Abstract
Aims
The prospect of weight gain discourages many cigarette smokers from quitting. Practice guidelines offer varied advice about managing weight gain after quitting smoking, but no systematic review and meta-analysis have been available. We reviewed evidence to determine whether behavioral weight control intervention compromises smoking cessation attempts, and if it offers an effective way to reduce post-cessation weight gain.
Methods
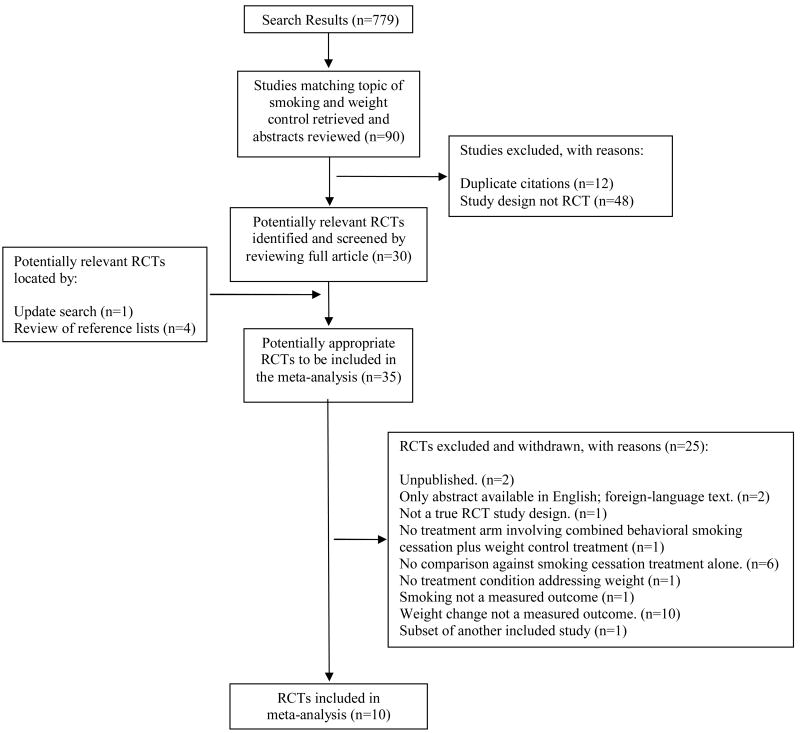
We identified randomized controlled trials that compared combined smoking treatment and behavioral weight control to smoking treatment alone for adult smokers. English-language studies were identified through searches of PubMed, Ovid MEDLINE, CINAHL, EMBASE, PsycINFO, Cochrane Central Register of Controlled Trials. Of 779 articles identified and 35 potentially relevant RCTs screened, 10 met criteria and were included in the meta-analysis.
Results
Patients who received both smoking treatment and weight treatment showed increased abstinence (OR=1.29, 95% CI=1.01,1.64) and reduced weight gain (g = -0.30, 95% CI=-0.63, -0.04) in the short term (<3 months) compared with patients who received smoking treatment alone. Differences in abstinence (OR=1.23, 95% CI=0.85, 1.79) and weight control (g= -0.17, 95% CI=-0.42, 0.07) were no longer significant in the long term (>6 months).
Conclusions
Findings provide no evidence that combining smoking treatment and behavioral weight control produces any harm and significant evidence of short-term benefit for both abstinence and weight control. However, the absence of long-term enhancement of either smoking cessation or weight control by the time-limited interventions studied to date provides insufficient basis to recommend societal expenditures on weight gain prevention treatment for patients who are quitting smoking.
Keywords: smoking cessation, weight gain, meta-analysis, systematic review
Introduction
Cigarette smoking remains the leading cause of preventable death in the world today [1, 2]. But fear of weight gain is a major barrier to smoking cessation [3-5]: 50% of female and 26% of male smokers state that concern about gaining weight discourages them from trying to quit smoking [6, 7]. Worries about weight gain also continue after quitting [8]. Most prospective epidemiological studies estimate that average post-cessation weight gain is between 3.0 to 5.5 kg [9, 10], although a few estimate double that amount [11]. Of adults who quit, 10% of men and 13% of women are at risk of major weight gain (>13 kg) [10].
The decreased mortality associated with smoking cessation almost always outweighs the health risk associated with weight gain [12]. However, the weight gain that follows smoking cessation does blunt several of the health benefits associated with quitting. Janzon et al observed that smoking cessation was associated with increased incidence of hypertension nine years after quitting, probably because weight gain was associated with elevated blood pressure [13]. Nilsson et al concluded that weight gain curtails a beneficial effect of cessation on glucose metabolism, consistent with the observation that quitting smoking increases waist circumference [8, 14]. Also, Chinn et al observed that post-cessation weight gain reduces the beneficial effect of quitting smoking on lung function [15]. Particularly against the backdrop of the global obesity epidemic [16], maximizing the health benefits due to smoking cessation requires finding a way to control weight gain.
Several pharmacotherapies (bupropion, varenicline, nicotine replacement) suppress post-cessation weight gain temporarily [17-19]. However, drug treatment only delays rather than prevents weight gain and is contraindicated for some medical comorbidities. Moreover, many patients prefer non-pharmacologic treatment [20-22].
Weight gain after quitting smoking results chiefly from increased dietary calorie intake and decreased metabolic rate [23, 24]. Accordingly, most behavioral interventions to minimize cessation-related weight gain endeavor to prevent positive energy balance. Primary strategies have been to limit calorie intake [25, 26], enhance energy expenditure via physical activity [27-31], or both [32-34]. Because foods and cigarettes may serve as substitutable reinforcers for smokers [35, 36], some dietary interventions preserve treat foods and implement only modest energy restriction [34]. A radically different approach involves cognitive intervention to minimize concern about weight [26]. We conducted a systematic review and meta-analysis to determine whether weight-related behavioral intervention offers an effective way to reduce post-cessation weight gain.
Methods
Data Sources and Systematic Searches
During January and February of 2007, one author (MB) searched the Cochrane Database of Systematic Reviews, the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Ovid MEDLINE, CINAHL, EMBASE, and PsycInfo. Appropriate controlled-vocabulary terms specific to each database and keyword searching within title and abstract fields were employed to retrieve meta-analyses, systematic reviews, and randomized controlled trials for behavioral interventions to promote smoking cessation and prevent weight gain. No date limits were placed on the searches. Articles in languages other than English were excluded, as were unpublished studies. To benefit from the quality control afforded by peer review, we did not search grey literature sources or unpublished conference abstracts, and did not obtain unpublished data from authors. The authors hand searched references from included studies and reviewed the list of included/excluded studies in a relevant Cochrane review [37]. An update search of literature databases was repeated, using the same methods with an added limit to retrieve only references published or added to the databases between January and August 2007. In July 2007 and again in November 2007, authors searched online for in-press articles appearing in journals that yielded included studies.
Study Selection
The study search protocol included randomized controlled trials and systematic reviews which compared behavioral interventions addressing both smoking cessation and weight gain prevention to behavioral interventions addressing solely smoking cessation. In accordance with systematic review methodology, explicit inclusion and exclusion criteria were established prior to the literature search and review process. Included studies were required to report data on both smoking cessation and weight gain outcomes and to incorporate a minimum of one month follow up. Studies addressing male or female adults (age 18-70) of any ethnic origin who self-identified as “regular” smokers were included. Behavioral interventions were operationalized as non-pharmacological, non-surgical treatments that either addressed both weight gain prevention and smoking cessation (in the intervention condition), or smoking cessation alone (in the comparison condition). Weight control interventions could act upon energy intake, energy expenditure, or attitudes about body weight. Energy intake interventions were those that aimed to modify eating behavior, for example by reducing calories, altering diet composition, or supplying meal replacements. Energy expenditure interventions aimed to increase physical activity or exercise. Other included interventions, such as cognitive-behavioral therapy (CBT) addressed weight concerns or eating attitudes. Studies involving nicotine replacement therapy (NRT) or other pharmacotherapies were included only if the identical drug treatment regimen was incorporated in both the behavioral intervention and control treatment arms.
