Abstract
The pathogenesis of Parkinson’s disease is not fully understood, but there is evidence that excitotoxic mechanisms contribute to the pathology. However, data supporting a role for excitotoxicity in the pathophysiology of the disease is controversial and sparse. The goal of this study was to determine whether changes in glutamate signaling and uptake contribute to the demise of dopaminergic neurons in the substantia nigra. Mice were treated chronically with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and probenecid or vehicle (probenecid or saline alone). Extracellular levels of glutamate in the substantia nigra were substantially increased, and there was an increase in the affinity, but no change in the velocity, of glutamate transport after MPTP/probenecid treatment compared to vehicle controls. In addition, the substantia nigra showed two types of programmed death, apoptosis (type I) and autophagic (type II) cell death. These data suggest that increased glutamate signaling could be an important mechanism for the death of dopaminergic neurons and trigger the induction of programmed cell death in the chronic MPTP/probenecid model.
Keywords: dopamine, probenecid, glutamate transport, tyrosine hydroxylase, apoptosis, autophagy, substantia nigra, glutamate, grid test, optical fractionator
Introduction
A formidable challenge in the field of Parkinson’s Disease (PD) research is to understand the processes that lead to the death of dopamine (DA)-containing neurons in the substantia nigra (SN). Neuronal loss is gradual and interrupting the process is a valid treatment goal, and there is good evidence that mitochondrial dysfunction and oxidative damage are contributing factors (Dawson and Dawson, 2002; Dawson and Dawson, 2003; Hunot and Hirsch, 2003; Meredith, 2004). Postmortem studies reveal a reduction in the activity of mitochondrial complex I by 30% to 40% (Schapira, 1993). Mitochondrial function is critical to the maintenance of adequate adenosine triphosphate (ATP) for basic cell processes. The loss of ATP disrupts functional ion pumps, augments the formation of radical oxygen species and reduces the plasma membrane potential, which in turn decreases the activation threshold for N-methyl-D-aspartate (NMDA) receptors (Novelli et al., 1988; Smith and Grace, 1992), thus increasing the neuron’s vulnerability to damage by glutamate (Blandini et al., 1996). Acute glutamate excitotoxicity can produce necrotic cell death (Dirnagl et al., 1999). However, programmed cell death, such as occurs in apoptosis or autophagy, are often delayed and happen as mitochondria become major targets following bulk increases in cytoplasmic Ca2+ (Hartley et al., 1994; Yue et al., 2002; Takacs-Vellai et al., 2006) from elevated glutamate signaling (Blandini et al., 1996). Glutamate levels in the SN are regulated by transporters and by local metabolism (see Plaitakis and Shashidharan, 2000), and any change in glutamate uptake would alter glutamate signaling, which could be deleterious for the cells.
Autophagy (also referred to as macroautophagy) is a degradative process, which can be protective for neurons (Kiffin et al., 2004). Under conditions of oxidative stress or injury, this pathway is activated and abnormal proteins and damaged organelles are engulfed by autophagic vacuoles which fuse with lysosomes (Komatsu et al., 2007). However, the recycling of organelles such as mitochondria is energetically expensive and under conditions of prolonged stress, autophagic death ensues (Alirezaei et al., 2008). One of the most widely used models of PD is produced by administering 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to mice. This toxin inhibits mitochondrial complex I and appears to kill neurons by necrosis or apoptosis, depending on the mode of administration (Nicotra and Parvez, 2000; Przedborski et al., 2004; Novikova et al., 2006). Autophagic cell death has been demonstrated in postmortem midbrain of PD patients (Anglade et al., 1997), and in vitro models demonstrate that autophagy is impaired by mutant alpha-synuclein protein (Cuervo et al., 2004). No study to date has explored glutamate function in the SN and its relationship to cell death in an MPTP model. Therefore we designed this investigation to test whether chronic treatment with MPTP in mice compromises glutamate function in the SN and whether changes in that function could trigger cell death via apoptotic or autophagic pathways.
Materials and methods
Animals and treatment
Adult, C57BL/6 male mice (Charles River Laboratories, Wilmington, MA, USA) weighing 20–30g were housed 2 to the cage and given food and water ad libitum. Once treatment regimens began, the diet of all mice was supplemented daily with a hydrating gel (Harlan Sprague Dawley, Inc., Indianapolis, IN). The protocols described below were conducted in accordance with the National Institutes of Health, “Guidelines for the Humane Care and Use of Laboratory Animals” (NIH Publications No. 80-23) and were approved by in-house Institutional Animal Care and Use Committees at the Chicago Medical School and the Portland VA Medical Center.
Mice were treated with 10 doses (2 ×week; 3.5 days apart) of MPTP hydrochloride (25 mg/kg in saline, s.c., Sigma-Aldrich, St Louis, MO) and probenecid (250 mg/kg in dimethyl sulfoxide, i.p., Sigma), or with probenecid (equal volume injected i.p.) or saline (equal volume injected s.c.) alone (control groups). The probenecid-treated animals were used as a control group, since previous studies have shown no change in the number of DA neurons or in the level of striatal DA after probenecid treatment alone (Lau et al., 1990, Petroske et al., 2001).
The functional impact of the toxin or vehicle was assessed using a novel grid test (Tillerson and Miller, 2003), which assesses coordination and rigidity. The grid was constructed of a horizontal square (15 cm2 with openings in the mesh of 0.5 cm2) attached to 4 cardboard walls. Each mouse was placed in the center of the grid and the grid was rotated 180o suspending the mouse approximately 30 cm above the floor. The mouse was allowed to move freely while upside down and was videotaped for 60 seconds. All steps with the forepaws were counted and each unsuccessful forepaw step was declared a fault. A ratio of total forepaw foot faults/total steps was established for each mouse. Animals had to take a minimum of 10 total steps to be included in the data set and must have stayed on the Grid for 10s for the trial to be recorded. A mean of 3 trials/mouse was used in the analysis. Data for each group was pooled and tested statistically with a one-way ANOVA followed by a Student-Newman-Keuls post hoc test.
