymers before or 4 weeks after transplantation into rat brain. Data were analyzed with a two sample t-test: t = 5.08, df = 10, p < 0.001.
| Pre-implantation | Post-implantation | |
|---|---|---|
| Polymer #1 | 33.9 | 51.2 |
| Polymer #2 | 30.4 | 52.6 |
| Polymer #3 | 48.1 | 52.8 |
| Polymer #4 | 43 | 46.4 |
| Polymer #5 | 48 | 46.7 |
| Polymer #6 | 43 | 55.8 |
|
| ||
| Average | 41.1 | 50.9 |
|
| ||
| Standard Deviation | 7.4 | 3.7 |
Discussion
The present study was designed to carefully assess anti-ictogenic and anti-epileptogenic properties of focal adenosine augmentation in kindled rat brain after implantation of silk-based polymers designed to release a constant and defined dose of adenosine during a limited time span. Based on a previous dose-response study (Wilz et al., 2008) we selected polymers releasing a target dose of 1000 ng adenosine per day. Focal adenosine augmentation therapies (AATs) are based on the neurochemical rationale that dysfunction of the adenosine system is a neuropathological hallmark of epilepsy and a contributing factor for seizure generation (Boison, 2008a; Boison, 2008b; Dulla et al., 2005; Li et al., 2008; Rebola et al., 2003). Remarkably, AAT was effective in suppressing seizures in mice that were refractory to standard antiepileptic drugs such as carbamazepine, valproate, and phenytoin (Gouder et al., 2003). Adenosine exerts its antiepileptic effects largely by activation of pre- and postsynaptic adenosine A1 receptors that are coupled to inhibitory G-proteins, decrease presynaptic glutamate release, stabilize the postsynaptic membrane potential, and inhibit adenylyl cyclase (Fredholm et al., 2005a; Fredholm et al., 2005b). A1 receptor activation is not only effective in seizure suppression (Jacobson and Gao, 2006), but also is essential in keeping an epileptogenic focus localized (Fedele et al., 2006). Based on these observations A1 receptor activation might combine anti-ictogenic with anti-epileptogenic effects. However, due to peripheral side effects of systemic A1 receptor activation (Güttinger et al., 2005), focal AATs become a necessity. Focal approaches for epilepsy therapy are generally well-tolerated and devoid of undue side effects (Nilsen and Cock, 2004) and include cell therapies (Boison, 2007b; Loscher et al., 2008; Raedt et al., 2007; Shetty and Hattiangady, 2007) and gene therapies (Foti et al., 2007; McCown, 2004; Raol et al., 2006; Vezzani, 2007). Here we demonstrate the therapeutic use of silk-based polymers engineered to release adenosine as a clinically viable therapeutic alternative to achieve focal AAT with the combined goals of anti-ictogenesis and anti-epileptogenesis.
Anti-ictogenic potential of adenosine
The anti-ictogenic properties of adenosine are well established (Boison, 2007a). Thus, direct focal injection of adenosine prevented seizures in rats (Anschel et al., 2004) and intraventricular implants of encapsulated adenosine-releasing cells provided robust seizure suppression in kindled rats (Boison, 2007a). Previously used rodent-cell based cell-therapy approaches are however not acceptable for future therapeutic approaches since they would involve xenografting. In addition, hitherto used cell based approaches precluded detailed dose response studies. As a first step to develop a novel AAT that is compatible with future clinical applications we combined the two FDA approved compounds silk and adenosine into one biocompatible, biodegradable focal delivery system for adenosine. Using this novel type of polymers a dose-response study was performed that demonstrated dose-dependent (target release rates of 0, 40, 200, and 1000 ng adenosine per day) retardation of kindling development in rats (Wilz et al., 2008). In this initial study it was not tested whether adenosine-releasing implants can suppress fully kindled seizures and anti-ictogenic and anti-epileptogenic effects were not differentiated. Here we made use of silk-based polymers that released a constant dose of around 1000 ng adenosine per day from day 4 to day 10, before gradually declining to non-detectable levels of adenosine (Fig. 1). The polymers were designed to release adenosine for a limited time to specifically assess seizure responses after expiration of adenosine release. In Experiment 1 we demonstrate complete suppression of fully kindled seizures during the first 10 days of polymer implantation (Fig. 2) in line with the specific release profile of the polymers (Fig. 1). Importantly, seizures begin to recur during expiration of adenosine release from the polymers (from day 14 to 21). This finding is of importance for two reasons: (i) recurrence of seizures after expiration of adenosine release from the polymers indicates that seizure suppression depends on implant-derived adenosine; (ii) the precise match of therapeutic effectiveness with the release properties of the polymer is a prerequisite for the anti-epileptogenesis studies of Experiment 2A & B.
