Abstract
The relationship of movement between different muscle groups has not been quantified before in the newborn period. Cerebral palsy (CP), which often occurs as a result of perinatal hypoxia-ischemia (H-I), is categorized depending on clinical presentation, brain region involvement and extent of involvement. In order to test different brain region involvement, this study investigates individual and multi-joint involvement in a rabbit model of CP. Pregnant rabbits at 70% gestation were subjected to 40-min uterine ischemia. Newborn rabbit kits were subjected to a swim test at 5 time points over the first eleven days of life. H-I kits were divided into hypertonic and non-hypertonic groups based on muscle tone at birth. The ranges and velocity of angular movement of the fore and hind-limb joints (wrist, elbow, shoulder, ankle, knee and hip) during supported swimming were determined. Severely impaired (hypertonic) animals have significantly reduced range and angular velocity of joint motion, which do not improve over time. The non-hypertonic group showed deficits in wrist and hind-limb movements that were not evident on prolonged observation. Preventive treatment with an inhibitor of neuronal nitric oxide synthase decreased the incidence of severely impaired kits; the non-hypertonic kits showed a different pattern of swimming. Supported swimming allows quantification of limb and joint motion in the principal plane of movement in the absence of weight bearing and decreases the need for balance control. Identification and quantification of milder deficits allows mechanistic studies in the causation of H-I injury as well as estimation of recovery with therapeutic agents.
INTRODUCTION
Hypoxic-ischemic brain injury results in cerebral palsy (CP), mental retardation or learning disabilities in surviving children (Robertson and Finer, 1985). Cerebral Palsy is a heterogeneous group of neurological disorders that appear in infancy or early childhood and permanently affect body movement and muscle coordination but do not worsen over time. It is now widely recognized that the majority of cases of term neonatal encephalopathy are related to hypoxic-ischemic brain injury that occurs in utero from a variety of intrapartum conditions (Badawi et al., 1998). Hypoxia-ischemia is also associated with subsequent cerebral injury in a disproportionately high percentage of the survivors of premature birth ((Schmidt et al., 2001); (Volpe, 2001)). The incidence of CP increases dramatically with the decline in gestational age at birth, such that 4–12% of infants with a birth weight less than 1000 grams are affected (Schmidt, Davis et al. 2001).
We have generated a rabbit model of antenatal hypoxia at 70% gestation resulting in survivors that manifest early signs consistent with cerebral palsy ((Derrick et al., 2004); (Derrick et al., 2007)). This model offers the distinct advantages that, similar to the human newborn, the newborn rabbit has immature locomotor function, and the cerebral hemispheres are not fully myelinated. Uterine ischemia in the dams resulted in a distinct neurobehavioral phenotype in the newborn kits, which was characterized by an increase in forelimb tone that was significantly correlated with histological evidence of persistent injury to subcortical motor pathways involving the basal ganglia and anterior thalamus. These studies provide the first animal model in which antecedent insult during development of the immature CNS results in a constellation of hypertonic motor deficits at term birth that are consistent with those observed in CP. We have previously shown that inhibition of nNOS in this animal model decreases reactive nitrogen species (Tan et al., 2001) and decreased the incidence of severely affected rabbit kits (Ji et al., 2009).
There is a spectrum of brain injury from H-I. In our original paper (Derrick et al., 2004) the neurobehavioral testing was categorical and somewhat subjective. The new test needed to better investigate the extent of involvement that would be 1) an objective test of locomotion for the postnatal rabbit, 2) quantifiably measure individual and multi-joint involvement and 3) determine whether the motor phenotype was static over time as in the case of cerebral palsy. Rabbits are lagomorphs and are thought to be phylogeneticly related to rodents. In the wild young rabbits are born below ground and remain in a warren for several weeks. In order to elicit motor movements in young rabbits we needed to subject them to an environment that stimulates movement. Submersing the kits in water reliably induces a reflex swimming motion unlike other attempts to induce movement. Swimming provides the advantage the limb motion can be assessed without the need to bear weight. The support that water provides is routinely used in humans with muscle weakness (Altan et al., 2004, Pariser et al., 2006). It is primarily used to improve cardiovascular performance (Hutzler et al., 1998) rather than improving mobility.(Fragala-Pinkham et al., 2008) Swimming has been used to assess the effect of weightlessness on the motor development of young rat (Walton et al., 2005).
The objective of this study was to investigate the extent of involvement of brain injury and assess the dynamic nature of movement deficits following H-I. The results validate the model as one of cerebral palsy by showing the movement deficits do not improve as one of the key features of cerebral palsy is that the motor deficits are not progressive. Furthermore, most of the kits not manifesting the cerebral palsy phenotype showed some subtle deficits that could not be easily identified using the neurobehavioral tests in our previous study. Treatment with a neuronal nitric oxide synthase inhibitor resulted in amelioration of severity of motor deficits.
MATERIALS AND METHODS
Ethical approval
All of the experiments were reviewed by the Institutional Animal Care and Use Committee (IACUC) of Northshore University Healthsystems, Evanston Il, USA.
Surgery Preparation
Timed pregnant New Zealand white rabbits (Myrtle’s Rabbits, Thompson Station Tennessee), 22 day gestation (70% term, E22), were subjected to in vivo uterine ischemia as previously described. (Tan et al., 1999); (Derrick et al., 2001); (Derrick et al., 2004) Briefly, the dams were anesthetized with intravenous fentanyl (75 (µg/kg/hr) and droperidol (3.75 mg/kg/hr). Bag and mask ventilation was provided to maintain normal arterial pH (7.35–7.45), pCO2 (32–45 torr) and pO2 (70–100 torr). Spinal anesthesia with 0.75% bupivicaine was administered to the dams through a 25G spinal needle in the L2–L5 intervertebral space. The fentanyl and droperidol dose was reduced by a fifth to allow the dam to breathe spontaneously through a mask. A 4F Fogarty arterial embolectomy catheter (Baxter Healthcare Corp, Santa Ana, CA) was inserted into the left femoral artery under aseptic conditions and advanced to 10 cm, into the descending aorta to above the uterine and below the renal arteries. Inflating the balloon with 300 µL of saline resulted in uterine ischemia. The absence of a recordable blood pressure in the contralateral (right) monitored continued aortic occlusion. At the end of the procedure the balloon was deflated and the catheter removed and the contralateral blood pressure returned. The femoral artery was repaired with 7-0 sutures to return some patency and the skin closed with 3-0 sutures. The dam was returned to her cage and allowed to deliver in a nest box, at term (31.5 days). Control dams were allowed to deliver spontaneously.