Immunohistochemistry and stereology
Mice were deeply anesthetized with pentobarbital (135 mg/kg) and transcardially perfused with physiologically buffered saline followed by fixative (3% paraformaldehyde in 0.1M phosphate buffer (PB) and 2.5% sucrose). Brains were sunk in 20% sucrose, blocked and then cut at 50µm on a freezing microtome. Sections were collected serially in well dishes for stereological studies of tyrosine hydroxylase (TH) -immunoreactive, Nissl-stained neurons in the substantia nigra pars compacta (SNc). Sections were incubated overnight at 4oC in mouse anti-TH sera (Diasorin, Stillwater, MN, USA), diluted 1:2000, using reagents from the Mouse-on-Mouse kit (Vector Laboratories, Burlingame, CA). This was followed by a room temperature incubation in biotinylated goat anti-mouse IgG, diluted 1:250, for 2 h. Sections were then incubated for 45 min in the reagents from the avidin-biotin complex (elite kit, Vector Laboratories) and reacted with 3,3’ diaminobenzidine hydrochloride (DAB). Sections were mounted serially on gelatinized slides, left to dry overnight, stained with cresyl violet, dehydrated and covered with coverslips.
Once dry, Nissl-stained, TH-immunopositive neurons were counted in the SNc following optical fractionator rules (see e.g., Petroske et al., 2001; Chan et al., 2007). Briefly, after a random selection of a starting section, neurons were counted in every third section for a total of 7 sections through the entire midbrain. Neurons were counted using dedicated software (StereoInvestigator, MicroBrightfield, Williston, VT) and hardware (Nikon E400 microscope with a motorized stage in 3 axes driven by the software. Using the optical disector, the total number of Nissl-stained, TH-immunoreactive neurons was estimated. The volume of the SNc was estimated following the Cavalieri method (Sterio, 1984).
In vivo microdialysis
Approximately four weeks after the last MPTP/p or vehicle injection, animals (n = 6/group) were anesthetized (1 ml/100 g of 2.5% ketamine, 1% xylazine and 0.5% acepromazine in normal saline), and placed in a mouse stereotaxic apparatus (Cartesian Research, Inc., Oregon). A small hole was drilled on the left side of the skull anterior: −3.2 mm; lateral: +1.7 mm (Franklin, 1997) and a stainless steel guide cannula (8 mm long, 21-gauge; Small Parts, Miami Lakes, FL) was lowered 1.5 mm from the surface of the skull. The animals were allowed to recover for one week prior to the start of the microdialysis experiment. Dialysis probes, 210 µm in diameter and 1 mm long, were prepared as previously described (Meshul et al., 1999). Details of the microdialysis procedure have been reported (Holmer et al., 2005). Briefly, the tip of the guide cannula was positioned within the cortex and the probe lowered so that the tip reaches the most ventral aspect of the SN. The probe was secured to the guide cannula and the other end was attached to a syringe containing artificial cerebrospinal fluid (aCSF). A pump (Orion, Model 365, Thermo Electron Corp, UK) pushed the aCSF through the probe overnight at a rate of 0.2 µl/min. The following morning, the pump speed was increased to 2 µl/min for 1 hour and then 4 samples were collected every 15 minutes. The mean probe recovery was 10.4 ± 1.2%.
Glutamate concentration in the dialysate samples was determined using a Hewlett Packard HPLC 1090 interfaced with a Hewlett Packard 1046A Programmable Fluorescence Detector. Dialysates were derivatized with o-phthalaldehyde and chromatographed as previously reported (Meshul et al., 1999, Meshul et al., 2002). Assay sensitivity was in the sub-picomole range. After collecting baseline glutamate levels, mice were injected with 15mg/kg levodopa (l-dopa ,i.p.) and then 6 samples were collected every 15 minutes. The animals were freely moving during the entire microdialysis procedure. At the conclusion of the experiment, the animals were transcardially perfused with glutaraldehyde fixative (2.5% glutaraldehyde/0.5% paraformaldehyde in 0.1 M HEPES, pH 7.3, containing 0.1% picric acid). Vibratome sections (100µm) were cut and stained with thionin to verify the site of the probe placement within the SN. Data were discarded from animals with incorrect placements.
Glutamate transporter assays
To establish possible alterations in glutamate transporter function, mice (n = 20/group) were treated chronically with MPTP/p or vehicle. All mice were killed 3 weeks later by cervical dislocation and decapitation. Following rapid removal of the brains, the midbrain was blocked and the paired SN were dissected. Crude synaptosomes were prepared from the midbrains of these animals and the SN from 5 mice were pooled for each group in each experiment.
The SN from each side of the brain was isolated within 2 min of decapitation. The SN from 5 mice per group (MPTP/p and vehicle groups) were pooled and homogenized in ice-cold medium of 0.32M sucrose in 50mM Tris, buffered to pH 7.5. The homogenate was centrifuged 5 min at 1200g at 4oC (Sorvall centrifuge, SS-34 rotor). The supernatant was then transferred to a fresh tube, centrifuged 12 min at 17,000g at 4oC and the pellet washed in ice-cold gradient medium and collected by centrifugation. The crude pellet was re-suspended in a final volume of 750 µl of homogenization medium. High affinity Na+-dependent transport was assayed at 4 different concentrations of D-[3H]aspartate increasing from 0.4 to 40 ×10-5M, as described previously (De Souza et al., 1999). D-aspartate was used instead of L-glutamate in these experiments, because D-aspartate is not metabolized by glutamate transporters (Davies and Johnston, 1976; Fyske et al., 1992). For the assay, resuspended synaptosomes were added to duplicate microcentrifuge tubes containing Krebs solution, pH 7.4. Following protein determination, the remaining synaptosomes were incubated at 37°C with 15 nCi of D -[2,3-3H]-aspartic acid (New England Nuclear, Downers Grove, IL). The reaction was stopped with 200 µl of ice cold 1 mM D-aspartate, followed by microcentrifugation at 15,700g at 4°C. The pellets were washed twice without re-suspending using Krebs medium followed by microcentrifugation. The washed pellets were dissolved by overnight incubation in 200 µl of 2% sodium dodecyl sulfate and suspended in 5 ml of Ecoscint A (National Diagnostics, Atlanta, GA, USA). The quantity of radioactivity was determined by liquid scintillation spectroscopy and the kinetic parameters Km and Vmax were determined by non-linear regression analysis. Data gathered were the means of results obtained from determination performed as triplicates in 4 experiments and the rate of transport was plotted as a function of substrate concentration.