Initial search of literature databases returned 779 journal articles. Two authors (BS and DH) independently evaluated studies for inclusion by reviewing the titles and abstracts. Ninety studies were judged to be eligible by at least one rater. After duplicate reports of the same research were removed, 78 unique studies remained. Full texts were obtained for all 78 of these studies. BS and DH reviewed the articles in detail and independently evaluated the studies for inclusion; differences were reconciled by consensus. After removing studies that were not RCTs, 30 studies remained. One additional study [38] was identified during the update search, and four additional studies were identified through the search of reference lists [28, 39-41]. No additional studies were identified through hand-searching. More discriminating full text review of the 35 candidate studies for inclusion [25-34, 38-62] decreased the yield to 10 studies (see Table 1, Included Studies) [25-34, 61]. A one-year follow-up to one of the included studies [61] was published in December 2007 [31], and the additional time point was added to the analysis. A Table of Excluded Studies, outlining reasons for exclusion of the remaining 25 studies, can be obtained from the first author upon request. The complete study selection process is shown in figure 1.
Table 1. Studies Included in Meta-Analysis [27, 29-38].
| Study | Sample | Intervention(s) | Outcome Measures | Hypothesized Best/Worst Treatment Arms | PEDRO Scale Quality Rating | |
|---|---|---|---|---|---|---|
| 9 Items* | Composite Score† | |||||
| Danielsson, et al. (1999) [25] |
N=287 Age: 30-60; m=46.9 (sd=7.0) Gender: 100% Female Ethnicity: not described Fagerstrom m=5.9 (sd=2.0) # cigarettes m=19.5 (sd=6.3) BMI m=26.8 (sd=2.3) Weight concerned? Yes |
Smoking + Weight Intervention: (N=137) Group behavioral cessation counseling + NRT§ (gum) + plus intermittent VLCD‡ meal replacement Smoking Control: (N=150) Group behavioral cessation counseling + NRT (gum) |
Smoking measures: CO-verified‖ continuous abstinence @ 16 & 52 weeks; Weight measures: Weight change in those continuously abstinent @ 12 weeks, in all randomized @ 52 weeks. |
Best: Smoking + Weight Intervention Worst: Smoking Control |
1, 1, ?, 1, ?, 0, 1, 1, 1 | 6 |
| Hall, et al. (1992) [32] |
N = 158 Age: m=40.36 (sd=8.85) Gender: 115 Female, 43 Male Ethnicity: 9.49% minority Fagerstrom: not reported # cigarettes m=27.44 (sd=11.73) Weight m=69.85 (sd=14.43) Weight concerned? No |
Innovative Smoking plus weight treatment: (N=53) Intensive group behavioral smoking cessation counseling + daily weight monitoring, individualized exercise and behavioral self-management Non-Specific Smoking plus weight treatment: (N=51) Intensive group behavioral smoking cessation counseling + weight gain prevention group Control: (N=54) Intensive group behavioral smoking cessation counseling. |
Smoking measures: CO-verified 7 day PP¶ abstinence @ 12 weeks; Cotinine verified PP abstinence @ 52 weeks Weight measures: All randomized weight change @ 6 weeks and 52 weeks |
Best: Innovative Smoking plus weight treatment Worst: Control |
1, 1, ?, 0, ?, 1, 1, 1, 1 | 6 |
| Marcus, et al. (1991) [28] |
N=20 Age: 20-50 (m=39, sd=13.6) Gender: 100 % Female Ethnicity: Not reported Fagerstrom: not reported # cigarettes m=28 (sd=18.4) Weight concerned? No |
Intervention: (N=10) Intensive group behavioral smoking cessation counseling + supervised exercise Control: (N=10) Intensive group behavioral smoking cessation counseling |
Smoking measures: Cotinine-verified 7 day PP @ 12 and 52 weeks Weight measures: All randomized weight change @ 15 weeks |
Best: Intervention Worst: Control |
1, 1, ?, 1, ?, 1, 1, 1, 1 | 7 |
| Marcus, et al. (1995) [29] |
N= 20 Age: 22-56 (m=37.5, sd=14.5) Gender: 100 % Female Ethnicity: Not reported Fagerstrom: not reported # cigarettes m=23 (sd=14.7) Weight concerned? No |
Intervention: (N=10) Intensive group behavioral smoking cessation counseling + supervised exercise Control: (N=10) Intensive group behavioral smoking cessation counseling |
Smoking measures: Cotinine-verified 7 day PP @ 12 and 52 weeks Weight measures: All randomized weight change @ 15 weeks |
Best: Intervention Worst: Control |
1, 1, ?, 0, ?, 0, 1, 1, 1 | 5 |
| Marcus, et al., (1999) [27] |
N = 281 Age: 18-65 (m=40.2, sd=8.9) Gender: 100% Female Ethnicity: Not reported Fagerstrom: m=6.3 (sd=1.9) # cigarettes m=22.4 (sd=9.4) BMI: m=25.4 (sd=5.0) Weight concerned? No |
Smoking + Weight Intervention: (N=134) Intensive group cognitive behavioral smoking cessation counseling + tailored exercise sessions Smoking Control: (N=147) Intensive group cognitive behavioral smoking cessation counseling + wellness sessions |
Smoking measures: Cotinine-verified continuous abstinence @ 8 and 60 weeks Weight measures: All randomized weight change @ 8 and 60 weeks |
Best: Smoking + Weight Intervention Worst: Smoking Control |
1, 1, 1, 0, ?, 0, 1, 1, 1 | 6 |
| Marcus, et al. (2005) [30] |
N = 217 Age: range 18-65; m=42.77 (sd=10.36) Gender: 100% Female Ethnicity: 82.5% White, 6.9% Black, 6.0% Hispanic, 4.6% Other minority Fagerstrom: m=4.85 (sd=2.55) # cigarettes m=20.60 (sd=9.36) BMI: m=26.22 (sd=5.57) Weight concerned? No |
Smoking + Weight Intervention: (N=109) Intensive group cognitive-behavioral smoking cessation counseling + supervised group and home-based moderate-intensity exercise Smoking Control: (N= 108) Intensive group cognitive behavioral smoking cessation counseling |
Smoking measures: Cotinine-verified continuous abstinence @ 8 and 60 weeks Weight measures: All randomized weight change @ 8 weeks |
Best: Smoking + Weight Intervention Worst: Smoking Control |
1, 1, ?, 1, 0, 1, 1, 1, 1 | 7 |
| Perkins, et al. (2001) [26] |
N=219 Age: range 18-65 Gender: 100% Female Ethnicity: not reported Fagerstrom (FTQ) m=5.0 (sd=2.1) Cigarettes/day: m=21.7 (sd=9.4) BMI m=25.6 (sd=4.9) Weight concerned? Yes |
Smoking + CBT**for Weight Concerns: (n=72) Intensive group cognitive behavioral smoking cessation counseling + CBT to reduce weight concerns (dieting discouraged) Smoking + Behavioral Weight Control: (n=72) Intensive group cognitive behavioral smoking cessation counseling + behavioral weight control counseling Smoking Control: (n=75) Intensive group cognitive behavioral smoking cessation counseling + non-specific social support |
Smoking measures: CO-verified continuous abstinence since quit day @ 12 weeks and 52 weeks Weight measures: weight change in those continuously abstinent from baseline @ 12 weeks and 52 weeks |
Best: Smoking + CBT for Weight Concerns Worst: Smoking Control |
1, 1, 0, 1, ?, 1, 1, 1, 1 | 7 |
| Pirie, et al. (1992) [33] |
N=417 Age: range 20-64 m=43.2 (sd=9.3) Gender: 100% Female Ethnicity: not reported Fagerstrom: not reported Cigarettes/day: m=26.2 (sd=10.8) BMI: m=24.1 (sd=3.4) Weight Concerned? No |
Smoking + NRT + Behavioral Weight Control: (N=98) Group cognitive behavioral smoking cessation counseling + 2mg. nicotine gum + behavioral weight control counseling Smoking + Behavioral Weight Control: (N=108) Group cognitive behavioral smoking cessation counseling + behavioral weight control counseling Smoking + NRT: (N=108) Group cognitive behavioral smoking cessation counseling + 2mg. nicotine gum Smoking Control: (N=103) Group cognitive behavioral smoking cessation counseling |