Another subset of dams was administered a very specific nNOS inhibitor, cis-N1-[4’-6”-amino-4”-methylpyridin-2”-ylmethyl)-pyrrolidin-3’-yl]-N2-[2’-(3”fluorophenyl)ethyl]ethane-1,2-diamine (JI-8), 30 minutes prior to and 30 minutes after 40 minutes of uterine ischemia (Ji et al., 2009) at E22.
Neurobehavioral testing
The hypoxic-ischemic kits underwent the previously published battery of neurobehavioral tests (Derrick et al., 2004) on postnatal day 1 and were divided into those kits with obvious hypertonia or posture (severe) or those with very mild or no hypertonia plus other subtle neurobehavioral deficits (mild) or normal groups. Kits in the severe group were unable to feed from the dam so they were removed and fed in a neonatal incubator. Gavage fed kits were fed rabbit breast milk at 250cc/kg/day divided into 2 or 3 feedings based on the observation that in the nature state rabbits feed once a day (Caba et al., 2003); (Tan et al., 2005). The rabbit milk was obtained using intermittent suction applied to teats of a restrained lactating rabbit. Mild and normal kits were fed by their dam as were the control kits.
Quantification of the forced swim test
Kits were marked with water resistant markers on their toes, ankle, knee, hip, shoulder, elbow, wrist, and finger tips. They were then placed into fish tank filled with 34–36°C water in a lane bordered by a clear Perspex sheet to limit perpendicular movement (see video). This was to keep the limb at right angles to the video camera and ensure movement in one plane parallel to the camera. Kits were placed in the water and supported with simple harness to prevent them from sinking and keep them stationary. Without the harness the young or severely affected kits are unable to keep their head above the surface of the water and aspirate water. Older kits swim from one end of the tank to the other resulting in a change in the angle on the video. The harness ensures they remain in the middle of the video frame. They were videotaped after swimming was initiated and when the limbs were at right angles to the camera to limit movement towards or away from the camera. The video was recorded for approximately 60 seconds. Kits were then dried and returned to their dam or the neonatal incubator. Kits were swum on P1, P5 and P11. 80–100 frames of the video digitized. The animals treated with the nNOS inhibitor were swum on P1 and P5. The digitized video was assessed using a custom program written using Matlab software. Each frame was assessed and the joint positions marked manually. The joint angles (degrees) for each frame were then calculated from the joint positions. (Figure 1B control and Figure 1C severely affected animal) The distribution was assessed and the start and end of each stroke marked manually to exclude regions where the kit was not swimming. The average of maximal, minimal, range, mean and median angle (degrees) and angular velocity (degrees/second) of each joint were calculated from at least 5–10 complete strokes and the stroke frequency (strokes/second) was also assessed for both limbs. The maximum and minimum angles were chosen to define maximal extension and flexion as these were hypothesized to be affected. The mean and median angle were chosen as measures of the mid joint motion. In severely affected animals differences between extensor and flexor tone could change the mid point of joint motion.
Figure 1. Rabbit kit Swimming.
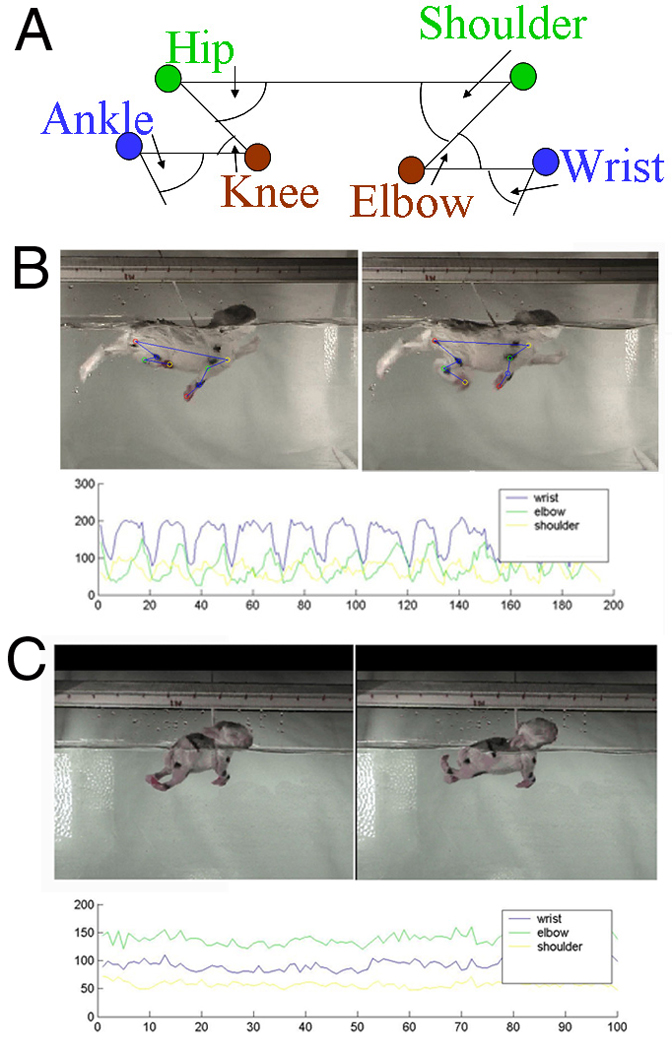
A Cartoon showing the joint positions and angles B Control kit still pictures showing one control kit swimming with the cartoon superimposed on the still picture. Below is the raw data output from the control kit. C Severely affected kit Swimming A Still pictures of a severely effected newborn rabbit kit. Below is the raw data output from severely effected animal.
To further refine the analysis of the joint motion, the swimming stroke was analyzed on a frame by frame basis and the joint angles compared between two joints on the same limb, for example the ankle and knee. The means of these values were obtained for all the recorded strokes.
Statistical testing
The maximum, minimum, mean and median joint angles were compared using a Wilcoxon test. Individual values of each rabbit kit were compared first between control animals and severe and then controls and mildly affected kits (non-hypertonic). Values were expressed as means±SEM. Comparisons were done by two-sample t-test. Two-joint values were expressed 5th and 95th percentiles (Figure 8). JI-8 treated animals were compared to controls and mildly affected (Figure 10) kits by ANOVA with post-hoc comparison of means with Bonferroni correction. All statistical analyses were done with SAS version 9.1 for Windows.