TUNEL Analysis
Mice (n = 5/group) were treated with MPTP/p or vehicle. Mice were killed by cervical dislocation and decapitation after 2, 3, 4 or 8 injections of MPTP/p or vehicle and at 3 days or 3 weeks post-treatment. Their brains were rapidly removed, frozen on dry ice (in isopentane) and the midbrains sectioned on a cryostat at a thickness of 20µm. Five sections (every 12th section) through the SN were directly mounted onto proteinase-resistant microscope slides, dried at room temperature, and stored in slide boxes containing desiccant at −80oC until processing. Slides were transferred from the freezer directly into a fixative solution (4% paraformaldehyde in 0.1M PB) for 30 min. They were then rinsed in PBS and labeled according to FD NeuroApop (FD NeuroTechnologies, Ellicott City, MD) kit instructions. Following the reaction in the chromogen, sections were rinsed, air dried, counterstained with methyl green or cresyl-violet, dehydrated and coverslipped. Apoptotic profiles were counted in all sections through the SNc and results were pooled for each group.
Electron microscopy studies of cell death
Mice (n = 5/group) treated with MPTP/p or vehicle, were anesthetized 1 or 3 weeks after treatment. Their brains were fixed by perfusion through the heart first with saline and then with a fixative containing 4% paraformaldehyde with 0.1% glutaraldehyde in 0.1 M PB. Coronal sections were cut through the midbrain at 70µm using a vibrating microtome (Leica, Milton Keynes, UK) and collected in vials containing the same buffer. Sections were flattened onto watch glasses then immersed in 1% osmium tetroxide in 0.1 M PB for 30 minutes. After a brief wash in water, they were dehydrated through an ascending series of alcohol concentrations, spending 40 minutes in a saturated solution of uranyl acetate in the 70% ethanol stage. Finally the sections were immersed in two changes of propylene oxide before being transferred to Durcupan resin (ACM Fluka, Sigma Aldrich, Poole UK) overnight. The resin was then gently warmed and the sections transferred to glass slides and a coverslip applied.
Areas of SNc were chosen randomly from each mouse to determine whether autophagosomes were present in intact neurons and whether MPTP/p treatment led to autophagic cell death. The coverslip was removed and a small piece of tissue excised and glued onto a pre-formed resin block using cyanoacrylate glue. Three blocks were cut from the SNc of each mouse. These blocks were then re-sectioned using an ultramicrotome (Ultracut E, Leica, Milton Keynes UK) at 60–70 nm and serial sections were mounted onto single slot, pioloform coated, copper grids. The sections were contrasted using lead stain and examined in a Philips 401 electron microscope. Images of dendrites showing signs of disruption and degenerating cell bodies were captured digitally (Multiscan, Gatan, Oxford UK).
Statistical Analysis
Data were first checked to verify a normal distribution. Non-normally-distributed data (number of TH-positive, Nissl-stained neurons) were analysed using Kruskal-Wallis followed by Dunnett’s post-hoc tests. The four baseline data points from the microdialysis study were averaged at each time point and then a grand mean determined. Values are expressed as the mean + S.E.M. in picomoles/µl of extracellular SN glutamate within the dialysate sample. Baseline levels and levels following acute l-dopa administration were compared using a one-way ANOVA followed by the Tukey-Kramer post-hoc for comparison of multiple means. Glutamate transport was plotted as a nonlinear regression. Only two groups - MPTP/p and vehicle (combined saline and probenecid controls) - were used for statistical tests in the glutamate transport and behavioral (grid test) experiments. Therefore, an unpaired Student’s t-test was used for both data sets.
Results
MPTP/p produces loss of DA neurons in the SNc
We estimated that MPTP/p treatment leads to a 68% loss of TH-immunoreactive, Nissl-stained neurons compared to vehicle-treated controls at 3 weeks after treatment (Table 1, Fig. 1). There was no change in midbrain volume between groups (Table 1). MPTP/p-treated (N = 10) mice also performed significantly worse on the grid test compared to vehicle-treated (N = 10, probenecid, and N = 10, saline) controls. By 3 weeks post-treatment, MPTP/p-treated mice demonstrated a significant increase (Student’s t-test, t = −4.98, p < 0.001 ) in the ratio of foot faults/total steps (MPTP/p = 0.19±0.23) compared to probenecid or saline controls (pooled vehicle: 0.09±0.02). There was no difference between control groups (p > 0.3).
Table 1.
Stereological estimates of the total number of Nissl-stained, TH-immunoreactive neurons in the SNc and reference volumes (Cavalieri method) of the region.
| Animal treatment N | Mean Vr ± SEM (mm3) | |||
|---|---|---|---|---|
| Total Number of SNc Neurons ± SEM CE | ||||
| MPTP/probenecid | 8 | 0.164 ± 0.003 | 3112 ± 100*§ | 0.11 |
| probenecid | 6 | 0.170 ± 0.004 | 9745 ± 254 | 0.09 |
| saline | 6 | 0.178 ± 0.008 | 9836 ± 100 | 0.08 |
Vr: reference volume; CE: coefficient of error for neuron estimates
significantly fewer neurons in SNc after MPTP/probenecid treatment compared to probenecid or saline
(Kruskal-Wallis test,* P < 0.001; Dunnets post hoc test, §P < 0.001 compared to probenecid control and §P < 0.001 compared to saline control).
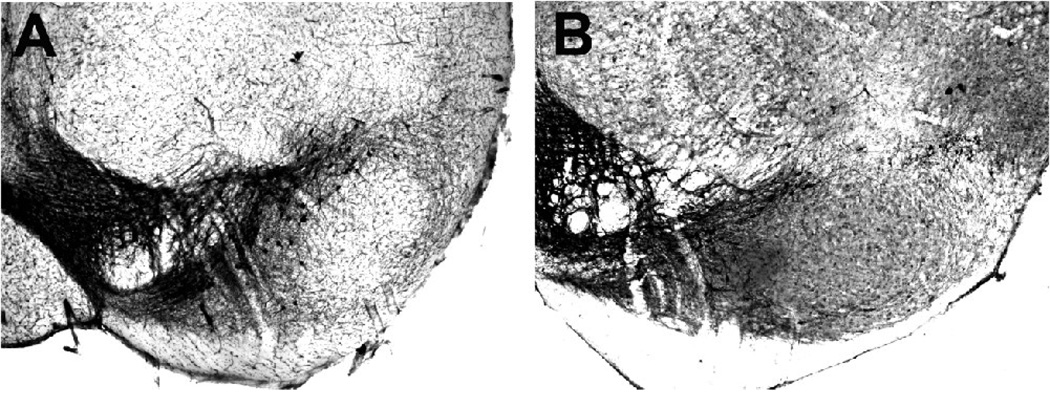
Fig. 1.