Anti-epileptogenic potential of adenosine
Several recent studies suggested a novel anti-epileptogenic role of focal AATs: (i) Both, stem cell derived (Li et al., 2007b), as well as silk-polymer based (Wilz et al., 2008), infrahippocampal implants designed to augment hippocampal adenosine retarded the progression of kindling epileptogenesis in rats. (ii) In a mouse model of CA3-selective epileptogenesis that includes astrogliosis and upregulation of ADK as pathological hallmarks of epileptogenesis, infrahippocampal stem cell derived adenosine-releasing implants reduced astrogliosis, prevented upregulation of ADK, and the occurrence of spontaneous seizures; in particular, the anti-astrogliotic effect of these cell-based implants can be interpreted as anti-epileptogenic effect.
In these previous studies, however, true anti-epileptogenic effects of focal AATs could not be studied separately from the anti-ictogenic effects of adenosine, since the possibility could not be excluded that epileptogenesis was masked by continuous seizure suppression by implant derived adenosine. To circumvent this problem, the current study was specifically designed to rigorously test possible anti-epileptogenic effects of implant-derived adenosine. To achieve this goal we engineered silk-based polymers to release a stable amount of adenosine over a limited time frame (1000 ng adenosine per day for up to 10 days). Two independent approaches were designed to demonstrate anti-epileptogenesis by these implants; in both approaches the polymers were implanted prior to the onset of kindling. In Experiment 2A implant recipients were kindled every other day from day 4 to 11 (corresponding to a total of 24 stimulations). Compared to control implant recipients, recipients of adenosine-releasing implants were characterized by marked retardation in the expression of kindled seizures (Fig. 2A). At this time point DPCPX failed to increase seizure scores in adenosine-releasing implant recipients indicating that the lack of higher seizure scores was not due to adenosine-based seizure suppression. These findings demonstrate an anti-epileptogenic effect of the adenosine releasing brain implants. Experiment 2B was designed to initiate kindling during the phase of constant high release of adenosine (1000 ng per day from day 4 to 8) (Fig. 1), and to resume kindling after a delay period of 9 days, a time frame during which adenosine release from the polymers had expired. This experimental paradigm is suited to quantify the degree of antiepileptogenesis (Silver et al., 1991). Drugs that do not have any anticonvulsant effects (e.g. carbamazepine in Silver et al., 1991) result in matching kindling curves between control and treatment groups, both during and after the drug phase; thus, carbamazepine did not affect kindling development during the drug-phase. Drugs that have partial antiepileptogenic effects (e.g. phenobarbital in Silver et al., 1991) display suppression of kindling development during the drug phase and resume kindling development at the same stage at which kindling was discontinued; however, in the case of phenbarbital the number of drug-free afterdischarges needed to elicit seizure stages corresponding to the control group was reduced, indicating partial antiepileptogenesis. Drugs that exert complete antiepileptogenic effects (e.g. valproate in Silver et al., 1991) display suppression of kindling development during the drug phase, resume kindling after discontinuation of the drug at the same stage as before discontinuation of the drug, but the numbers of drug-free afterdischarges to elicit corresponding seizures in drug-treated and control animals is the same. According to these considerations, our data (Fig. 3B) demonstrate almost complete suppression of kindling development during the first 24 kindling stimulations in recipients of adenosine-releasing implants. After the 30th stimulation delivered at day 8, recipients of adenosine-releasing implants were still strongly protected with seizure scores around 1. At the same time, recipients of control implants were completely kindled. When kindling was resumed at day 18, recipients of adenosine-releasing implants were still protected and in the absence of implant-derived adenosine gradually developed kindled seizures. The progression of seizure development in these animals was in parallel to kindling development in control animals, however the number of drug-free afterdischarges needed to elicit seizure stages corresponding to those in control animals was reduced. According to McNamara’s considerations our findings demonstrate that the transient release of adenosine during the first kindling sessions (day 4 to 8) provided partial prevention of epileptogenesis. If lack of seizures during that time were due to adenosine-based seizure suppression (masking epileptogenesis), then animals should have reacted with stage 5 seizures according to the control animals at day 18. It is important to note that despite the lack of behavioral seizures, an electrographic afterdischarge was always elicited even in recipients of adenosine releasing implants (Fig. 4), indicating that animals were kindled with supra-threshold stimulations that are expected to deliver an epileptogenesis-relevant trigger.