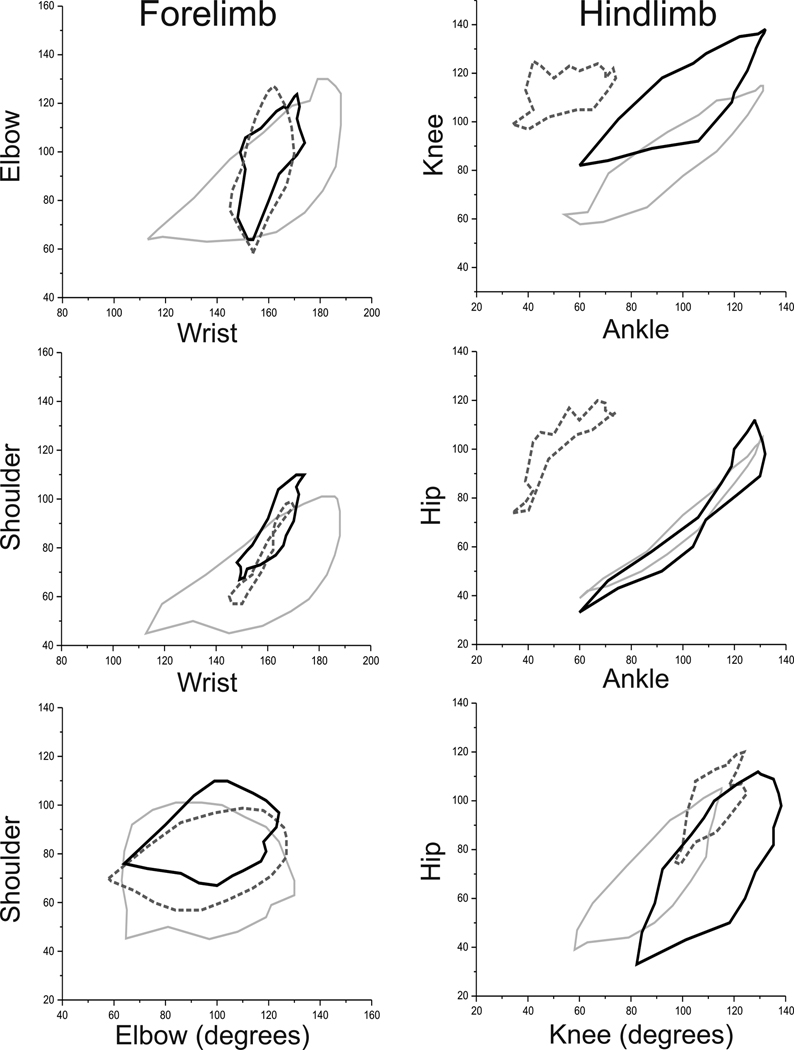
Figure 8. Two Joint Plots Comparing JI 8 treated kits with Control and Mild Groups at P1.
On cursory behavioral examination, JI-8 kits almost look normal. On two joint plots, JI-8 treated kits revealed subtle deficits but did show improvement towards control in the hind limbs compared to the mildly group. However, the two joint plots also show that the JI-8 treated kits behaved differently from both the control and mild kits in some respects. Control kits are shown in light gray, hypoxia with dashed line and JI-8 kits with black line.
RESULTS
Normal Postnatal Motor Development of the Newborn Rabbit
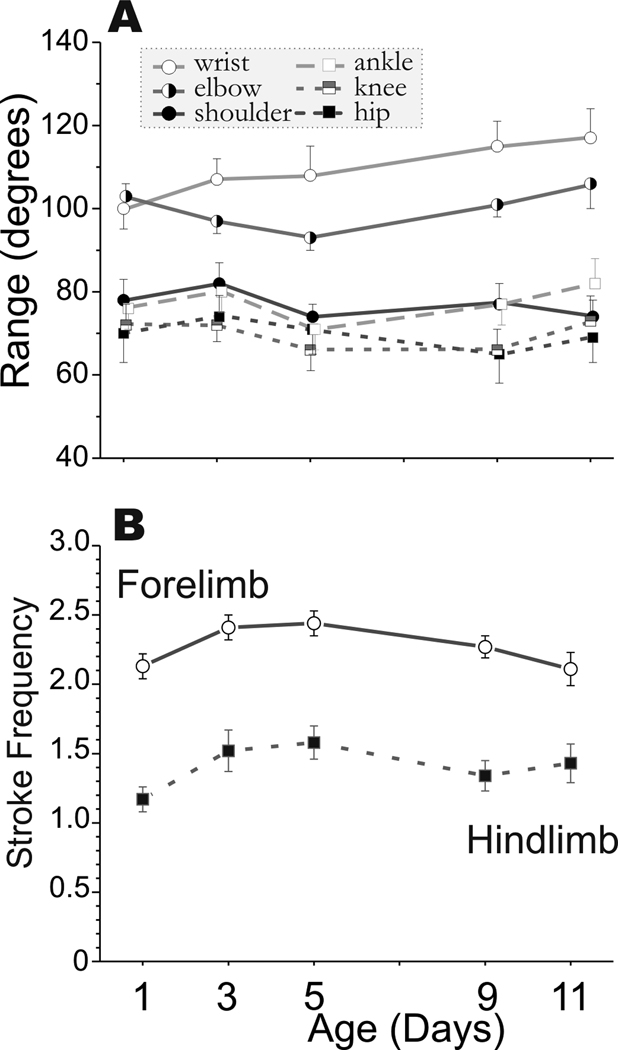
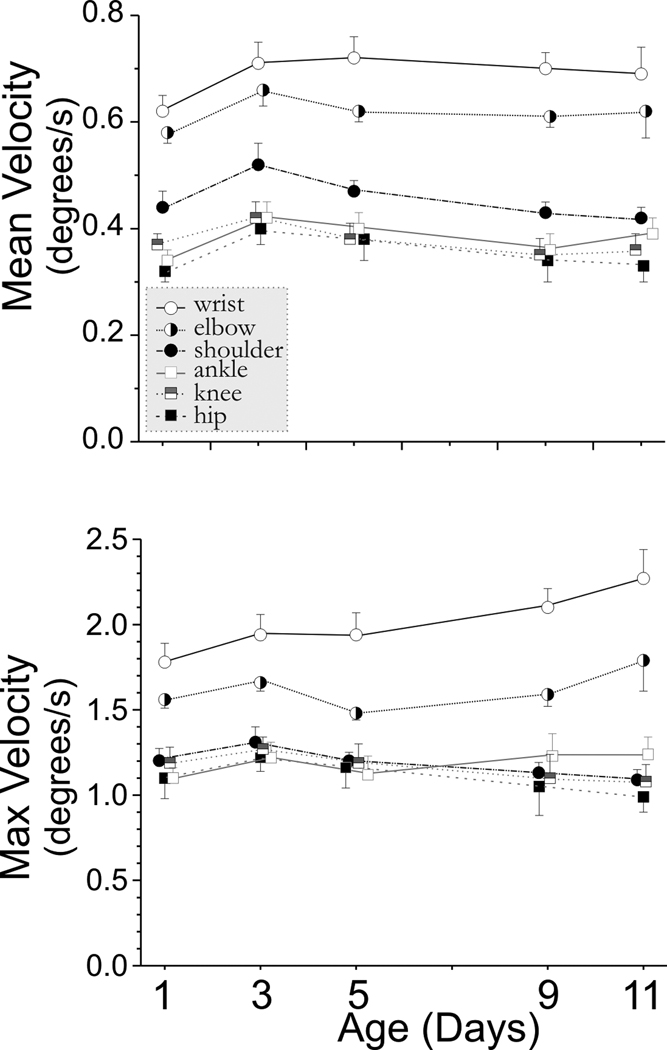
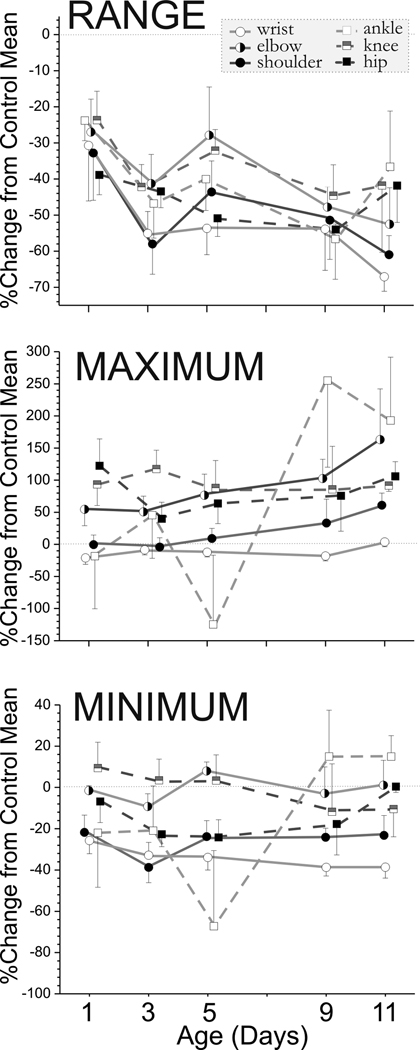
We systematically investigated the development of newborn rabbit kits at P1, P3, P5, P9, and P11. Control non-hypoxic kits showed an increase in the range of joint movement from P1 to P11 (see Figure 2a). This increase in range was largely due to increased flexion as shown by a decrease in the minimal joint angle (supplemental materials Figure 1). The joint motion was symmetrical as the mean joint angle (supplemental materials Figure 1) was equal to the median angle (data not shown). The wrist and elbow joints had the greatest range of motion. The ankle, knee, shoulder and wrist were able to flex more over time. We had previously observed that the forelimbs moved more than the hind limbs, and the tone was also higher in the forelimbs (Derrick et al., 2004). This discrepancy was reflected in the stroke frequency on the swimming test in control kits (Figure 2b). The forelimbs moved faster than hind limbs, and the stroke frequency increased sharply from P1 to P3 and then decreased to P1 values by P11 as compared to the hind limbs stroke frequency, which remained constant from P3 to P11. The change in mean and maximum velocity over time is seen in Figure 3. These results show that quantifiable parameters of joint movement can be assessed for normal newborn rabbit motor performance.
Figure 2. Range of angular Joint Motion in Control Animals from P1-P11.