Smoking measures: Bioverified†† continuous abstinence from quit day @ 4 weeks post quit and 52 weeks Weight measures: weight change in those continuously abstinent from baseline @ 8 weeks and 52 weeks‡‡ |
Best: Smoking + NRT + Behavioral Weight Control Worst: Smoking Control |
1, 1, ?, 1, ?, 1, 0, 1, 1 | 6 |
| Spring, et al. (2004) [34] |
N=315 Age: range 20-75 m=42.69 (sd=10.30) Gender: 100% Female Ethnicity: 66% Caucasian, 31% African American, 3% other Fagerstrom (FTQ): m=5.95 (sd=1.97) Cigarettes/day: m=20.40 (sd=9.47) BMI: m=27.43 (sd=5.46) Weight Concerned? No |
Smoking + Late Behavioral Weight Control: (N=104) Intensive group cognitive behavioral smoking counseling + weight control treatment final 8 weeks (prepackaged meal plan, physical activity program) Smoking + Early Behavioral Weight Control: (N=104) Intensive group cognitive behavioral smoking counseling + weight control treatment first 8 weeks (prepackaged meal plan, physical activity program) Smoking Control: (N=107) Intensive group cognitive behavioral smoking counseling |
Smoking measures: CO-verified 7 day PP @ 12 weeks and 36 weeks Weight measures: All randomized absolute weight change @ 12 weeks and 36 weeks |
Best: Smoking + Late Behavioral Weight Control Worst: Smoking Control |
1, 1, 1, 1, 0, 1, 1, 1, 1 | 8 |
| Ussher, et al. (2003, 2007) [61, 31] |
N=299 Age: range 18-65 m=42.9 (sd=11.1) Gender: 63% Female Ethnicity: 88% White Fagerstrom (FTND): m=5.6 (sd=2.1) Cigarettes/day: m=21.9 (sd=9.0) BMI: m=25.6 (sd=4.6) Weight Concerned? No |
Smoking + NRT + Exercise Counseling: (N=154) individual brief cognitive behavioral smoking cessation counseling + 15mg. 16hr. transdermal nicotine patch + brief exercise counseling Smoking + NRT + Health Education Counseling: (N=145) individual brief cognitive behavioral smoking cessation counseling + 15mg. 16hr. transdermal nicotine patch + brief health education counseling |
Smoking measures: CO-verified continuous abstinence @ 6 weeks and 52 weeks Weight measures: Weight change from baseline in those continuously abstinent @ 6 weeks and 52 weeks |
Best: Smoking + NRT + Exercise Counseling Worst: Smoking + NRT + Health Education Counseling |
1, 1, ?, 1, 0, 1, 1, 1, 1 | 7 |
| Total | N=2233 | |||||
PEDro quality rating items: (1) eligibility criteria were specified; (2) participants randomly allocated to groups; (3) allocation concealed; (4) groups similar at baseline on main prognostic signs; (5) blinding of assessors who measured at least one key outcome; (6) adequacy of follow-up; (7) intent to treat analysis; (8) between group statistical comparison of outcomes; (9) study gives both point estimates and variability for an outcome [64]. A score of 1=meets criteria, 0=does not meet criteria, ?=unclear from manuscript whether study meets criteria or not. Two PEDro items regarding blinding of subjects and blinding of treatment providers were not scored, as blinding is not feasible in this type of behavioral intervention study.
Composite Score from PEDro quality ratings (range = 0-9)
NRT = nicotine replacement therapy
VLCD = very low calorie diet
CO-verified = bioverified via smokelyzer measurement of carbon monoxide
PP abstinence = point prevalence abstinence
CBT = Cognitive Behavioral Therapy
Bioverification is CO and/or cotinine in non-NRT; CO and/or thiocyanate in NRT groups
Short term weight gain sample limited to those participants who remained continuously abstinent for the 52 weeks of the protocol.
Figure 1. Study Flow Diagram.
Inter-rater reliability was calculated during the title/abstract review phase. Thirty nine studies were categorized as eligible by both reviewers, and 689 studies were categorized as ineligible by both reviewers. The proportion of agreement (percent concordant) between the two authors was 93% (728 out of 779; 95% CI, 92% to 95%).
Data Extraction and Quality Assessment
Data from the ten studies were extracted using a data extraction form. Extraction was checked by two authors, and discrepancies were resolved by consensus among four authors (BS, BH, DH, HGM). The sample characteristics extracted were: study quality, sample size, age, gender, ethnicity, number of cigarettes/day, Fagerstrom score [63], BMI, weight, and weight concern. Smoking cessation outcome was classified as either short-(≤ 3 months) or long-term (≥6 months) based on recommendations made by the Society for Research on Nicotine and Tobacco Subcommittee on Abstinence Measures [64]. If a study did not provide a short-term data point, we extended the time criterion up to 4 months in order to capture one. For the long-term outcome, we chose the latest follow-up assessment after six months that was available. The same procedure was followed for the classification of weight gain outcomes. To maintain the assumption of independent sets of effect sizes, we included only one effect size estimate from each study for each category of outcome. When a study offered more than one assessment that met our criteria for either short-(≤ 3 months) or long-term cessation (≥ 6 months), we selected the latest assessment time point that fit within our definition. The same procedure was followed for selection of weight outcomes.
For smoking, abstinence status was coded as continuous (abstinence between initial quitting and a follow-up time point) if available, or as 7 day point-prevalence (abstinence during the week prior to assessment). If both continuous and point prevalence abstinence were available, we analyzed the former as it affords the more rigorously defined measure of abstinence. In all cases, abstinence status was measured using the combination of self-report of smoking and biochemical verification. Weight gain outcome was coded according to the sample on which it was based: either all participants randomized to treatment or abstainers only. If both all randomized and abstainers only weight gain were available, we analyzed the all randomized as the more comprehensively representative outcome of the sample. Standard deviation was selected as the unit of distribution dispersal measurement and kg was selected as the unit of weight for between-study analytic comparison. In instances where included studies reported different units (e.g., confidence interval, pounds), the reported units were converted for uniformity prior to analysis.
Quality of studies was assessed using the validated PEDro scale [65]. This scale was developed using physiotheraphy studies and, accordingly, acknowledges that blinding is important, but not always feasible. Conducive to scoring of behavioral interventions, the scale rewards studies that cannot be double-blinded due to the study question but otherwise have a high internal validity [66, 67]. Two items from the PEDro Scale regarding blinding of subjects and therapists were not scored, as these were not feasible given the interventions studied.