The beneficial mechanisms how chronic augmentation of brain adenosine, as achieved by the implants described here, might at least partially contribute to the prevention of epileptogenesis need to be distinguished from mechanisms of acute rises in adenosine to micromolar levels (Fredholm et al., 2005a) as a response to injury that are thought to trigger astrogliosis (Boison, 2008b). More work is needed to fully understand the mechanistic differences of opposing downstream-effects of chronic implant-based increases in adenosine versus acute high-level increases in adenosine during brain injury. Several hypothetical mechanisms may be involved: (i) A moderate increase in adenosine levels as achieved by brain implants might not be sufficient to trigger receptor expression changes on astrocytes, might preferentially activate astrocytic A1 receptors, and thus promote anti-epileptogenic effects via astrocyte modulation. (ii) In contrast, acute high levels of adenosine inhibited ADK (Mimouni et al., 1994); thus high levels of adenosine after acute injury have the potential to trigger upregulation of ADK as a compensatory mechanism that is linked to epileptogenesis (Li et al., 2008). (iii) An acute rise in adenosine to micromolar levels (i.e. as occurs after injury or during prolonged status epilepticus) is expected to lead to changes in astrocytic adenosine receptors, most notably downregulation of A1 receptors that are involved in regulating astrocyte proliferation; changes in astrocytic adenosine receptors could then trigger astrogliosis as part of the epileptogenic cascade.
Therapeutic potential of biodegradable adenosine-releasing polymers
The degradation of the scaffolds demonstrated here (Fig. 5; Table 1) suggests the presence of silk-degrading proteases in rat brain. For example, chymotrypsin has been shown to degrade silk (Li et al., 2003) and a number of chymotrypsin-like proteases have been identified in rat brain. Thus, caldecrin (a chymotrypsin-like protease) was demonstrated to be expressed within the hippocampus of adult rat brain (Tomomura et al., 2002). Further investigation will be needed to assess the specific proteases involved in the process and modes to regulate the degradation lifetime of this type of implant. The biodegradability of silk-based polymeric implants demonstrated here constitutes a major advantage for the preventive use of this type of brain implants. Partial anti-epileptogenic effects of adenosine-releasing silk-scaffolds as investigated here here would permit the preventive use of such implants in patients of high risk in developing epilepsy, e.g. after traumatic brain injury. Thus, silk-based adenosine-releasing scaffolds could be implanted into a traumatized brain area shortly after the injury, making synergistic use of the neuroprotective (Cunha, 2005), anti-ictogenic, and possible anti-epileptogenic properties of adenosine. Sustained delivery of adenosine might improve the therapeutic outcome in these patients and eventually the polymer would be completely degraded and resorbed without leaving any residues.