Rabbit kit joint motion during swimming was video taped on day 1,3,5,9 and 11 of life. Fourteen control kits from 3 litters were studied. Joint position and angles were calculated from 80–100 frames of digitized video. A Matlab program was used to calculate the range, mean, minimum and maximum angles for the 3 major joints in the fore (wrist, elbow, shoulder) and hind limbs (ankle, knee, hip). Panel A the mean ± SEM for the range. The range increases are due to increased flexion. The decrease in minimum and mean angles is shown in Supplemental Figure 1. The forelimb joints are represented by circles and the hind limb by squares. Some of the symbols have been offset slightly at each time point for clarity. Panel B shows the stroke frequency is different in fore- and hind limbs. The forelimb is represented by circles and the lower limb by squares.
Figure 3. Angular velocity in Control Animals from P1–P11.
Rabbit kit joint motion during swimming was video taped on day 1,3,5,9 and 11 of life (n=14 control kits). Joint position and angles were calculated from 80–100 frames of digitized video. A Matlab program was used to calculate the range, mean, minimum and maximum angular velocity (degrees/sec) for the 3 major joints in the fore (wrist, elbow, shoulder) and hind limbs (ankle, knee, hip). This figure shows the mean± SEM for the mean and maximum angular velocity. The forelimb is represented by circles and the hindlimb by squares. Some of the symbols have been offset slightly at each time point for clarity.
Two-Joint Analysis
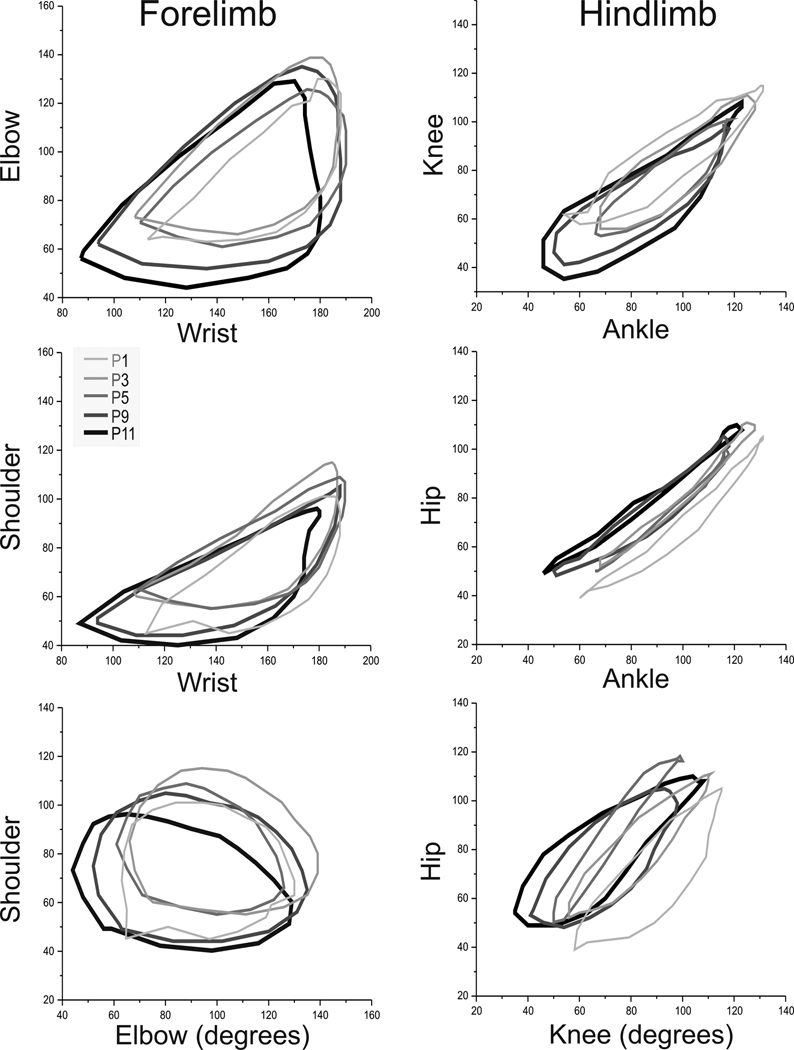
We next investigated whether the relationship of multiple muscle groups changed over time by plotting the motion of one joint to another. The two-joint analysis of joint angle was conducted separately for fore- and hind limbs. Over time, the two joint analysis revealed a slight directional shift with increasing age as seen in Figure 4. This shift is different for the forelimbs compared to hind limbs. The maturational shift increases with age for the hip but decreases for all other joints. We next wanted to investigate the swimming behavior of the affected groups following hypoxia-ischemia subdivided into severe and mild on the neurobehavioral scoring system.