Data Synthesis and Meta-Analysis
Study effects were combined using the Comprehensive Meta-analysis software version 2.2.046 [68]. When a study had multiple smoking/weight intervention or control groups, the effect size reflects the difference in outcome (either smoking abstinence or weight gain) between the pooled experimental arms and the pooled control arms. For the dichotomous outcome of smoking cessation, the effect is measured by the odds ratio, which is the odds of cessation in the intervention group divided by the odds of cessation in the control group. Odds ratios greater than 1 favor the intervention group. For the continuous measure of weight gain, the effect is measured by Hedge's g, which is the treatment minus control difference in group means divided by the pooled within-group standard deviation [69]. Negative values of g favor the intervention group. For each analysis, the combined effect size (odds ratio or g) is reported together with a two-sided 95% confidence interval, a z statistic for the test that the combined effect is zero, and a 2-tailed p-value for this test. Forest plots are included with each analysis, where squares represent individual studies, the size of the square represents the study size, and a diamond represents the combined effect.
Because tests of homogeneity were done and found to be statistically significant for some outcomes, the random effects model was used [70-72]. This model takes into account the between study variability in estimating the standard error of the outcome measures and enables generalization beyond the observed datasets. Heterogeneity was explored by performing sensitivity analyses for short- and long-term outcomes. Similar analyses examined treatment effects on smoking cessation separately for studies that evaluated continuous abstinence and for those that evaluated point prevalence to see if results differed. Subgroup analyses were also performed for studies that evaluated weight gain for all randomized participants and for those that analyzed abstainers only. Meta-regression was conducted to examine associations between treatment effects and study quality.
Funnel plots [73] of odds ratio and standardized Hedge's g against standard error were used to assess ascertainment (i.e., publication) bias. Ascertainment bias would be evidenced by asymmetry in the funnel plot. Weighted linear regression-based [74] methods were used to test the asymmetry of funnel plots. The method of Rosenthal [75] was used to estimate the number of unpublished studies (fail-safe N) that would be required to turn an overall significant effect into a nonsignificant effect. PEDro scales were correlated with effect sizes for all outcomes using the Spearman correlation coefficient.
Results
Experimental Versus Control Treatments
Smoking Cessation
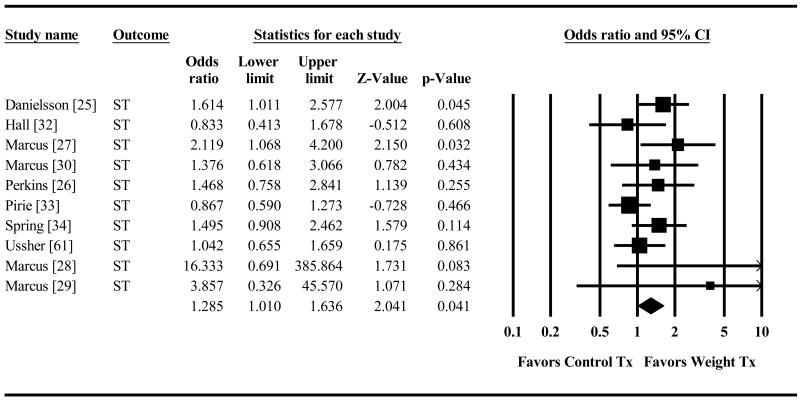
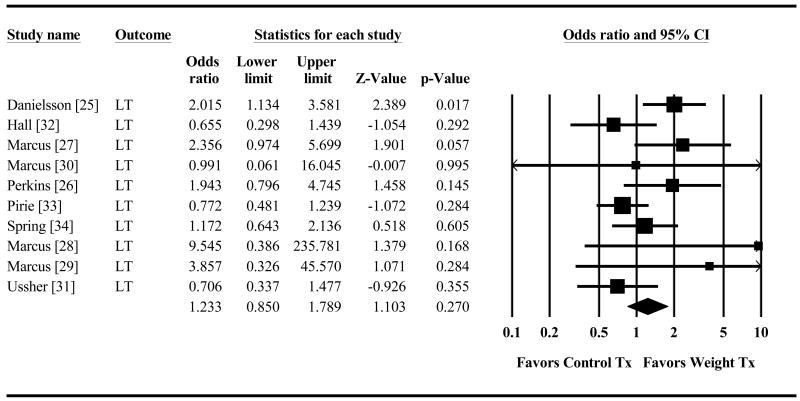
Combined smoking plus weight treatment produced significantly higher short-term abstinence (OR=1.29, 95% CI=1.01,1.64 p=.041, Figure 2) than did smoking treatment alone. The weight intervention advantage was no longer significant for long-term abstinence (OR=1.23, 95% CI=0.85,1.79 p=.27, Figure 3). The direction of the effect size was similar across both types of abstinence measures (continuous abstinence (CA) and point prevalence (PP) for the short-term (CA OR=1.27, 95% CI=0.96,1.68 p=.088; PP OR=1.40, 95% CI=.72,2.75 p=.33) and the long-term outcomes (CA OR=1.31 95% CI=0.80,2.14 p=.29; PP OR=1.11 95% CI=.56,2.20 p=.76). (Raw data for all analyses is provided in the supporting information that accompanies this paper (see details at the end).
Figure 2. Effect of Control Treatment (Smoking Cessation Only) versus Weight Treatment (Smoking Cessation + Weight Control) on Odds of Short Term Smoking Cessation.
Diamond indicates overall short-term effect. The width of the diamond indicates the 95% confidence interval. The size of the square for each individual effect is proportional to the study's weight in the analysis.
Figure 3. Effect of Control Treatment (Smoking Cessation Only) versus Weight Treatment (Smoking Cessation + Weight Control) on Odds of Long Term Smoking Cessation.
Diamond indicates overall long-term effect. The width of the diamond indicates the 95% confidence interval. The size of the square for each individual effect is proportional to the study's weight in the analysis.
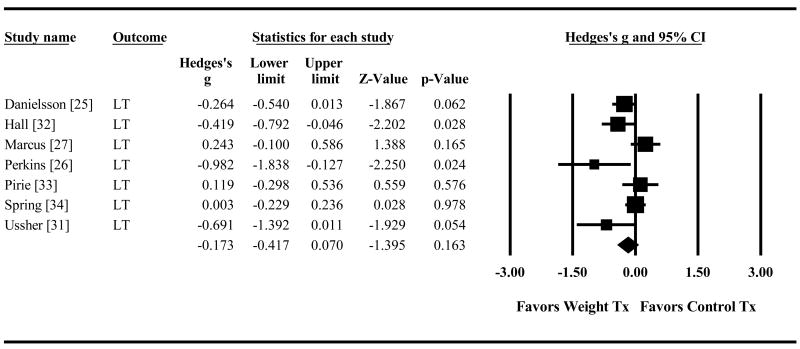
Post-cessation Weight Gain
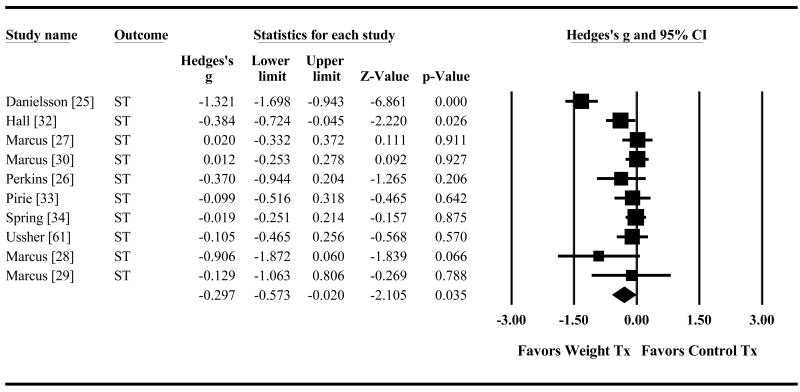
Combined smoking plus weight treatment also reduced short-term weight gain significantly compared to smoking treatment alone (g=-0.30 95% CI=-0.57,-0.02 p=.035, Figure 4). The advantage was no longer significant for long-term weight control (g=-0.17 95% CI=-0.42,0.07 p=.16, Figure 5). Direction of effects was similar regardless of whether weight gain was measured for all randomized participants (AR) or abstainers only (AO) for the short term (AR g=-0.11, 95% CI=-0.28,0.07 p=.24; AO g=-0.48, 95% CI=-1.10,0.15 p=.13) and the long-term (AR g=-0.10, 95% CI=-0.36,0.15 p=.43; AO g=-0.45, 95% CI=-1.16,0.27 p=.23).