Representative photomicrographs of TH-immunoreactive neurons in the SNc and ventral tegmental area (VTA) in probenecid (N = 10)- (A) and MPTP/p (N = 10)- (B) treated mice, 3 weeks after treatment. Note the loss of TH-immunoreactive neurons in (B) compared to (A). Scale bar in A equals 250µm and is valid for B.
MPTP/p increases midbrain glutamate levels
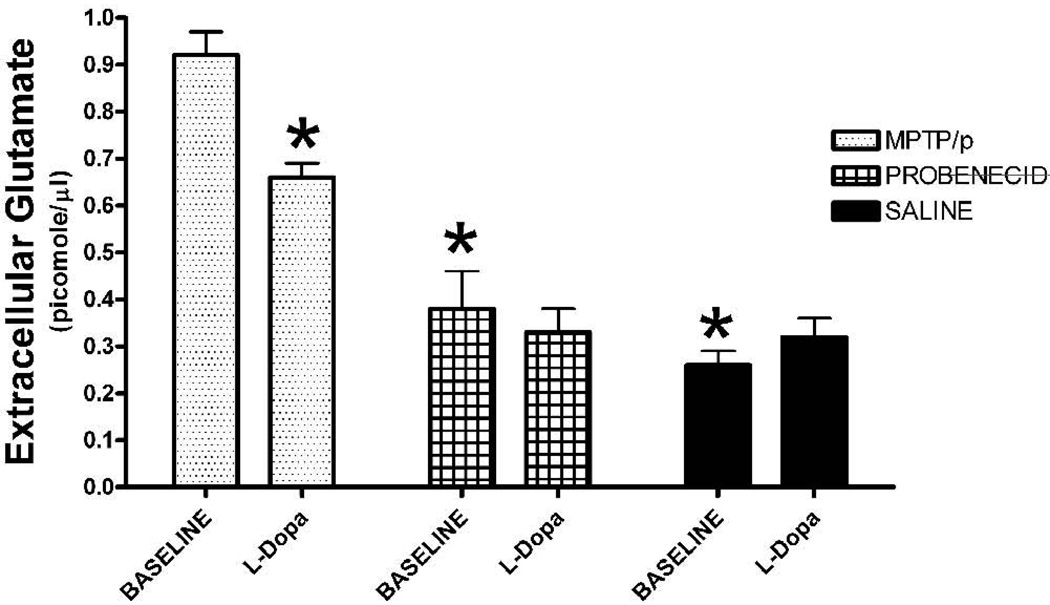
In vivo microdialysis of the midbrain showed a significant, three-fold increase in the basal levels of extracellular glutamate in the SN for the MPTP/p-treated mice compared to vehicle-treated controls (Fig. 2; one-way ANOVA, F (2,15) = 36.0, p < .0001). There was no difference in baseline levels of extracellular glutamate between probenecid- and saline- treated controls (Fig. 2; p > 0.3). A challenge injection of l-dopa significantly lowered the level of extracellular glutamate (0.66 ± 0.03 picomoles/µl) in the SN compared to baseline (0.91 ± 0.07 picomoles/µl) for MPTP/p-treated mice (one-way ANOVA, F (1,10) = 22.1, p < 0.001), but had no effect on glutamate levels of the vehicle groups (p > 0.3).
Fig. 2.
In vivo microdialysis of the SN. Four baseline samples were collected from mice treated with MPTP/p (N = 6), probenecid (N = 6) or saline (N = 6). There was a significant increase in the baseline level of extracellular glutamate for the MPTP/p group compared to the probenecid- or saline-treated groups. Following acute administration of l-dopa (15 mg/kg, ip), there was a decrease in SN extracellular glutamate levels in only the MPTP/p versus the other two control groups. Values are means ± SEM. *p < 0.001 compared to the baseline value for the MPTP/p group.
MPTP/p increases the affinity for substrate but does not change the rate of transport
High affinity sodium-dependent uptake of D-aspartate was assayed in midbrains taken from mice 4–5 weeks after chronic MPTP treatment or vehicle. The mean Km value for synaptosomes preparations from MPTP/p mice was 3.55 ± 0.18, which was significantly decreased (−26%, Student’s t-test, t = 3.27, p < 0.05) over the affinity for substrate in controls (vehicle groups combined: Probenecid: 4.78 ± 0.31; saline: 4.56 ± 0.28). The maximal rate of transport (Vmax) was unaffected (MPTP/p group: 0.49 ± 0.07 nmol/min/mg; vehicle group: 0.66 ± 0.06 nmol/min/mg). However, there was a trend for the velocity of the reaction to be reduced (p = 0.1), suggesting that there could have been a reduction in transporters. There was no difference between the saline and probenecid groups in either measure (p > 0.2).
Apoptosis and autophagic cell death of SNc neurons
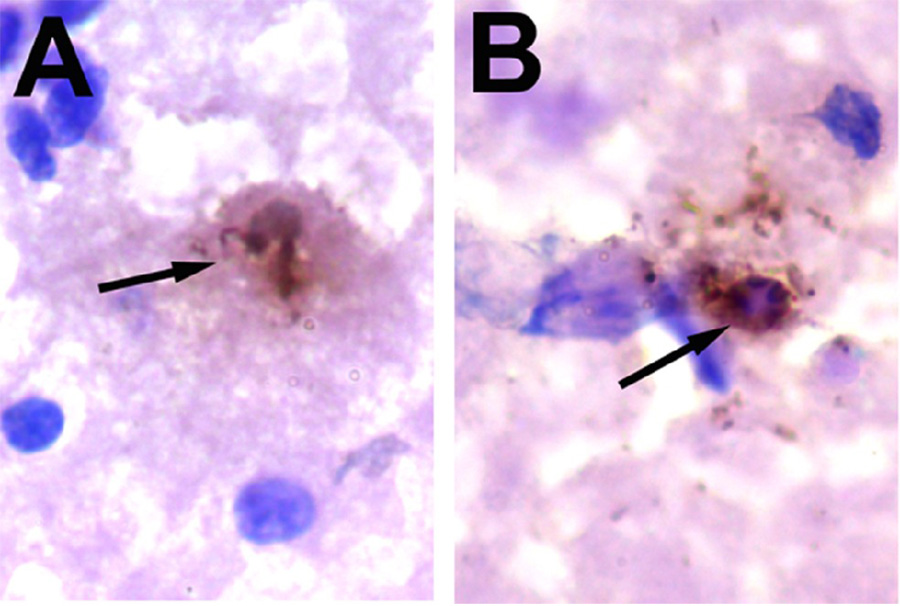
Apoptotic profiles were never detected in the SNc of mice treated with probenecid or saline. However, TUNEL-positive cells with dark nuclei and shrunken cytoplasm were seen in the SNc of mice treated with MPTP/p (Fig. 3). We found no TUNEL-positive cells until 8 injections of MPTP/p had been completed and in these mice we found an average of two TUNEL-positive cells. At 3 days after completed of the MPTP/p regimen, there were between 8 and 14 TUNEL-positive cells/per MPTP/p-treated mouse, which is less than 1% of the total number of neurons in the SNc (Table 1). The cells were primarily located in the ventral tier and at mid to caudal levels of the SNc. No TUNEL-positive cells were seen at the rostral end of the SNc.