Figure 4. Two joint plots of control rabbit kits from P1–P11.
Rabbit kit joint motion during swimming was video taped on day 1,3,5,9 and 11 of life (n=14 control kits). The 80–100 frames of digitized swimming video were used to plot the angles of joints in the fore- and hind limbs and then mean angles obtained for each animal. Only mean values are shown for clarity for each pair of joints on day 1,3,5,9 and 11 (3 pairs each for fore- and hind limb).
P1 Light gray thin line, P3 Dark-light gray and thicker line, P5 Dark gray, P9 Dark-dark gray, P11 Black and thick line.
Panel A Wrist-Elbow, Panel B Wrist-Shoulder Panel C Elbow-Shoulder, Panel D Ankle-Knee, Panel E Ankle Hip and Panel F Knee-Hip.
There is a maturational shift to lesser angles in all joints except the hip with increasing age from P1 to P11.
Effect of Hypoxia on Joint Motion in the Severely Affected Kits
In the severe group, newborn kits were clearly different from controls and a representative of the severe group is shown in Figure 1C. There was a significant and obvious decrease in the range of joint motion (Figure 5). The swimming test revealed that the differences were due to decreased maximal extension in the wrist, hip and ankle and decreased flexion at the elbow (Figure 5). We had previously observed that the limbs in the most severely affected animals hardly moved and what movement was observed was due to movement of the back. This compensatory trunk movement sometimes obscured our ability to discern the movement of the limbs. With the swimming test the full action of the limbs could be determined (see video). In the severe group, the angular velocity for each joint was different from controls at different ages (see Table 1). From Figure 1c it can be seen that it is difficult to define the start and end of the swimming stroke which makes calculation of the frequency problematic. The severe group animals were easy to distinguish from the control animals.
Figure 5. Difference in range maximum and minimum joint angles over time between control and severely affected kits from P1–P11.
The severely hypoxia kits were swum on Day 1–11 and the joint range, maximum angle and minimum angles were compared to the mean of the control kits at each time point. The mean± SEM of the percentage decrease of the severely effected kits compared to controls are shown over time (n= 14 control kits, n=7 severe kits but only 2 survived to p11). A line that would indicate no change is shown zero difference on the y axis. The differences persist with age, indicating the non-changing nature of the motor deficits in the severe group.
Table 1. Hypertonic Group Vs Control Angular Velocity from P1-P11.
Table shows age at which the joint showed significant differences from control
| Joint | Range | Minimum Velocity |
Maximum Velocity |
Mean Velocity |
Median Velocity |
|---|---|---|---|---|---|
| Wrist | 3,5,7,11 | 1,3,5 | 3,5,9,11 | 1,3,5,9,11 | 1,3,5,9,11 |
| Elbow | 1,3,5 | 1,3,5,9 | 1,3,5 | 1,3,5,9 | 1,3,5,9,11 |
| Shoulder | 1,3,5 | 1,3,6,9 | 1,3,5,11 | 1,3,5,9,11 | 1,3,5,9,11 |
| ankle | 3 | 3 | 3 | ||
| knee | 3,5 | 5 | 3,5 | ||
| hip | 3,5 | 3,5 | 1,3,5,11 | 1,3,5 |
Effect of Hypoxia on Joint Motion in the Mildly Affected Kits
We combined kits showing either single or multiple subtle neurobehavioral deficits in the mildly affected group. Some of these kits were hard to score on the ordinal scale as being abnormal in our previous neurobehavioral scoring system developed for newborn kits (Derrick et al., 2004) and now accepted by others (Saadani-Makki et al., 2008). Some of these kits showed subtle motor abnormalities. Sub-analysis of the mild group on the swimming test showed significant deficits compared to controls. The most sensitive deficit was the range of joint motion in the wrist and ankle on day 1. Figure 6 shows the comparison of joint angles using 95% confidence intervals. Panels A–C show the changes in the forelimb and show that the wrist is the primary joint that is affected in the forelimb. Panels D–E show the hind-limb show that all three joints are affected. These results showed that the two-joint analysis was a powerful tool in investigating subtle motor abnormalities.
Figure 6. Two joint plots of control and mild group at P1.