Figure 4. Effect of Control Treatment (Smoking Cessation Only) versus Weight Treatment (Smoking Cessation + Weight Control) on Short-Term Post-Quit Weight Gain.
Diamond indicates overall short-term effect. The width of the diamond indicates the 95% confidence interval. The size of the square for each individual effect is proportional to the study's weight in the analysis.
Figure 5. Effect of Control Treatment (Smoking Cessation Only) versus Weight Treatment (Smoking Cessation + Weight Control) on Long-Term Post-Quit Weight Gain.
Diamond indicates overall long-term effect. The width of the diamond indicates the 95% confidence interval. The size of the square for each individual effect is proportional to the study's weight in the analysis.
Publication bias and quality scores
In the tests for publication bias, Egger's two-tailed p-values ranged from 0.06 to 0.33 for the four main analyses, giving evidence against the possibility that small negative studies may have been excluded. For the short term results that were statistically significant, the fail-safe N was 13 for smoking cessation and 38 for weight gain, indicating that many null studies would be required to reverse the statistical significance of these results.
PEDro scales ranged from 5 to 8 for the studies analyzed. There was no significant correlation between the PEDro scale and the effect sizes for Figures 2 through 5, with Spearman correlation coefficients ranging from -0.42 to 0.17 with p>0.35 for all correlations.
Discussion
Country-specific tobacco treatment guidelines differ in their clinical recommendations regarding management of post-cessation weight gain, and no quantitative systematic review of the evidence has previously been available. Despite the salience of weight gain for patients, the topic is not discussed in tobacco practice guidelines for England [76, 77], Scotland [78, 79]; and Northern Ireland [80]. Guidelines from New Zealand caution that attempting to achieve weight control simultaneously with smoking cessation could undermine tobacco abstinence. The New Zealand Ministry of Health advises that “Dieting at the same time as stopping smoking may increase the risk of relapse, therefore people should concentrate on achieving and maintaining abstinence from smoking first and then tackle the issue of weight gain” [81]. Similarly, the 2000 United States Public Health Services (USPHS) tobacco guideline recommended that patients concentrate primarily on smoking cessation, not weight control, until ex-smokers are confident that they will not return to smoking” [1]. The USPHS 2008 guideline update advises clinicians to help patients quit smoking first and then address weight gain either personally or by referral [82].
The concern that weight control efforts might undermine tobacco abstinence originated from an early clinical trial by Hall, Tunstall, Vila, and Duffy [30] that suggested detrimental effects of weight control efforts on smoking cessation outcome. Some subsequent theory [83] and evidence [34, 84] indicates that trying to change multiple behaviors simultaneously is difficult and can undermine efforts to change any single behavior. However, other evidence indicates that changing multiple behaviors simultaneously is feasible [85]. Results of this systematic review and meta-analysis do not confirm worries that adding behavioral weight control treatment to behavioral smoking cessation intervention undermines tobacco abstinence. On the contrary, the findings indicate that combined smoking cessation + weight control treatment, compared to smoking cessation treatment alone, enhances tobacco abstinence and also reduces post-cessation weight gain significantly in the short-term. Although combined smoking plus weight treatment still lacked any adverse effect on abstinence, the advantageous effect on weight control was no longer significant after six months.
Since the trends were for weight control treatment to produce positive long-term effects on both abstinence and weight control, we considered whether the study was adequately powered to detect meaningful long-term benefits. If the intra-study correlations in the outcome measures are taken into account then, using a two-tailed test with a Type I error rate of 5%, the cumulated sample had 80% power to detect an odds ratio advantage of 1.36 for short term smoking cessation and 1.60 for long-term abstinence. This translates into a difference in cessation rates of 32% in the control group versus 39% in the treatment group for short term abstinence and 16% versus 22% for long term abstinence. There was 80% power to detect a standardized mean difference of 0.16 for short-term weight control and 0.19 for long-term weight control. This translates into a mean short term weight gain of 1.42 kg in the control group versus 0.98 kg in the treatment group and a mean long term weight gain of 3.88 kg versus 3.21 kg. Therefore, power appears to have been adequate to detect long-term benefits on both smoking cessation and weight control.
The results did not suggest publication bias, nor was there evidence that results differed as a function of different methods of assessing abstinence (continuous versus point prevalence) or weight gain (all randomized participants versus continuously abstinent participants). Only half of the studies tested for differential attrition, but of those, four found no evidence of it (31, 33, 35, 36); one found greater retention for patients randomized to weight control treatment (34). Participants were randomized to the experimental (smoking + weight) or control (smoking only) conditions, limiting the likelihood that third variables account for observed benefits of adding weight control to smoking cessation treatment. Intervention and control conditions were reasonably well-matched on intensity and duration for most studies (see Table 1), making it unlikely that greater attention or support explained an advantage of combined smoking and weight treatment. Of the specific studies that showed significant benefit of combined treatment on short-term [25, 27] and long-term [25] abstinence, and short-term [25, 32] and long-term [26, 32] weight control, only one [32] extended greater therapist support to the combined treatment group.
We conclude that, rather than undermining either short- or long-term abstinence, adding weight control to smoking treatment improves both abstinence and weight control in the short-term. In the longer term and at the present stage of treatment development, adding weight control to smoking cessation treatment does not appear to produce either benefit or harm.
Most combined behavioral smoking and weight treatments tested to date have been unsuccessful at significantly suppressing long-term weight gain. Although several guidelines advise that exercise reduces post-cessation weight gain [81, 90, 91], we did not find any trial testing exercise alone as the weight control intervention that showed significantly reduced long-term, or even short-term weight gain. The two behavioral interventions that did produce significant long-term suppression of weight gain were very different from each other. One implemented a strict regimen of dieting, exercise, and daily weighing [32]; the other discouraged dieting and encouraged weight acceptance [26]. These two studies had no other unique commonalities that we could discern. Women in one study were weight-concerned [26]; those in the other study were not [32]. Both interventions entailed a group treatment format, but so did the interventions in all but one other trial [31]. The two trials were done in different decades and in different parts of the United States.
The finding of a significant intervention-related weight control benefit in short-term but not yet long-term follow-up parallels findings for weight control treatment more generally [86] and may suggest a need to extend the duration of treatment. The observed success at preventing short-term weight gain surpasses that obtained for weight gain prevention in children [87]. Disappointing results for long-term weight control parallel those for weight gain prevention among adult women [88-89]. Obesity intervention has seen more than a doubling of weight control benefit in three decades largely as a result of extending treatment duration from an average of 8 to 32 weeks [92]. The longest treatment duration in the present sample of studies was 16 weeks. Improvement in the management of recurrent disorders, such as depression, has been achieved by extending the duration of medication. Likewise, chronic vulnerability to behavioral problems like smoking or obesity may also require long-term intervention.
As is often the case, the conclusions that can be drawn from the present systematic review are limited by the relatively restricted number of high-quality randomized clinical trials available to address the study questions. Constraints on the availability of high quality primary research are a problem that characterizes the evidence base for many interventions, particularly behavioral ones [93-96]. Findings may only be able to be generalized to females, in view of the fact that 2079 participants in the included trials were females and only 154 were males. Conclusions may apply only to formal programs that promote smoking cessation and weight gain prevention, since it is not clear that the findings would extend to smokers attempting to quit and limit their weight gain on their own. The nature of the smoking cessation and weight control interventions, the timing of assessments, and the metrics for abstinence in the relevant studies were heterogeneous. To its credit, the tobacco research community has called for greater consistency in the conduct, analysis, and reporting of smoking cessation trials [64]. Increased availability of transparently and consistently reported trials [97] and research syntheses will help to advance evidence-based practice in smoking cessation with weight gain prevention.