Fig. 3.
(A–B) Typical TUNEL-positive neurons in the SNc 3 days after MPTP/p (N = 5) treatment. No TUNEL-positive neurons were present in the SNc of probenecid (N = 5) or saline (N = 5) controls. Sections were counterstained with cresyl violet. Scale bar in (A) equals 10µm and is valid for (B).
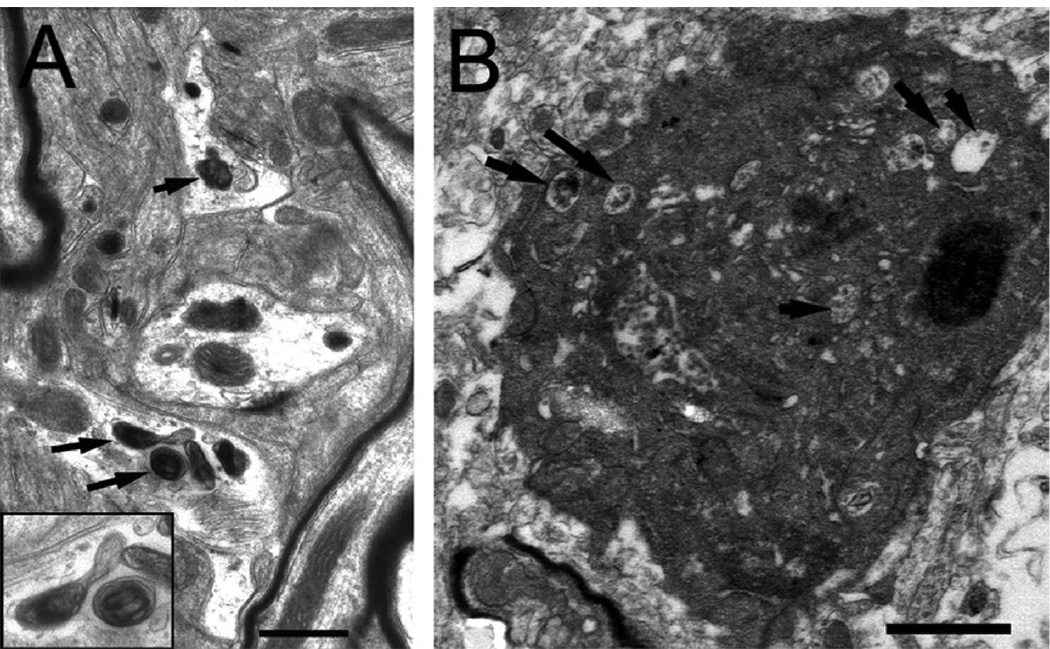
Qualitative, ultrastructural examination revealed prominent, organelle- and proteinaceous- filled secondary lysosomes in the SNc of MPTP/p-, but not vehicle-treated, mice. Although rare, autophagosomes were detected by their double membrane structure surrounding proteinaceous deposits and lipid in all MPTP/p-treated mice at 1-week post treatment (Fig. 4A). Autophagic cell death, however, was found in the SNc of one mouse at 3 weeks post-MPTP/p treatment (Fig. 4B). No apoptotic nuclei were observed with the EM, but the SN was only studied at one time point, when others have shown that the number of apoptotic profiles have severely declined (Novikova et al., 2006).
Fig. 4.
Neuronal signs of autophagy in the SNc of mice treated with MPTP/p (N = 5). (A) small dendrites with autophagic vacuoles (arrows). In the lower left corner is an inset showing the two vacuoles at a higher power. (B) SNc cell showing signs of autophagic cell death including vacuolization of the cytoplasm (arrows). Scale bar in (A) equals 0.5µm and in (B) equals 1.0µm.
Discussion
This study is the first to demonstrate a significant increase in the extracellular level of glutamate and a change in the affinity of glutamate for its transporters in the SN of MPTP/p-treated mice. Both apoptotic and autophagic cell death accompany this elevation in glutamate, indicating that these programmed forms of death may be triggered by excitotoxicity. Such novel observations could be relevant to understanding cell death in this model of PD.
Origin of extracellular glutamate and role of the transporters
It is important to question whether the extracellular glutamate that we measured in the SN is derived from the Ca2+ dependent vesicular pool, Ca2+ independent, cytoplasmic pool associated with the glutamate/cystine antiporter, or glial pool (Timmerman and Westerink, 1997; Baker et al., 2002). We and others have reported that about 30% of extracellular glutamate is Ca2+-dependent (Xue et al., 1996; Baker et al., 2002; Meshul et al., 2002) and that over 60% of the K+-depolarized extracellular level of glutamate is Ca2+ dependent (Meshul et al., 1999), suggesting a role for the synaptic vesicle pool within the nerve terminal. Replacement of calcium with the divalent chelating agent, EGTA and increasing the aCSF concentration of magnesium results in a decrease (25%) in the basal level of glutamate, suggesting that a significant proportion of the resting level of glutamate is of neuronal, and not, glial origin (Meshul et al., 2002). Moreover, 60% of the basal extracellular level of glutamate is due to exchange with the glutamate/cystine antiporter, which is Ca2+-insensitive but exchanges glutamate from the cytoplasm of the nerve terminal (Baker et al., 2002). However, the relevance and contribution of the glutamate-cystine antiporter remains controversial (Cavelier and Attwell, 2005).