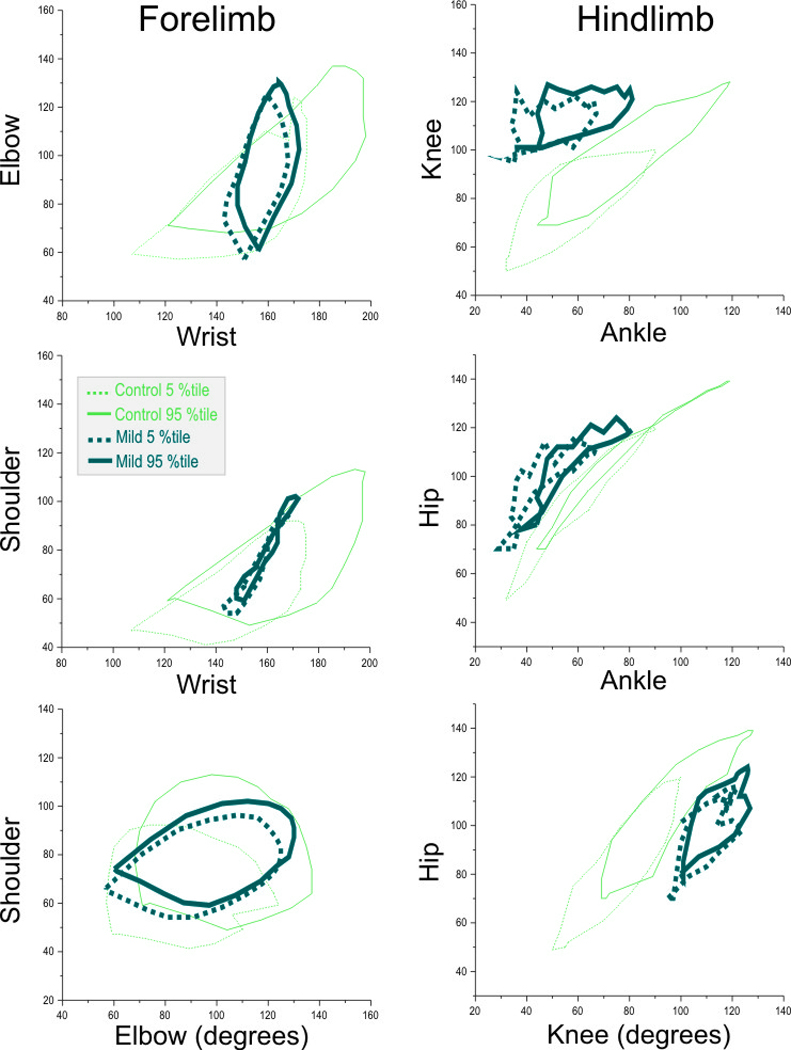
Plots of the 3 pairs of joints in the fore- and hind limbs of P1 kits in the control and mildly affected group (n=18 control, n=9 mild group). The plots depict the 5th and 95th percentiles. Panel A Wrist-Elbow, Panel B Wrist-Shoulder Panel C Elbow-Shoulder, Panel D Ankle-Knee, Panel E Ankle Hip and Panel F Knee-Hip. Using the two-joint analysis, one can easily identify the mildly affected kits. In the forelimb, the wrist is predominantly affected and thus Panels A and B are useful in identifying the mild group, while in the hind limb, there are deficits in all joints and thus all Panels D–F show clear cut differences.
We were only able to follow a subset of very mildly affected newborn kits from postnatal day 1 to 11 following intrauterine hypoxia-ischemia. The kits in this group were fed by their mothers and did not include the severely or mild-moderately affected newborn kits principally because the intensive care needed for these kits was too labor-intensive. The two-joint analysis for the subgroup of mildly affected animals that were studied at ages 1, 5 and 11 days are shown in the supplemental materials. (supplemental Figure 2) This group showed normalization over time.
Effect of nNOS Inhibitor on Joint Motion in the Mildly Affected Kits
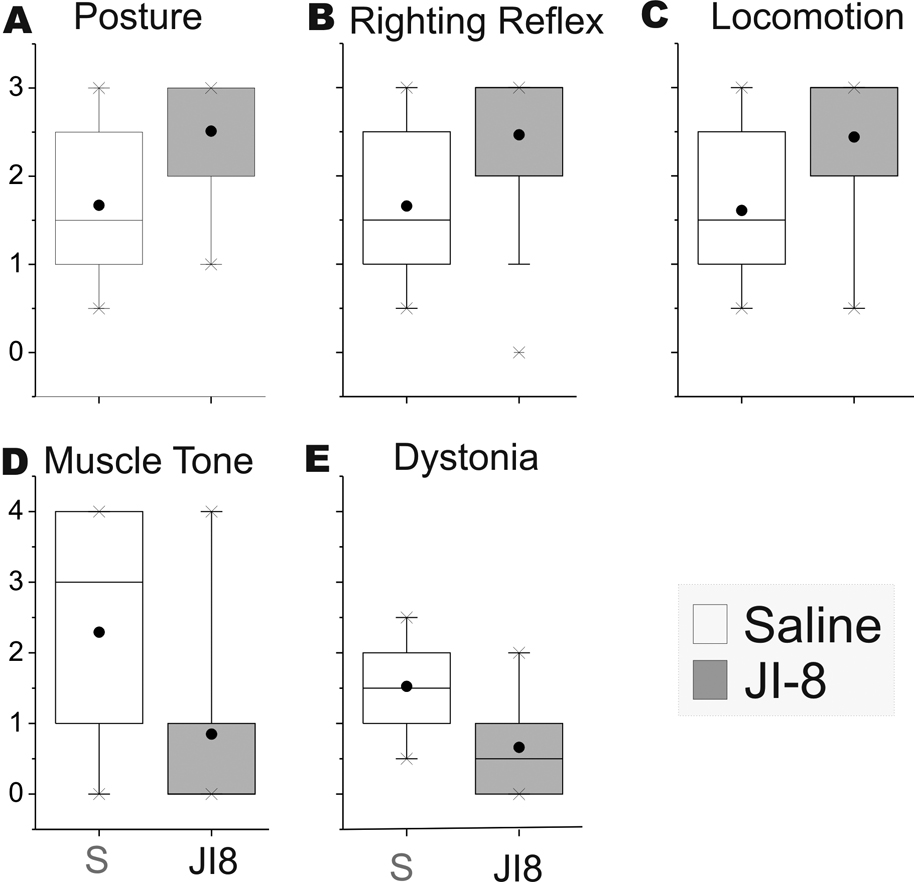
We next investigated whether treatment with a neuroprotective agent would improve motor performance following hypoxia-ischemia. Administration of the nNOS inhibitor resulted in significant decrease in the number of perinatal deaths to 9% from 39% in the saline control group (overall n=46, 69 respectively). Of the survivors, the number of severely affected kits decreased from 45% to 21% in the JI-8 group. These results are similar to our published (Ji et al., 2009) outcomes with the same nNOS inhibitor. JI-8 treatment also resulted in improvement in posture, righting reflex and locomotion scores and a decrease in hypertonia and dystonia scores (Figure 7 A–E). We next investigated whether the mildly affected JI-8 treated group showed any differences with the forced swim test in the mildly affected kits. We chose a cohort of newborn kits that were not categorized as severe following antenatal maternal treatment with JI-8. This selected population would thus include some of the animals that would either have been severe or dead if no treatment was given. In the mildly affected kits in the JI-8 treated group, the pattern of swimming was not exactly similar to that in the saline group (Figure 8). There was an increase in the stroke frequency in the forelimb but the minimum angle was greater, indicating that the stroke was smaller. This would suggest that the quantitative differences in the swimming test could reveal potential avenues of investigation of the mechanisms of protection.