Our appraisal of the research provides no suggestion that combining formal smoking treatment and behavioral weight control produces any harm and significant evidence that combined treatment produces short-term benefit for both abstinence and weight control. However, the absence of long-term enhancement of either smoking cessation or weight control by the time-limited interventions studied to date fails to justify expenditures on weight gain prevention treatment for patients who are quitting smoking.
Acknowledgments
This review was supported in part by NIH grants P30 CA060553, R25 CA100600, and HL0756451. The funding organization had no role in the review process or preparation of this manuscript.
References
- 1.Fiore MC, Bailey WC, Cohen SJ, Dorfman SF, Goldstein MG, Gritz ER, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2000. [Google Scholar]
- 2.World Health Organization Research for International Tobacco Control. WHO report on the global tobacco epidemic, 2008: the MPOWER package. Geneva: World Health Organization; 2008. [Google Scholar]
- 3.Alberg AJ, Carter CL, Carpenter MJ. Weight gain as an impediment to cigarette smoking cessation: a lingering problem in need of solutions. Prev Med. 2007;44:296–7. doi: 10.1016/j.ypmed.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Klesges RC, Brown K, Pascale RW, Murphy M, Williams E, Cigrang JA. Factors associated with participation, attrition, and outcome in a smoking cessation program at the workplace. Health Psychol. 1988;7:575–89. doi: 10.1037//0278-6133.7.6.575. [DOI] [PubMed] [Google Scholar]
- 5.White MA, Mckee SA, O'Malley SS. Smoke and mirrors: magnified beliefs that cigarette smoking suppresses weight. Addict Behav. 2007;32:2200–10. doi: 10.1016/j.addbeh.2007.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark MM, Hurt RD, Croghan IT, Patten CA, Novotny P, Sloan JA, et al. The prevalence of weight concerns in a smoking abstinence clinical trial. Addict Behav. 2006;31:1144–52. doi: 10.1016/j.addbeh.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Cooper TV, Dundon M, Hoffman BM, Stoever CJ. General and smoking cessation related weight concerns in veterans. Addict Behav. 2006;31:722–5. doi: 10.1016/j.addbeh.2005.05.045. [DOI] [PubMed] [Google Scholar]
- 8.Pisinger C, Jorgensen T. Weight concerns and smoking in a general population: the Inter99 study. Prev Med. 2007;44:283–9. doi: 10.1016/j.ypmed.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 9.O'Hara P, Connett JE, Lee WW, Nides M, Murray R, Wise R. Early and late weight gain following smoking cessation in the Lung Health Study. Am J Epidemiol. 1998;148:821–30. doi: 10.1093/oxfordjournals.aje.a009706. [DOI] [PubMed] [Google Scholar]
- 10.Williamson DF, Madans J, Anda RF, Kleinman JC, Giovino GA, Byers T. Smoking cessation and severity of weight gain in a national cohort. N Engl J Med. 1991;324:739–45. doi: 10.1056/NEJM199103143241106. [DOI] [PubMed] [Google Scholar]
- 11.Eisenberg D, Quinn BC. Estimating the effect of smoking cessation on weight gain: an instrumental variable approach. Health Serv Res. 2006;41:2255–66. doi: 10.1111/j.1475-6773.2006.00594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kawachi I, Colditz GA, Stampfer MJ, Willett WC, Manson JE, Rosner B, et al. Smoking cessation in relation to total mortality rates in women. A prospective cohort study. Ann Intern Med. 1993;119:992–1000. doi: 10.7326/0003-4819-119-10-199311150-00005. [DOI] [PubMed] [Google Scholar]
- 13.Janzon E, Hedblad B, Berglund G, Engstrom G. Changes in blood pressure and body weight following smoking cessation in women. J Intern Med. 2004;255:266–72. doi: 10.1046/j.1365-2796.2003.01293.x. [DOI] [PubMed] [Google Scholar]
- 14.Nilsson P, Lundgren H, Soderstrom M, Fagerstrom KO, Nilsson-Ehle P. Effects of smoking cessation on insulin and cardiovascular risk factors--a controlled study of 4 months' duration. J Intern Med. 1996;240:189–94. doi: 10.1046/j.1365-2796.1996.16844000.x. [DOI] [PubMed] [Google Scholar]
- 15.Chinn S, Jarvis D, Melotti R, Luczynska C, Ackermann-Liebrich U, Anto JM, et al. Smoking cessation, lung function, and weight gain: a follow-up study. Lancet. 2005;365:1629–35. doi: 10.1016/S0140-6736(05)66511-7. [DOI] [PubMed] [Google Scholar]
- 16.Branca F, Nikogosian H, Lobstein T, World Health Organization Regional Office for Europe . The challenge of obesity in the WHO European Region and the strategies for response. Copenhagen, Denmark: WHO Regional Office for Europe; 2007. [Google Scholar]
- 17.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, et al. Varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, et al. Efficacy of varenicline, an alpha4beta2 nicotinic acetylcholine receptor partial agonist, vs placebo or sustained-release bupropion for smoking cessation: a randomized controlled trial. JAMA. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 19.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. Effect of maintenance therapy with varenicline on smoking cessation: a randomized controlled trial. JAMA. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 20.Givens JL, Datto CJ, Ruckdeschel K, Knott K, Zubritsky C, Oslin DW, et al. Older patients' aversion to antidepressants. A qualitative study. J Gen Intern Med. 2006;21:146–51. doi: 10.1111/j.1525-1497.2005.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lowe B, Schulz U, Grafe K, Wilke S. Medical patients' attitudes toward emotional problems and their treatment. What do they really want? J Gen Intern Med. 2006;21:39–45. doi: 10.1111/j.1525-1497.2005.0266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pound P, Britten N, Morgan M, Yardley L, Pope C, Daker-White G, et al. Resisting medicines: a synthesis of qualitative studies of medicine taking. Soc Sci Med. 2005;61:133–55. doi: 10.1016/j.socscimed.2004.11.063. [DOI] [PubMed] [Google Scholar]
- 23.Filozof C, Fernandez Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 24.Klesges R, Meyers AW, Klesges LM, La Vasque ME. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol Bull. 1989;106:204–30. doi: 10.1037/0033-2909.106.2.204. [DOI] [PubMed] [Google Scholar]
- 25.Danielsson T, Rossner S, Westin A. Open randomised trial of intermittent very low energy diet together with nicotine gum for stopping smoking in women who gained weight in previous attempts to quit… including commentary by Jones K. BMJ. 1999;319:490–4. doi: 10.1136/bmj.319.7208.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perkins KA, Marcus M, Levine D, D'amico D, Miller A, Broge M, et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J Consult Clin Psychol. 2001;69:604–13. [PubMed] [Google Scholar]
- 27.Marcus BH, Albrecht AE, King TK, Parisi AF, Pinto BM, Roberts M, et al. The efficacy of exercise as an aid for smoking cessation in women: a randomized controlled trial. Arch Intern Med. 1999;159:1229–34. doi: 10.1001/archinte.159.11.1229. [DOI] [PubMed] [Google Scholar]
- 28.Marcus BH, Albrecht AE, Niaura RS, Abrams DB, Thompson PD. Usefulness of physical exercise for maintaining smoking cessation in women. Am J Cardiol. 1991;68:406–7. doi: 10.1016/0002-9149(91)90843-a. [DOI] [PubMed] [Google Scholar]
- 29.Marcus BH, Albrecht AE, Niaura RS, Taylor ER, et al. Exercise enhances the maintenance of smoking cessation in women. Addict Behav. 1995;20:87–92. doi: 10.1016/0306-4603(94)00048-4. [DOI] [PubMed] [Google Scholar]
- 30.Marcus BH, Lewis BA, Hogan J, King TK, Albrecht AE, Bock B, et al. The efficacy of moderate intensity exercise as an aid for smoking cessation in women: A randomized controlled trial. Nicotine Tob Res. 2005;7:871–80. doi: 10.1080/14622200500266056. [DOI] [PubMed] [Google Scholar]