The neuronal source of glutamate measured in the present study is not known, but it could come from axon terminals in the SN provided by the subthalamic, cortical and/or pedunculopontine nuclei (Bevan et al., 2002). The effect of l-dopa on extracellular glutamate levels (present results) could be due to a direct affect of dopamine on glutamate release. It has been reported that striatal glutamate nerve terminals contain dopamine D-2 receptors (Sesack et al., 1994) and activation of these receptors by dopamine agonists or their inhibition by dopamine antagonists can decrease or increase glutamate release, respectively (Calabresi et al., 1992; Yamamoto and Davy, 1992; Bamford et al., 2004). We have reported that systemic administration of l-dopa results in a decrease in the basal extracellular levels of striatal glutamate compared to the vehicle-treated group in nigrostriatal lesioned animals (Holmer et al., 2005). Our present results suggest that acute administration of l-dopa in the MPTP/p group led to an increase in tissue levels of dopamine, as reported for the dopamine-deficient mouse (Bamford et al., 2004). This increase in either extracellular dopamine or enhanced dopamine release could negatively feed back onto glutamate nerve terminals, resulting in a decrease in extracellular striatal glutamate (Fig. 2). No such increase in dopamine tissue levels presumably occurs in the control groups. It has also been reported that either local infusion (in the SN) or systemic administration of a dopamine D-2 antagonist results in augmentation of extracellular glutamate within the SN (Hatzipetros and Yamamoto, 2006; Hatzipetros et al., 2007). Local infusion of the dopamine D-2 agonist, quinpirole, attenuates this increase in glutamate.
Interestingly, our results show that the affinity of glutamate transporters for their substrate increases after MPTP/p treatment without changing the rate of transport. This increase could be due to a compensatory mechanism that blocks putative desensitization of glutamate receptors (Nieoullon et al., 1982). We would therefore expect that glutamate uptake would increase and lower extracellular levels of the excitotoxin. However, glutamate levels in this model are elevated at the same time that the affinity for the excitatory amino acid is raised. An alternative interpretation could be that the increase in extracellular glutamate is due to the reversal of the glutamate transporter (Kaneko et al., 2002), increased release through a transporter-independent mechanism via the astrocytes (Ohta et al., 2002) and/or cytoplasmic release via the cystine-glutamate antiporter (Baker et al., 2002). Enhancements in extracellular glutamate following a nigrostriatal lesion could therefore involve a combination of nerve terminal and transporter- independent and dependent release, with the increased affinity of the transporters to limit the diffusion of excess glutamate.
Glutamate-induced excitotoxicity and cell death
It is well known that free radicals, generated by increases in excitotoxins such as glutamate, contribute to the cytosolic changes leading to delayed types of programmed cell death, i.e. apoptosis (type I) or autophagy (type II; for review, see (Rubinsztein et al., 2005). When neurons are stressed, damage to complex I in the mitochondria lowers vital energy levels, alters membrane potentials, and cells lose their ability to sequester or pump out Ca2+ (Perier et al., 2005). Cellular stress also leads to a loss of integrity of mitochondrial membranes, resulting in their depolarization (Chinopoulos et al., 1999). Oxidative radicals released by the mitochondria serve as important signaling molecules that trigger increases in inflammation and apoptosis, i.e. cell death initiated by mitochondrial swelling and the release of cytochrome c (Perier et al., 2005).
In the present study, we employed an assay of apoptosis in the SN of MPTP/p and vehicle-treated mice at different time points during the treatment regimen and shortly thereafter. The assay we used was based on the principle of in situ DNA nick-end labeling technique (TUNEL), in which terminal deoxynucleotidyl transferase catalyzes the incorporation of biotinylated deoxyuridines onto the free 3’-hydroxyl termini of DNA breaks (Arends et al., 1990). Although a useful technique, positive staining with this assay cannot be regarded as irrefutable evidence of apoptotic cell death (Bicknell and Cohen, 1995; Grasl-Kraupp et al., 1995). Nevertheless, It seems reasonable that our results demonstrate apoptosis. Even if some cells have died of necrosis, our data support the findings of apoptosis by others studying the same model at the same time period after treatment (Novikova et al., 2006). Indeed, the number of cells with apoptotic nuclei surge one day after MPTP/p treatment, a peak that is followed by a steady decline in these profiles in the first week after treatment (Novikova et al., 2006). Thus, apoptosis may prevail when maintenance of mitochondrial and other normal cytosolic functions can no longer be supported (Kazuno et al., 2006). We have also found a second type of programmed death (type II: autophagic cell death), in the MPTP/p model. Initially, autophagic processes are presumably neuroprotective, perhaps even preventing apoptotic cell death. This is because autophagy actively recycles damaged proteins and organelles, such as mitochondria (Kiffin et al., 2004, Komatsu et al., 2007). Our study detected autophagosomes a week after MPTP/p treatment and autophagic cell death at 3 weeks post-treatment. Although this type of cell death was only seen in one MPTP/p-treated mouse, it may be linked to the proteinaceous inclusions we reported earlier (Meredith et al., 2002). Autophagy has been associated with abnormal protein accumulations and the engulfing of damaged mitochondria in PD (Zhu et al., 2003, Butler et al., 2005), and human postmortem studies report both apoptosis and autophagy in the melanin-containing neurons in the SN (Anglade et al., 1997). In the MPTP/p model, two fundamentally different types of programmed cell death, each of which is induced by mitochondrial stress (Rubinsztein et al., 2005), are associated with strong elevations of a potent stressor, glutamate.