Limitations and outlook
The intention of the current design of polymers was to provide adenosine release during a restricted time window. This specific design of the implant allowed us to evaluate possible anti-epileptogenic effects of adenosine and to demonstrate seizure suppression in fully kindled rats. Our present data, but also those derived from previous studies (Li et al., 2007a; Li et al., 2007b; Li et al., 2008; Wilz et al., 2008) suggest at least partial anti-epileptogenic effects of focal AATs. Additional studies, including dose-response studies and the use of different models of epileptogenesis, are warranted to further address possible anti-epileptogenic effects of adenosine.
The current study design did not allow us to perform long-term seizure suppression studies. For the aim of long-term seizure suppression the design of the implants can be modified to allow sustained long-term delivery of adenosine. We have demonstrated recently that 3D porous matrices similar to those used here can be processed to function in vivo from weeks to a year or more depending on the mode of processing (Wang et al., 2007b). Eventually, to provide further extended release of adenosine, silk-based 3D scaffolds could be combined with human mesenchymal stem cells (hMSCs) engineered to release adenosine. We previously published an RNAi-based lentiviral method to engineer hMSCs for therapeutic adenosine release (Ren et al., 2007). Infrahippocampal implants of these cells reduced acute kainic acid induced brain injury and seizures (Ren et al., 2007). Eventually, hMSCs taken from a patient and engineered to release adenosine could be used as autologous brain implants in combination with biodegradable silk-based scaffolds.
Acknowledgments
This project was supported by grant R01NS058780 from the National Institute of Neurological Disorders and Stroke and by the Epilepsy Research Foundation through the generous support of the Arlene & Arnold Goldstein Family Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- Anschel DJ, Ortega EL, Kraus AC, Fisher RS. Focally injected adenosine prevents seizures in the rat. Exp Neurol. 2004;190:544–547. doi: 10.1016/j.expneurol.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Boison D, Huber A, Padrun V, Deglon N, Aebischer P, Mohler H. Seizure suppression by adenosine-releasing cells is independent of seizure frequency. Epilepsia. 2002;43:788–796. doi: 10.1046/j.1528-1157.2002.33001.x. [DOI] [PubMed] [Google Scholar]
- Boison D. Adenosine-based cell therapy approaches for pharmacoresistant epilepsies. Neurodegener Dis. 2007a;4:28–33. doi: 10.1159/000100356. [DOI] [PubMed] [Google Scholar]
- Boison D. Cell and gene therapies for refractory epilepsy. Current Neuropharmacology. 2007b;5:115–125. doi: 10.2174/157015907780866938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine as a modulator of brain activity. Drug News Persp. 2007c;20:607–611. doi: 10.1358/dnp.2007.20.10.1181353. [DOI] [PubMed] [Google Scholar]
- Boison D. Astrogliosis and adenosine kinase: a glial basis of epilepsy. Future Neurology. 2008a;3:221–224. [Google Scholar]
- Boison D. The adenosine kinase hypothesis of epileptogenesis. Progress in Neurobiology. 2008b;84:249–262. doi: 10.1016/j.pneurobio.2007.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D. Adenosine as a neuromodulator in neurological diseases. Curr Opin Pharmacol. 2008c;8:2–7. doi: 10.1016/j.coph.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha RA. Neuroprotection by adenosine in the brain: from A1 receptor activation to A2A receptor blockade. Purinergic Signaling. 2005;1:111–134. doi: 10.1007/s11302-005-0649-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link P-CO2 to cortical excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, Masino SA. The role and regulation of adenosine in the central nervous system. Annu Rev Neurosci. 2001;24:31–55. doi: 10.1146/annurev.neuro.24.1.31. [DOI] [PubMed] [Google Scholar]
- Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Exp Neurol. 2006;200:184–190. doi: 10.1016/j.expneurol.2006.02.133. [DOI] [PubMed] [Google Scholar]
- Foti S, Haberman RP, Samulski RJ, McCown TJ. Adeno-associated virus-mediated expression and constitutive secretion of NPY or NPY13–36 suppresses seizure activity in vivo. Gene Ther. 2007;14:1534–1536. doi: 10.1038/sj.gt.3303013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Cunha RA, Svenningsson P, Vaugeois JM. Adenosine and brain function. Int Rev Neurobiol. 2005a;63:191–270. doi: 10.1016/S0074-7742(05)63007-3. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, Chen JF, Masino SA, Vaugeois JM. Actions of adenosine at its receptors in the CNS: Insights from knockouts and drugs. Annu Rev Pharmacol Toxicol. 2005b;45:385–412. doi: 10.1146/annurev.pharmtox.45.120403.095731. [DOI] [PubMed] [Google Scholar]
- Gouder N, Fritschy JM, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia. 2003;44:877–885. doi: 10.1046/j.1528-1157.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- Güttinger M, Padrun V, Pralong W, Boison D. Seizure suppression and lack of adenosine A1 receptor desensitization after focal long-term delivery of adenosine by encapsulated myoblasts. Exp Neurol. 2005;193:53–64. doi: 10.1016/j.expneurol.2004.12.012. [DOI] [PubMed] [Google Scholar]
- Hofmann S, Foo CT, Rossetti F, Textor M, Vunjak-Novakovic G, Kaplan DL, Merkle HP, Meinel L. Silk fibroin as an organic polymer for controlled drug delivery. J Control Release. 2006;111:219–227. doi: 10.1016/j.jconrel.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Horan RL, Antle K, Collette AL, Wang Y, Huang J, Moreau JE, Volloch V, Kaplan DL, Altman GH. In vitro degradation of silk fibroin. Biomaterials. 2005;26:3385–3393. doi: 10.1016/j.biomaterials.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Huber A, Padrun V, Deglon N, Aebischer P, Mohler H, Boison D. Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc Natl Acad Sci USA. 2001;98:7611–7616. doi: 10.1073/pnas.131102898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson KA, Gao ZG. Adenosine receptors as therapeutic targets. Nat Rev Drug Discov. 2006;5:247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin HJ, Kaplan DL. Mechanism of silk processing in insects and spiders. Nature. 2003;424:1057–1061. doi: 10.1038/nature01809. [DOI] [PubMed] [Google Scholar]
- Kim UJ, Park J, Kim HJ, Wada M, Kaplan DL. Three-dimensional aqueous-derived biomaterial scaffolds from silk fibroin. Biomaterials. 2005;26:2775–2785. doi: 10.1016/j.biomaterials.2004.07.044. [DOI] [PubMed] [Google Scholar]
- Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RS, Homanics GE, Dixon CE, Schnermann J, Jackson EK. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. J Cereb Blood Flow Metab. 2006;26:565–575. doi: 10.1038/sj.jcbfm.9600218. [DOI] [PubMed] [Google Scholar]
- Li C, Vepari C, Jin HJ, Kim HJ, Kaplan DL. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124. doi: 10.1016/j.biomaterials.2006.01.022. [DOI] [PubMed] [Google Scholar]
- Li M, Ogiso M, Minoura N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials. 2003;24:357–365. doi: 10.1016/s0142-9612(02)00326-5. [DOI] [PubMed] [Google Scholar]
- Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007a;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brustle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007b;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W. Current status and future directions in the pharmacotherapy of epilepsy. Trends Pharmacol Sci. 2002;23:113–118. doi: 10.1016/S0165-6147(00)01974-X. [DOI] [PubMed] [Google Scholar]
- Loscher W, Gernert M, Heinemann U. Cell and gene therapies in epilepsy - promising avenues or blind alleys? Trends in Neurosciences. 2008;31:62–73. doi: 10.1016/j.tins.2007.11.012. [DOI] [PubMed] [Google Scholar]