- 31.Ussher M, West R, Mcewen A, Taylor A, Steptoe A. Randomized controlled trial of physical activity counseling as an aid to smoking cessation: 12 month follow-up. Addict Behav. 2007;32:3060–4. doi: 10.1016/j.addbeh.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Hall SM, Tunstall CD, Vila KL, Duffy J. Weight gain prevention and smoking cessation: cautionary findings. Am J Public Health. 1992;82:799–803. doi: 10.2105/ajph.82.6.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pirie PL, Mcbride CM, Hellerstedt W, Jeffery RW, Hatsukami D, Allen S, et al. Smoking cessation in women concerned about weight. Am J Public Health. 1992;82:1238–43. doi: 10.2105/ajph.82.9.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spring B, Doran N, Pagoto S, Schneider K, Pingitore R, Hedeker D. Randomized Controlled Trial for Behavioral Smoking and Weight Control Treatment: Effect of Concurrent Versus Sequential Intervention. J Consult Clin Psychol. 2004;72:785–96. doi: 10.1037/0022-006X.72.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheskin LJ, Hess JM, Henningfield J, Gorelick DA. Calorie restriction increases cigarette use in adult smokers. Psychopharmacology (Berl) 2005;179:430–6. doi: 10.1007/s00213-004-2037-x. [DOI] [PubMed] [Google Scholar]
- 36.Spring B, Pagoto S, Mcchargue D, Hedeker D, Werth J. Altered reward value of carbohydrate snacks for female smokers withdrawn from nicotine. Pharmacol Biochem Behav. 2003;76:351–60. doi: 10.1016/j.pbb.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 37.Ussher M. Exercise interventions for smoking cessation. Cochrane Database Syst Rev. 2005:CD002295. doi: 10.1002/14651858.CD002295.pub2. [DOI] [PubMed] [Google Scholar]
- 38.Prapavessis H, Cameron L, Baldi JC, Robinson S, Borrie K, Harper T, et al. The effects of exercise and nicotine replacement therapy on smoking rates in women. Addict Behav. 2007;32:1416–32. doi: 10.1016/j.addbeh.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 39.Hill JS. Effect of a program of aerobic exercise on the smoking behaviour of a group of adult volunteers. Can J Public Health. 1985;76:183–6. [PubMed] [Google Scholar]
- 40.Hill RD, Rigdon M, Johnson S. Behavioral smoking cessation treatment for older chronic smokers. Behav Ther. 1993;24:321–9. [Google Scholar]
- 41.Martin JE, Calfas KJ, Patten CA, Polarek M, Hofstetter CR, Noto J, et al. Prospective evaluation of three smoking interventions in 205 recovering alcoholics: one-year results of Project SCRAP-Tobacco. J Consult Clin Psychol. 1997;65:190–4. doi: 10.1037//0022-006x.65.1.190. [DOI] [PubMed] [Google Scholar]
- 42.Albrecht AE, Marcus BH, Roberts M, Forman DE, Parisi AF. Effect of smoking cessation on exercise performance in female smokers participating in exercise training. Am J Cardiol. 1998;82:950–5. doi: 10.1016/s0002-9149(98)00511-6. [DOI] [PubMed] [Google Scholar]
- 43.Andersen LB, Klausen K, Nisbeth O. One-year effect of health counselling on life-style and risk factors for heart disease. Ugeskr Laeger. 2002;164:1814–8. [PubMed] [Google Scholar]
- 44.Becona E, Vazquez FL. Smoking cessation and weight gain in smokers participating in a behavioral treatment at 3-year follow-up. Psychol Rep. 1998;82:999–1005. doi: 10.2466/pr0.1998.82.3.999. [DOI] [PubMed] [Google Scholar]
- 45.Bock B, Marcus BH, King TK, Borrelli B, Roberts MR. Exercise effects on withdrawal and mood among women attempting smoking cessation. Addict Behav. 1999;24:399–410. doi: 10.1016/s0306-4603(98)00088-4. [DOI] [PubMed] [Google Scholar]
- 46.Buchanan L, El-Banna M, White A, Moses S, Siedlik C, Wood M. An exploratory study of multicomponent treatment intervention for tobacco dependency. J Nurs Scholarsh. 2004;36:324–30. doi: 10.1111/j.1547-5069.2004.04059.x. [DOI] [PubMed] [Google Scholar]
- 47.Clark MM, Hays J, Vickers KS, Patten CA, Croghan IT, Berg E, et al. Body image treatment for weight concerned smokers: A pilot study. Addict Behav. 2005;30:1236–40. doi: 10.1016/j.addbeh.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 48.Copeland AL, Martin PD, Geiselman PJ, Rash CJ, Kendzor DE. Smoking cessation for weight-concerned women: group vs individually tailored, dietary, and weight-control follow-up sessions. Addict Behav. 2006;31:115–27. doi: 10.1016/j.addbeh.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 49.Gomel M, Oldenburg B, Simpson JM, Owen N. Work-site cardiovascular risk reduction: a randomized trial of health risk assessment, education, counseling, and incentives. Am J Public Health. 1993;83:1231–8. doi: 10.2105/ajph.83.9.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grinstead O. Behavioral Treatment Approaches to Prevent Weight Gain Following Smoking Cessation. University of California, Los Angeles; 1982. [Google Scholar]
- 51.Grove JR, Wilkinson A, Dawson B. Exercise and the smoking withdrawal process. Sports Med News. 1991;9 [Google Scholar]
- 52.Manley RS, Boland FJ. Side-effects and weight gain following a smoking cessation program. Addict Behav. 1983;8:375–80. doi: 10.1016/0306-4603(83)90038-2. [DOI] [PubMed] [Google Scholar]
- 53.Otterstad JE. Influence on lifestyle measures and five-year coronary risk by a comprehensive lifestyle intervention programme in patients with coronary heart disease. Eur J Cardiovasc Prev Rehabil. 2003;10:429–37. doi: 10.1097/01.hjr.0000107024.38316.6a. [DOI] [PubMed] [Google Scholar]
- 54.Patten CA, Martin JE, Calfas KJ, Brown SA, Schroeder DR. Effect of three smoking cessation treatments on nicotine withdrawal in 141 abstinent alcoholic smokers. Addict Behav. 2000;25:301–6. doi: 10.1016/s0306-4603(98)00129-4. [DOI] [PubMed] [Google Scholar]
- 55.Russell PO, Epstein LH, Johnston JJ, Block DR, Blair E. The effects of physical activity as maintenance for smoking cessation. Addict Behav. 1988;13:215–8. doi: 10.1016/0306-4603(88)90016-0. [DOI] [PubMed] [Google Scholar]
- 56.Saban-Ruiz J, Fabregate Fuente R, Fabregate Fuente M, Marquez Nieto J, Bernal E. Sequential lifestyle change in patients with metabolic syndrome. Edothelial impact. Investigacion Cardiovascular. 2004;7:131–72. [Google Scholar]
- 57.Sorensen G, Stoddard A, Hunt MK, Hebert JR, Ockene JK, Avrunin JS, et al. The effects of a health promotion-health protection intervention on behavior change: the WellWorks Study. Am J Public Health. 1988;88:1685–90. doi: 10.2105/ajph.88.11.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steptoe A, Doherty S, Rink E, Kerry S, Kendrick T, Hilton S. Behavioural counselling in general practice for the promotion of healthy behaviour among adults at increased risk of coronary heart disease: randomised trial. BMJ. 1999;319:943–7. doi: 10.1136/bmj.319.7215.943. discussion 7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Taylor CB, Houston-Miller N, Haskell WL, Debusk RF. Smoking cessation after acute myocardial infarction: the effects of exercise training. Addict Behav. 1988;13:331–5. doi: 10.1016/0306-4603(88)90039-1. [DOI] [PubMed] [Google Scholar]
- 60.Tonstad S, Sundfor T, Seljeflot I. Effect of lifestyle changes on atherogenic lipids and endothelial cell adhesion molecules in young adults with familial premature coronary heart disease. Am J Cardiol. 2005;95:1187–91. doi: 10.1016/j.amjcard.2005.01.047. [DOI] [PubMed] [Google Scholar]
- 61.Ussher M, West R, Mcewen A, Taylor A, Steptoe A. Efficacy of exercise counselling as an aid for smoking cessation: a randomized controlled trial. Addiction. 2003;98:523–32. doi: 10.1046/j.1360-0443.2003.00346.x. [DOI] [PubMed] [Google Scholar]
- 62.Ward T. Using psychological insights to help people quit smoking. J Adv Nurs. 2001;34:754–9. doi: 10.1046/j.1365-2648.2001.01805.x. [DOI] [PubMed] [Google Scholar]
- 63.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 64.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5:13–25. [PubMed] [Google Scholar]
- 65.PEDro Scale (Sydney (Australia), Centre for Evidence-Based Physiotherapy).