Figure 7. Neurobehavioural testing of P1 animals comparing saline and nNOS inhibitor treated mild groups.
A–E: Box plots of 5 components of the neurobehaviuoral testing Posture, righting reflex, Locomotion score, muscle tone and dystonia. Normal control animals score 3 on posture, righting reflex and locomotion and 1 on muscle tone and dystonia. The JI-8 treated animals are closer to controls than the saline treated animals. F. On the swim test, the angular velocity decreased after the Dam was treated with a potent nNOS inhibitor, JI-8. The x axis shows the joint and treatment with P1 kits in white and P5 in hatched bars. The Y axis shows maximum angular velocity of the joint.
DISCUSSION
In the study of hypoxia-ischemic brain injury to the developing brain, animal models that show hypertonic deficits are needed. Evaluation of the deficits over time is essential to understand the evolution of injury and recovery. Understanding the relationship of different muscle groups in movement disorders is also essential for translation from the animal model to human infants. Our laboratory has developed a rabbit model that shows hypertonia at birth. This paper showed for the first time that the movement disorder in newborn rabbit kits can be not only quantified by a simple non-invasive test that does not harm the kits, but analysis of multi-joint movement reveals the details of the motor deficits. The testing allows longitudinal assessment in individual kits using quantitative markers and can be used to study evolution of joint motion in hypertonic kits. This swimming test complements the previous neurobehavioral scoring system devised by us (Derrick et al., 2004). The major improvement was the sophisticated analysis of limb movements not influenced by trunk movements. This paper also showed that minor deficits can be discovered and quantified. More importantly, the pattern of injury was different in different kits, which implies that different pathways or regions of the brain were affected in individual kits. In the severely hypertonic animals we quantified deficits of voluntary movement which complement the qualitative tone deficits previously shown (Derrick et al., 2004). These deficits were found in both the absolute angles and the angular velocity. The deficits were more marked in the forelimb compared to the hind-limb. The deficits did not show significant recovery over the first 11 days of life, and the non-changing nature of the deficits further validates our model as one of cerebral palsy.
Quantification of joint motion has been performed extensively in rodents (Balduini et al., 2000, Grow et al., 2003). This quantification sometimes includes elements of cognition, tests the integration of the sensory and motor systems, or looks at the limb as a whole. Fischer et al (Fischer et al., 2002) performed an analysis of joint motion that was similar to our analysis in multiple species using serial X-rays, which is much more expensive and time consuming and furthermore, was not looking at injured animals. In addition, weight bearing will have an influence on movement when there is muscle weakness. Walton et al (Walton et al., 2005) used swimming rats in a similar manner to our rabbit kits but their injury was prolonged weight zero gravity. Using water to reduce weight bearing has also been performed in human patients with cerebral palsy (Galli et al., 2007) and after strokes (Kamper et al., 2001). Our swimming test is a quantifiable test of voluntary limb motion in the absence of weight bearing that allows evaluation of each joint individually.
When normal animals from non-water dwelling species are placed in water, the reflex action is to swim and attempt to escape the water and keep their head out of the water. The animals will use their maximum effort in an attempt to escape, thus allowing quantification of voluntary joint movement. Since any new test needs to define normal development, we show in rabbits that the joint motion of the limbs was symmetrical and increases in range over time. We also showed that the movement of the fore- and hind- limbs was separate. Forced swimming has been used for 30 years as a screening test for depression (Porsolt et al., 1977) and efficacy of antidepressant medications. The major differences are that in this paper we were looking at the initial period of vigorous swimming where as in the depression literature the test waits until vigorous swimming ceases.
In the hypoxia exposed animals that were in the mild group on Day 1 this modified forced swim test allows more in-depth analysis of subtle deficits. These animals did not have severe tone problems and the motor deficits were mild or subtle. This group was more heterogeneous than the severely hypertonic animals. The two-joint analysis showed that the largest effects were on the hind-limb and the wrist joint. We would speculate that this was due to the relative importance of those joints to the rabbit which would imply that a larger portion of the rabbit brain was dedicated to their movement. As shown in Figure 7, the hind-limb movement was more disordered than the forelimb. This implies that the injury in the brain is not limited to the motor cortex and the coordination of limb motion is multifaceted.
An area of future study will be the effect of training on the ability to perform the swimming task. It has been shown that forced swimming of rats for 15 minutes will result in rapid histone modification (Chandramohan et al., 2008). A 12-week program of aquatic exercise in children with a spectrum of neurodevelopmental disabilities resulted in improved endurance but no significant improvement in mobility(Fragala-Pinkham et al., 2008) Given that the kits were only submerged for 60–90 seconds every other day, it is probably unlikely that repetitive testing resulted in improvement in joint mobility due to training, but this will be investigated in the future.
This paper also shows for the first time the effect of neuroprotectants on joint motion. Inhibition of neuronal nitric oxide synthase during the acute period of hypoxia-ischemia by antenatal maternal administration decreases the incidence of severe hypertonia and dead animals (Ji et al., 2009). We show for the first time that JI-8 also changes the pattern of swimming in animals that were not severely hypertonic and were mildly affected. This change in pattern implies that different areas of the brain will have different patterns of protection based on the expression of nNOS within each region. It is speculated that JI-8 affects motor control more than motor strength, or more central than peripheral mechanisms. The swimming screening method could give clues as to the site of neuroprotection of other agents.
In summary this paper presents the first in-depth analysis of the relationship of multiple motor groups in the newborn period and suggests a new way of investigating the motor deficits of cerebral palsy patients. We present the swimming test as a new test of joint motion in the absence of weight bearing. Our findings validate the previous neurobehavioral deficits in our rabbit model of antenatal hypoxic-ischemia as one mimicking the deficits of cerebral palsy. The main advantage of using quantifiable measures of motor deficits in both fore-and hind limbs allows the delineation of the motor deficits and suggests possible involvement of different brain regions. Such analysis will allow the use of tailor-made physical therapy and rehabilitation programs. Furthermore, not only can we quantifiably measure the improvement by neuroprotectants, we show that the neuroprotectant JI-8 probably acts in a specific manner to maintain motor function.