- McCown TJ. The clinical potential of antiepileptic gene therapy. Expert Opinion on Biological Therapy. 2004;4:1771–1776. doi: 10.1517/14712598.4.11.1771. [DOI] [PubMed] [Google Scholar]
- McNamara JO. Emerging insights into the genesis of epilepsy. Nature. 1999;399:A15–22. doi: 10.1038/399a015. [DOI] [PubMed] [Google Scholar]
- Mimouni M, Bontemps F, Van den Berghe G. Kinetic studies of rat liver adenosine kinase. J Biol Chem. 1994;269:17820–17825. [PubMed] [Google Scholar]
- Nilsen KE, Cock HR. Focal treatment for refractory epilepsy: hope for the future? Brain Res Brain Res Rev. 2004;44:141–153. doi: 10.1016/j.brainresrev.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Racine R. Kindling: the first decade. Neurosurg. 1978;3:234–252. doi: 10.1227/00006123-197809000-00018. [DOI] [PubMed] [Google Scholar]
- Raedt R, Van Dycke A, Vonck K, Boon P. Cell therapy in models for temporal lobe epilepsy. Seizure-European Journal of Epilepsy. 2007;16:565–578. doi: 10.1016/j.seizure.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Raol YH, Lund IV, Bandyopadhyay S, Zhang G, Roberts DS, Wolfe JH, Russek SJ, Brooks-Kayal AR. Enhancing GABA(A) receptor alpha 1 subunit levels in hippocampal dentate gyrus inhibits epilepsy development in an animal model of temporal lobe epilepsy. J Neurosci. 2006;26:11342–11346. doi: 10.1523/JNEUROSCI.3329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. Eur J Neurosci. 2003;18:820–828. doi: 10.1046/j.1460-9568.2003.02815.x. [DOI] [PubMed] [Google Scholar]
- Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208:26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld SS, Taylor EW. Reactions of 1-N6-ethenoadenosine nucleotides with myosin subfragment 1 and acto-subfragment 1 of skeletal and smooth muscle. J Biol Chem. 1984;259:11920–11929. [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B. Concise review: Prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25:2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver JM, Shin C, McNamara JO. Antiepileptogenic effects of conventional anticonvulsants in the kindling model of epilespy. Ann Neurol. 1991;29:356–363. doi: 10.1002/ana.410290404. [DOI] [PubMed] [Google Scholar]
- Sofia S, McCarthy MB, Gronowicz G, Kaplan DL. Functionalized silk-based biomaterials for bone formation. J Biomed Mater Res. 2001;54:139–148. doi: 10.1002/1097-4636(200101)54:1<139::aid-jbm17>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Tomomura A, Yamada H, Itagaki K, Fujimoto K, Katoh S. Rat brain expresses serum calcium-decreasing factor (caldecrin) Neurosci Lett. 2002;317:17–20. doi: 10.1016/s0304-3940(01)02409-0. [DOI] [PubMed] [Google Scholar]
- Vajda FJE. Pharmacotherapy of epilepsy: New armamentarium, new issues. Journal of Clinical Neuroscience. 2007;14:813–823. doi: 10.1016/j.jocn.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Vezzani A. The promise of gene therapy for the treatment of epilepsy. Expert Rev Neurother. 2007;7:1685–1692. doi: 10.1586/14737175.7.12.1685. [DOI] [PubMed] [Google Scholar]
- Wang X, Kim HJ, Xu P, Matsumoto A, Kaplan DL. Biomaterial coatings by stepwise deposition of silk fibroin. Langmuir. 2005;21:11335–11341. doi: 10.1021/la051862m. [DOI] [PubMed] [Google Scholar]
- Wang X, Wenk E, Hu X, Castro GR, Meinel L, Wang X, Li C, Merkle H, Kaplan DL. Silk coatings on PLGA and alginate microspheres for protein delivery. Biomaterials. 2007a;28:4161–4169. doi: 10.1016/j.biomaterials.2007.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wenk E, Matsumoto A, Meinel L, Li C, Kaplan DL. Silk microspheres for encapsulation and controlled release. J Control Release. 2007b;117:360–370. doi: 10.1016/j.jconrel.2006.11.021. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008;29:3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcik WJ, Neff NH. Adenosine measurement by a rapid HPLC-fluorimetric method: induced changes of adenosine content in regions of rat brain. J Neurochem. 1982;39:280–282. doi: 10.1111/j.1471-4159.1982.tb04736.x. [DOI] [PubMed] [Google Scholar]