Acknowledgments
The authors thank Drs. Adrian Dervan, Mimi Mo and Modjgan Keyghobadi for their technical assistance. This work was supported by an USPHS grant, NS41799 (to GEM and ST) and the Department of Veterans Affairs Merit Review program (to CKM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alirezaei M, Kiosses WB, Flynn CT, Brady NR, Fox HS. Disruption of neuronal autophagy by infected microglia results in neurodegeneration. PLoS ONE. 2008;3:e2906. doi: 10.1371/journal.pone.0002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anglade P, Vyas S, Javoy-Agid F, Herrero MT, Michel PP, Marquez J, Mouatt-Prigent A, Ruberg M, Hirsch EC, Agid Y. Apoptosis and autophagy in nigral neurons of patients with Parkinson's disease. Histol. Histopathol. 1997;12:25–31. [PubMed] [Google Scholar]
- Arends MJ, Morris RG, Wyllie AH. Apoptosis. The role of the endonuclease. Am. J. Pathol. 1990;136:593–608. [PMC free article] [PubMed] [Google Scholar]
- Baker DA, Xi ZX, Shen H, Swanson CJ, Kalivas PW. The origin and neuronal function of in vivo nonsynaptic glutamate. J. Neurosci. 2002;22:9134–9141. doi: 10.1523/JNEUROSCI.22-20-09134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J. Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevan MD, Magill PJ, Terman D, Bolam JP, Wilson CJ. Move to the rhythm: oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002;25:525–531. doi: 10.1016/s0166-2236(02)02235-x. [DOI] [PubMed] [Google Scholar]
- Bicknell GR, Cohen GM. Cleavage of DNA to large kilobase pair fragments occurs in some forms of necrosis as well as apoptosis. Biochem. Biophys. Res. Commun. 1995;207:40–47. doi: 10.1006/bbrc.1995.1150. [DOI] [PubMed] [Google Scholar]
- Blandini F, Porter RH, Greenamyre JT. Glutamate and Parkinson's disease. Mol. Neurobiol. 1996;12:73–94. doi: 10.1007/BF02740748. [DOI] [PubMed] [Google Scholar]
- Butler D, Brown QB, Chin DJ, Batey L, Karim S, Mutneja MS, Karanian DA, Bahr BA. Cellular responses to protein accumulation involve autophagy and lysosomal enzyme activation. Rejuvenation Res. 2005;8:227–237. doi: 10.1089/rej.2005.8.227. [DOI] [PubMed] [Google Scholar]
- Calabresi P, De Murtas M, Mercuri NB, Bernardi G. Chronic neuroleptic treatment: D2 dopamine receptor supersensitivity and striatal glutamatergic transmission. Ann. Neurol. 1992;31:366–373. doi: 10.1002/ana.410310404. [DOI] [PubMed] [Google Scholar]
- Cavelier P, Attwell D. Tonic release of glutamate by a DIDS-sensitive mechanism in rat hippocampal slices. J. Physiol. 2005;564:397–410. doi: 10.1113/jphysiol.2004.082131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. 'Rejuvenation' protects neurons in mouse models of Parkinson's disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Chinopoulos C, Tretter L, Adam-Vizi V. Depolarization of in situ mitochondria due to hydrogen peroxide-induced oxidative stress in nerve terminals: inhibition of alpha-ketoglutarate dehydrogenase. J. Neurochem. 1999;73:220–228. doi: 10.1046/j.1471-4159.1999.0730220.x. [DOI] [PubMed] [Google Scholar]
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305:1292–1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- Davies LP, Johnston GAR. Uptake and release of D- and L-aspartate by rat brain slices. J. Neurochem. 1976;26:1007–1014. doi: 10.1111/j.1471-4159.1976.tb06485.x. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Neuroprotective and neurorestorative strategies for Parkinson's disease. Nat. Neurosci. 2002;5 Suppl:1058–1061. doi: 10.1038/nn941. [DOI] [PubMed] [Google Scholar]
- Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson's disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- De Souza IE, McBean GJ, Meredith GE. Chronic haloperidol treatment impairs glutamate transport in the rat striatum. Eur. J. Pharmacol. 1999;382:139–142. doi: 10.1016/s0014-2999(99)00589-0. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Fyske EM, Iversen EG, Fonnum F. Inhibition of glutamate uptake into synaptic vesicles. Neurosci. Lett. 1992;135:125–128. doi: 10.1016/0304-3940(92)90151-v. [DOI] [PubMed] [Google Scholar]
- Grasl-Kraupp B, Ruttkay-Nedecky B, Koudelka H, Bukowska K, Bursch W, Schulte-Hermann R. In situ detection of fragmented DNA (TUNEL assay) fails to discriminate among apoptosis, necrosis, and autolytic cell death: a cautionary note. Hepatology. 1995;21:1465–1468. doi: 10.1002/hep.1840210534. [DOI] [PubMed] [Google Scholar]
- Hartley A, Stone JM, Heron C, Cooper JM, Schapira AH. Complex I inhibitors induce dose-dependent apoptosis in PC12 cells: relevance to Parkinson's disease. J. Neurochem. 1994;63:1987–1990. doi: 10.1046/j.1471-4159.1994.63051987.x. [DOI] [PubMed] [Google Scholar]
- Hatzipetros T, Raudensky JG, Soghomonian JJ, Yamamoto BK. Haloperidol treatment after high-dose methamphetamine administration is excitotoxic to GABA cells in the substantia nigra pars reticulata. J Neurosci. 2007;27:5895–5902. doi: 10.1523/JNEUROSCI.5260-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzipetros T, Yamamoto BK. Dopaminergic and GABAergic modulation of glutamate release from rat subthalamic nucleus efferents to the substantia nigra. Brain Res. 2006;1076:60–67. doi: 10.1016/j.brainres.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Holmer HK, Keyghobadi M, Moore C, Meshul CK. l-dopa-induced reversal in striatal glutamate following partial depletion of nigrostriatal dopamine with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine. Neuroscience. 2005;136:333–341. doi: 10.1016/j.neuroscience.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Hunot S, Hirsch EC. Neuroinflammatory processes in Parkinson's disease. Ann. Neurol. 2003;53 Suppl. 3:S49–S58. doi: 10.1002/ana.10481. discussion S58–60. [DOI] [PubMed] [Google Scholar]
- Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J. Comp. Neurol. 2002;444:39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- Kazuno AA, Munakata K, Nagai T, Shimozono S, Tanaka M, Yoneda M, Kato N, Miyawaki A, Kato T. Identification of mitochondrial DNA polymorphisms that alter mitochondrial matrix pH and intracellular calcium dynamics. PLoS Genet. 2006;2:e128. doi: 10.1371/journal.pgen.0020128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol. Biol. Cell. 2004;15:4829–4840. doi: 10.1091/mbc.E04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu M, Ueno T, Waguri S, Uchiyama Y, Kominami E, Tanaka K. Constitutive autophagy: vital role in clearance of unfavorable proteins in neurons. Cell Death Differ. 2007;14:887–894. doi: 10.1038/sj.cdd.4402120. [DOI] [PubMed] [Google Scholar]