- 66.Bhogal SK, Teasell RW, Foley NC, Speechley MR. The PEDro scale provides a more comprehensive measure of methodological quality than the Jadad scale in stroke rehabilitation literature. J Clin Epidemiol. 2005;58:668–73. doi: 10.1016/j.jclinepi.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 67.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83:713–21. [PubMed] [Google Scholar]
- 68.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis. Englewood, NJ: Biostat Inc.; 2005. [Google Scholar]
- 69.Hedges LV. Distribution theory for Glass's estimator of ES and related estimators. J Educ Stat. 1981;6:107–28. [Google Scholar]
- 70.Dersimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 71.Hedges LV, Vevea JL. Fixed- and random-effects models in meta-analysis. Psychol Methods. 1998;3:486–504. [Google Scholar]
- 72.Sutton AJ, Abrams KR, Jones DR, Sheldon TA, Song F. Methods for meta-analysis in medical research. Chichester, England: J. Wiley; 2000. [Google Scholar]
- 73.Light RJ, Pillemer DB. Summing up the science of reviewing research. Cambridge, Mass: Harvard University Press; 1984. [Google Scholar]
- 74.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosenthal R. The file drawer problem and tolerance for null results. Psychol Bull. 1979;86:638–41. [Google Scholar]
- 76.National Institute for Health and Clinical Excellence. Brief interventions and referral for smoking cessation in primary care and other settings. NICE; 2006. [Google Scholar]
- 77.West R, Mcneill A, Raw M. Smoking cessation guidelines for health professionals: an update. Health Education Authority. Thorax. 2000;55:987–99. doi: 10.1136/thorax.55.12.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.NHS Health Scotland, Ash Scotland. Smoking Cessation Guidelines for Scotland: 2004 Update. Glasgow: NHS Health Scotland; 2004. [Google Scholar]
- 79.NHS Health Scotland, Ash Scotland. Smoking Cessation Update 2007; Supplement to the 2004 Smoking Cessation Guidelines for Scotland. Glasgow: NHS Health Scotland; 2007. [Google Scholar]
- 80.Department of Health Social Services and Public Safety. Training Framework for Smoking Cessation Services in Northern Ireland. Belfast: DHSSPS; 2003. [Google Scholar]
- 81.New Zealand Ministry of Health. New Zealand Smoking Cessation Guidelines. Wellington: Minstry of Health; 2007. [Google Scholar]
- 82.Fiore MC, Jaén CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. [Google Scholar]
- 83.Baumeister RF, Vohs KD, Tice DM. The Strength Model of Self-Control. Curr Dir Psychol Sci. 2007;16:351–5. [Google Scholar]
- 84.Joseph AM, Willenbring ML, Nugent SM, Nelson DB. A randomized trial of concurrent versus delayed smoking intervention for patients in alcohol dependence treatment. J Stud Alcohol. 2004;65:681–91. doi: 10.15288/jsa.2004.65.681. [DOI] [PubMed] [Google Scholar]
- 85.Hyman DJ, Pavlik VN, Taylor WC, Goodrick GK, Moye L. Simultaneous vs sequential counseling for multiple behavior change. Arch Intern Med. 2007;167:1152–8. doi: 10.1001/archinte.167.11.1152. [DOI] [PubMed] [Google Scholar]
- 86.National Heart Lung and Blood Institute. Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults: The Evidence Report. 1998 [Google Scholar]
- 87.Summerbell CD, Waters E, Edmunds LD, Kelly S, Brown T, Campbell KJ. Interventions for preventing obesity in children. Cochrane Database ; :Syst Rev. 2005:CD001871. doi: 10.1002/14651858.CD001871.pub2. [DOI] [PubMed] [Google Scholar]
- 88.Levine MD, Klem ML, Kalarchian MA, Wing RR, Weissfeld L, Qin L, et al. Weight gain prevention among women. Obesity. 2007;15:1267–77. doi: 10.1038/oby.2007.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Simkin-Silverman LR, Wing RR, Boraz MA, Kuller LH. Lifestyle intervention can prevent weight gain during menopause: results from a 5-year randomized clinical trial. Ann Behav Med. 2003;26:212–20. doi: 10.1207/S15324796ABM2603_06. [DOI] [PubMed] [Google Scholar]
- 90.Agence Nationale D'accréditation Et D'evaluation En Santé (National Agency of Accreditation and Evaluation in Health) Texte des recommandations. Conférence de Consensus sur l'Arrêt de la consommation du tabac (Consensus Conference on smoking cessation).1998. [Google Scholar]
- 91.Department of Defense VA. VA/DoD clinical practice guideline for the management of tobacco use. Washington, DC: Department of Veteran Affairs; 2004. [Google Scholar]
- 92.Wadden TA, Butryn ML, Wilson C. Lifestyle modification for the management of obesity. Gastroenterology. 2007;132:2226–38. doi: 10.1053/j.gastro.2007.03.051. [DOI] [PubMed] [Google Scholar]
- 93.Maciosek MV, Coffield AB, Edwards NM, Flottemesch TJ, Goodman MJ, Solberg LI. Priorities among effective clinical preventive services: results of a systematic review and analysis. Am J Prev Med. 2006;31:52–61. doi: 10.1016/j.amepre.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 94.Moyer VA, Klein JD, Ockene JK, Teutsch SM, Johnson MS, Allan JD. Screening for overweight in children and adolescents: where is the evidence? a commentary by the childhood obesity working group of the US Preventive Services Task Force. Pediatrics. 2005;116:235–8. doi: 10.1542/peds.2005-0305. [DOI] [PubMed] [Google Scholar]
- 95.Spring B. Evidence-based practice in clinical psychology: what it is, why it matters; what you need to know. J Clin Psychol. 2007;63:611–31. doi: 10.1002/jclp.20373. [DOI] [PubMed] [Google Scholar]
- 96.Spring B, Pagoto S, Kaufmann PG, Whitlock EP, Glasgow RE, Smith TW, et al. Invitation to a dialogue between researchers and clinicians about evidence-based behavioral medicine. Ann Behav Med. 2005;30:125–37. doi: 10.1207/s15324796abm3002_5. [DOI] [PubMed] [Google Scholar]
- 97.Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel-group randomised trials. Lancet. 2001;357:1191–4. [PubMed] [Google Scholar]