Supplementary Material
Abbreviations
- CP
Cerebral palsy
- H-I
Hypoxia-ischemia
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Altan L, Bingol U, Aykac M, Koc Z, Yurtkuran M. Investigation of the effects of pool-based exercise on fibromyalgia syndrome. Rheumatol Int. 2004;24:272–277. doi: 10.1007/s00296-003-0371-7. [DOI] [PubMed] [Google Scholar]
- Badawi N, Kurinczuk JJ, Keogh JM, Alessandri LM, O'Sullivan F, Burton PR, Pemberton PJ, Stanley FJ. Antepartum risk factors for newborn encephalopathy: the Western Australian case-control study. Bmj. 1998;317:1549–1553. doi: 10.1136/bmj.317.7172.1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balduini W, De Angelis V, Mazzoni E, Cimino M. Long-lasting behavioral alterations following a hypoxic/ischemic brain injury in neonatal rats. Brain Res. 2000;859:318–325. doi: 10.1016/s0006-8993(00)01997-1. [DOI] [PubMed] [Google Scholar]
- Caba M, Rovirosa MJ, Silver R. Suckling and genital stroking induces Fos expression in hypothalamic oxytocinergic neurons of rabbit pups. Brain Res Dev Brain Res. 2003;143:119–128. doi: 10.1016/s0165-3806(03)00064-6. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y, Droste SK, Arthur JS, Reul JM. The forced swimming-induced behavioural immobility response involves histone H3 phosphoacetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-D-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur J Neurosci. 2008;27:2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- Derrick M, Drobyshevsky A, Ji X, Tan S. A model of cerebral palsy from fetal hypoxia-ischemia. Stroke. 2007;38:731–735. doi: 10.1161/01.STR.0000251445.94697.64. [DOI] [PubMed] [Google Scholar]
- Derrick M, He J, Brady E, Tan S. The in vitro fate of rabbit fetal brain cells after acute in vivo hypoxia. J Neurosci. 2001;21:RC138. doi: 10.1523/JNEUROSCI.21-07-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derrick M, Luo NL, Bregman JC, Jilling T, Ji X, Fisher K, Gladson CL, Beardsley DJ, Murdoch G, Back SA, Tan S. Preterm fetal hypoxia-ischemia causes hypertonia and motor deficits in the neonatal rabbit: a model for human cerebral palsy? J Neurosci. 2004;24:24–34. doi: 10.1523/JNEUROSCI.2816-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer MS, Schilling N, Schmidt M, Haarhaus D, Witte H. Basic limb kinematics of small therian mammals. J Exp Biol. 2002;205:1315–1338. doi: 10.1242/jeb.205.9.1315. [DOI] [PubMed] [Google Scholar]
- Fragala-Pinkham M, Haley SM, O'Neil ME. Group aquatic aerobic exercise for children with disabilities. Dev Med Child Neurol. 2008;50:822–827. doi: 10.1111/j.1469-8749.2008.03086.x. [DOI] [PubMed] [Google Scholar]
- Galli M, Cimolin V, Valente EM, Crivellini M, Ialongo T, Albertini G. Computerized gait analysis of botulinum toxin treatment in children with cerebral palsy. Disabil Rehabil. 2007;29:659–664. doi: 10.1080/09638280600948136. [DOI] [PubMed] [Google Scholar]
- Grow JL, Liu YQ, Barks JD. Can lateralizing sensorimotor deficits be identified after neonatal cerebral hypoxia-ischemia in rats? Dev Neurosci. 2003;25:394–402. doi: 10.1159/000075665. [DOI] [PubMed] [Google Scholar]
- Hutzler Y, Chacham A, Bergman U, Szeinberg A. Effects of a movement and swimming program on vital capacity and water orientation skills of children with cerebral palsy. Dev Med Child Neurol. 1998;40:176–181. doi: 10.1111/j.1469-8749.1998.tb15443.x. [DOI] [PubMed] [Google Scholar]
- Ji H, Tan S, Igarashi J, Li H, Derrick M, Martasek P, Roman LJ, Vasquez-Vivar J, Poulos TL, Silverman RB. Selective neuronal nitric oxide synthase inhibitors and the prevention of cerebral palsy. Ann Neurol. 2009;65:209–217. doi: 10.1002/ana.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamper DG, Schmit BD, Rymer WZ. Effect of muscle biomechanics on the quantification of spasticity. Ann Biomed Eng. 2001;29:1122–1134. doi: 10.1114/1.1424918. [DOI] [PubMed] [Google Scholar]
- Pariser G, Madras D, Weiss E. Outcomes of an aquatic exercise program including aerobic capacity, lactate threshold, and fatigue in two individuals with multiple sclerosis. J Neurol Phys Ther. 2006;30:82–90. doi: 10.1097/01.npt.0000282572.63297.3d. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- Robertson C, Finer N. Term infants with hypoxic-ischemic encephalopathy: outcome at 3.5 years. Dev Med Child Neurol. 1985;27:473–484. doi: 10.1111/j.1469-8749.1985.tb04571.x. [DOI] [PubMed] [Google Scholar]
- Saadani-Makki F, Kannan S, Lu X, Janisse J, Dawe E, Edwin S, Romero R, Chugani D. Intrauterine administration of endotoxin leads to motor deficits in a rabbit model: a link between prenatal infection and cerebral palsy. Am J Obstet Gynecol. 2008;199(651):e1–e7. doi: 10.1016/j.ajog.2008.06.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt B, Davis P, Moddemann D, Ohlsson A, Roberts RS, Saigal S, Solimano A, Vincer M, Wright LL. Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med. 2001;344:1966–1972. doi: 10.1056/NEJM200106283442602. [DOI] [PubMed] [Google Scholar]
- Tan S, Bose R, Derrick M. Hypoxia-ischemia in fetal rabbit brain increases reactive nitrogen species production: quantitative estimation of nitrotyrosine. Free Radic Biol Med. 2001;30:1045–1051. doi: 10.1016/s0891-5849(01)00499-3. [DOI] [PubMed] [Google Scholar]
- Tan S, Drobyshevsky A, Jilling T, Ji X, Ullman LM, Englof I, Derrick M. Model of cerebral palsy in the perinatal rabbit. J Child Neurol. 2005;20:972–979. doi: 10.1177/08830738050200120801. [DOI] [PubMed] [Google Scholar]
- Tan S, Zhou F, Nielsen VG, Wang Z, Gladson CL, Parks DA. Increased injury following intermittent fetal hypoxia-reoxygenation is associated with increased free radical production in fetal rabbit brain. J Neuropathol Exp Neurol. 1999;58:972–981. doi: 10.1097/00005072-199909000-00007. [DOI] [PubMed] [Google Scholar]
- Volpe JJ. Neurobiology of periventricular leukomalacia in the premature infant. Pediatr Res. 2001;50:553–562. doi: 10.1203/00006450-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Walton KD, Benavides L, Singh N, Hatoum N. Long-term effects of microgravity on the swimming behaviour of young rats. J Physiol. 2005;565:609–626. doi: 10.1113/jphysiol.2004.074393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.