- Lau YS, Trobough KL, Crampton JM, Wilson JA. Effects of probenecid on striatal dopamine depletion in acute and long-term 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated mice. Gen. Pharmacol. 1990;21:181–187. doi: 10.1016/0306-3623(90)90898-v. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Halliday GM, Totterdell S. A critical review of the development and importance of proteinaceous aggregates in animal models of Parkinson's disease: New insights into Lewy body formation. Parkinsonism Relat. Disord. 2004;10:191–202. doi: 10.1016/j.parkreldis.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Totterdell S, Petroske E, Santa Cruz K, Callison RC, Jr, Lau YS. Lysosomal malfunction accompanies alpha-synuclein aggregation in a progressive mouse model of Parkinson's disease. Brain Res. 2002;956:156–165. doi: 10.1016/s0006-8993(02)03514-x. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Kamel D, Moore C, Kay TS, Krentz L. Time-dependent changes in striatal glutamate synapses following a 6-OHDA lesion. Neuroscience. 1999;88:1–16. doi: 10.1016/s0306-4522(98)00189-4. [DOI] [PubMed] [Google Scholar]
- Meshul CK, Kamel D, Moore C, Kay TS, Krentz L. Nicotine alters striatal glutamate function and decreases the apomorphine-induced contralateral rotations in 6-OHDA lesioned rats. Exp. Neurol. 2002;175:257–274. doi: 10.1006/exnr.2002.7900. [DOI] [PubMed] [Google Scholar]
- Nicotra A, Parvez SH. Cell death induced by MPTP, a substrate for monoamine oxidase B. Toxicology. 2000;153:157–166. doi: 10.1016/s0300-483x(00)00311-5. [DOI] [PubMed] [Google Scholar]
- Nieoullon A, Kerkerian L, Dusticier N. Inhibitory effects of dopamine on high affinity glutamate uptake from rat striatum. Life Sci. 1982;30:1165–1172. doi: 10.1016/0024-3205(82)90658-0. [DOI] [PubMed] [Google Scholar]
- Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-D-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988;451:205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- Novikova L, Garris BL, Garris DR, Lau YS. Early signs of neuronal apoptosis in the substantia nigra pars compacta of the progressive neurodegenerative mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine/probenecid model of Parkinson's disease. Neuroscience. 2006;140:67–76. doi: 10.1016/j.neuroscience.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Ohta K, Nomura T, Kanno T, Nagai K, Yamamoto S, Yajima Y, Kondoh T, Kohmura E, Saito N, Nishizaki T. L-trans-PDC enhances hippocampal neuronal activity by stimulating glial glutamate release independently of blocking transporters. Biochem. Biophys. Res. Commun. 2002;295:376–381. doi: 10.1016/s0006-291x(02)00668-x. [DOI] [PubMed] [Google Scholar]
- Perier C, Tieu K, Guegan C, Caspersen C, Jackson-Lewis V, Carelli V, Martinuzzi A, Hirano M, Przedborski S, Vila M. Complex I deficiency primes Bax-dependent neuronal apoptosis through mitochondrial oxidative damage. Proc. Natl. Acad. Sci. U.S.A. 2005;102:19126–19131. doi: 10.1073/pnas.0508215102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petroske E, Meredith GE, Callen S, Totterdell S, Lau YS. Mouse model of Parkinsonism: a comparison between subacute MPTP and chronic MPTP/probenecid treatment. Neuroscience. 2001;106:589–601. doi: 10.1016/s0306-4522(01)00295-0. [DOI] [PubMed] [Google Scholar]
- Plaitakis A, Shashidharan P. Glutamate transport and metabolism in dopaminergic neurons of substantia nigra: implications for the pathogenesis of Parkinson's disease. J. Neurol. 2000;247 Suppl 2:II25–II35. doi: 10.1007/pl00007757. [DOI] [PubMed] [Google Scholar]
- Przedborski S, Tieu K, Perier C, Vila M. MPTP as a mitochondrial neurotoxic model of Parkinson's disease. J. Bioenerg. Biomembr. 2004;36:375–379. doi: 10.1023/B:JOBB.0000041771.66775.d5. [DOI] [PubMed] [Google Scholar]
- Rubinsztein DC, DiFiglia M, Heintz N, Nixon RA, Qin ZH, Ravikumar B, Stefanis L, Tolkovsky A. Autophagy and its possible roles in nervous system diseases, damage and repair. Autophagy. 2005;1:11–22. doi: 10.4161/auto.1.1.1513. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial complex I deficiency in Parkinson's disease. Adv. Neurol. 1993;60:288–291. [PubMed] [Google Scholar]
- Sesack SR, Aoki C, Pickel VM. Ultrastructural localization of D2 receptor-like immunoreactivity in midbrain dopamine neurons and their striatal targets. J Neurosci. 1994;14:88–106. doi: 10.1523/JNEUROSCI.14-01-00088.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ID, Grace AA. Role of the subthalamic nucleus in the regulation of nigral dopamine neuron activity. Synapse. 1992;12:287–303. doi: 10.1002/syn.890120406. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J. Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Takacs-Vellai K, Bayci A, Vellai T. Autophagy in neuronal cell loss: a road to death. Bioessays. 2006;28:1126–1131. doi: 10.1002/bies.20489. [DOI] [PubMed] [Google Scholar]
- Tillerson JL, Miller GW. Grid performance test to measure behavioral impairment in the MPTP-treated-mouse model of parkinsonism. J. Neurosci. Meth. 2003;123:189–200. doi: 10.1016/s0165-0270(02)00360-6. [DOI] [PubMed] [Google Scholar]
- Timmerman W, Westerink BH. Brain microdialysis of GABA and glutamate: what does it signify? Synapse. 1997;27:242–261. doi: 10.1002/(SICI)1098-2396(199711)27:3<242::AID-SYN9>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Xue CJ, Ng JP, Li Y, Wolf ME. Acute and repeated systemic amphetamine administration: effects on extracellular glutamate, aspartate, and serine levels in rat ventral tegmental area and nucleus accumbens. J. Neurochem. 1996;67:352–363. doi: 10.1046/j.1471-4159.1996.67010352.x. [DOI] [PubMed] [Google Scholar]
- Yamamoto BK, Davy S. Dopaminergic modulation of glutamate release in striatum as measured by microdialysis. J. Neurochem. 1992;58:1736–1742. doi: 10.1111/j.1471-4159.1992.tb10048.x. [DOI] [PubMed] [Google Scholar]
- Yue Z, Horton A, Bravin M, DeJager PL, Selimi F, Heintz N. A novel protein complex linking the delta 2 glutamate receptor and autophagy: implications for neurodegeneration in lurcher mice. Neuron. 2002;35:921–933. doi: 10.1016/s0896-6273(02)00861-9. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Guo F, Shelburne J, Watkins S, Chu CT. Localization of phosphorylated ERK/MAP kinases to mitochondria and autophagosomes in Lewy body diseases. Brain Pathol. 2003;13:473–481. doi: 10.1111/j.1750-3639.2003.tb00478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]